?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The abundance, distribution, and diversity of fishes in Ribb Reservoir were studied based on a total of 1629 fish samples collected from February to October 2021. Fish samples were collected using gillnets of different stretched mesh sizes. Except for temperature and pH, most physico-chemical variables revealed significant spatial variation (ANOVA, p < 0.05). Fishes were highly correlated with the concentration of dissolved oxygen (r ≈ 0.8, p < 0.01) and electrical conductivity (r ≈ 0.89, p < 0.01). Three fish species include Oreochromis niloticus (Linnaeus, 1758), Clarias gariepinus (Burchell, 1822) and Labeobarbus intermedius (Rüppell, 1835) were identified (H' = 1.06). The diversity indices did not show variations among seasons (H' = 0.98–0.907) and sites (H' = 1.04–1.07). All the fish species occurred in all sampling sites and seasons. Oreochromis niloticus (41.9%) was the most important and abundant fish species followed by C. gariepinus (38.2% by %IRI). The relative importance index showed O. niloticus (45.4%) was the most abundant species in the dry season. However, C. gariepinus (73.3%) was the most abundant species in the wet season. Before the construction of the Ribb Dam, the Ribb River was dominated by about eight species. However, in the current study (after the construction of the Ribb Dam), only three species were found. Results revealed that the diversity of fishes has decreased primarily due to the dam effect followed by anthropogenic impacts such as poor land management systems, high sedimentation load, excessive irrigation and illegal fishing. Therefore, an appropriate dam design with a fish ladder and effective watershed management setup is needed to sustain fish resource utilization in the country for food security.
Public interest statement
Irrigation activities are important to feed the fast-growing world population. However, these activities can affect fish distribution and diversity. For instance, along the Lake Tana Sub-basin, the construction of dams and weirs are expanding within the rivers without considering fisheries aspects. In Ethiopian drainage basins, there are more than 200 fish species are inhabited. However, this fish resource is not well utilized due to the scientific knowledge gap. Based on this, the present finding was focused on the abundance, distribution, and diversity of fishes in the Ribb Reservoir. The Ribb River is one of the tributaries of Lake Tana. The movement of spawning Labeobarbus fish species was impacted by the damming of Ribb River. The results revealed that the diversity, distribution, and abundance of fishes are highly impacted by the Ribb Dam followed by anthropogenic activities such as high sedimentation load, poor land management systems, excessive irrigation practices, and fishing pressure.
1. Introduction
It is known that Lake Tana is the largest waterbody in Ethiopia and covers an area of 3600 km2 (De Graaf et al., Citation2004; Reyntjens & Wudneh, Citation1998) as cited in Shumye (Citation2016). This highland lake accommodates four fish families Cichlidae, Clariidae, Balitoridae, and Cyprinidae. The Cichlidae (Oreochromis niloticus Linnaeus, 1758), Clariidae (Clarias gariepinus Burchell, 1822), and Balitoridae (Nemacheilus abyssinicus Boulenger, 1902) are represented by a single species. However, Cyprinidae (Barbus, Garra, Labeobarbus, and Varicorhinus) are represented by four genera in the lake (Dejen, Citation2003; Getahun, Citation2000; Nagelkerke & Sibbing, Citation2000). Of these four genera, Labeobarbus is the most abundant and consists of 17 species endemics to Lake Tana (Nagelkerke & Sibbing, Citation2000). Rivers are known for the availability of longitudinal fish communities (Ganassin et al., Citation2021). Nine Labeobarbus species from Lake Tana spawn in the inflowing rivers (S.A. Gebremedhin et al., Citation2017). Based on this, L. brevicephalus, L. intermedius, L. megastoma, L. nedgia, L. truttiformis, and L. tsanensis travel more than 60 km up to the Ribb River for reproduction purposes (Anteneh et al., Citation2013). Ribb River flows into Lake Tana (Shumye, Citation2016). For this reason, the Labeobarbus fish species of Lake Tana migrate upstream of the Ribb River for the breeding grounds from July to November (Getahun et al., Citation2008; De Graaf et al., Citation2005). However, their movement is negatively affected by the dams. The effects of large weirs and dams on the abundance and diversity of fishes were studied by several authors (Anteneh et al., Citation2013; Getahun et al., Citation2008; Mequanent et al., Citation2021) in the Ribb River (S.A. Gebremedhin et al., Citation2017), feeding rivers of Lake Tana (Haipeng et al., Citation2019), Yangtze River (Francisca et al., Citation2019), Guadiana River and Betani River (Limbu et al., Citation2021).
