?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The efficiency of squid by-product hydrolysate supplementation in the diet of carnivorous black tiger shrimp (Penaeus monodon) has been evaluated for 8 weeks. A low fish meal diet, containing 5% fish meal (unsupplemented control), was formulated and supplemented with graded levels of squid by-product hydrolysate at 0.5% and 1%. A positive control with 22% fish meal as the main protein source was also prepared. The growth in terms of weight gain (WG) and specific growth rate (SGR), nutrient retentions, digestive enzyme activities and muscle growth-related genes expression has been evaluated. Results revealed that WG and SGR of shrimp fed on diets supplemented with 0.5% and 1% squid by-product hydrolysate (WG:534.02–555.07%, SGR:3.30–3.36% day−1) were comparable (p > 0.05) to the fish meal control (WG:544.03%, SGR:3.33% day−1) and higher (p < 0.05) than the unsupplemented control group (WG:434.68%, SGR:2.99% day−1). Similar trend has been observed in protein efficiency ratio (0.16–0.20), nutrient retentions (protein retention:10.95–13.42%, lipid retention:2.71–3.15%) and muscle growth-related genes expression (GLUT1:1.05–1.50, MHC:0.78–1.33, tor:1.09–1.43, 4e-bp:1.17–1.33; s6k:1.17–1.53). Total feed intake (988.11–1268.97 mg), feed conversion ratio (1.63–2.05) and survival (84.87–92.11%) were not significantly different (p > 0.05) among treatments. Moreover, digestive enzyme activities (protease:1010.13–1503.45 Units mg−1protein, amylase:168.51–223.45 Units mg−1protein, lipase:4510.93–5380.82 Units mg−1protein) were not influenced (p > 0.05) by hydrolysate supplementations. These results suggest that the growth performance of P. monodon fed on a plant protein-based diet (with 5% fish meal) has improved upon the supplementation of at least 0.5% squid by-product hydrolysate in the formulated feed and is comparable to that of the shrimp fed the fish meal-based diet.
PUBLIC INTEREST STATEMENT
Utilization of low fish meal or plant protein-based diets in shrimp are widely studied to lessen the fish meal dependence of aquafeed formulations. However, the use of plant proteins resulted in growth reduction in the cultured organisms and a decrease in harvestable biomass. To address this issue, squid processing by-product hydrolysate is supplemented in a low fish meal diet for carnivorous shrimp with the primary intention of evaluating if the hydrolysate could improve the feed value, comparable to a full fish meal diet, of a plant protein-based diet. Our results indicate that squid by-product hydrolysate supplementation improved the feed value of the plant protein-based diet, and enhanced shrimp growth and feed conversion efficiency comparable to a full fish meal-based diet. Collectively, our study suggests that the use of locally produced hydrolysate as a dietary supplement to plant protein-based diets can lessen the fish meal dependence of feed formulations.
1. Introduction
Recently, there is a surge of interest in the development of dietary formulations with minimal fish meal content for cultured aquatic animals since fish meal supply is limited and considered unsustainable. The most extensively studied alternative protein sources are plant proteins (Samocha et al., Citation2004). However, it was observed that the use of plant proteins in aquaculture diets is linked to reduced animal growth and has limitations including diet palatability and attractability (Dossou et al., Citation2018; García-Ortega et al., Citation2016; Yaghoubi et al., Citation2016). These observations suggest that formulated plant protein-based diets, even all essential nutrients are provided, could not equate the benefits of fish meal-based dietary formulations for shrimp. Fish meal appears to contain growth promoting factors that could not be provided or not present in most plant-based dietary formulations. However, previous studies showed that inclusion or supplementation of protein hydrolysates enhanced the quality of low fish meal or plant-based diets for shrimp (Gonzalez et al., Citation2007; Li et al., Citation2018). Fish protein hydrolysates were found to enhance growth of aquatic animals by improving protein and lipid accretion, enhancing digestive enzyme activities and promote muscle growth by regulating the expression of muscle growth-related genes (Canada et al., Citation2018; Niu et al., Citation2014; Shao et al., Citation2018; Wei et al., Citation2020).
It is estimated that only about 50–60% of the total seafood catch worldwide is utilized for direct human consumption and the rest is processed since they are main sources of inedible by-products (Venugopal, Citation2009). Due to the expansion of the aquatic animal processing industry, a significant volume of underutilized and unutilized processing industry by-products are generated and are becoming an environmental hazard (Arvanitoyannis & Kassaveti, Citation2008; Uddin et al., Citation2010). Currently, the squid processing industry has intensified its operations and is expanding rapidly. In the squid processing industry, about 52–60% of by-products are produced and these include heads, viscera, pens, ink, skin, fins, tentacles, arms, and damaged mantles (Ezquerra-Brauer & Aubourg, Citation2019; Joseph et al., Citation1987). To efficiently utilize this biological aquatic resource, practical bio-conversion and utilization of these processing by-products are currently being explored (Halim et al., Citation2016; Wang et al., Citation2019). Conversion of this squid industry by-product into a hydrolysate has been suggested to generate a potential bioactive feed supplement for aquaculture.
Squid by-product hydrolysate is a prospective attractant due to its high protein content and balanced essential amino acid profile. Lian et al. (Citation2005) reported that squid hydrolysate contains attractant amino acids such as glycine and alanine. It also contains a significant amount of taurine which is a strong feeding stimulant (González-Félix et al., Citation2014). Furthermore, an unknown growth promoting factor termed as squid factor has also been reported to be present in this hydrolysate (Guillaume et al., Citation1989). Squid protein has been widely utilized as a fish feed ingredient. It has been estimated that the squid products’ market size within aquaculture feeds is 25,000−75,000 tonnes for squid meal, 35,000–100,000 tonnes for squid liver powder and 10,000–50,000 for squid liver oil (Tacon et al., Citation2006). Squid meal as a feed ingredient has been known to promote growth of cultured shrimps (Cruz-Suárez et al., Citation1992; Sánchez et al., Citation2012). Several authors have also documented the antioxidant properties of cephalopods protein produced from cuttlefish and squid (Bousopha et al., Citation2016; Siahpoosh & Alikhani, Citation2016; Soufi-Kechaou et al., Citation2015; Suárez-Jiménez et al., Citation2015). Moreover, González-Félix et al. (Citation2014) documented that jumbo squid protein hydrolysates supplemented at 5% to a fish meal-based diet was found to promote the growth of Penaeus vannamei. These earlier studies have shown the potential of squid protein hydrolysate as a supplement to promote growth of cultured shrimp. However, in these studies the squid hydrolysate was used as a supplement to fish meal-based experimental diets and most of these studies were done in P. vannamei. To date, there has been limited information on the influence on growth and nutrient utilization of squid by-product hydrolysate as a dietary supplement to a plant protein-based diet for a highly carnivorous shrimp, Penaeus monodon. The present study investigates the influence of squid by-product hydrolysate supplementation to plant protein-based diet on growth performance and nutrient utilization of P. monodon. This investigation also documents the effects of squid by-product hydrolysate supplementation on the digestive enzyme activities and muscle-growth related genes expression in P. monodon.
