?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The consumption of vegetables and particularly zucchini (Cucurbita pepo ssp) is subject of nutritional recommendations at the world level. However, like other foodstuffs, zucchini can be subject to many microbial alterations causing poor post-harvest practices that could affect its organoleptic and marketable characteristics. The aim of this study was to highlight the capacity of Pseudomonas fluorescens F19 to inhibit the germs of alteration of zucchini sold in Abidjan city (Côte d’Ivoire), in order to improve their shelf life. A sample of 60 zucchini including 30 spoiled and 30 healthy of the Coucourzelle variety was used for this study. The phenotypic identification of the spoilage germs was carried out on Potato Dextrose Agar medium and the inhibition of P. fluorescens F19 was demonstrated in vitro and in vivo. Six species of fungi belonging to three genera (Aspergillus sp., Phytophtora sp., Candida sp.) were isolated from the altered zucchini. Among these spoilage agents, three species including Phytophtora sp, Candida sp and Aspergillus sp (c) showed significant spoilage of zucchini. Phytophtora sp is the main spoilage agent of zucchini with a total spoilage within three days. However, the in vitro biological activity of the supernatant of P. fluorescens F19 showed a significant inhibition of the growth of the fungi responsible of zucchini spoilage with an average of 73.33%. In vivo the use of P. fluorescens F19 allowed to extend the shelf life of Coucourzelle zucchini from 3 to 10 days. P. fluorescens F19 has antifungal properties.
PUBLIC INTEREST STATEMENT
Because of their potential source of nutrients, fruits consumption is recommended for people well-being. For very long time, preservation of agricultural products and mainly food crops against pests has been made through the use of chemical substances. However, it has been demonstrated that these products are sources of environmental pollution and public health problems. They are responsible for acute or chronic contaminations at the consumer’s level. Because of harmful effects of pesticides, consumers are requesting food products with good sanitary qualities. At the same time, it was found that certain microorganisms such as Pseudomonas and Bacillus produce metabolites, which are safe for consumer’s health and the environment. These microorganisms are used as biopesticides to prevent fruit alteration. This study aims to highlight the ability of certain microorganisms to preserve zucchini vegetables against alteration due to microorganisms such as Phytophtora sp., Aspergillus sp. and Candida sp.
1. Introduction
Fruits and vegetables constitute a real source of energy, mineral salts and vitamins that are abundant and low cost (Ife & Kuipers, Citation2003; Yaouba & Mpounze, Citation2017). Indeed, the consumption of fruit and vegetables is considered by many international organizations as a public health issue and by the way nutritional recommendations at the global level by the World Health Organization and the National Nutrition and Health Program (FAO, Citation1995). Thus, the consumption of four to five fruits and vegetables is recommended per person per day (Tassadit, Citation2012). In Côte d’Ivoire, fruits and vegetables occupy a prominent place in national economy with 5th rank in terms of foreign exchange generating sector. Among these fruits and vegetables, Zucchini is the one, generally found in the diet of several households (Yankel, Citation2017). Zucchini (Cucurbita pepo ssp) is a plant belonging to cucurbitaceae family. It is a fruit rich in potassium, calcium, phosphorus, vitamins (A, B3, C, D and K) and zinc, present in the seeds (Mathieu et al., Citation2009).
However, fruit production in Côte d’Ivoire is facing many challenges including post-harvest spoilage due to the recrudescence of pathogens. These parasitic infestations are linked to difficulties in applying good agricultural practices, especially to inadequate post-harvest fruit handling (Yaouba & Mpounze, Citation2017).
Among these storage diseases mentioned, fungal spoilage is one of the main challenges to the quality of fresh fruits in Côte d’Ivoire (Alloue-Boraud et al., Citation2015), and their availability throughout the year. Fruit contamination by fungal infections can occur in the field or during post-harvest packaging operations, storage and sometimes after purchase by the consumer (Kouadia et al., Citation2019).
The diversity of pathogens that caused these conditions makes their control difficult. Indeed, the chemical control methods used for their reduction constitute one of the constraints of growing pathogen resistance and the maximum limits of pesticide residues in fruits imposed by importing countries.
