Abstract
Sitophilus zeamais and Prostephanus truncatus can cause weight loss of about 20 to 90% of untreated stored maize seeds. This study assesses four plants (Lantana camara, Moringa oleifera, Citrus sinensis and Hyptis suaveolens) extracts as seed maize protectants against the two insects in Ghana. The study was laid out in a CRD with three replications. Dried powders (5 and 10% w/w) and aqueous extracts (0.05 and 0.1 g/mL) of the botanicals were evaluated for their insecticidal activity with untreated control and Actellic included as checks. Oviposition and survivorship of insects decreased in grains treated with plant extracts. The phytochemical analysis revealed that compounds such as alkaloids, saponins, tannins and phenolic, steroids, flavonoids, anthraquinones, phlobatannins, cardiac glycosides and terpenoids were recorded in all four plant extracts. These compounds may have caused lower progeny emergence, inhibitory effect, repellent action and antifeedant effect to S. zeamais and P. truncatus in grains treated with the botanicals. Maize seeds treated with botanicals after 10 weeks in cribs recorded a reduction in the percentage of seeds damaged and weight loss caused by the two insects compared to the untreated seeds. The study proposes that the botanicals tested, especially H. suaveolens have the potential to enhance quality seed production thereby boosting growth in the seed industry. The botanicals are recommended for use by seed producers and farmers to control P. truncatus and S. zeamais in stored maize seeds.
PUBLIC INTEREST STATEMENT
The adverse effect of the application of chemical pesticides on the environment, human and non-target organisms has necessitated the search for alternative management strategies such as botanicals. Several botanicals have been tested and proven to be effective in controlling post- harvest pests. These botanicals are harmless on human health and environment by reducing the chemical remains on food grains as in the case of pesticides. The current study shows that extracts from Lantana camara, Moringa oleifera, Citrus sinensis and Hyptis suaveolens are effective in controlling Sitophilus zeamais and Prostephanus truncatus, which are the two most important storage insect pests of maize. The botanicals tested, especially H. suaveolens have the potential of enhancing quality seed production thereby boosting growth in the seed industry.
1. INTRODUCTION
Maize (Zea mays L.) is a major cereal grain crop in terms of output. World-wide production is predicted to increase by nearly 370 MT through the subsequent 10 years, considering a growth of 15% by 2023 (OECD/Food and Agriculture Organization of the United Nations, Citation2014). By 2050, the global demand for maize could rise by 50% as reported by Ignaciuk (2014). The increasing maize demand and its global advance indicates that by 2023, maize will account for the greatest share (34%) of the total harvested crop area (OECD/Food and Agriculture Organization of the United Nations, Citation2014). Africa produces around 7% of the total world production (FAOSTAT, Citation2014; Verheye, Citation2010). FAO (Citation2015) also reported that West African countries have experienced an increased total land area cultivated to maize from 3.2 to 8.9 million ha “between” 1961 to 2005. In Ghana, there is a general increase in the area under maize cultivation which stands at 970,000 ha as at 2017 according to MoFA (NDPC, Citation2018). In 2020, maize was estimated to increase to 3,071,000 tonnes in Ghana (FAO, Citation2021). Maize plays an enormous role in the sub-region as it has substituted other staple crops such as sorghum and millet in terms of quantity consumed per household and also, has been a main source of income for smallholder farmers (Smith et al., Citation1997). NDPC (Citation2018) reported that the trend of productivity of maize in Ghana as at 2016 was 1.99 MT/Ha which increased slightly to 2.05 MT/Ha in 2017. The Grains and Legumes Development Board in view of this, under the national seed support produced 55 tons of foundation seed maize and processed 500 tons of certified maize seed to address the low maize productivity issue (ISSER, Citation2007). Despite this intervention at the production level, there is evidence of seed and food insecurity arising from storage losses. One of the elements contributing to high storage losses is the problem of storage insect pests such as the maize weevil, Sitophilus zeamais (Motschulsky); rice weevil, Sitophilus oryzae (L.); Angoumois grain moth, Sitotroga cerealella (Olivier); and the larger grain borer, Prostephanus truncatus (Horn) (Tefera et al., Citation2011). They can cause 20–40% losses during cultivation and 30–90% postharvest and storage losses (Odendo et al., Citation2001). In West Africa, an estimated 25–40% of grain crop is lost in shops every 12 months due to weevil menace (Costa, Citation2014). The insects have the capacity to infest intact kernels and they are known as primary storage pests of maize (Throne & Eubanks, Citation2002). Maize weevil, show cosmopolitan distribution, occurring in numerous warm and humid regions worldwide (López-Castillo et al., Citation2018. According to Markham et al. (Citation1994); CAB International (Citation2005), both adult weevils and larvae feed on undamaged grains and reduce them to powder. The pest creates holes in whole previously undamaged grains causing the grain to lose its viability and market value. The seed whose germ has been attacked will not germinate. The main effect of S. zeamais infestation on stored grains is the damage through feeding activities of the adult weevils and the development of immature stages within the grain (Longstaff, Citation1981). The use of stored grains as seeds accounts for almost 80% of the seeds used by small-scale farmers (Crissman et al., Citation1993; Louwaars & De Boef, Citation2012). The larger grain borer (LGB) Prostephanus truncatus Horn is the single most important field and storage pest of dried cassava and maize in Africa (Farrell & Schulten, Citation2002). The LGB causes a wide range of grain losses in maize, which include: weight loss, nutritional loss, loss in grain quality, loss in seed viability and loss of commercial value (McFarlane et al., Citation1990). Postharvest losses in susceptible varieties can range from 40 to 100% (CIMMYT, Citation1999; Denning et al., Citation2009; Mushi, Citation1990). However, according to APHLIS (Citation2015), in Africa, between 2003 and 2014 postharvest weight loss of maize ranged from 16.8 to 19.9%. Many farmers sell their produce just after harvesting in order to mitigate postharvest loss. Farmers forfeit prospective earnings that they would have attained if grains stored are sold later (Stephens and Barrett, Citation2011Pest infestation starts from the field before crops are harvested and kept in warehouses and therefore requires control measures right from the field through to storage. The management measures relies heavily on the use of pesticides which have adverse effects on humans and the environment. There is therefore the call to explore other better, dependable, environmentally and human friendly and inexpensive options to manage storage pests that attack this crop (Dayan et al., Citation2009). Plant-based products may provide attractive alternatives to inorganic insecticides for pests control because plant-based products pose little danger to the ecosystem. Weinzierl and Henn (Citation1992) justified that, orange peel oil and powder has fumigant action against fleas. Karr and Coats (Citation1988) also accounted that, orange peel powder and oil have fumigant action against household insects and rice weevils. Preliminary research showed that the leaves of L. camara possess a rich source of bioactive molecules (Sharma et al., Citation1988). The use of botanicals to control pests at storage will have the potential in the growth of the seed industry to enhance quality seed production. The current study proposes that the botanicals tested have the potential in the growth of the seed industry against, P. truncatus and S. zeamais as the major pest of stored grains. The study also evaluated the effects of seed treatment with botanicals on seed maize germination and recommended its use to farmers to control P. truncatus and S. zeamais in stored seed maize.