2. Materials and methods
2.1. Test diets
Basal plant protein-based diets, containing 5% fish meal and formulated to satisfy the nutrient requirement of P. monodon, were prepared following the formulation of (González-Félix et al., Citation2014) with modifications. Three experimental diets were prepared by supplementing the basal diet with increasing levels of squid by-product hydrolysate at 0%, 0.5% and 1%. Another dietary treatment containing fish meal (22%) as the main protein source was formulated to serve as the fish meal control group (FM Control). The ingredients and composition of the experimental diets are presented in .
Table 1. Composition of the control and experimental diets (g 100 g−1 diet, dry weight)
All dry ingredients were sieved in a 100 µm mesh and were mixed thoroughly prior to the addition of fish oil. Wheat gluten served as the binder, feed ingredients were mixed for 15 min, formed into a dough, and extruded in a 2 mm die laboratory pelletizer. The pelleted diets were oven-dried at 60°C for 24 h. Then, the formulated diets were cooled down at room temperature for 30 min and stored at −20°C. Pellets were ground to appropriate sizes prior to feeding. Proximate composition of the diets which include protein, fiber, ash, and moisture were analyzed following AOAC (Citation1990) while diet lipid was determined according to Bligh and Dyer (Citation1959).
2.2. Test animal and maintenance
Specific pathogen-free black tiger shrimp post larvae were sourced from a local hatchery and transported to the experimental rearing facilities of the Institute of Aquaculture, University of the Philippines Visayas, Philippines. The shrimp were acclimated in a recirculating set-up for 2 weeks and fed with the commercial diet prior to the start of the experiment. Optimum water physico-chemical parameters were maintained throughout the culture period (salinity: 21.9–23.7 ppt, temperature: 28.5–30.9°C, dissolved oxygen: >5 ppm, ammonia: 0–0.25 ppm, nitrite: 0 ppm, pH 8.0–8.2). All experiments were conducted in adherence to all the applicable guidelines (institutional, national, and international) on the care and use of animals for research.
2.3. Growth trial
During the feeding trial, P. monodon (ABW: 0.11 ± 0.01 g) were stocked randomly into 12 units of 60 L tanks at a density of 25 individuals tank−1. Experimental animals were fed with test diets in excess to allow ad libitum feeding at 0800, 1200 and 1600 h for 8 weeks. Excess feeds were collected 1 h after every feeding, dried at 60°C and weighed to account the total feed intake. Periodic sampling was done every 15 days to monitor shrimp growth, survival, feeding ration adjustment and tank cleaning. Upon the termination of the growth trial, % weight gain, specific growth rate, protein efficiency ratio, feed conversion ratio and survival were computed. The following equations were used in the computation of growth parameters and nutrient retention (Hardy & Barrows, Citation2002).
where: CNC – carcass nutrient content
From each treatment, shrimp samples were also collected for the analysis of the digestive enzyme activities and muscle growth-related genes expression. Initial and final carcass composition (moisture, protein, ash) of the experimental animals were measured using AOAC (Citation1990) method while lipid composition was determined according to Bligh and Dyer (Citation1959).
2.4. Digestive enzyme activity analyses
Shrimp hepatopancreas were collected and homogenized in a buffer for the determination of total protease, α-amylase, and lipase activities. Protein content of the tissue homogenate extract was measured according to Bradford (Citation1976) using bovine serum albumin as a standard. Protease activity was analyzed according to Burokerkilgore and Wang (Citation1993) using casein as a substrate. Whereas starch was utilized as substrate in the α-amylase activity assay (Bernfeld, Citation1951 as modified by Areekijseree et al., Citation2004). Moreover, the protocols of Pinsirodom and Parkin (Citation2001) were followed in analyzing digestive lipase activity of the shrimp. One enzyme unit was equivalent to change in absorbance of the samples without and with crude extract min−1 mg−1 protein, μg glucose produced min−1 mg−1 protein and µM fatty acid produced min−1 mg−1 protein for protease, α-amylase and lipase activities, respectively. Enzyme activities were expressed as units mg−1 protein.
2.5. Muscle growth-related gene expression analyses
First abdominal muscle segment of shrimp was dissected, excised aseptically and stored in RNAlater (Invitrogen) prior to analysis. The collected tissue was blotted dry and homogenized in TRIzol Reagent (Life Technologies) to isolate the total RNA. The purity and integrity of the isolated RNA were analyzed in Nanodrop spectrophotometer and in 1% agarose gel electrophoresis (Nayak et al., Citation2010), respectively. Complementary DNA was synthesized using GoScriptTM Reverse Transcription System (Promega). Transcription patterns of muscle growth-related genes such as glucose transporter 1 (GLUT 1), myosin heavy chain (MHC), and TOR signaling pathway related genes (tor, s6k and 4e-bp) were determined by real-time reverse transcription polymerase chain reaction. The amplifications were carried out in a 96-well plate with a total volume of 10 µL using 1 µL of cDNa, 200 nM of each gene-specific primer pairs with 1X ViPrimePLUS Taq qPCR Green Master Mix I (Vivantis) in a Real-Time PCR system (CFX Connect, Bio-Rad). Relative gene expression was analyzed according to Tomy et al. (Citation2016) relative to a reference gene (shrimp elongation factor −1 alpha, EF-1α). The primer and PCR conditions are shown in () and a non-template control was also run for each primer pair to confirm the specificity of the reaction and to avoid non-template amplification.
Table 2. Primers and conditions used in real-time reverse transcription polymerase chain reaction for muscle growth-related genes
2.6. Statistical analysis
Data are reported as mean ± standard error of the mean (SEM). Growth, nutrient retention, digestive enzyme activity, and muscle growth-related gene expression data were analyzed using One-Way Analysis of Variance and the differences between means were determined using Tukey Test at 0.05 significance level. Prior to the conduct of One-Way Analysis of Variance, homogeneity of variances was tested using Levene’s test while normality of the data was analyzed using Shapiro-Wilk’s test.