These chemicals, considered as the most effective treatment to deal with these problems, cause harmful consequences on the environment through the accumulation of residues and soil pollution (Kouassi, 2001; Thakore, Citation2006). The emergence and generalization of resistance mechanisms in pathogens, ecological imbalance, due to the fact that many of these synthetic compounds have a broad spectrum of action, destroying not only the harmful agents, but also other populations in the ecosystem, and health problems for users and consumers. In view of these drawbacks, it is important to find alternative solutions to fight against phytopathogens while preserving the environment and consumer health. Thus, biological control through the use of Generaly Recognize As Safe (GRAS) bacteria has been widely studied. Among biocontrol agents, Bacillus subtilis has long been used as a biopesticide due to its antibacterial and antifungal properties (Ongena, Citation2014). The work of Alloue-Boraud et al. (Citation2015) and Ban Koffi et al. (Citation2017) showed that the use of Bacillus subtilis GA1 as a biopesticide made the inhibition of various mango pathogenic microorganisms possible, thus improving their shelf life. Studies by Koffi et al. (Citation2016) inhibited the growth of spoilage germs in pineapple and extended its shelf life by 14 days. Also, Koua et al. (Citation2020) reported that the Bacillus subtilis species isolated from the rhizosphere of the cocoa tree had the capacity to inhibit the spoilage germs of the pods but also to induce resistance against fungal and viral phytopathogens, in particular Cocoa Shollen Shoot Virus (CSSV). Antibiotic production by some bacteria plays a major role in disease suppression (Raaijmakers et al., Citation2002). So far, Gram-negative bacteria, especially Pseudomonas strains, have been intensively investigated with regard to the production of antimicrobial metabolites (Koffi et al., Citation2016; Whipps, Citation2001). Pseudomonas fluorescens F19 has been tested in vitro and in vivo for the inhibition of the growth of Geotrichum sp., a proven pathogen of tomato (Ban Koffi et al., Citation2015). The main part of the present study was to find the ability of P. fluorescens F19 to stop or reduce growth and development of microorganisms responsible of zucchini alteration in order to improve their shelf life.
2. Material and methods
2.1. Bacterial strains
The zucchini samples consisted of healthy and altered zucchini of the variety courcouzelle sold in markets of Abidjan (Côte d’Ivoire) (). The strain of P. fluorescens F19 provided from the Collection of Bioindustry Unit to University of Liège Gembloux agro biotech (Belgium). Strains were storage in cryotubes in presence of 20% of glycerol at −80°C.
2.2. Isolation and phenotypic pathogen identification
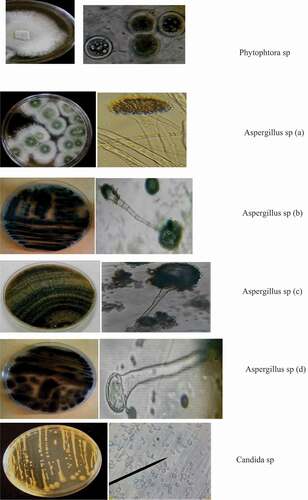
The isolation of fungi was carried out according to the method described by Chiejina (Citation2008). Altered zucchini were rinsed with distilled water and then disinfected with 70°C ethanol during 1 min. Then, the infested areas are removed, cut and deposited aseptically on the Potato Dextrose Agar (PDA) (Merck, Germany) medium. The Petri dishes were incubated at 28 ± 1°C (Ewekeye et al., Citation2013) for five days. Pure cultures were obtained by several transfers of colony growth from PDA plates to clean PDA plates aseptically. Isolates were identified based on the growth patterns, color of mycelia and microscopic examination of vegetative and reproductive structures according to Botton et al. (Citation1990).
2.3. Pathogenicity test
This test was performed according to the method described by Okigbo et al. (Citation2009). Thus, unaltered zucchini are washed with tap water, rinsed three times with sterile distilled water and disinfected using ethanol-soaked paper towels at 70°C. Three wells 3 mm in diameter and 5 mm deep were made on each zucchini. Then, 100 µl of a suspension prepared from colonies of the presumptive pathogen isolated were introduced into the wells. After 3 to 7 days, an observation was made on the presence or absence of disease symptoms and also the type of symptoms.
2.4. Pathogen incrimination test
The implication of the microorganism in the alteration of the zucchini was confirmed by a re-isolation test. Its realization consisted in taking part of the mycelium from the altered zucchini after the artificial inoculation and to cultivate it on the PDA medium. After incubation at 28°C for three days, macroscopic and microscopic observations were made to establish a correspondence with the previous isolated fungus.