Gebru et al. (Citation2019) described those reservoirs have a substantial function in the community such as drinking, industrial use, irrigation, run-off control, electric generation, fishing purposes, and for aesthetic purposes. However, the reservoirs can cause a decrease in biodiversity when challenged by contamination (Basavaraja et al., Citation2014). The abundance, distribution, and diversity of fishes, indicate the well-being of waterbodies like the amount and eminence of food items present (Gebru et al., Citation2019). The decline in the abundance of freshwater fish around the globe has been a sign of water pollution, overfishing, and agricultural activities (Vijverberg et al., Citation2012).
In addition to this, habitat modifications such as transportation of rocks, cobbles, pebbles, sand mining, dams, weirs, big dams without a fish ladder or fish corridor, dynamiting near waterbodies, prohibited electro-fishing and use of toxic plants leading to declining fish abundance and diversity in the rivers (Limbu & Prasad, Citation2020; Mishra & Baniya, Citation2016). In Ethiopia, dam and weir construction are increasing at an alarming rate since 2010 for irrigation purposes to improve the livelihoods of the community during the dry season. Particularly, in the Lake Tana watershed, there are diversion weirs built on more than 15 tributary rivers, while several dams and weirs are under construction on perennial rivers flowing into Lake Tana (S.A. Gebremedhin et al., Citation2017).
Consequently, Ribb Reservoir is a recently constructed dam for irrigation purposes in the South Gondar Zone, Lake Tana basin. Apart from irrigation, it has potential for fish production of the livelihoods in the community. Previously, Getahun et al. (Citation2008) reported 18 fish species from the longitudinal side of Ribb River. On other the hand, eight fish species were documented by Mequanent et al. (Citation2021) below the Ribb Dam after the dam was constructed. However, no study has been carried out on fish abundance, diversity, and distribution in relation to habitat differences and seasonal with references to water environmental parameters in the reservoir. Therefore, this study was aimed to fill the gap by assessing the diversity, distribution, and relative abundance of fish species for a better conservation strategy in Ribb Reservoir, Tana basin, Ethiopia.
2. Materials and methods
2.1. The study area
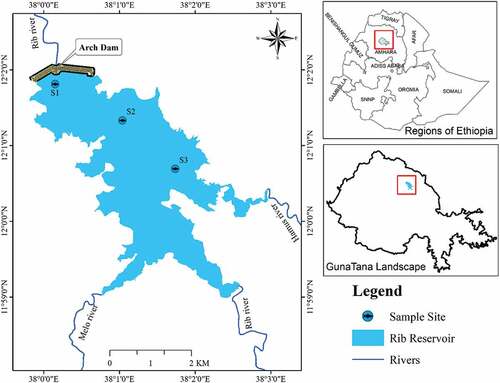
The construction of the Ribb Dam was completed in 2018. It has a height of 73 m and 800 m length (Ministry of Water and Energy, MWE, Citation2010). The Ribb Reservoir is situated in the Ribb River in the South Gondar Zone on the Eastern sides of Lake Tana sub-basin. The Ribb River covers about 130 km length and has a drainage area of closely 1790 km2 with an annual average discharge of 14.6331 m3/s (Bezabih, Citation2021). The Ribb Reservoir is surrounded by four kebeles including Medeb Gubda, Jarashikra, and Ayvaniva from Farta woreda and Amstya kebele from Ebnat woreda. The reservoir has three inflow rivers namely Melo, Hamus and Ribb to fill enough. It has a catchment area of about 685 km2 on the dam site. Based on the Universal Transverse Mercator (UTM), the Ribb Reservoir is also located between 392,174.64 E, 1,330,225.76 N, and 390,813.45 E, 1,330,018 N, at altitudes between 1,880 and 1,970 m (Ayalew & Bharti, Citation2020). The basin climate is also categorized as the rainy season which starts from May to October. The monthly precipitation varied from 65 mm in May to 411 mm in July (Ministry of Water and Energy, MWE, Citation2010).