3. Results
3.1. Growth performance
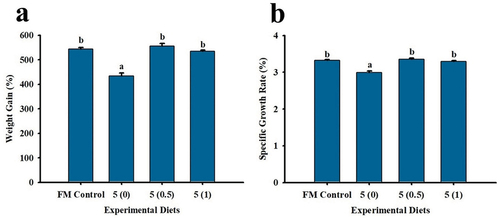
Upon the termination of the growth trial, % weight gain and specific growth rate were found significantly lower in the low fish meal diet control, without the hydrolysate supplementation. Shrimp fed with FM Control and diets supplemented with squid by-product hydrolysate at 0.5% and 1% exhibited similar growth rates and % weight gain values (). The total feed intake, feed conversion ratio and survival were not significantly different among treatments. Similar to weight gain and specific growth rate, protein efficiency ratio was lower in the unsupplemented control group as compared to the other treatments ().
Table 3. Zootechnical parameters of P. monodon fed with low fish meal diets supplemented with squid by-product hydrolysate for 8 weeks
3.2. Carcass composition
Proximate composition of shrimp carcass revealed that protein and lipid contents were not affected by the dietary treatments (). However, carcass ash content was significantly lower in 5 (0) in comparison to the fish meal control and diets supplemented with the hydrolysate.
Table 4. Carcass proximate composition (%, dry weight basis) P. monodon fed with low fish meal diets supplemented with squid by-product hydrolysate for 8 weeks
3.3. Nutrient retention
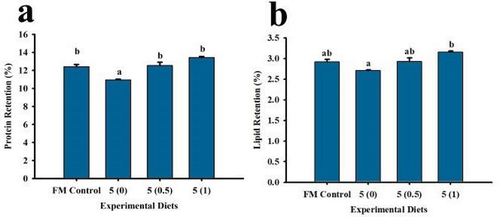
The protein retention of shrimp fed with the FM Control, 5 (0.5) and 5 (1) were found not significantly different (). The lowest protein retention was recorded in 5 (0) treatment group. Furthermore, lipid retention was also found lowest in 5 (0) treatment group but was not significantly different from the FM Control and 5 (0.5) treatment groups. The highest lipid retention was observed in the 5 (1) group.
3.4. Digestive enzymes
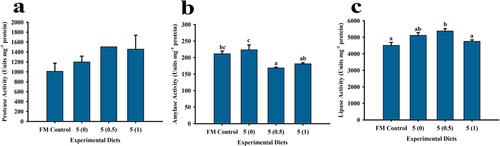
Digestive enzyme activities showed that protease was not affected by the treatments () but α-amylase was highest in treatment 5 (0) that was not significantly different from the FM control group. Lowest α-amylase activity was observed in treatment 5 (0.5) that was statistically similar to the value obtained in treatment at 5 (1). In addition, α-amylase activity in treatment 5 (1) did not differ significantly from FM Control treatment. Lipase activities in treatments FM Control, 5 (0) and 5 (1) groups were found to be similar. However, the treatment group at 5 (0.5) exhibited the highest lipase activity but did not differ significantly with that of the treatment at 5 (0).
3.5. Muscle growth-related genes expression levels
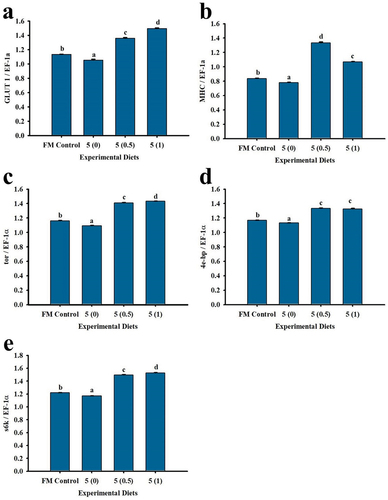
Most of the muscle growth-related genes were observed to be up-regulated in response to the dietary treatments (). Results showed highest transcription of GLUT 1, tor, 4e-bp and s6k genes except for MHC in treatment 5 (1). Highest MHC gene expression was found in 5 (0.5). Alternatively, the lowest gene expression was recorded in the unsupplemented control group at 5 (0).
Figure 4. Muscle growth-related genes expression of P. monodon fed with low fish meal diets supplemented with squid by-product hydrolysate for 8 weeks. (a) GLUT 1, (b) MHC (c) tor (d) 4e-bp (e) s6k. Values are mean ± SEM. Different superscript letters indicate significant differences among treatments.
4. Discussion
Cumulative information suggests that plant-based dietary formulations could not equal the benefits attained in using fish meal-based shrimp diets wherein it appears to contain growth promoting factors. However, earlier works demonstrated that protein hydrolysate supplementation improved the feed value of plant-based diets for shrimp (Forster et al., Citation2011; Li et al., Citation2018; Mendoza et al., Citation2001; Nunes et al., Citation2019; Suresh et al., Citation2011; Zhou et al., Citation2016). Squid by-product hydrolysate is another potential feed supplement that is highly suitable for aquaculture use (Lian et al., Citation2005) and might improve the value of plant protein-based diets. However, until now the use of squid protein hydrolysate to improve the feed value of a plant-based, low fish meal, dietary formulation has not been evaluated in a carnivorous shrimp, P. monodon. The present work is the first time to document the influence of a squid by-product hydrolysate to improve the feed value of a plant protein-based diet for P. monodon. Moreover, based on comprehensive review of existing literature, our present findings on the comparable growth of P. monodon, fed the fish meal-based diet and those fed with plant based-diet supplemented with squid by-product hydrolysate is considered unprecedented.
In the present study, no significant difference in survival () among the treatments has been noted indicating that the nutrient requirement of the experimental shrimp has been met (Alvarez et al., Citation2007). The depressed growth exhibited in the group without squid by-product hydrolysate supplementation ()) might be due to the presence of anti-nutritional factors and indigestible non-starch polysaccharides associated with the high soybean meal inclusion levels (Bulbul et al., Citation2016; Li et al., Citation2018). These negative effects of high soybean meal inclusion appear to have been offset by the supplementation of squid by-product hydrolysate as evident on the improved growth performance of shrimp in treatments supplemented with 0.5–1% squid by-product hydrolysate ()). This dietary inclusion level of squid by-product hydrolysate improved the feed value of a plant protein-based diet and promoted shrimp growth equivalent to those that received the full fish meal-based diet. The present results corroborate with the earlier findings suggesting that supplementation of protein hydrolysates enhances the growth performance of cultured shrimp including P. vannamei (Li et al., Citation2018; Soares et al., Citation2020) and Litopenaeus schmitti (Gonzalez et al., Citation2007).