2.5. In vitro antagonism
Pseudomonas fluorescens F19 were examined for its inhibitory effect against isolated fungi which was obtained from spoiled zucchini. In vitro antifungal activity was assessed according to the method of Ahmed et al. (Citation2007). Briefly, strains were cultured diametrically on Potato Dextrose Agar (PDA) and one mm-diameter mycelia disk of a pure culture of the pathogen were spotted at each part of the strains of P. fluorescens F19. As control, a disk of the pathogen was placed at the centre of PDA medium in a Petri dish. The Petri dish were incubated for one week at 28°C in darkness and the radius of each fungal growth was measured. Relative growth inhibitions were expressed as a percentage. The experiment was conducted twice.
Inhibition rate = [] × 100 (Erdogan & Benlioglu, Citation2010)
r: Radius of microorganism growth in presence of Pseudomonas fluorescens F19
R: Radius of microorganism growth without treatment
2.6. In vivo antagonism test of Pseudomonas fluorescens F19
Mature zucchini vegetables (variety Courcouzelle) used in all experiments were carefully selected without disease or wounding symptoms. Vegetable surface was disinfected by dipping ethanol 70% for 3 min, rinsed three times with sterile distilled water and dried under filter-sterilized air flow. The 2 cm wide and 1.5 cm deep wells were then artificially created with a sterile knife. Zucchini were treated with the bacterial antagonist by adding 100 μl of cell or endospore suspension containing either 107 endospores/ml obtained at 600 nm depending on the experiment, in each wounded site 24 hours prior to pathogen challenge. Infection with pathogen was realized in all cases 24 hours after treatment with P. fluorescens F19, by adding the same volume of a conidial suspension prepared as described above in order to introduce 105 conidia/ml per site. The controls were directly inoculated with the fungal strains isolated from the spoiled zucchini. Treated vegetables were incubated in a laminar air-flow cabinet at 22°C and disease incidence was evaluated 2, 3, 5, 7 and 10 days after pathogen challenge based on the diameter of spreading grey mould lesions that developed around infected sites. The experiment was repeated twice.
2.7. Conservation test of zucchini by Pseudomonas fluorescens F19
2.7.1. Supernatant of Pseudomonas fluorescens F19 production
Preparation of the pre-culture
A pure colony (24 hours) of Pseudomonas fluorescens F9 were inoculated in Erlenmeyer flasks of 250 ml containing 65 ml of Yeast Peptone Glucose (YPG) medium. These medium were incubated at 30°C in orbital shaker (shaking incubator, Biobase) at 105 rpm during 8 hours. These pre-cultures were used to inoculate 2 L Erlenmeyer flasks containing 500 ml of YPG medium.
Production and harvest of supernatant
The production of supernatant was conducted in two Erlenmeyer flasks of 2 L. Each Erlenmeyer containing 500 ml of sterile YPG medium was inoculated with 65 ml of the pre-culture and incubated at 30°C in orbital shaker (shaking incubator, Biobase) at 105 rpm during 48 hours. After incubation, the medium were centrifuged at 4°C at 5000 rpm for 10 minutes. The pellet, which represents the biomass was separated from the supernatant and washed three times with physiological water (9 ‰ NaCl). The supernatant were stored at 4°C for storage tests.
2.7.2. Conservation test
This test consisted in immersing the zucchini vegetables in a container containing the supernatant of P. fluorescens F19 according to the method described by Cissé (Citation2012). Therefore, the healthy zucchini were washed in tap water and rinsed three times with sterile distilled water. They were disinfected with a paper towel soaked in 70% ethanol during 1 min. They were then sliced and immersed in the supernatant and then air dried. Controls were not treated with the supernatant. Zucchini after immersion were stored at room temperature (25°C). After the appearance of mycelia on zucchini, an observation is made.
2.8. Statistical analysis
Analysis of variance (ANOVA) was used to analyze the data. The difference between the treatments was considered significant at the 5% level (P = 0.05). Tukey test was carried out to determine the various classes of homogeneity through software R x64 3.5.2 for Windows.
3. Results
3.1. Fungi isolated from zucchini
The macroscopic and microscopic study revealed molds and a kind of yeast isolated on the spoiled zucchini. The moulds involved in spoilage of zucchini are made up of the genera Aspergillus, Candida and Phytophthora. Thus the genus Aspergillus is the most dominant with four species including Aspergillus sp (a), Aspergillus sp (b), Aspergillus sp (c) and (d). The other genera were reported with one species for each genus. The isolated moulds are mentioned in .