2. MATERIALS AND METHODS
2.1. Collection and culturing of maize weevil & larger grain borer
Stock of S. zeamais and P. truncatus used for the experimental set up was collected from the Entomology Laboratory of the Department of Crop Science, University of Ghana. About 250 sexed adult each of S. zeamais and P. truncatus were introduced into two different 2 L kilner jars containing 500 g of maize grain samples and kept in the laboratory at 28 ± 2°C, 65% relative humidity and 12 h light: 12 h dark (Osafo, Citation1998; Weaver et al., Citation1997; Epidi et al., Citation2009). The S. zeamais sexes can be distinguished with the male having rough, distinctively shorter and wider rostrum while female weevil has smooth, shiny, longer and narrower rostrum. The sexes of the P. truncatus were determined according to the method by Shires and McCarthy (Citation1976). The culture was kept on the shelf of the laboratory for 1 week to allow for oviposition. The adult insects were sieved out and emerging generations were used to set up the experimental cultures.
2.2. Selection of plants for extract preparation
The selection of botanicals used in the storage of grains in this research was based on the following factors which include; previous research carried on the plants, effectiveness of the botanical against stored insects and availability. Four plants (Lantana camara, Hyptis suaveolens, Citrus sinensis and Moringa oleifera) were identified for extract preparations (). A reference synthetic pesticide—Actellic was selected based on the fact that it is one of the most commonly used synthetic chemical of stored grains in Ghana and a control (untreated grains).
Table 1. Botanicals used for the experiment
2.3. Collection and preparation of plant powders
Sweet oranges (C. sinensis) were bought from the Amasaman market in the Greater Accra region and were peeled for use for the experimental work. Fresh leaves of L. camara, H. suaveolens and M. oleifera were collected from bushes at Pokuase and Amasaman all in the Ga—West District in the greater Accra region in clearly labelled sack bags. They were brought into the Crop Science Laboratory of the University of Ghana—Legon where they were prepared for the confirmation of their identity at the Herbarium in the Department of Plant and Environmental Biology of the University. The plant specimens were then washed with tap water to remove sand and other unwanted particles and air dried under room temperature for 14 days (Wambua et al., Citation2011). The selected botanicals were pounded using mortar and pestle after which they were ground to give a fine powder with grinder. The powders were sieved with Impact Test Sieve of a mesh size 70μ to give a uniform size powder. The ground powders were stored in four different airtight containers in a cool place away from sunlight before being used for the treatment of the grains against the insects.
2.4. Preparation of methanol extract of plants
About 100 g each of the plant powders were weighed into six different conical flasks containing 430 mL each of 100% methanol. The flasks were covered with Para film and placed in a shaker for 48 h. The solution was strained with a net of 2.5μ and concentrated using rotary evaporator at 60°C after which the residues were dissolved in acetone to give a concentration of 0.05 g/mL and 0.1 g/mL for the various bioassays. The extracts were then transferred into conical flasks, corked and then kept in a refrigerator.
2.5. Phytochemical constituent analysis of the plant methanol extracts
1 mg/mL of stock solution of each of the methanol extract of the plants were prepared and used for qualitative analysis in triplicates.
2.6. Data collected
2.6.1. Effect of plant powders on adult insects
Sterilized whole maize grains (100 g) were put into glass jars and four botanical powders of two sets (5 and 10%) were admixed to the grains. Actellic 25 EC was applied at 2 ml/L (0.5 g/L) of acetone while the control treatment was without any botanical powder. The setups were left to stand for an hour before infesting with 20 unsexed adults (5–10 days old) of S. zeamais and P. truncatus. The treatments were replicated three times. Daily mortality of insects was recorded for 7 days. Insects were considered dead if they did not respond to probing of by a blunt probe.
2.6.2. Effect of methanol extracts on adult insect in treated grains
Sterilized maize grains (50 g) were put in kilner glass jars and the four botanical extracts at two concentrations (0.05 g/mL and 0.1 g/mL) and Actellic (2 ml/L) were applied to the grains in each jar. The control was treated with only acetone. The treated grains were air dried for an hour to evaporate the solvent following which 20 adult (5–10 days old) S. zeamais and P. truncatus were introduced into the jars which were then covered with muslin cloth held with rubber bands. The treatments were replicated four times and left under controlled room at 28 ± 2°C and 65% relative humidity for 7 days. Dead insects were counted after 48 hours for 7 days.
2.6.3. Contact toxicity by topical application
In this test, the method adopted by Obeng-Ofori and Reichmuth (Citation1997) was used. Ten adults of S. zeamais and P. truncatus (5–10 days old) each were placed in a separate petri dish lined with moist filter paper. To the notum of the insects, 1 µL each of four botanical extracts (stated above), actellic and a control (methanol) were applied using micro—pipette. The experiment was replicated four times. The mortality of insects was taken for 96 hours.
2.6.4. Effect of methanol extracts on oviposition
Maize grains (50 g) were weighed into glass jars and treated with four different botanicals each. Another jar was treated with Actellic at 2 ml/L whilst the control was treated with acetone. The treated grains were left for one hour after which the grains were infested with mixed sexes of 20 adult S. zeamais and P. truncatus (5–10 days old). The adult insects were sieved on the eighth day and the number of eggs laid was determined using the egg plug staining techniques (acid fuchsin method) (FAO, Citation2008).
2.6.5. Seed germination test
Seed germination test was conducted by randomly picking one hundred (100) seeds from the maize before and after the seeds were treated with methanol extracts of four botanicals. The test was conducted with four (4) replicates of twenty five (25) seeds per replicate. The selected seeds were placed on a wetted blotter paper in Petri dishes. After 7 days, the number of germinated seeds were counted and recorded. The percentage germination (viability index) was calculated using the formula:
GP = NSG/TNS ×100;
where, GP = germination percentage, NSG = number of seeds germinated from each Petri dish and TNS = total number of seeds tested in each Petri dish (Ogendo et al., Citation2004; Zibokere, Citation1994).