Further in the present study, feed intake and feed conversion ratio were not affected by the supplementation of squid by-product hydrolysate (). This lack of effect of squid by-product hydrolysate supplementation on the feed intake and feed conversion ratio indicates that the growth promoting effect of this supplement is not associated with the feed attractability and efficiency of feed utilization. However, the observed enhancement of shrimp growth ()) associated with squid by-product hydrolysate supplementation in the present study can be attributed to the improvement in protein efficiency ratio (). Nguyen et al. (Citation2012) stated that protein efficiency ratio is commonly used as a criterion to evaluate the protein quality of a feed ingredient for aquatic feed suggesting that squid by-product hydrolysate supplementation improved the capability of the shrimp to efficiently utilize plant-based dietary proteins. Similar results have been reported by Quinto et al. (Citation2018) indicating that supplementation with protein hydrolysate in P. vananmei can elicit a protein efficiency ratio similar to diets with full fish meal as the protein source. Also, similar findings were reported by Valle et al. (Citation2015) and suggested that the significant improvement in protein efficiency ratio in response to protein hydrolysate supplementation is attributed to the presence of free amino acids and highly digestible peptides which can improve protein absorption (Hernández et al., Citation2011).
Furthermore, the observed enhancement of protein efficiency ratio and growth of shrimp receiving the squid by-product hydrolysate supplement is associated with the enhancement of nutrient retentions. Protein retention of shrimp fed with 5 (0.5) and 5 (1) diets were similar to that of the FM Control and significantly lower protein retention has been observed in the group without the squid by-product supplementation ()). Bulbul et al. (Citation2016) stated that this decreasing protein retention in shrimp fed with a low fish meal diet is due to the increased rate of protein catabolism in the experimental animal. Alternatively, Hernández et al. (Citation2011) observed that apparent nitrogen utilization was enhanced in shrimp fed with diets containing 5% tuna by-product hydrolysate supplement in comparison to the control diet without supplementation. Comparable results were also reported by Soares et al. (Citation2020) indicating improved nitrogen retention in P. vannamei receiving diets supplemented with poultry by-product and swine liver protein hydrolysates. It has been also shown in Salmo salar that dietary supplementation with fish protein hydrolysate is strongly correlated with body protein accretion and muscle tissue growth but with less impact on overall growth (Hevrøy et al., Citation2006). It has been suggested that the enhanced protein retention observed in aquatic animals receiving protein hydrolysate supplement is associated with the enhancement of tissue protein synthesis rates that are triggered by the presence of significant amounts of free amino acids (Hernández et al., Citation2011).
Moreover, lipid retention was found higher in shrimp fed with 5 (1) diet ()) indicating that squid by-product hydrolysate also promotes lipid retention and supports the growth promoting effects of this dietary supplement on P. monodon. Similar to our present findings, Niu et al. (Citation2014) also noted the enhancement of body fat content with increasing fish protein hydrolysate inclusion in P. vannamei diets. Similar observation has also been reported by Leal et al. (Citation2010) on Nile tilapia fed with shrimp head hydrolysates as fish meal replacement. However, the mechanism on how squid by-product hydrolysate promotes lipid retention is still unclear and is a good subject for further investigations.
In the present study, the significant enhancement of body protein retention ()) associated with improved growth in the shrimp fed the low fish meal diets supplemented with squid by-product hydrolysate ()) is supported by significant enhancement in the transcription of genes related to muscle growth and recruitment. In the present study, significant up-regulation of the expression of myosin heavy chain (MHC; )), a major protein component of muscle fibers, is associated with the dietary supplementation with squid by-product hydrolysate. Similar to our findings, it has been reported in salmon that dietary supplementation of fish protein hydrolysate promoted better body protein accretion and up-regulated the transcription of MHC that is linked to significant muscle growth (Hevrøy et al., Citation2006). Also, in an in vitro study with cultured myotubes, it has been elucidated that peptides and soluble proteins from soybean hydrolysates were able to activate the transcription of myosin heavy chain (Saneyasu et al., Citation2018). It is tempting to speculate that a similar mechanism may be at work in the activation and up-regulation of MHC in shrimp in response to the presence of peptides and amino acids in the squid by-product hydrolysate as observed in the present study.
The enhancement of body protein accretion ()) in the present study is also associated with the significant up-regulation of the genes encoding the target of rapamycin (tor), the ribosomal s6 kinase 1 (s6k) and the eukaryotic translation initiation factor 4e-binding protein 1 (4e-bp; )) which comprises the component of the intercellular signaling pathways involved in muscle protein synthesis and growth (Gao et al., Citation2019; Seiliez et al., Citation2008). In earlier works, it has been shown that the activation of tor pathway is strongly correlated with enhanced growth performance in several cultured fish including grouper (Wu et al., Citation2017), sea bream (Irm et al., Citation2020) and turbot (Song et al., Citation2016). It has been also shown in fish that feeding with diets containing low fish meal and high plant protein ingredients could result to tor down-regulation and significant depression in growth (Yuan et al., Citation2019). Similar to our findings, Dai et al. (Citation2020) and Li et al. (Citation2021) have shown that dietary supplementation of a composite of marine protein hydrolysates could decrease the use of fish meal, up-regulate the transcription of tor and s6k, and improves the growth performance and feed utilization of largemouth bass, Micropterus salmoides. Growth improvement associated with the up-regulation of the 4e-bp genes was also reported in tiger puffer, Takifugu rubripes, fed with plant protein-based diets supplemented with 12% fish protein hydrolysate (Wei et al., Citation2021). In addition, in a study with turbot (Scophthalmus maximus), it has been shown that growth inhibition due to high dietary content of plant protein was alleviated with the supplementation of fish protein hydrolysate that up-regulated the transcription of muscle protein accretion genes including the 4e-bp gene (Wei et al., Citation2020). The present results are in agreement with these earlier findings on the transcription patterns of muscle growth-related pathway genes (tor, 4e-bp and s6k) and provide additional evidences that in P. monodon the significant improvement in growth and body protein accretion associated with squid protein hydrolysate supplementation is linked with the up-regulation of these genes.
In the present study with P. monodon, genes encoding proteins involved in the transport of glucose, (GLUT) as metabolic fuels for muscle cell growth are also up-regulated ()) in response to dietary supplementation with squid by-product hydrolysate. Up-regulation of this glucose transporter gene supports the up-regulation of muscle protein accretion genes and may account for the better growth and higher protein retention in animals fed with the squid by-product hydrolysate. Our present findings concur with the earlier findings of Leduc et al. (Citation2018) on European seabass (Dicentrarchus labrax) suggesting that glucose transporter proteins in the intestinal cells are significantly up-regulated. In addition, it was reported that overall growth performance is improved in the fish receiving a very low fish meal diet (5%) supplemented with mixed tilapia and shrimp protein hydrolysates (Leduc et al., Citation2018). Moreover, it was also shown in wistar rats that feeding with whey protein hydrolysate activates the glucose transporter-4 genes that stimulate glucose uptake to the rat muscle cells. The activation of this glucose transporter has been suggested as an effect of peptides and free amino acids that are present in the hydrolysates (Morato et al., Citation2013). These earlier findings conform to our present results that squid by-product protein hydrolysate supplementation improves the growth of shrimp, fed with low fish meal diets, through the up-regulation of muscle protein synthesis and muscle glucose transporter genes.