3.2. Fungi implication in zucchini alteration
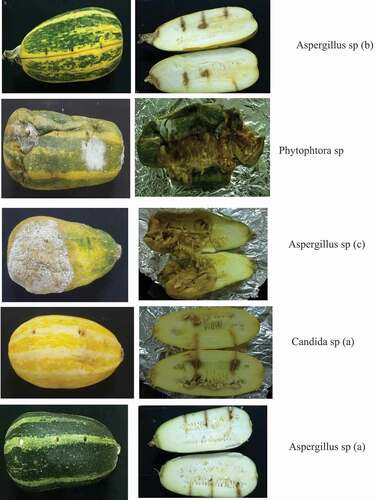
Aspergillus sp (a) and Aspergillus sp (b) caused alterations that are less visible outdoors after 10 days. However, they are observed indoors through brown areas. Zucchini inoculated by Phytophtora sp. showed symptoms of rottenness which manifested themselves by wilting of the zucchini, the appearance of brown spots and white mycelium. The zucchini showed signs of spoilage as early as the 3rd day and the rot was complete by the 10th day. Alteration related to Aspergillus sp. (c) resulted in a whitish film on the inoculated areas. The zucchini showed signs of spoilage 4 days after the artificial inoculation. This alteration progressed until the 10th day with the almost total rotting of the zucchini. Zucchini artificially inoculated with Candida sp. showed a medium degree of alteration, which manifested itself as an almost intact zucchini. Considering the level of involvement of each strain in the spoilage of the zucchini, observations revealed that the strains of Phytophtora sp. Candida sp. and Aspergillus sp. (c) caused more damage than the others. For this reason, these three (3) strains were selected for further work ().
3.3. Biological activities of Pseudomonas fluorescens F19
3.3.1. Antifungal activity of P. Fluorescens F19
The inhibition zones varied, respectively, from 45.83% to 85.38% when P. fluorescens F19 was cultivated 24 H before the confrontation and from 64.58% to 92.30% when it was cultivated 72 H before the confrontation with the pathogens ().
Table 1. Inhibition rates of Pseudomonas fluorescens F19 versus fungal isolated
3.3.2. In vivo antagonist test of P. fluorescens F19
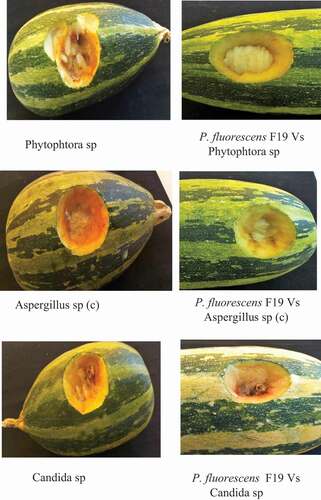
P. fluorescens F19 biomass reduced the incidence of pathogens in zucchini vegetable spoilage. This efficiency resulted in an absence of rot on the walls of the openings ().
3.3.3. Test of zucchini vegetable conservation
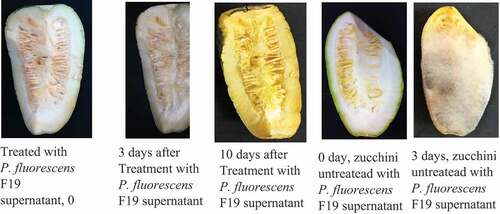
The zucchini untreated with the Pseudomonas fluorescens F19 supernatant recorded alterations on the third day of storage. However, in spite of the color change on the zucchini treated with P. fluorescens F19 supernatant, they showed no signs of deterioration after 10 days of conservation ().
4. Discussion
Zucchini, like any fruit and vegetable, suffer significant post-harvest losses which would contribute to their scarcity on the markets. During the transport of fruits and vegetables, losses can be caused by physical shocks although the packaging often helps to mitigate the blows, but also when the refrigeration conditions are not respected. The microbiological analyzes carried out on zucchini of the Coucourzelle variety have shown that the spoilage fungal flora consists mainly of Aspergillus sp., Phytophthora sp and Candida sp.