2.6.6. Damage assessment
Grain damage was assessed using the method adopted by Cornelius et al. (Citation2008). Sterilized whole maize grains (2 kg) each was treated with two concentrations (0.05 g/mL and 0.1 g/mL) of methanol extracts of the four botanicals. The control was treated with methanol only. The treated grains were air dried for three hours after which the grains were introduced into 30 × 40 cm sacks. One hundred adult (5–10 days old) S. zeamais and P. truncatus of mixed sexes were released into the two different bags respectively. Each treatment was replicated three times laid in Complete Randomized Design (CRD). The bags were then kept in a crib at the University of Ghana farm for 10 weeks after which loss was assessed using count and weigh method. Samples of over 500 grains each were taken from each bag and were separated into damaged and undamaged grains. These were counted and weighed. Percentage weight loss was calculated using the method by FAO (1985).
Percent Weight Loss = (UNd)-(DNu)/U (Nd + Nu) X 100
Where Nu is the number of undamaged grains
Nd is the number of damaged grains
U is the weight of undamaged grains
D is the weight of damaged grains.
2.6.7. Qualitative analysis of phytochemicals
2.6.7.1. Detection of alkaloids
Dragendorff’s and Mayer’s test: Each sample (0.5–1 mL) was taken into a test tube. A few drops of Mayer’s reagent were added; it was shaken well and allowed to settle for some time. Cream colour precipitate indicates the presence of alkaloids in the sample.
2.6.7.2. Detection of saponins
The method described by Wall et al. (1951; Wall et al., Citation1954) was used. 0.5 g of each plant extract was shaken with water in a test tube. Frothing which persisted after heating was taken as a preliminary evidence for the presence of saponins. Few drops of olive oil was added to 0.5 g of the extract and vigorously shaken. Formation of soluble emulsion in the extract indicates the presence of saponin (Ngbede et al., Citation2008; Odebiyi & Sofowora, Citation1990).
2.6.7.3. Detection of tannins and phenolic compounds
Ferric chloride test: Few drops of ferric chloride was added to 0.5 mL of test solution in a test tube. Appearance of blue-green colour confirms the presence of tannins and phenolic compounds in the sample.
2.6.7.4. Detection of phlobatannins
0.5 g of each plant extract was boiled with 1% aqueous hydrochloric acid. A deposition of a red precipitate was taken as evidence for the presence of phlobatannins (Trease and Evans 1978).
2.6.7.5. Detection of anthraquinones
Borntranger’s test: 0.5 g of plant extract was shaken with 5 mL benzene, filtered and 5 mL of 10% ammonia solution was added to the filtrate. The mixture was shaken and the presence of a pink, red, or violet colour indicated the presence of free anthraquinones (Trease and Evans, 1978)
2.6.7.6. Detection of cardiac glycosides
Keller-Killani test: The extracts (5 mL each) were treated with 2 mL of glacial acetic acid containing one drop of ferric chloride solution. It is treated with concentrated tetraoxosulphate (VI) acid (H2SO4). A greenish colour confirms the presence of cardiac glycosides.
2.6.7.7. Detection of steroids and terpenoids
Salkowski test: 0.5–1 mL of test solution was treated with chloroform in a test tube. A few drops of concentrated sulphuric acid were added and shaken well. A red colour appearing at the lower layer indicates the presence of steroids and the formation of yellow layer also indicates the presence of steroids and terpenoids.
2.6.7.8. Detection of flavonoids
Shinoda test: 1 mL of test solution was mixed with few fragments of magnesium ribbon and concentrated hydrochloric acid was added drop wise. A Pink scarlet colour which appeared after a few minutes confirms the presence of flavonoids in the sample.
2.7. Data analysis
Data on percentages were arcsine transformed whereas data on counts were square root transformed so as to stabilize the variance. A general analysis of variance (ANOVA) for adult weevil mortality, number emerging as adults, percentage seed weight loss, percentage seed damage and percentage seed germination were conducted using GenStat statistical package 12th Edition. Mean separation was done by using Fisher’s protected LSD to compare the significant differences between the treatments at 5% level of significance.
3. RESULTS
3.1. Effect of plant powder on survival of P. truncatus and S. zeamais in treated maize
The response of P. truncatus and S. zeamais to the four plant powders at 5 and 10% after 7 days is presented in . Treatments significantly (P < 0.001) influenced the survival of the insects.
Table 2. Percentage means survival of P. truncatus and S. zeamais in treated maize after 7 days
The results from on the survivorship of insects indicated that maize seeds treated with higher concentrations of powder reduced the survivorship of both P. truncatus and S. zeamais after 7 days. The four powders were potent against the two insect species but survivorship at 10% application was least with L. camara treatment in both insects after 7 days.
3.2. Contact toxicity by topical application
The results indicated that the toxicity of methanol extract of the various plants was considerably (p < 0.001) influenced by the type of plant and concentration (0.05 g/mL and 0.1 g/mL) of extract administered as compared to the control after 96 h. Mortality also varied with species of insect. After four days, maize treated with the botanicals caused mortality of the insects from 35.2 to 66.1% and 45.0–61.7% in S. zeamais and P. truncatus respectively (, , , ). Mortality of S. zeamais was most pronounced in M. oleifera treatment compared to the other botanicals, while H. suaveolens was most effective against P. truncatus.