Furthermore, in the present study, the digestive enzyme activities of the shrimp appeared to be not influenced by the squid by-product hydrolysate supplementation ()). In contrast to the present findings, the works of Córdova-Murueta and García-Carreño (Citation2002) which used 3–15% hydrolysate, and Niu et al. (Citation2014) that incorporated 9–36% hydrolysate in the diet have shown significant enhancement of digestive protease activities of the experimental shrimp receiving the supplemented diets. However, in the present study, the low inclusion level of squid by-product hydrolysate as a supplement and the high content of plant-based ingredients in the experimental diets may explain the differences in the results obtained. Similar to the present findings, Shao et al. (Citation2018) reported that P. vannamei digestive enzymes were not influenced by supplementation with dietary fish protein hydrolysates. The differences in the responses obtained in these studies could be due to the differences in the quantity and quality of the hydrolysate used, the dietary formulation and the cultured species used in these tests.
5. Conclusions
Overall, the use of squid by-product hydrolysate supplementation at 0.5% of the diet can offset the negative effects of high plant protein inclusion in shrimp diets in terms of growth and nutrient utilization. This growth promotion associated with squid by-product hydrolysate supplementation in a plant-based diet of shrimp is linked to improved protein efficiency ratio, nutrient retentions, and up-regulation of the muscle growth-related and muscle glucose transporter gene expressions. The use of squid by-products hydrolysate as a feed supplement could enhance the efficiency and maximize the utilization of squid as biological resource and promotes the sustainability of P. monodon aquaculture by decreasing the utilization of fish meal in the formulated diets.
Acknowledgements
The DOST-PCAARRD-Institute of Aquaculture, College of Fisheries and Ocean Sciences, UP Visayas project on the Improvement of Philippine Penaeus vannamei for Enhanced Growth and White Spot Syndrome Virus Resistance through Selective Breeding is acknowledged for the technical and operational support to this work. Ms. Rowena E. Cadiz, Ms. Emelyn Joy Mameloco and Mr. Alan N. Failaman are also appreciated for the technical assistance during the conduct of the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors

Maila V. Pan
Maila V. Pan, faculty of Zamboanga State College of Marine Sciences and Technology and Ph.D. student at University of the Philippines Visayas (UP Visayas). She works on bioconversion and utilization of industry by-products into bioactive feed additives for aquaculture.
Rex Ferdinand M. Traifalgar
Rex Ferdinand M. Traifalgar, Ph.D., Professor at UP Visayas. He is involved in research on the production of alternative feed materials and additives from underutilized marine invertebrates and fermented agricultural biomass for aquaculture use.
Victor Marco Emmanuel N. Ferriols
Victor Marco Emmanuel N. Ferriols, Ph.D., Professor at UP Visayas, with research interests in molecular biology, genetics and their applications in the aquaculture of bivalves and seaweeds.
Liberato V. Laureta
Liberato V. Laureta, Ph.D., Professor Emeritus at UP Visayas, with research interests in aquaculture energetics, marine benthos ecology and systematics.
Jose P. Peralta
Jose P. Peralta, Ph.D., Professor at UP Visayas, with research interests in fish hydrolysates as bioactive additives in human food and aquafeeds.
References
- Alvarez, J. S., Hernández-Llamas, A., Galindo, J., Fraga, I., García, T., & Villarreal, H. (2007). Substitution of fishmeal with soybean meal in practical diets for juvenile white shrimp Litopenaeus schmitti (Pérez-Farfante & Kensley 1997). Aquaculture Research, 38(7), 689–16. https://doi.org/10.1111/j.1365-2109.2007.01654.x
- AOAC. (1990). Official Methods of Analysis 15th. K. Helrich, Ed. Vol. 1. Association of Official Analytical Chemists, Inc.
- Areekijseree, M., Engkagul, A., Kovitvadhi, U., Thongpan, A., Mingmuang, M., Rungruangsak-Torrisen, P. P., & Rungruangsak-Torrissen, K. (2004). Temperature and pH characteristics of amylase and proteinase of adult freshwater pearl mussel, Hyriopsis (Hyriopsis) bialatus Simpson 1900. Aquaculture, 234(1–4), 575–587 https://doi.org/10.1016/j.aquaculture.2003.12.008.
- Arvanitoyannis, I. S., & Kassaveti, A. (2008). Fish industry waste: Treatments, environmental impacts, current and potential uses. International Journal of Food Science & Technology, 43(4), 726–745 https://doi.org/10.1111/j.1365-2621.2006.01513.x.
- Bernfeld, P. (1951 Enzymes of starch degradation and synthesis Nord, F. F.). . Advances in Enzymology and Related Subjects of Biochemistry, 12 (Interscience Publishers, Inc.), 379–428 https://doi.org/10.1002/9780470122570.ch7.
- Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8), 911–917. https://doi.org/10.1139/o59-099
- Bousopha, S., Nalinanon, S., & Sriket, C. (2016). Production of collagen hydrolysate with antioxidant activity from pharaoh cuttlefish skin. Chiang Mai University Journal of Natural Sciences, 15(2), 151–162. https://doi.org/10.12982/cmujns.2016.00012
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254. https://doi.org/10.1016/0003-2697(76)90527-3
- Bulbul, M., Kader, M. A., Asaduzzaman, M., Ambak, M. A., Chowdhury, A. J. K., Hossain, M. S., Ishikawa, M., & Koshio, S. (2016). Can canola meal and soybean meal be used as major dietary protein sources for kuruma shrimp, Marsupenaeus japonicus? Aquaculture, 452 (1 February 2016) , 194–199. https://doi.org/10.1016/j.aquaculture.2015.10.036
- Burokerkilgore, M., & Wang, K. (1993). A Coomassie Brilliant Blue G-250-based colorimetric assay for measuring activity of calpain and other proteases. Analytical Biochemistry, 208(2), 387–392 https://doi.org/10.1006/abio.1993.1066.
- Canada, P., Engrola, S., Mira, S., Teodosio, R., Yust, M., Sousa, V., Pedroche, J., Fernandes, J., Conceicao, L., & Valente, L. (2018). Larval dietary protein complexity affects the regulation of muscle growth and the expression of DNA methyltransferases in Senegalese sole. Aquaculture, 491 (1 April 2018) , 28–38 https://doi.org/10.1016/j.aquaculture.2018.02.044.
- Córdova-Murueta, J. H., & García-Carreño, F. L. (2002). Nutritive value of squid and hydrolyzed protein supplement in shrimp feed. Aquaculture, 210(1–4), 371–384 https://doi.org/10.1016/S0044-8486(02)00011-X.