Indeed, according to Rispail (Citation2008), the genus Candida sp. usually has mucous membranes, skin and integuments as its habitat. It could infect zucchini during picking or during handling by traders and customers. Koua in 2015 isolated the genus Candida sp. on Kéith variety mangoes in Côte d’Ivoire. The presence of Candida sp. could reflect a lack of hygiene in zucchini. Indeed, yeasts of the genus Candida sp. are found in the digestive tract of animals and humans. Their presence on zucchini would reveal poor hygienic conditions observed during their handling. The presence of Aspergillus sp. on zucchini would have occurred during storage as those molds encountered during food storage. According to Pitt and Hocking (Citation1977) and Cahagnier et al. (Citation1998), Aspergillus is one of the dominant moulds of mouldy flora. This genus was also isolated by Djossou (Citation2011) on coffee in Côte d’Ivoire. Lamrani (Citation2009) isolated Aspergillus niger, Aspergillus flavus, Aspergillus fumigatus and Aspergillus ochraceus, on olives in Morocco. The presence of Aspergillus sp. on zucchini is undesirable due to their toxicological potential. Under favourable conditions, some Aspergillus sp. are responsible for the production of mycotoxins such as aflatoxins and ochratoxin A (Abarca et al., Citation2003). The presence of Phytophthora sp. on zucchini could be associated with contamination from the field. Indeed, some species of Phytophthora are soil germs and are responsible for the spoilage of many fruits and vegetables in the field. This is the case with Phytophthora capsici, the agent responsible for crown and fruit rots in cucurbits (Couture, Citation2008). This species is feared around the world in cucurbit crops because of the enormous post-harvest losses it causes, as was the case in Mexico in 1922 (Couture, Citation2008). According to Messiaen et al. (Citation1991), At least four phytophthora (P. megasperma, P. cryptogea, P. dreschleri, P. capsici) have been reported as susceptible to attacking the planar crown of cucumbers, melons or zucchini. In south of France, the most to be feared will be P. capsici, the agent of zucchini fruit rot. The biological activity inhibited the growth of different isolates. P. fluorescens F19 has an antifungal character capable of inhibiting the growth of Candida sp., Phytophthora sp. and Aspergillus sp. (c) This activity was detected in vitro on solid medium. P. fluorescens F19 had a mean inhibition of 73.33% of all isolates. This could be due to the action of an enzyme called chitinase excreted by Pseudomonas fluorescens F19 in the medium. According to Kishor et al. (Citation2005), the cell wall of phytopathogenic fungi consists of chitin (linear polymer of β- (1–4) -N acetylglucosamine), and that the production of chitinases by fluorescent Pseudomonas disintegrates this polymer. Naik and Sakthivel (Citation2006) also reported that the chitinolytic activity of Pseudomonas fluorescens by production of chitinases hydrolyzes chitin, leading to distortion of the hyphae of pathogenic fungi. This result could also be explained by the extraction by Pseudomonas fluorescens F19 of several antimicrobial metabolites with antifungal activity. According to, Weller et al. (Citation2007), Pseudomonas fluorescens produce several antifungal metaoblites in the stationary phase such as phenazines, pyrrolnitrine, pyoluteorin and DAPG (2,4-diacetylphloroglucinol), which are frequently detected in Pseudomonas fluorescens. In vitro analyzes carried out at different incubation times of the biopesticide agent showed that the percentage inhibition of the spoilage agent increased with the cultivation time of P.fluorescens F19. This could be explained by the fact that after 72 hours of culture in agar medium, the majority of secondary metabolites responsible for the inhibition of pathogens are produced and diffused in the agar. Thus, the latter already present in the agar would effectively inhibit the proliferation of spoilage germs in zucchini. The reference strain (Pseudomonas fluorescens F19) showed a high level of efficacy against pathogens on zucchini. This reduction in the incidence of Phytophthora sp and Aspergillus sp (c) in zucchini spoilage is believed to be primarily due to one of the properties of Pseudomonas fluorescens F19 which is the rapid and extensive colonization of injury sites. This would hamper the establishment of the pathogen at the injury sites by reducing the space and nutrients available. Authors such as Droby et al. (Citation2006) and Zhao et al. (Citation2008) reported that, application of the antagonist to injury sites prior to pathogen infection is necessary to ensure better colonization