At the Ribb Reservoir, the mean and the minimum precipitation are about 1,400 and 1,200 mm per annum, respectively. The temperature varies from 19°C (in December) to 23°C (in May). Moreover, the maximum and minimum in the area temperatures are about 30°C and 11.5°C, respectively (Getahun et al., Citation2008). The wind speed is low and decreases the possible evapotranspiration values between December (95 mm/month) and April (140 mm/month). The amount of sunlight is reduced to 6.0 h in July and 6.5 h in August (Bezabih, Citation2021). Based on the fish species composition, three families Cyprinidae (6 species), Cichlidae (O. niloticus), and Clariidae (C. gariepinus) were harbored in the Ribb River at the dam site before the construction of the dam (Getahun et al., Citation2008; Table ).
Table 1. Fish species were previously reported in the longitudinal section of the Ribb River (Getahun et al., Citation2008)
2.2. Study site selection
The study was undertaken from February to October 2021. An inspection was conducted with the fisheries research team of Guna Tana Integrated Field Research and Development Center, Debre Tabor University in December 2020–January 2021. Pilot observation is important to identify different habitats in relation to fishing pressures and human-induced impacts from the Ribb Reservoir. Thus, three sites were selected by considering suitability in setting gillnets, commercial fishing landing sites, water depth, fishing effort vulnerability areas, anthropogenic effects, and nursery habitats. The sites were S1 (dam, near the Ribb Dam), S2 middle of the reservoir, and S3 (inlet, entrance of Hamus River; Figure ). Global Positioning System (GPS) was recorded to fix the locations of the sampling sites (Table ).
Table 2. Characteristics of the different habitats from Ribb Reservoir
2.3. Physico-chemical parameters
Water quality parameters were measured once per month from February to March 2021 and May to October 2021 for the dry and the wet seasons, respectively. The parameters were noted in the late morning between 9:00 and 11:00 a.m. (Shukla & Singh, Citation2013) using HANNA Technologies analytical equipment. Therefore, the physico-chemical water quality parameters such as pH, TDS, electrical conductivity, and temperature were measured seasonally using HANNA multi-meter model (HI-98129) equipment (specifications: pH range 0.00–14.00, temperature range 0–50°C (32–122°F); RH max 100%). Dissolved oxygen (DO) was measured using a digital DO meter (DO-520) (specifications: range 0–20.0 mg l−1, accuracy ± 0.4 mg l−1 (23 ± 5°C)). Turbidity was measured using a Turbidity meter SGZ-200BS (specification: measuring range NTU:0 ~200, minimum display NTU:0.1, repeatability: ≤2%, display accuracy: 6%).
2.4. Fish sample collection and measurements
Fish sampling was done once per month from February to March 2021 and from May to October 2021 for the dry and the wet seasons, respectively. Single filament gillnets with a mesh size of 3.5, 5, 6, 8, and 10 cm and 25 m length were used. Besides this, hooks and long lines were used to catch larger fishes from three sampling sites in Ribb Reservoir. Gillnets were positioned in the late afternoon (3:00 p.m.) and inspected the next morning (9:00 a.m.). Moreover, fish samples were collected below the Ribb Dam to know the effect of river damming on the fish migration.
All the fish specimens were identified at the field to species level based on the guidebook of (Habteselassie, Citation2012). Total length (TL), fork length (FL), and standard length (TL) were measured using a measuring board 0.1 cm. The total weights of fish (TW) were measured using a sensitive balance with a sensitivity of 0.1 g.
2.4. Data analysis
SPSS software version 20.0 was used to analyze the collected data. A one-way ANOVA was employed to compare the variations in water quality parameters among the sampling sites. It was also used to compute the abundance and diversity of fishes among the sampling sites and seasons. Pearson correlation matrix was also used to investigate the association of fish species abundance and distribution with the water quality parameters. Furthermore, Shannon Diversity Index (H') was computed to know fish diversity in the reservoir (Shannon & Weaver, Citation1963).
H' = implies fish diversity index
N = total number of fishes
Pi = ni/N = number of fishes in (ni)
According to Kolding (Citation1989), IRI (index relative importance) among fish species is important to compute the relative abundance of fishes by using the number, weight, and frequency of occurrence fish individuals in the total catch.
%Wi = weight percentage, %Ni = number of fish in percent, %Fi = percentage occurrence fish in the total number of settings, %Wj = percentage of weight, %Nj = percentage of fish number in total species, %Fj = Frequency percentage occurrence within total species in the total number of settings.