- Cruz-Suárez, L. E., & Ricque, D., & AQUACOP. (1992). Effect of squid meal on growth of Penaues monodon juveniles reared in pond pens and tanks. Aquaculture, 106(3–4), 293–299 https://doi.org/10.1016/0044-8486(92)90261-I.
- Dai, M., Li, S., Fu, C., Qiu, H., & Chen, N. (2020). The potential role of marine protein hydrolyzates in elevating nutritive values of diets for largemouth bass, Micropterus salmoides. Frontiers in Marine Science, 7(April), 1–11 https://doi.org/10.3389/fmars.2020.00197.
- Dossou, S., Koshio, S., Ishikawa, M., Yokoyama, S., Dawood, M. A. O., El Basuini, M. F., El-Hais, A. M., & Olivier, A. (2018). Effect of partial replacement of fish meal by fermented rapeseed meal on growth, immune response and oxidative condition of red sea bream juvenile, Pagrus major. Aquaculture, 3(November2017), 228–235 https://doi.org/10.1016/j.aquaculture.2018.02.010.
- Ezquerra-Brauer, J. M., & Aubourg, S. (2019). Recent trends for the employment of jumbo squid (Dosidicus gigas) by-products as a source of bioactive compounds with nutritional, functional and preservative applications: A review. International Journal of Food Science and Technology, 54(4), 987–998. https://doi.org/10.1111/ijfs.14067
- Forster, I. P., Bechtel, P., Dominy, W. G., Lane, S., Avena, R., Ju, Z. Y., & Conquest, L. (2011). Use of fish hydrolysates and fish meal byproducts of the Alaskan fishing industry in diets for pacific white shrimp Litopenaeus vannamei. North American Journal of Aquaculture, 73(3), 288–295. https://doi.org/10.1080/15222055.2011.598371
- Gao, Y., Lu, S., Wu, M., Yao, W., Jin, Z., & Wu, X. (2019). Effects of dietary protein levels on growth, feed utilization and expression of growth related genes of juvenile giant grouper (Epinephelus lanceolatus). Aquaculture, 504(September2018), 369–374 https://doi.org/10.1016/j.aquaculture.2019.02.023.
- García-Ortega, A., Kissinger, K. R., & Trushenski, J. T. (2016). Evaluation of fish meal and fish oil replacement by soybean protein and algal meal from Schizochytrium limacinum in diets for giant grouper Epinephelus lanceolatus. Aquaculture, 452 (1 February 2016) , 1–8 https://doi.org/10.1016/j.aquaculture.2015.10.020.
- Gonzalez, D., Cordoba, J., & Buitrago, F. (2007). Estudios preliminares en la formulacion de dietas para camaron blanco (Litopenaeus schmitti) utilizando ensilado de pescado. Revista Cientifica, 17(2), 166–172 http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0798-22592007000200010.
- González-Félix, M. L., Perez-Velazquez, M., Ezquerra-Brauer, J. M., Bringas-Alvarado, L., Sánchez-Sánchez, A., & Torres-Arreola, W. (2014). Evaluation of jumbo squid (Dosidicus gigas) byproduct hydrolysates obtained by acid-enzymatic hydrolysis and by autohydrolysis in practical diets for Pacific white shrimp (Litopenaeus vannamei). Food Science and Technology (Campinas), 34(3), 552–558 https://doi.org/10.1590/1678-457x.6414.
- Guillaume, J., Cruz-Ricque, E., Cuzon, G., Van Wormhoudt, A., & Revol, A. (1989). Growth factors in penaeid shrimp feeding Advances in Tropical Aquaculture February 20-Marcch 4, 1989 Tahiti, French Polynesia. , 9 (IFREMER), 327–338 https://archimer.ifremer.fr/doc/1989/acte-1464.pdf.
- Halim, N. R. A., Yusof, H. M., & Sarbon, N. M. (2016). Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review,”. Trends in Food Science and Technology, 51 (May 2016), 24–33 https://doi.org/10.1016/j.tifs.2016.02.007.
- Hardy, R., & Barrows, F. (2002). Diet formulation and manufacture. In J. Halver & R. Hardy (Eds.), Fish Nutrition (3rd ed., pp. 505–600). Academic Press.
- Hernández, C., Olvera-Novoa, M. A., Smith, D. M., Hardy, R. W., & Gonzalez-Rodriguez, B. (2011). Enhancement of shrimp Litopenaeus vannamei diets based on terrestrial protein sources via the inclusion of tuna by-product protein hydrolysates. Aquaculture, 317(1–4), 117–123 https://doi.org/10.1016/j.aquaculture.2011.03.041.
- Hevrøy, E. M., Jordal, A. E. O., Hordvik, I., Espe, M., Hemre, G. I., & Olsvik, P. A. (2006). Myosin heavy chain mRNA expression correlates higher with muscle protein accretion than growth in Atlantic salmon, Salmo salar. Aquaculture, 252(2–4), 453–461 https://doi.org/10.1016/j.aquaculture.2005.07.003.
- Irm, M., Taj, S., Jin, M., Timothée Andriamialinirina, H. J., Cheng, X., & Zhou, Q. (2020). Influence of dietary replacement of fish meal with fish soluble meal on growth and TOR signaling pathway in juvenile Black Sea bream (Acanthopagrus schlegelii). Fish & Shellfish Immunology, 101((June 2020)), 269–276 https://doi.org/10.1016/j.fsi.2020.03.053.
- Joseph, J., Prabhu, P. V., & Madhavan, P. (1987). Utilization of squid waste as meal. Fishery Technology, 24 (1) , 41–43 https://aquadocs.org/handle/1834/33976.
- Leal, A. L. G., Castro, P. F., Lima, J. P. V., Souza Correia, E., & Souza Bezerra, R. (2010). Use of shrimp protein hydrolysate in Nile tilapia (Oreochromis niloticus, L.) feeds. Aquaculture International, 18(4), 635–646 https://doi.org/10.1007/s10499-009-9284-0.
- Leduc, A., Zatylny-Gaudin, C., Robert, M., Corre, E., Corguille, G. L., Castel, H., Lefevre-Scelles, A., Fournier, V., Gisbert, E., Andree, K. B., & Henry, J. (2018). Dietary aquaculture by-product hydrolysates: Impact on the transcriptomic response of the intestinal mucosa of European seabass (Dicentrarchus labrax) fed low fish meal diets. BMC Genomics, 19(1), 1–20 https://doi.org/10.1186/s12864-018-4780-0.