and, maximum rate of protection by reducing the incidence of infection of pathogen. Moreover, this reduction in the incidence of pathogens in spoilage of zucchini could also be attributed to the phenomenon of resistance induction in zucchini fruits. Indeed, in the case of post-harvest diseases, several studies have shown that certain antagonists can establish particular interactions with the injured tissues, which would allow the fruits to accelerate the processes of wound healing and induce the processes of resistance to fruit pathogens (Droby & Chalutz, Citation1994). The studies of Zhao et al. (Citation2008) showed that inoculation of tomatoes with a strain of P. fluorescens had generated peroxidase (POD), polyphenoloxidase (PPO), superoxide dismutase (SOD) and catalase (CAT), phenynl alanine ammonia-lyase (PAL), chitinase (CHI) and β-1,3-glucanase correlated with induction of resistance in tomato fruits to Rhizopus nigricans. Immersion of zucchini in P. fluorescens F19 supernatant extended the shelf life of zucchini by 7 days. Beyond this time, spots appeared on the treated fruits. On the other hand, the control not having been treated with the supernatant of P. fluorescens F19 was kept for a period of 3 days. Inhibition of the growth of pathogens using the culture supernatant of P. fluorescens F19 demonstrates the presence in the supernatant of antimicrobial agents. In fact, Bérdy (Citation2005) showed in a study that the P. fluorescens strains produced in their culture medium about 800 compounds, of which 610 are antifungals. This would confirm the ability of P. fluorescens F19 to protect zucchini against yeasts and molds. This protection has been observed by Touré et al. (Citation2004) in the storage of apples with B. subtilis for 10 days. Also, according to Koua (Citation2015), the use of B. subtilis GA1 biomass made it possible to extend the storage time of the mango of the Kéith variety from 3 to 12 days.
5. Conclusion
The objective of this study was to contribute to the control of fungal alterations of Zucchini in Côte d’Ivoire using P. fluorescens F19. The results of microbiological analyzes have shown that zucchini from Côte d’Ivoire area are contaminated by molds of the genus Aspergillus, Phytophtora and Candida. P. fluorescens F19 has been demonstrated to have antifungal properties on isolated moulds through in vivo using the supernatant. This suggests that P. fluorescens F19 or its metabolites could be a means of biological control against fungal alterations of zucchini and thus increase the conservation period.
Additional information
Funding
Notes on contributors

Eric Y. Yapi
Eric Yapi Yapi is a researcher and Microbiologist at National Center for Agronomic Research, Technological Research Station, Abidjan, Côte d’Ivoire.
Fulgence Y. Koffi
Fulgence Yao Koffi is Microbiologist and a teacher, researcher at Department of Biochemistry-Genetics, UFR-Biological Sciences, Peleforo GON COULIBALY University, Korhogo, Côte d’Ivoire.
References
- Abarca, M. L., Accensi, F., Bragulat, M. R., Castella, G., & Cabañes, F. J. (2003). Aspergillus carbonarius as the main source of ochratoxin A contamination in dried vine fruits from the Spanish market. Journal of Food Science, 66(3), 504–12. DOI: https://doi.org/10.4315/0362-028x-66.3.504
- Ahmed, I. H., Labuschagne, N., & Korsten, L. (2007). Screening rhizobacteria for biological control of Fusarium root and crown rot of sorghum in Ethiopia. Biological Control, 40(1), 97–106. https://doi.org/https://doi.org/10.1016/j.biocontrol.2006.07.017
- Alloue-Boraud, M., Ban-Koffi, L., Dadie, T., Dje, K., & Ongena, M. (2015). Utilisation de Bacillus subtilis GA1 pour la lutte contre les germes d’altération de la mangue en Côte d’Ivoire. Journal of Animal and Plant Sciences, 25, 3954–3965. http://www.m.elewa.org/JAPS
- Ban Koffi, L., Alloue-boraud, W. A. M., Assamoi, J., Koussemon, M., & Ongena, M. (2015). Study of protective effect of Pseudomonas fluorescens F19 against microorganisms responsible of tomato fruits (Lycopersicon esculentum Mill) spoilage in Côte d’Ivoire. Journal of Innovative Research, 3(9), 9–16.
- Ban Koffi, L., Alloue-Boraud, W. A. M., Dadie, A. T., Koua, S. H., & Ongena, M. (2017). Enhancement of mango fruit preservation by using antimicrobial properties of Bacillus subtilis GA1. Cogent Food & Agriculture, 3(1), 1394249. https://doi.org/https://doi.org/10.1080/23311932.2017.1394249
- Bérdy, J. (2005). Bioactive microbial metabolites. The Journal of Antibiotics, 58(1), 1–26. https://doi.org/https://doi.org/10.1038/ja.2005.1
- Botton, B., Breton, A., Fèvre, M., Gauthier S., Vayssier Y. et Veau P. (1990). Moisissures utiles et nuisibles (Importance industrielle ed., pp. 512p). Masson.