3. Results
3.1. Physico-chemical characteristics
The (mean ± SE) recorded values of the physico-chemical characteristics are presented in Table . Of these, environmental variables DO, electrical conductivity, turbidity and TDS revealed significant variations (ANOVA, p < 0.05) among the sampling sites in the reservoir. However, pH and temperature values did not show variation among the sampling sites (p > 0.05). The concentration of DO ranged from 3.2 to 5 mg/L with a total mean of 3.9 ± 0.2 mg/L in the reservoir. The value of the electrical conductivity was found between 173 and 200 μS/cm during the sampling seasons with a total mean of 183.3 ± 2.7 μS/cm. The turbidity of the reservoir ranged between 4.5 and 6.4 NTU with a mean of 5.2 ± 0.2 NTU of the total sampling sites in the reservoir. The total dissolved solids (TDS) were found between 88.3 and 145.2 ppm with a total mean of 107.8 ± 7.5 ppm in all sampling sites. The minimum and the maximum water surface temperatures of 21.6°C and 23.1°C with a mean of 22.5°C ± 0.2°C recorded in the reservoir. The pH ranged from 8.3 to 8.8 with a mean of 8.4 ± 0.1.
Table 3. One-way ANOVA of the mean environmental variables in different habitats from Ribb Reservoir
3.2. Fish diversity
A total of 1629 samples (769 males and 860 females) were collected in the reservoir during the study period. Of these total catches, three families, three genera, and three species were collected from the dam, middle, and inlet sites of the Ribb Reservoir. The family Cichlidae contributed the maximum percentage (46.2%), followed by Cyprinidae (30.6%), and Clariidae (23.3%) which had contributed the lowest of the total species catch in the reservoir. From the Cichlidae family, O. niloticus was the most dominant species accounting for (46.2%) of the total catch in the reservoir. Labeobarbus intermedius was the second dominant fish species (30.6%) of the Cyprinidae family. Whereas C. gariepinus was the third available commercially important species from the reservoir (Table ).
Table 4. Fish species composition from Ribb Reservoir
3.3. Fish diversity indices
The spatial and temporal Shannon diversity indices for fishes are presented in Table ) The mean Shannon diversity index (H') was 1.06. The values of the Shannon diversity indices during the dry (H' = 0.98) and the wet (H' = 0.91') seasons also did not reveal significant differences between seasons (ANOVA, p > 0.05). Similar fish species were obtained in the dry, wet seasons and at sampling sites (dam, middle, and inlet). The fish diversity indices among the sampling sites were 1.04), 1.05) and 1.07) for middle, dam and inlet, respectively, and did not show significant variation (ANOVA, p > 0.05). Based on the season-based Shannon diversity indices with respect to the sampling sites ranged from H' = 1.07 to 0.9 and did not show significant variation (ANOVA, p > 0.05).
Table 5. Fish diversity indices among the sampling seasons and sites from the Ribb Reservoir
3.4. Fish species distribution in relation to physico-chemical parameters
In this study, the distribution of all fishes showed a strong positive correlation with the concentration of dissolved oxygen (r ≈ 0.8, p < 0.01) and electrical conductivity (r ≈ 0.89, p < 0.01) in the reservoir. The temperature availability and the distributions of the three fish species revealed a medium positive correlation (r ≈ 0.5, p > 0.01). The relationship between pH and the fish species showed a weak positive correlation (r ≈ 0.3, p > 0.01). The association of turbidity (r ≈ -0.7, p < 0.01) and total dissolved solids (TDS) (r ≈ -0.5, p > 0.01) showed a negative correlation with the three fish species in Ribb Reservoir (Table ).
Table 6. Pearson correlation matrix analysis of fish species distribution in relation to physico-chemical water quality parameters from Ribb Reservoir
3.5. Relative abundance of fish species
The relative importance index was indicated in (Table ). In terms of number percentage (%No.), O. niloticus (46.2) was the most abundant within the total catch followed by L. intermedius (30.6). While the contribution of C. gariepinus (23.3) in the catch. In the case of weight percentage contribution (%W) C. gariepinus (54.4) dominated the catch followed by O. niloticus (29.6) and L. intermedius (15.9). In terms of frequency occurrence, O. niloticus (100%), C. gariepinus (88.9%), and L. intermedius (77.8%) were the foremost frequently occurred species of the total settings. Based on the rank of percentage importance relative index (%IRI), the present study revealed that O. niloticus was the most important and abundant fish species followed by C. gariepinus(41.9%) and (38.2%) of the total catch, respectively. However, L. intermedius had less contribution which consisted only (19.98%) of the total catch in the reservoir.