Figure 1. Contact toxicity of methanol extract of four botanicals by topical application to S. zeamais at 0.05 g/mL after 96 hours
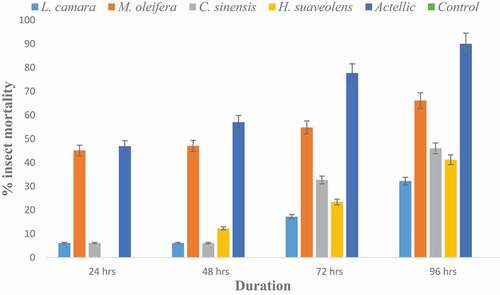
Figure 2. Contact toxicity of methanol extract of four botanicals by topical application to P. truncatus at 0.05 g/mL
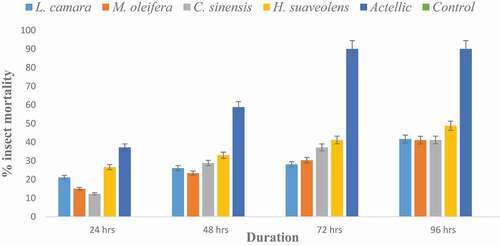
Figure 3. Contact toxicity of methanol extract of four botanicals by topical application to Prostephanus truncatus at 0.1 g/mL
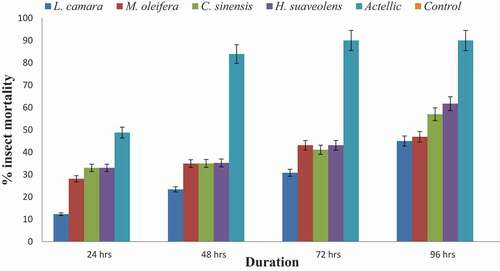
Figure 4. Contact toxicity of methanol extract of four botanicals by topical application to S. zeamais at 0.1 g/mL
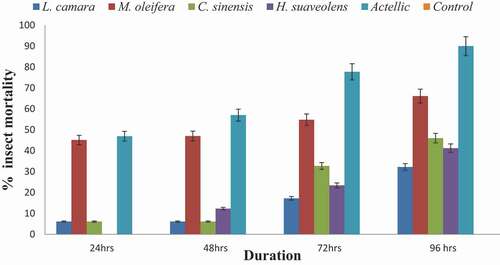
From , M. oleifera induced mortality of 66% followed by C. sinensis recording 41%. All the insects survived in the control after 96 hours. The reference synthetic Actellic caused mortality of almost all the insects after 96 hours.
All the botanicals also caused insect mortality of between 41 and 48% after 96 hours while Actellic recorded 90% mortality at 0.05 g/l. All insects survived under control ().
From , all P. truncatus survived at the control whilst the Actellic caused 90% mortality after 96 hours. Maize treated with botanicals caused mortality rate of 45–62 % with H. suaveolens recording the highest mortality.
The four botanical extracts caused insect (S. zeamais) mortality of 32–66 % with M. oleifera recording the highest while L. camara recorded the least (32%). All the insects survived in the control after 96 hours ().
3.3. Effect of methanol extract of botanicals on oviposition of P. truncatus and S. zeamais
The number of eggs laid by P. truncatus and S. zeamais on grains (50 g) treated with L. camara, M. oleifera, C. sinensis, H. suaveolens at the rate of 0.05 g/mL and 0.1 g/mL and Actellic at 2 mL/L is presented in .
Table 3. Mean number of eggs laid by the insects in treated seed maize after 7 days
Of the four botanicals used (), H. suaveolens and L. camara extracts recorded the least number of eggs laid by the two insects after 7 days at both concentrations (0.05 g/mL and 0.1 g/mL) applied as compared to the control where a higher number of eggs were laid on the untreated grains.
3.4. Effect of pre-planting seed treatment on seed maize germination
Seeds treated with L. camara and M. oleifera recorded a percentage viability of 94.0% each which, unlike other treatments, was significantly higher than the values obtained in untreated seeds ().
Table 4. Effect of pre-planting seed treatment on maize seed germination after 7 days
3.5. Insect damage assessment
All seeds treated with botanicals gave higher protection (P ≤ 0.05) against insect damage compared to untreated grains. All tested botanicals were more effective at higher (0.1 g/mL) dosage than at lower (0.05 g/mL) dosage in terms of reducing weight loss. Comparatively, P. truncatus caused a higher weight loss in seeds treated with botanical extracts compared with S. zeamais in both rates of application. Hyptis suaveolens recorded a percentage weight loss of 1.49–2.32% and was more effective in reducing damage caused by the two insects than the three other botanicals. The control recorded about 13% damage weight loss caused to the maize seeds by the two insects. The reference Actellic recorded the least damage weight loss (0.13%) caused by the insect ().
Table 5. Mean percentage seed weight loss by P. truncatus and S. zeamais following treatment with methanol extracts of botanicals after 10 weeks of treatment
From , it could be observed that all the four botanicals could reduce damage and weight loss caused by the two insects of study as compared to control which recorded percentage mean weight loss of between 11 and 13% after 10 weeks. Of the four botanicals, maize seeds treated with H. suaveolens were least attacked by the two insects and at concentrations of 0.05 g/mL and 0.1 g/mL followed by L. camara, C. sinensis and M. oleifera. Comparing the level of damage caused by the two insects, P truncatus was able to cause more damage and percentage weight loss to maize seeds in all the treatments compared to S. zeamais. The reference Actellic could curtail damage caused by the two insects of study than all the other treatments.
3.6. Phytochemical constituents of plants
The results showed that the compounds alkaloids, saponins, tannins and phenol, steroids and flavonoids were present in all the botanicals used. It was revealed that anthraquinones was present in only M. oleifera. Phlobatannins was also present in L. camara and C. sinensis. Cardiac glycosides and terpenoids were present in all the botanicals except H. suaveolens. shows the compounds present in the four botanicals.
Table 6. Phytochemical constituents of the four experimental botanicals
Key: + = Present; — = Absent
4. DISCUSSION
4.1. Effect of plant powders on survival of Prostephanus truncatus and Sitophilus zeamais
The ground powders of L. camara, M. oleifera, C. sinensis and H. suaveolens showed different levels of effectiveness against P. truncatus and S. zeamais in treated grains after 7 days. Survival of insects reduced with increasing quantity of powder from 5 to 10%. The survival of P. truncatus and S. zeamais in grains administered with L. camara powder at 10% showed that the botanical is a promising control agent against the two insects since it contained alkaloids (Ameyaw & Duker-Eshun, Citation2009). These compounds are toxic (Mithöfer & Boland, Citation2012) to both insects and might have been responsible for the low survivorship of insects in the treated grains. The results of this work have confirmed the protectant potential of L. camara and H. suaveolens powders against the two insect species that attack stored maize grains (Ojo & Ogunleye, Citation2013). Bioactive: tannins, terpenes and steroids reportedly present in the plant might have caused the reduction in survival of the insects in treated grains as reported by Dibua et al. (Citation2000). This may explain the efficacy of L. camara in this study. The present investigation revealed that the powder has the potential to reduce the survival of S. zeamais and P. truncatus to 59% when applied at 5% concentration. The survival of insects further reduced to 48.8% as the dosage of the powder increased to 10%. Shifa Vanmathi et al. (Citation2010) earlier observed that a higher amount of plant extracts were more effective than lower concentrations in reducing oviposition and increasing the mortality of the target insect pests. The leaf powder of L. camara might have also contained other chemical compounds preventing insects from feeding on grains that have been treated with the powder. The use of M. oleifera and C. sinensis powder as grain protectants against S. zeamais and P. truncatus caused higher survivorship than the other two botanicals discussed earlier. Although, M. oleifera and C. sinensis contain alkaloids, terpenoid, morphine and phenol, they may be in smaller concentration in the plants parts that have been used for the experiment compared to other two botanicals (Akinkurolere, Citation2012). A suggested possible reason of adult mortality might be the effective adhesion of dust particles to spiracles of pest and their death due to suffocation.