- Cahagnier, B. S., Dragacc, C., Frayssinet, J. M., et Frayssinet, C. (1998). Moisissures des aliments peu hydrates. Lavoisier Tec&Doc.
- Chiejina, N. V. (2008). Mycoflora of some salad vegetables. Biological Research, 6(2), 392–395.
- Cissé, M. (2012). Immobilisation d’un système lactoperoxyde dans un enrobage de chitosane dans le but de prolonger la conservation des mangues [Thèse de doctorat]. Université Montpellier Supagro, 159p
- Couture, I. (2008). Agronomie, Phytophthora capsici: Une maladie de sol redoutable, Ministère de l’Agriculture, des Pêcheries et de l’Alimentation 1355, rue Gauvin, Bureau 3300, St-Hyacinthe (Québec), 7 p.
- Djossou, O. (2011). Microflores post-récolte du café robusta et utilisation des bactéries pour le contrôle des moisissures mycotoxynogènes et de l’Ochratoxine A [Thèse de doctorat]. Université Paul Cezanne AIX Marseille III, 123p.
- Droby, S., & Chalutz, E. (1994). Mode of action of biocontrol agents of postharvest disease. In C. L. Wilson & Wisniewski (Eds.), Biological Control of postharvest disease. Theory and practice (pp. 63–75). CRC Press.
- Droby, S. (2006). Improving quality and safety of fresh fruits and vegetables after harvest by the use of biocontrol agents and natural materials. Acta Horticulturea, 709, 45–51
- Erdogan, O., & Benlioglu, K. (2010). Biological control of Verticillium wilt on cotton by the use of fluorescent Pseudomonas spp. Under field conditions. Biological Control, 53(1), 39–45. https://doi.org/https://doi.org/10.1016/j.biocontrol.2009.11.011
- Ewekeye, T. S., Oke, O. A., Quadri, A. I., Isikalu, A. O., Umenwaniri, M. O., & Durosinmi, M. L. (2013). Studies on post harvest deterioration of some fruits and vegetables in selected markets in Lagos State, Nigeria. American Journal of Research Communication, 1(10), 209–223. www.usa-journals.com
- FAO. (1995). Fruit and vegetable processing. Agricultural Services Bulletin 119. Rome, 50(3), 245–255. www.fao.org/3/v5030e/V5030E00.htm
- Ife, F. J., & Kuipers, B. (2003). La conservation des fruits et légumes, Agrodok 3, Fondation Agromisa, Wageningen, ISBN: 90-77073-32-9 , 94p. Agromisa.
- Kishor, G. K., Pande, S., & Podile, A. R. (2005). Biological control of collar rot disease with broadspectrum antifungal bacteria associated with groundnut. Canadian Journal of Microbiology, 51(2), 123–132. https://doi.org/https://doi.org/10.1139/w04-119
- Koffi, Y. F., Alloue-Boraud, W. A. M., Koffi, L. B., Adohi, A. F., Dje, M. K., & Ongena, M. (2016). Highlighting Bacillus subtilis GA1 antifungi potentialities for pineapple (Ananas comosus) conservation in Côte d’Ivoire. International Journal of Agronomy and Agricultural Research, 9(1), 100–108. http://www.innspub.net
- Koua, S. H. (2015). Determination des propriétés inhibitrices de Bacillus subtilis GA1 sur la croissance des germes d’altération des mangues [Mémoire de Master]. université NANGUI ABROGOUA, 59 p.
- Koua, S. H., Coulibaly, N. D., Alloue-Boraud, W. M., Konan, F., & Dje, K. M. (2020). Bacillus subtilis strains isolated from cocoa trees (Theobroma cacao L.) Rhizosphere for their use as potential plant growth promoting rhizobacteria in Côte d’Ivoire. Current Microbiology, 77(9), 2258–2264. https://doi.org/https://doi.org/10.1007/s00284-020-02027-x
- Kouadia, A. M.-J., Abo, K., & Kouadio, K. T. (2019). Evolution des infections naturelles sur les mangues, les avocats et les bananes en Côte d’Ivoire et principaux champignons responsables. Journal of Applied Biosciences, 134(1), 13710–13721. https://doi.org/https://doi.org/10.4314/jab.v134i1.8
- Lamrani, K. (2009). Etude de la biodiversité des moisissures nuisibles et utiles isolées à partir des Maâsra du Maroc [Thèse de doctorat] Université Mohamed V – Agdal faculté des sciences Rabat, Maroc, 213p.