Table 7. Number of fish species and their index of relative importance (IRI) in Ribb Reservoir
3.6. Spatial and seasonal fish distribution
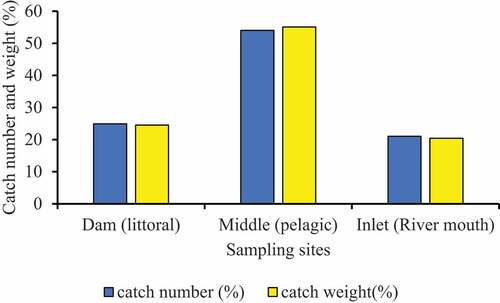
The highest catch of the fish species was recorded at the middle site followed by the dam site. Accordingly, the middle of the reservoir contributed (54.02% numerically) and (55.08% in terms of weight) to the overall specimen. The dam site contributed (24.92%) and (24.53%) of catch by number and weight, respectively. The inlet contributed (21.06%) and (20.4%) numerically and weight of the total catch, respectively. The three fish species were harbored in all sampling sites. However, proportions of catch in number (%) and weight (%) varied from habitat to habitat. The present study showed variations in the catches of the fish species among the sampling sites in Ribb Reservoir (ANOVA, p < 0.05; Figure ).
Figure 2. Percentage fish catch composition by number and weight for each sampling site in Ribb Reservoir.
Of the total fish samples (1629), 1329 (81.6%) and 300 (18.42%) were collected during the dry and the wet seasons of the year, respectively. In the dry season, O. niloticus contributed the highest catch by number (53.6%) followed by L. intermedius (31.97%). However, C. gariepinus contributed (46.8%) followed by O. niloticus (37.12%) caught by weight. O. niloticus (100%), C. gariepinus (80%), and L. intermedius (60%) frequently occurring fish species during the dry season. Considering the index of relative importance, O. niloticus (45.4%) was the most abundant species followed by C. gariepinus (30.6%) and L. intermedius (24.0%) in the dry season. In the wet season, C. gariepinus comprised (62.33%) and (80.74%) of the highest catch by number and weight followed by L. intermedius (24.33%) and (13.55%), respectively. The catch of O. niloticus (13.33%) and (5.72%) had a minor contribution by number and weight in the wet season. The percentage of importance of the relative index revealed that C. gariepinus (73.3%) was the most abundant species in the wet season (Table ). Oreochromis niloticus was the most abundant species at the dam site (50.1%) followed by the middle site (42.3%). Labeobarbus intermedius was the most abundant fish species at the inlet site (42.3%) followed by O. niloticus (35.7%). A high abundance of C. gariepinus was observed at the middle site (40.7%) followed by the dam site (34.9%). Labeobarbus intermedius was the least abundant species at the dam site (15.1%) followed by the middle site (16.9%). The results revealed that there was a significant variation in fish abundance and distribution among the sampling sites and over the seasons (ANOVA, p < 0.05; Table ).
Table 8. Temporal and spatial distributions of fishes in Ribb Reservoir
4. Discussion
Sustainable fish utilization ultimately depends on the environmental variables in waterbodies. All fish species do not resist the fluctuations of the environmental variables beyond their limit (Bhatnagar & Devi, Citation2013). The physico-chemical parameters from the Ribb Reservoir varied significantly (ANOVA, p < 0.05) among the sampling sites. In this study, the obtained mean value of DO concentration (3.9 mg/L) is the least with that in the Ribb River at the dam site (planned site for a dam construction) (6.8 mg/L; Getahun et al., Citation2008), Tekeze Reservoir (4.32–6.37 mg/L; Gebru et al., Citation2019), Lake Langeno (5.2 mg/L; M. Temesgen et al., Citation2016), Lake Zeway (3.46–6.01 mg/L; Abera et al., Citation2018), Lake Tana (6.71–7.78 mg/L; S. Gebremedhin et al., Citation2014), Lake Chamo (10.26–17.42 mg/L; Fenta & Kidanemariam, Citation2016), Tekeze and Abay basins (6.25–9.9 mg/L; Tewabe et al., Citation2010) and Lake Hawassa (11.2–20.55 mg/L; Woldesenbet, Citation2015). In the present study, the variation of the concentration of dissolved oxygen might be due to the availability of the irrigation dam. This might increase human-induced activities near and around the reservoir such as poor land-use practices, poor vegetation cover, high sedimentation load and extensive agricultural activities. Moreover, Ribb Reservoir is a newly constructed waterbody; its watershed lacks vegetation cover, which may also cause soil erosion discharge into the reservoir that could alter the water quality. This may cause a decline in the concentration of dissolved oxygen. Gebru et al. (Citation2019) also confirmed that the expansion of hydropower or irrigation dams affects water quality and quantity and blocks fish migration as well.