4.2. Toxicity of extracts applied topically to insects
In this study, all the botanical extracts were toxic to insects at different levels compared to the control after 96 hours of treatment by topical application. In all the treatments, the higher concentration (0.1 g/mL) was more effective to both insects than the lower concentration (0.05 g/mL). Hyptis suaveolens and M. oleifera were highly toxic to P. truncatus (61.7%) and S. zeamais (66.1%), respectively. Compounds such as flavonoids, saponins, tannins and phenol have been reported to be present in these botanicals (Irvine, Citation1961) and might be responsible for the observed mortalities. Sitophilus zeamais was more predisposed to methanol extract than P. truncatus and this might be attributed to its more robust nature, high feeding ability and highly sclerotized cuticle which might have decreased the absorption of component of the extract through the cuticle.
4.3. Effect of methanol extracts of botanicals on oviposition of Prostephanus truncatus and Sitophilus zeamais
All the botanicals were effective at 0.1 g/mL to both insects. This means that the botanicals might possess repellent and/or oviposition deterrent action which might have resulted in the changes prompted by physiology and behaviours in the adult insects as reflected by their egg laying capacity. This confirms the research reported by Adebayo and Gbolade (Citation1994) that L. camara which contains caryophyllene and germacrene D in large quantities exhibited some ovipositional suppression on Callosobruchus maculatus. Also Schmutterer (Citation1990) and Ndomo et al. (Citation2009) confirmed that that botanical extracts and their essential oils have anti-oviposition and fertility reducing effect on a host of insects.
4.4. Toxicity of extracts to Sitophilus zeamais and Prostephanus truncatus in treated maize grain
The methanol extracts at both concentrations applied to adult insects in treated seeds after seven days significantly (P < 0.001) reduced the survival of both insects compared to the control. The toxicity of the extract applied to adult insects in treated seed was influenced by the type of plant, concentration applied and contact duration (days). The most effective botanical on S. zeamais was L. camara. Earlier study has reported that leaves of L. camara is active against insects (Ogendo et al., 2003; Dua et al., Citation2010) whilst the least effective was M. oleifera and the most effective botanical on P. truncatus was L. camara. Although M. oleifera also contains similar chemical components such as saponins, flavonoids, alkaloids, steroids, tannins and phenolic compounds, it might have not been potent enough to kill insect pests as compared to L. camara and H. suaveolens which contains other additional compounds. However, they can be used to prevent fungi attack in stored grains (Oudhia, Citation2008). The higher concentration of the botanicals induced lower insect survivorship. Lantana camara at 0.1 g/mL recorded 40% survivorship in S. zeamais after 5 days of treatment. Therefore, the extracts were slower in killing insects than the synthetic chemical (Actellic). This confirms earlier report by Obeng-Ofori and Dankwah (Citation2004) that Actellic has rapid knock down action which instantly killed adult insects on contact.
4.5. Germination
Seeds treated with botanical extracts was significantly higher in germination than the values obtained in untreated seeds. The result generally indicated that seeds were viable and had a good germination percentage which was still in certification limits for seed maize (70%) (ISTA, Citation2007). The use of plant products by farmers to store their grains does not have any negative influence on germination of the treated seeds. The use of leaf extracts of M. oleifera as indicated by Foidl et al. (Citation2001) results in enhanced growth of plant.
4.6. Assessment of seed damage
In all the four botanicals tested, the mean percentage weight loss and seed damage was lower at higher (0.1 g/mL) concentration than in the lower (0.05 g/mL) concentration. This confirms the findings of Parwada et al. (Citation2018) that botanicals result in lowering the occurrence in the weevil attack if the concentration is increased. The research work showed that maize seeds treated with H. suaveolens, L. camara, C. sinensis and M. oleifera, prevented emergence and suppressed insect’s activities. This also corroborates the findings of Wahedi (Citation2012) where maize grains treated with neem seed oil was able to decrease the percentage of weight loss caused by the insects. Maize seed without treatment with botanicals experienced higher insects’ damage (11.1–12.8% weight loss) than those treated with botanicals (1.5–4.2% weight loss). The extracts of L. camara were used to protect grain against almond moth as the extracts exhibited fumigant and contact activity (Gotyal et al., Citation2010). Research carried out by Parwada et al. (Citation2018) proved that the efficacy of botanical pesticides decreases with time as shown by the reduced mortality percentages after six weeks of its application. This suggests that botanical need constant reapplications for them to offer continual protection of the grain against P. truncatus and S. zeamais.
5. CONCLUSIONS AND RECOMMENDATIONS
The experiment conducted was to assess the efficacy of four plant extracts as maize seed storage protectant against S. zeamais and P. truncatus in Ghana. The present findings showed that the plant extracts exhibited contact toxicity on the insects of study. The powders of the four botanicals were deleterious to the insects thereby reducing insect survival when applied at 5% and 10% concentration. Hyptis suaveolens and Lantana camara were observed to be the most promising botanicals in protecting maize grains against the two insects. Maize seeds treated with methanol extracts of the botanicals after 10 weeks, recorded a reduction in percentage seeds damaged and weight loss caused by the two insects as compared to the untreated seeds which recorded higher number of damaged seeds and weight loss. Apart from exhibiting the anti-oviposition, lowering survivorship and inhibiting reproduction inhibition effects, the methanol extracts of the botanicals demonstrated repellent properties on the two insects. Pre-planting seed treatment with the four botanicals enhanced good germination and seedling emergence. Maize seed treated with M. oleifera recorded the highest with 94% seedling emergence. This suggests that grains treated with M. oleifera will enhance good germination of seeds for a good field emergence and establishment. However, the long-term effect of seed treatment with the extracts needs further investigations.