- Li, X., Wang, L., Zhang, C., Rahimnejad, S., song, K., & Yuan, X. (2018). Effects of supplementing low-molecular-weight fish hydrolysate in high soybean meal diets on growth, antioxidant activity and non-specific immune response of Pacific white shrimp (Litopenaeus vannamei). Turkish Journal of Fisheries and Aquatic Sciences, 18(5), 717–727. https://doi.org/10.4194/1303-2712-v18_5_07
- Li, S., Dai, M., Qiu, H., & Chen, N. (2021). Effects of fishmeal replacement with composite mixture of shrimp hydrolysate and plant proteins on growth performance, feed utilization, and target of rapamycin pathway in largemouth bass, Micropterus salmoides. Aquaculture, 533 (25 February 2021) , 736185 https://doi.org/10.1016/j.aquaculture.2020.736185.
- Lian, P. Z., Lee, C. M., & Park, E. (2005). Characterization of squid-processing byproduct hydrolysate and its potential as aquaculture feed ingredient. Journal of Agricultural and Food Chemistry, 53(14), 5587–5592 https://doi.org/10.1021/jf050402w.
- Mendoza, R., De Dios, A., Vazquez, C., Cruz, E., Ricque, D., Aguilera, C., & Montemayor, J. (2001). Fishmeal replacement with feather-enzymatic hydrolyzates co-extruded with soya-bean meal in practical diets for the Pacific white shrimp (Litopenaeus vannamei). Aquaculture Nutrition, 7(3), 143–151 https://doi.org/10.1046/j.1365-2095.2001.00164.x.
- Morato, P. N., Lollo, P. C. B., Moura, C. S., Batista, T. M., Camargo, R. L., Carneiro, E. M., Amaya-Farfan, J., & Nadal, A. (2013). Whey protein hydrolysate increases translocation of GLUT-4 to the plasma membrane independent of insulin in Wistar rats. PLoS ONE, 8(8), 8 https://doi.org/10.1371/journal.pone.0071134.
- Nayak, S., Singh, S. K., Ramaiah, N., & Sreepada, R. A. (2010). Identification of upregulated immune-related genes in Vibrio harveyi challenged Penaeus monodon postlarvae. Fish & Shellfish Immunology, 29(3), 544–549 https://doi.org/10.1016/j.fsi.2010.05.010
- Nguyen, H. T. M., Perez-Galvez, R., & Berge, J. P. (2012). Effect of diets containing tuna head hydrolysates on the survival and growth of shrimp Penaeus vannamei. Aquaculture, 324–325 (12 January 2012) , 127–134 https://doi.org/10.1016/j.aquaculture.2011.11.014
- Nguyen, C., Nguyen, T. G., Van, N. L., Pham, H. Q., Nguyen, T. H., Pham, H. T., Nguyen, H. T., Ha, T. T., Dau, T. H., Vu, H. T., Nguyen, D. D., Nguyen, N. T. T., Nguyen, N. H., Van Quyen, D., Chu, H. H., & Dinh, K. D. (2016). De novo assembly and transcriptome characterization of major growth-related genes in various tissues of Penaeus monodon. Aquaculture, 4641 , 545–553 https://doi.org/10.1016/j.aquaculture.2016.08.003.
- Niu, J., Zhang, Y. Q., Liu, Y. J., Tian, L. X., Lin, H. Z., Chen, X., Yang, H. J., & Liang, G. Y. (2014). Effects of graded replacement of fish meal by fish protein hydrolysate on growth performance of early post-larval Pacific white shrimp (Litopenaeus vannamei, Boone). Journal of Applied Animal Research, 42(1), 6–15. https://doi.org/10.1080/09712119.2013.795897
- Nunes, A. J. P., Sabry-Neto, H., Oliveira-Neto, S., & Burri, L. (2019). Feed preference and growth response of juvenile Litopenaeus vannamei to supplementation of marine chemoattractants in a fishmeal-challenged diet. Journal of the World Aquaculture Society, 50(6), 1048–1063. https://doi.org/10.1111/jwas.12648
- Pinsirodom, P., & Parkin, K. (2001). Lipase assays. In J. Whitaker (Ed.), Current protocols in food analytical chemistry (pp. C3.1.1–C3.1.13). John Wiley & Sons, Inc.
- Quinto, B. P. T., Albuquerque, J. V., Bezerra, R. S., Peixoto, S., & Soares, R. (2018). Replacement of fishmeal by two types of fish protein hydrolysate in feed for postlarval shrimp Litopenaeus vannamei. Aquaculture Nutrition, 24(2), 768–776. https://doi.org/10.1111/anu.12605
- Samocha, T. M., Allen Davis, D., Saoud, I. P., & DeBault, K. (2004). Substitution of fish meal by co-extruded soybean poultry by-product meal in practical diets for the Pacific white shrimp, Litopenaeus vannamei. Aquaculture, 231(1–4), 197–203. https://doi.org/10.1016/j.aquaculture.2003.08.023
- Sánchez, D. R., Fox, J. M., Gatlin, D. M., III, & Lawrence, A. (2012). Dietary effect of squid and fish meals on growth and survival of Pacific white shrimp Litopenaeus vannamei in the presence or absence of phytoplankton in an indoor tank system. Aquaculture Research, 43(12), 1880–1890. https://doi.org/10.1111/j.1365-2109.2011.02997.x
- Saneyasu, T., Shindo, H., Honda, K., & Kamisoyama, H. (2018). The extract of soybean protein increases slow-myosin heavy chain expression in C2C12 myotubes. Journal of Nutritional Science and Vitaminology, 64(4), 296–300. https://doi.org/10.3177/jnsv.64.296
- Seiliez, I., Gabillard, J. C., Skiba-Cassy, S., Garcia-Serrana, D., Gutiérrez, J., Kaushik, S., Panserat, S., & Tesseraud, S. (2008). An in vivo and in vitro assessment of TOR signaling cascade in rainbow trout (Oncorhynchus mykiss). American Journal of Physiology - Regulatory Integrative and Comparative Physiology, 295(1), 1. https://doi.org/10.1152/ajpregu.00146.2008
- Shao, J., Zhao, W., Liu, X., & Wang, L. (2018). Growth performance, digestive enzymes, and TOR signaling pathway of Litopenaeus vannamei are not significantly affected by dietary protein hydrolysates in practical conditions. Frontiers in Physiology, 9(Aug), 1–8. https://doi.org/10.3389/fphys.2018.00998
- Siahpoosh, A., & Alikhani, K. (2016). Evaluation of antioxidant capacity and free radical scavenging activities of pepsin extract of cuttlefish (Sepia pharaonis) from Persian Gulf. Indian Journal of Traditional Knowledge, 15(4), 604–610.