- Mathieu, C., Gérald, C., & Ronan, B. (2009). La production biologique de courgettes en Bretagne, (PAIS/Inter Bio Bretagne), 5p http://www.maraibio.fr
- Messiaen, C.-M., Dominique, B., & Francis, R. (1991). Les maladies des plantes maraîchères (3e ed., pp. 552 pages). Quae.
- Naik, P. R., & Sakthivel, N. (2006). Functional characterization of a novel hydrocarbonoclastic Pseudomonas sp. strain PUP6 with plant-growth-promoting traits and antifungal potential. Research in Microbiology, 157(6), 538–546. https://doi.org/https://doi.org/10.1016/j.resmic.2005.11.009
- Okigbo, R. N., Ramesh, P., & Achusi, C. T. (2009). Post-harvest deterioration of cassava and its control using extracts of Azadirachtaindica and Aframomummelegueta. E-Journal of Chemistry, 6(4), 1274–1280. https://doi.org/https://doi.org/10.1155/2009/680519
- Ongena, M. (2014). Biopesticides: Une protection plus naturelle pour les cultures. Université de Liège, 10p http://reflexions.ulg.ac.be
- Pitt, J. I., & Hocking, A. D. (1977). Influence of solute and hydrogen ion concentration on the waterrelations of some xerophilic fungi. Journal of General Microbiology, 101(1), 35–40. https://doi.org/https://doi.org/10.1099/00221287-101-1-35
- Raaijmakers, J. M., Vlami, M., & De Souza, J. T. (2002). Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek, 81(1/4), 537–547. https://doi.org/https://doi.org/10.1023/A:1020501420831
- Rispail, P. (2008). Epidémiologie et diagnostic biologique des candidoses muqueuses et cutanéo-phanériennes. Module Intégré 5 Dermatologie. Faculté de Médecine Montpellier-Nîmes, 10p
- Tassadit, D. (2012). Amélioration de la conservation des mangues 4ème gamme par application de traitements thermiques et utilisation d’une conservation sous atmosphère modifiée [Thèse de doctorat]. Université d’Avignon et des pays de vaucluse, 149p.
- Thakore, Y. (2006). The biopesticide market for global agricultural use. Industrial Biotechnology, 2(3), 294–298. https://doi.org/https://doi.org/10.1089/ind.2006.2.194
- Touré, Y., Ongéna, M., Jacques, P., Guiro, A., & Thonart, P. (2004). Role of lipopeptides produced by Bacillus subtilis GA1 in the reduction of grey mould disease caused by Botrytis cinerea on apple. Journal of Applied Microbiology, 96(5), 1151–1160. https://doi.org/https://doi.org/10.1111/j.1365-2672.2004.02252.x
- Weller, D. M., Raaijmakers, J. M., Gardener, B. B., & Thomashow, L. S. (2007). Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annual Review of Phytopathology, 40(1), 309–348. https://doi.org/https://doi.org/10.1146/annurev.phyto.40.030402.110010
- Whipps, J. M. (2001). Microbial interactions and biocontrol in the rhizosphere. Journal of Experimental Botany, 52(suppl_1), 487–5119. https://doi.org/https://doi.org/10.1093/jxb/52.suppl_1.487
- Yankel, S. (2017). Retrieved may 17, 2018, from https://culturemaraichage.com/2017/09/11/la-culture-de-la-courgette-en-cote-divoire/
- Yaouba, A., & Mpounze, E. G. P. (2017). Isolation and pathogenicity evaluation of postharvest fungal of some fruits in Cameroun. International Journal of Environmental Research, 2(1), 56–60. https://doi.org/http://dx.doi.org/10.22161/ijeab/2.1.9
- Zhao, Y., Tu, K., Shao, X., Jing, W., & Su, Z. (2008). Effects of the yeast Pichiaguilliermondii against Rhizopus nigricans on tomato fruit. Postharvest Biology and Technology, 49(1), 113–120. https://doi.org/https://doi.org/10.1016/j.postharvbio.2008.01.001