The reductions in percentage of grain weight loss as well as in damaged seeds have important implications for the use of the botanicals as maize seed storage protectants against P. truncatus and S. zeamais by farmers. It is recommended that these four botanicals can be used as maize seed storage protectants against storage insect pests. Further study is needed using other storage insect pests in order to broaden its spectrum of effect. The use of pre-planting seed treatments with botanicals is recommended especially for commercial seed producers to enhance the production of quality insect-free and disease-free seed. It is also recommended that a further trial should be conducted on the field by planting maize seed treated with these botanicals for definitive conclusions of the effectiveness of the extracts on plant establishment and yield.
Acknowledgements
This study was partially supported by the World Bank-Africa Centers of Excellence for Development Impact (ACE Impact) project through the West Africa Centre for Crop Improvement (WACCI), University of Ghana, Legon. We are also grateful to the University of Ghana for providing laboratory space and facilities as well as equipment to conduct this research in the Entomology Laboratory of the Department of Crop Science.
Disclosure Statement
The authors declare no competing interests.
Additional information
Funding
Notes on contributors
Daniel Kwadjo Dzidzienyo
Samuel Y. Gariba has completed his Master of Philosophy (MPhil) degree in Seed Science and Technology from the West Africa Centre for Crop Improvement (WACCI), College of Basic and Applied Sciences, University of Ghana.
Daniel K. Dzidzienyo is a Lecturer at the Biotechnology Centre and also an Associate Faculty at the West Africa Centre for Crop Improvement (WACCI), College of Basic and Applied Sciences, University of Ghana.
Vincent Y. Eziah is a Lecturer at the Department of Crop Science and also an Associate Faculty at the West Africa Centre for Crop Improvement (WACCI), College of Basic and Applied Sciences, University of Ghana.
References
- Adebayo, T. A., & Gbolade, A. A. (1994). Protection of stored cowpea from callosobruchus maculatus using plant products. International Journal of Tropical Insect Science, 15, 185–16. https://link.springer.com/article/10.1017/S1742758400015435#citeas
- Akinkurolere, R. O. (2012). Comparative effects of three plant powders and pirimiphos-methyl against the infestation of Callosobruchus Maculatus (F.) (Coleoptera: Bruchidae) in cowpea seeds (pp. 116). Federal University of Technology.
- Ameyaw, Y., & Duker-Eshun, G. (2009). The alkaloid content of the ethano-plant organs of three anti-malarial medicinal plant species in the eastern region of Ghana. International Journal of Chemistry, 7(1), 48–58. https://www.researchgate.net/publication/266888350_The_alkaloid_contents_of_the_ethnoplant_organs_of_three_anti_malarial_medicinal_plant_species_in_the_eastern_region_of_Ghana
- APHLIS. (2015). African post harvest losses information system. APHLIS. Available at http://www.aphlis.net. (Accessed on 6 March 2015).
- CAB International. (2005). Crop Protection Compedium Global Module (2nded., pp. 2).
- CIMMYT. (1999). CIMMYT 1997/98. World maize facts and trends. Maize production in drought-stressed environments: Technical options and research resource allocation.
- Cornelius, E. W., Bani, R. J., Boateng, B. A., Egyir, I. S., Josaih, M. N., Obeng- Ofori, D., Ofosu- Anim, J., & Timpo, S. E. (2008). Postharvest Science and Technology (pp. 11–137). Smartline Ltd. University of Ghana.
- Costa, S. J. (2014). Reducing Food Losses in Sub-Saharan Africa (Improving Post-Harvest Management and Storage Technologies of Smallholder Farmers. In An ‘Action Research’ evaluation trial from Uganda and Burkina Faso. UN World Food Programme. August 2013 – April 2014. https://documents.wfp.org/stellent/groups/public/documents/special_initiatives/WFP265205.pdf
- Crissman, C. C., Crissman, L. M., & Carli, C. (1993). Seed Potato Systems in Africa: A Case Study (pp. 52). International Potato Center (CIP).
- Dayan, F. E., Cantrell, C. L., & Duke, S. O. (2009). Natural products in crop protection. Bioorganic & Medicinal Chemistry, 17(12), 4022–4034. https://doi.org/https://doi.org/10.1016/j.bmc.2009.01.046
- Denning, G., Kabambe, P., Sanchez, P., Malik, A., & Flor, R. (2009). Input subsidies to improve smallholder maize productivity in Malawi: toward an African green revolution. Public Library of Science Biology, 7(5), e1000023. https://doi.org/https://doi.org/10.1371/journal.pbio.1000114
- Dibua, G. I., Greg, E. O., Ozioma, F. N., & Uju, E. A. (2000). Deterrent effects of some botanical products on oviposition of the cowpea bruchid callosobruchus maculatus (F.) (coleoptera: bruchidae). International Journal of Pest Manage, 46(2), 109–113. https://doi.org/https://doi.org/10.1080/096708700227462
- Dua, V. K., Pandey, A. C., & Dash, A. P. (2010). Adulticidal activity of essential oil of Lantana Camara leaves against mosquitoes. Indian Journal of Medical Research, 131, 434–439, https://pubmed.ncbi.nlm.nih.gov/20418559/
- Epidi, T. T., Udo, I. O., & Osakwe, J. A. (2009). Susceptibility of Sitophilus zeamais Motsch. and Callosobruchus maculatus F. to plant parts of Ricinodendron heudelottii. Journal of Plant Protection Research, 491, 411–415. https://doi.org/https://doi.org/10.2478/v10045-009-0065-6
- FAO. (2008). Household metal silo: Key allies in FAO’s fight against hunger; Agricultural and Food Engineering Technologies Service.
- FAO. (2015). FAO Statistical Pocketbook 2015. Food and Agriculture Organization of the United Nations.
- FAO. (2021). Food and agricultural organisation (FAO) country analysis: global information and early warning system (GIEWS). FAO GIEWS Country Brief on Ghana. FAO/GIEWS. http://www.fao.org/giews/countrybrief/country.jsp?code=GHA
- FAOSTAT. (2014). Africa Maize production- 2012/13. http://faostat3.fao.org/browse/Q/QC/E (Accessed on June 10, 2015). https://scholar.google.com/scholar?hl=en&q=FAOSTAT+2014.+Africa+Maize+Production-+2012%2F13.+From+http%3A%2F%2Ffaostat3.fao.org%2Fbrowse%2Fq%2Fqc%2Fe.+%28Retrieved+on+19+January+2016%29.