- Soares, M., Rezende, P. C., Corrêa, N. M., Rocha, J. S., Martins, M. A., Andrade, T. C., Fracalossi, D. M., & Do Nascimentovieira, F. (2020). Protein hydrolysates from poultry by-product and swine liver as an alternative dietary protein source for the Pacific white shrimp. Aquaculture Reports, 17(April), 100344. https://doi.org/10.1016/j.aqrep.2020.100344
- Somboonwiwat, K., Supungul, P., Rimphanitchayakit, V., Aoki, T., Hirono, I., & Tassanakajon, A. (2006). Differentially expressed genes in hemocytes of vibrio harveyi-challenged shrimp Penaeus monodon. Journal of Biochemistry and Molecular Biology, 39(1), 26–36. https://doi.org/10.5483/bmbrep.2006.39.1.026
- Song, F., Xu, D., Mai, K., Zhou, H., Xu, W., He, G., & Yin, Z. (2016). Comparative study on the cellular and systemic nutrient sensing and intermediary metabolism after partial replacement of fishmeal by meat and bone meal in the diet of turbot (Scophthalmus maximus L.). PLoS ONE, 11(11), 1–19. https://doi.org/10.1371/journal.pone.0165708
- Soonthornchai, W., Rungrassamee, W., Karoonuthaisiri, N., Jarayabhand, P., Klinbunga, S., Söderhäll, K., & Jiravanichpaisal, P. (2010). Expression of immune-related genes in the digestive organ of shrimp, Penaeus monodon, after an oral infection by Vibrio harveyi. Developmental and Comparative Immunology, 34(1), 19–28. https://doi.org/10.1016/j.dci.2009.07.007
- Soufi-Kechaou, E., Berge, J. P., Jaouen, P., & Amar, R. B. (2015). Optimization of common cuttlefish (Sepia officinalis) protein hydrolysate using pepsin by response surface methodology. Journal of Aquatic Food Product Technology, 24(3), 270–282. https://doi.org/10.1080/10498850.2013.773116
- Suárez-Jiménez, G. M., Robles-Sánches, R. M., Yépiz-Plascencia, G., Burgos-Hernández, A., & Ezquerra-Brauer, J. M. (2015). In vitro antioxidant, antimutagenic and antiproliferative activities of collagen hydrolysates of jumbo squid (Dosidicus gigas) byproducts. Food Science and Technology, 35(3), 421–427. https://doi.org/10.1590/1678-457X.6658
- Suresh, A. V., Kumaraguru Vasagam, K. P., & Nates, S. (2011). Attractability and palatability of protein ingredients of aquatic and terrestrial animal origin, and their practical value for blue shrimp, Litopenaeus stylirostris fed diets formulated with high levels of poultry byproduct meal. Aquaculture, 319(1–2), 132–140. https://doi.org/10.1016/j.aquaculture.2011.06.039
- Tacon, A., Hasan, M., & Subasinghe, R. 2006. Use of fishery resources as feed inputs to aquaculture development: Trends and policy implications FAO Fisheries Circular No. 1018 (99 pp). Food and Agriculture Organization of the United Nations.
- Tomy, S., Saikrithi, P., James, N., Balasubramanian, C. P., Panigrahi, A., Otta, S. K., Subramoniam, T., & Ponniah, A. G. (2016). Serotonin induced changes in the expression of ovarian gene network in the Indian white shrimp, Penaeus indicus. Aquaculture, 452 (1 February 2016) , 239–246. https://doi.org/10.1016/j.aquaculture.2015.11.003
- Uddin, M. S., Ahn, H.-M., Kishrimura, H., & Chun, B.-S. (2010). Production of valued materials from squid viscera by subcritical water hydrolysis. Journal of Environmental Biology, 31(5), 675–679 https://pubmed.ncbi.nlm.nih.gov/21387921/
- Valle, B. C. S., Dantas, E. M., Silva, J. F. X., Bezerra, R. S., Correia, E. S., Peixoto, S. R. M., & Soares, R. B. (2015). Replacement of fishmeal by fish protein hydrolysate and biofloc in the diets of Litopenaeus vannamei postlarvae. Aquaculture Nutrition, 21(1), 105–112. https://doi.org/10.1111/anu.12149
- Venugopal, V. (2009). Marine product for health care. In V. Venugopal (Ed.), Marine product for health care (pp. 185–214). CRC Press.
- Wang, C.-H., Doan, C. T., Nguyen, V. B., Nguyen, A. D., & Wang, S.-L. (2019). Reclamation of fishery processing waste: A mini-review. Molecules, 24(2234), 17. https://doi.org/10.3390/molecules24122234
- Wei, Y., Liang, M., & Xu, H. (2020). Fish protein hydrolysate affected amino acid absorption and related gene expressions of IGF-1/AKT pathways in turbot (Scophthalmus maximus). Aquaculture Nutrition, 26(1), 145–155. https://doi.org/10.1111/anu.12976
- Wei, Y., Wang, J., Zhang, X., Duan, M., Jia, L., Xu, H., Liang, M., & Liu, J. (2021). Fish protein hydrolysate supplementation in plant protein based diets for tiger puffer (Takifugu rubripes) is an effective strategy of fish meal sparing. Aquaculture Reports, 20(July 2021) , 100720. https://doi.org/10.1016/j.aqrep.2021.100720
- Wu, M., Lu, S., Wu, X., Jiang, S., Luo, Y., Yao, W., & Jin, Z. (2017). Effects of dietary amino acid patterns on growth, feed utilization and hepatic IGF-I, TOR gene expression levels of hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) juveniles. Aquaculture, 468(1 February 2017) , 508–514. https://doi.org/10.1016/j.aquaculture.2016.11.019
- Yaghoubi, M., Mozanzadeh, M. T., Marammazi, J. G., Safari, O., & Gisbert, E. (2016). Dietary replacement of fish meal by soy products (soybean meal and isolated soy protein) in silvery-black porgy juveniles (Sparidentex hasta). Aquaculture, 464(1 November 2016) , 50–59. https://doi.org/10.1016/j.aquaculture.2016.06.002
- Yuan, X. Y., Liu, M. Y., Cheng, H. H., Huang, Y. Y., Dai, Y. J., Bin, L. W., & Jiang, G. Z. (2019). Replacing fish meal with cottonseed meal protein hydrolysate affects amino acid metabolism via AMPK/SIRT1 and TOR signaling pathway of Megalobrama amblycephala. Aquaculture, 510(15 August 2019) , 225–233. https://doi.org/10.1016/j.aquaculture.2019.05.056
- Zhou, Y., Thirumurugan, R., Wang, Q., Lee, C. M., & Davis, D. A. (2016). Use of dry hydrolysate from squid and scallop product supplement in plant based practical diets for Pacific white shrimp Litopenaeus vannamei. Aquaculture, 465(1 December 2016), 53–59. https://doi.org/10.1016/j.aquaculture.2016.08.028