The temperature variation among the sampling sites did not reveal a significant difference (p > 0.05). The mean value water surface temperature of the current study (22.5°C) is comparable with the water temperature reported from Ribb River at the dam site (23.8°C; Getahun et al., Citation2008), Lake Ziway (23.0°C; Abera et al., Citation2018), Lake Langeno (23.54°C; M. Temesgen et al., Citation2016) but lower than in Tekeze Reservoir (26.93–28.53°C; Gebru et al., Citation2019).
The mean value pH (8.4) of this finding showed little variation from the previous report of Getahun et al. (Citation2008) (7.5) Ribb River at the dam site. It is comparable, Tekeze Reservoir (7.72–8.19), Lake Zeway (8.84–8.66; Tilahun & Ahlgren, Citation2010). However, the value is lower than Lake Langeno (9.45), as reported by Temesgen et al. (Citation2016).
The mean electrical conductivity (EC) of the present study (183.3 μS/cm) is the least when compared within Tekeze Reservoir (194.0–221.67 μS/cm; Gebru et al., Citation2019), Lake Langeno (1782.05 μS/cm; Temesgen et al., Citation2016), Lake Zeway (361.5–484.5 μS/cm; Abera et al., Citation2018), Lake Hawassa (701–756 μS/cm; Woldesenbet, Citation2015) and Koka Reservoir (357–561 μS/cm; Gebre-Mariam et al., Citation2002).
The total dissolved solids in the present study (107.8 ppm) is the lowest of the reports of Tekeze Reservoir (126.65–144.08 ppm; Gebru et al., Citation2019), Lake Langeno (1512.99 μS/cm), (Temesgen et al., Citation2016), in Lake Hawassa (455.6 mg/L; Woldesenbet, Citation2015) and Lake Chamo (656 mg/L; Fenta & Kidanemariam, Citation2016).
The mean value of the turbidity (5.2 NTU) was also found in the acceptable range. However, less than the report in Lake Tekeze (6.07–8.11 NTU; Gebru et al., Citation2019).
Before the construction of the dam, in the same study area, the concentration of dissolved oxygen (DO) declined from 6.8 to 3.9 mg/L. This could be due to less water circulation when it becomes standing water and since it is the new reservoir there is less availability of phytoplankton and aquatic vegetations. However, the water temperature and pH showed slight variation (Getahun et al., Citation2008). In conclusion, all water quality variables were in the optimum range to support the aquatic life in the reservoir.
In the present finding, three genera, three families, and three species (O. niloticus, L. intermedius, and C. gariepinus) were studied. The family Cichlidae (O. niloticus) (46.2%) had contributed the highest percentage of the total species followed by Cyprinidae (L. intermedius) (30.6%), and the family Clariidae (23.3%). However, in the Lake Langeno family, Cyprinidae (5 species) contributed 55% of the catch (M. Temesgen et al., Citation2016), Tekeze Reservoir Cyprinidae (10 species) consisted 66% of the catch (Gebru et al., Citation2019; Teame et al., Citation2016), Ribb River at dam site (planned site for a dam construction) six migratory Labeobarbus species (Anteneh et al., Citation2013; Getahun et al., Citation2008), below the dam nine Labeobarbus species (Mequanent et al., Citation2021) were reported. The family Cyprinidae is the most dominant fish species. In the present study, L. intermedius (among the migratory spawning Labeobarbus species, Table ), riverine origin (O. niloticus and C. gariepinus) species were investigated. This is probably due to the dam’s effect on the fish movement. The height of the Ribb Dam is 73 m, without a fish ladder, and with a strong water current at the outlet side. The spawning migratory Labeobarbus fishes from Lake Tana could not jump this high, with a high water current to join the main reservoir. This might be the cause of to decline in fish diversity in the reservoir. Similarly, before the construction of small dams or weirs, L. intermedius, L. brevicephalus, L. tsanensis, L. nedgia, L. truttiformis, L. crassibarbis, L. megastoma, L. macrophtalmus, L. platydorsus, and L. acutirostris were distributed throughout Gelda and Shinfa Rivers of Lake Tana tributaries (Anteneh et al., Citation2012; Getahun et al., Citation2008). However, after the construction of small dams except for the L. intermedius none of the species were found above the weirs rather they were found below the weirs (S.A. Gebremedhin et al., Citation2017). In the same way, the study conducted from Lake Tana tributaries such as Gelda, Tekon, Shini, and Chibrna Rivers showed high catch per unit effort in biomass of the Labeobarbus species below the weirs than above the weirs (S.A. Gebremedhin et al., Citation2017). The effect of river damming on fish communities was also reported. For instance, according to Hilborn (Citation2013), the Columbia Dam located in the U.S. state of Washington has blocked the upstream migration of fishes and aquatic mammals including striped bass (Morone saxatilis), American shad (Alosa sapidissima), salmon, Chinese sturgeon (Acipenser sinensis), and Chinese paddlefish (Psephurus gladius). Moreover, Haipeng et al. (Citation2019) reported that the dams in the upper Yangtze River have blocked 35 migratory fish species, leading to an increased risk of extinction of Corieus guichenoti and reduce the likelihood of successful recruitment of Acipenser dabryanus and Psephurus gladius.