- Farrell, G., & Schulten, G. M. (2002). Larger grain borer in Africa; A history of efforts to limit its impact. Integrated Pest Management Reviews, 7(2), 67–84. https://doi.org/https://doi.org/10.1023/A:1026345131876
- Foidl, N., Makkar, H. P. S., & Becker, K. (2001). The potential of Moringa oleifera for Agricultural and industrial uses. In: What development potential for Moringa products? Dar Es Salaam. October 20th -November 2nd 2001.
- Gotyal, B. S., Srivastava, C., Walia, S., Jain, S. K., & Reddy, D. S. (2010). Efficacy of wild sage (Lantana camara) extracts against almond moth (cadra cautella) in stored wheat (triticum aestivum) seeds. Indian Journal of Agricultural Sciences, 80(5), 433–436. https://scholar.google.com/scholar?cluster=6540031242760661927&hl=en&as_sdt=0,5
- Irvine, F. R. (1961). Woody plants of Ghana (pp. 720–722). Oxford University Press.
- ISSER. (2007). The state of the Ghanaian economy 2006. Institute of Statistical Social and Economic Research. University of Ghana, Legon.
- ISTA. (2007). International Rules for Seed Testing. International Seed Testing Association (ISTA). Bassersdorf, Switzerland: ISTA
- Karr, L. L., & Coats, J. R. (1988). Insecticidal Properties of d-Limonene. Department of Entomology. Iowa State University, Ames.
- Longstaff, B. C. (1981). Biology of the grain pest species of the genus sitophilus (coleoptera: curculionidae): A critical review. Protection Ecology, 3(2), 83–130. https://scholar.google.com/scholar?cluster=14108386794687051142&hl=en&as_sdt=0,5
- López-Castillo, L. M., Silva-Fernández, S. E., Winkler, R., Bergvinson, D. J., Arnason, J. T., & García-Lara, S. (2018). Postharvest insect resistance in maize. Journal of Stored Products Research, 77, 66–76. https://doi.org/https://doi.org/10.1016/j.jspr.2018.03.004
- Louwaars, N. P., & De Boef, W. S. (2012). Integrated seed sector development in Africa: A conceptual framework for creating coherence between practices, programs, and policies. Journal of Crop Improvement, 26(1), 39–59. https://doi.org/https://doi.org/10.1080/15427528.2011.611277
- Markham, R. H., Bosque-Perez, N. A., Borgemeister, C., & Meikle, W. (1994). Developing pest management strategies for sitophilus zeamais and prostephanus truncatus in the tropics. FAO Plant Protection Bulletin, 42(3), 97–116. https://scholar.google.com/scholar?cluster=3547985020553010016&hl=en&as_sdt=0,5
- McFarlane, J. A., Sherman, C., & Walker, A.K. (1990). Differences between some strains of stored –grain beetles in their capacity to cause grain damage: Possible implications for the management of pesticide resistance. Tropical Science Series, 30(4), 357–371. https://scholar.google.com/scholar?cluster=9059051723408773657&hl=en&as_sdt=0,5
- Mithöfer, A., & Boland, W. (2012). Plant defense against herbivores: Chemical aspects. Annu Rev Plant Biology, 63(1), 431–450. https://doi.org/https://doi.org/10.1146/annurev-arplant-042110-103854
- Mushi, A. M. (1990). Damage caused by larger grain borer, prostephanus trancatus in Tanzania and its control measures. Proceedings: intergrated pest management in tropical and sub-tropical cropping systems, Bad Durkheim, Germany, 3, 961–976.
- Ndomo, A. F., Tapondjou, A. L., Tendonkeng, F., & Tchouanguep, F. M. (2009). Evaluation des propriétés insecticides des feuilles de Callistemon viminalis (Myrtaceae) contre les adultes d’Acanthoscelis obtectus (Say) (Coleoptera: Bruchidae). Tropicultura, 27(3), 137–143. https://scholar.google.com/scholar?cluster=14800888052139373402&hl=en&as_sdt=0,5
- NDPC. (2018) Implementation of the Ghana shared growth and development agenda (GSGDA) II, 2014–2017. 2017 Annual Progress Report. National Development Planning Commission (NDPC). Government of Ghana. https://s3-us-west-2.amazonaws.com/new-ndpc-static1/CACHES/PUBLICATIONS/2019/05/31/2017+APR.pdf
- Ngbede, J., Yakubu, R. A., & Nyam, D. A. (2008). Phytochemical screening for active compounds in canarium scheinfurthii (atile) leaves from Jos North, Plateau State Nigeria. Research Journal of Biological Sciences, 3(9), 1076–1078. https://medwelljournals.com/abstract/?doi=rjbsci.2008.1076.1078
- Obeng-Ofori, D., & Dankwah, J. A. (2004). Biological effects of four protectants applied to store bambara groundnut against infestation by callosobruchus maculatus (fab) (coleoptera: bruchidae). Journal of the Ghana Science Association, 37(1), 33-42. DOI:https://doi.org/10.4314/gjas.v37i1.2077, https://www.ajol.info/index.php/gjas/article/view/2077
- Obeng-Ofori, D., & Reichmuth, C. H. (1997). Bioactivity of eugenol, a major component of essential oil of ocimum suave (wild.) against four species of stored-product Coleoptera. International Journal of Pest Management, 43(1), 89–94. https://doi.org/https://doi.org/10.1080/096708797229040
- Odebiyi, A., & Sofowora, A. E. (1990). Phytochemical screening of Nigerian medicinal plants. Part III. Lloydia, 41, 234–246. https://scholar.google.com/scholar?cluster=15412544397966293667&hl=en&as_sdt=2005&sciodt=0,5
- Odendo, M., De Groote, H., & Odongo, O. M. (2001). Assessment of farmers’ preferences and constraints to maize production in Moist Midaltitude Zone of Western Kenya. Paper presented at the 5th international conference of the African crop science society, Lagos, Nigeria.
- OECD/Food and Agriculture Organization of the United Nations. (2014) . OECD-FAO Agricultural Outlook 2014. OECD Publishing.
- Ogendo, J. O., Deng, A. L., Belmain, S. R., Walker, D. J., & Musandu, A. A. O. (2004). Effect of insecticidal plant materials lantana camara and tephrosia vogelii hook, on the quality parameters of stored maize grains. Journal of Food Technology, 9(1), 29–36. https://scholar.google.com/scholar?cluster=926598064288643641&hl=en&as_sdt=0,5
- Ojo, D. O., & Ogunleye, R. F. (2013). Comparative Effectiveness of the Powders of Some Underutilized Botanicals for the Control of Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae).International Journal of Pure and Applied Sciences and Technology, 16(2), 55-62. https://scholar.google.com/scholar?cluster=3985930255631089856&hl=en&as_sdt=2005&sciodt=0,5.