Table 9. The presence and absence of upstream riverine spawning migratory fish species in Ribb River before and after irrigation dams
4.1. Impact of the dam on fish migration
In the present study, the Ribb Dam negatively affects the fish species composition, particularly the migratory spawning Labeobarbus species from Lake Tana. Nine migratory spawning Labeobarbus species were originally found in Lake Tana. However, when the breeding time approaches, they move upstream to find a favorable condition for breeding grounds. Accordingly, during the rainy season, these species can travel up to 60 km (Anteneh et al., Citation2013). However, the expansion of agricultural irrigation dams and weirs without a fish ladder (corridor) affects these fish species (Anteneh et al., Citation2013; Getahun et al., Citation2008; Mequanent et al., Citation2021). Before the construction of the dam, a total of about eight fish species were reported while in the current (after the construction of the dam) study three species were found due to the dam effect. Previous and current findings indicate the presence and absence of Labeobarbus species with respect to the dam and weirs (Table ).
The H' value of the current study (3 species: H' = 1.058) is smaller when compared to the value reported in the H' value described in Lake Langeno (7 species: H' = 1.264; M. Temesgen et al., Citation2016), Tekeze Reservoir (15 species: H' = 1.447–1.697; Gebru et al., Citation2019), Lake Zeway (9 species: H' = 1.5127 to 1.6681; Abera et al., Citation2018), Blue Nile river (8 species: H' = 1.23–1.64; Tewabe et al., Citation2010), Alwero dam (10 species: H' = 1.99), Baro River white Nile basin (14 species: H' = 2.21; Gebreselassie et al., Citation2021), major river basins of Ethiopia (Awoke, Citation2015), in Gelda, Tekon, Shini, and Chibrna Rivers (H' = 1.4, 1.39–1.52, 1.27 and 1.40–1.55), respectively (S.A. Gebremedhin et al., Citation2017), Omo Turkana basin (31 species: H' = 2.20–2.43; Wakjira & Getahun, Citation2017). However, the value of H' was greater than the report in the Upper Awash River (3 species: H' = 0–0.8; Temesgen et al., Citation2021). The present study reflects the poorest fish diversity could be due to the dam effect (Table ).
In this study, similar species were recorded in the dry and wet seasons in the reservoir. However, the proportion of catch varied (higher in the dry season than the wet). Many authors documented that almost similar fish species were caught within the dry and wet seasons but, a higher catch in the wet season than in the dry. For example, in Lake Langeno (Temesgen et al., Citation2016), Tekeze Reservoir (Gebru et al., Citation2019), upper Awash River (Temesgen et al., Citation2021), Lake Zeway (Abera et al., Citation2018), Lake Tana (Tewabe, Citation2014), and Omo Turkana River System (Wakjira & Getahun, Citation2017). The proportion of fish caught was higher in the dry season than in the wet season. This is probably due to water level fluctuations. For instance, in the dry season, when the water level decreases the fish communities assemble in a specific area of the reservoir. This makes the fish vulnerable to the gillnet. On the other hand, in the wet season, when the water level increases, the fish species caught were low. This might be due to the unsuitable bottom habitat (fragmented) where the fishes hide in the hole at the time of catch. Moreover, when the water level increases it makes difficult the gillnet setting for the fishermen. Furthermore, the littoral region vegetations of a given reservoir are important for the shelter (refuge) of fish. However, in the Ribb Reservoir, there is no littoral vegetation because it is newly constructed. This may also affect the proportion of catches. Different authors also confirmed that water level fluctuations could affect the proportion of fish caught (Abera et al., Citation2018; Gebru et al., Citation2019; Temesgen et al., Citation2016).