- Osafo, W. F. (1998). Extracts of three plant materials as post- harvest grain protectants against Sitophilus zeamais (Most) (Coleoptera: Curculionidae) (pp. 32). University of Ghana.
- Oudhia, P. (2008). Phyllanthus amarus Prota 11(1): Medicinal plants/Plantes médicinales 1. [CD-Rom]. PROTA.
- Parwada, C., Chikuvire, T. J., Kamota, A., Mandumbu, R., & Mutsengi, K. (2018). Use of botanical pesticides in controlling sitophilus zeamais (maize weevil) on stored zea mays (maize) grain. Modern Concepts & Developments in Agronomy, 1(4). DOI: https://doi.org/10.31031/MCDA.2018.01.000517
- Schmutterer, H. (1990). Properties and potential of natural pesticides from the neem tree, azadirachta indica. Annual Review of Entomology, 35(1), 271–297. https://doi.org/https://doi.org/10.1146/annurev.en.35.010190.001415
- Sharma, O. P., Makkar, H. P. S., & Dawara, R. K. (1988). A review of the noxious plant lantana camara. Toxicon, 26(11), 975–987. https://doi.org/https://doi.org/10.1016/0041-0101(88)90196-1
- Shifa Vanmathi, J., Padmalatha, C., Ranjit Singh, A. J. A., & Suthakar Isaac, S. (2010). Efficacy of selected plant extracts on the oviposition deterrent and adult emergence activity of callosobruchus maculatus (bruchidae; coeleoptera). Global Journal of Science Frontier Research, 10(8), 2–8. https://scholar.google.com/scholar?cluster=15011928489851502847&hl=en&as_sdt=2005&sciodt=0,5
- Shires, S. W., & McCarthy, S. (1976). A character for sexing live adults of prostephanus truncatus (horn) (Bostrichidae. Coleoptera). Journal of Stored Products Research, 12(4), 273–275. https://doi.org/https://doi.org/10.1016/0022-474X(76)90044-8
- Smith, J., Weber, G., Manyong, V. M., & Fakorede, M. A. B. (1997). Fostering sustainable increase in maize productivity in Nigeria. Chapter 8. In D. Byerlee & C. K. Eicher (Eds.), Africa’s emerging maize revolution (pp. 107–124). lynne Rienner Publishers.
- Stevens, E. C., & Barrett, C. B. (2011). Incomplete Credit markets and Commodity marketing behavior. Journal of Agricultural Economics, 62(1), 1–24, https://doi.org/https://doi.org/10.1111/j.1477-9552.2010.00274.x
- Tefera, T., Mugo, S., Likhayo, P., & Beyene, Y. (2011). Resistance of three-way cross experimental maize hybrids to post-harvest insect pests, the larger grain borer (prostephanus truncatus) and maize weevil (sitophilus zeamais). International Journal of Tropical Insect Sciences, 63(1–2), 3–12. https://doi.org/https://doi.org/10.1017/S1742758411000075
- Throne, J. E., & Eubanks, M. W. (2002). Resistance of tripsacorn to sitophilus zeamais and oryzaephilus surinamensis. Journal of Stored Product Research, 38(3), 239–245. https://doi.org/https://doi.org/10.1016/S0022-474X(01)00018-2
- Trease, G. E., & Evans, W. C. (1978). A textbook of Pharmacognosy (11th Edn. ed.). Bailliere Tindall London, pp.530. .
- Verheye, W. (2010). Growth and Production of Maize: Traditional Low-Input Cultivation. In Land Use, Land Cover and Soil Sciences. Encyclopedia of Life Support Systems (EOLSS), UNESCO-EOLSS Publishers, Oxford, UK. https://biblio.ugent.be/publication/1009134/file/6718222
- Wahedi, J. A. (2012). Laboratory evaluation of neem (azadirachta indica linn (meliaceae)) seed powder and seed oil for the control of sitophilus zeamais (coleoptera: curculionidea) on stored maize. Adamawa State University Journal of Scientific Research, 2(2), 110–115. https://scholar.google.com/scholar?cluster=9583383411345318204&hl=en&as_sdt=2005&sciodt=0,5
- Wall, M. E., Krider, M. M., Krewson, C. F., Eddy, C. R., Willaman, J. J., Corell, D. S., & Gentry, H. S. (1954). Steroidal sapogenins. VII. Survey of plants for steroidal sapogenins and other constituents. Journal of the American Pharmaceutical Association (Scientific Ed.), 43(1), 1–7. https://doi.org/https://doi.org/10.1002/jps.3030430102
- Wambua, L. M., Deng, A. L., Ogendo, J. O., Owuoche, J., & Bett, P. K. (2011). Toxic, antifeedant and repellent activity of aqueous crude extracts of tephrosia vogelii hook on the larval stages of helicoverpa armigera hubner. Baraton Interdisciplinary Research Journal, 1, 19–29. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Wambua%2C+L.+M.%2C+Deng%2C+A.+L.%2C+Ogendo%2C+J.+O.%2C+Owuoche%2C+J.%2C+and+Bett%2C+P.+K.+%282011%29.+Toxic%2C+antifeedant+and+repellent+activity+of+aqueous+crude+extracts+of+Tephrosia+vogelii+Hook+on+the+larval+stages+of+Helicoverpa+armigera+Hubner.+Baraton+Interdisciplinary+Research+Journal+1%3A+19+-+29&btnG=
- Weaver, D. K., Zettler, J. L., Wells, C. D., Baker, J. E., Bertsch, W., & Throne, J. E. (1997). Toxicity of fractionated and degraded Mexican marigold floral extract to adult SItophilus zeamais (coleoptera: curculionidae). Journal of Economic Entomology, 90(6), 1678–1683. https://doi.org/https://doi.org/10.1093/jee/90.6.1678
- Weinzierl, R., & Henn, T. (1992). Alternatives in insects’ management: Biological and Biorational Approaches. University of Illinois, Urban-Champaign, North Central Regional Extension publication 401.
- Zibokere, D. S. (1994). Insecticidal potency of red pepper (capsicum annuum) on pulse beetle (callosobruchus maculatus) infesting cowpea (vigna anguiculata) seeds during storage. Indian Journal of Agricultural Sciences, 64(10), 727–728. https://scholar.google.com/scholar?cluster=15681107677218251353&hl=en&as_sdt=2005&sciodt=0,5