Furthermore, the reason for a higher catch in the dry season is probably due to fishermen having worked full time as a major work. Moreover, there is road infrastructure access for the fish supply chain. Although, in the wet season, since all the fishermen were part-timers (farmers) they relied on agricultural activities during the rainy season. In addition to that, Debre Tabor to Ebnat bridge (Melo River bridge) was broken; therefore, in the wet season, it was difficult to cross the Melo River. This leads to the freezing of the fish market. For this reason, the supply of fish has decreased during the wet season. Furthermore, the author stated the same occasion the catch of the fish depends on the fishermen’s activity. For example, the dry season is taken as primary work due to the absence of rainfall and the high demand for fish in the market (Abera et al., Citation2018). In conclusion, the overall fish caught was small. This might be due to there being no motorized and wooden boats, the reservoir is occupied by only a limited number of reed boats made from papyrus and all fishermen are farmers who do not have skill how to fishing (personal communication). Temesgen et al. (Citation2016) also mentioned the problems of Ethiopian fishery as the types of fishing gears, fishing methods, socioeconomic factors, lack of facilities, infrastructure, lack of expertise, and poor land-use practices around fish habitats. These all might be applicable in the Ribb Reservoir.
The Pearson correlation matrix of the present study showed that O. niloticus, L. intermedius, and C. gariepinus were distributed with high concentrations of DO (Table ). The fish growth would slow with a low amount of DO in water systems. In the present study, the abundance of the three fish species in the reservoir was strongly correlated with DO and electrical conductivity but medium correlation with water temperature. Similarly, in Tekeze Reservoir, the abundance and distribution of O. niloticus, L. intermedius, and C. gariepinus showed a positive strong correlation with high DO, electrical conductivity, and TDS (Gebru et al., Citation2019). While, in Lake Langeno, the distribution of O. niloticus showed a positive correlation with DO, temperature, TDS, conductivity, and pH; however, the relationship was very weak. However, C. gariepinus and L. intermedius were highly correlated with chlorophyll content in the same study area (Temesgen et al., Citation2016). Abera et al. (Citation2018) also reported on Lake Ziway in contrast with the present study. Around the watershed of the reservoir, irrigation activities are excessively undertaken; therefore, an urgent call is required for management to keep sustaining the water quality.
Based on the spatial distribution, similar fish species were caught in all sampling sites. However, the proportion of catches varied. In this study, the middle of the reservoir contributed the highest catch. In contrast, the littoral and river mouth habitats represented a high number of fish caught in Lake Langeno (Temesgen et al., Citation2016), Tekeze Reservoir (Gebru et al., Citation2019). The variation might be due to most fish species preferring river mouths and littoral habitats for spawning grounds (Gebru et al., Citation2019). Furthermore, these habitats have plenty of nutrients and vegetation cover for breeding purposes (Temesgen et al., Citation2016). However, in this study, the S1 (dam) and S3 (inlet) had a relatively low proportion of catch. This might be due to the inlet river mouth where there were anthropogenic impacts (agricultural activities) that cause sedimentation and siltation load due to poor land management systems and excessive irrigation as documented by Mequanent et al. (Citation2021). Therefore, these lead to the uneven distribution of fish species. The dam site (littoral) also contributed relatively low catch. This might be due to most fish species are moving downstream through the outlet opening when the water resource needed for irrigation purposes. To avoid this, the iron mesh is important to prevent the movement of fishes downstream; this will reduce the fish stock and diversity in the reservoir. Generally, the variations in the proportion of fish caught among the sampling sites might be due to high sedimentation load, poor land-use practices, excessive irrigation practices and illegal fishing pressures. In addition to this, meteorological factors such as solar radiation, wind, precipitation and seasonal fluctuations affect fish abundance, diversity and distribution (Mequanent et al., Citation20211).
Regarding percentage contribution in number, O. niloticus was the most abundant species followed by L. intermedius in all studied habitats. This implies that all water quality parameters were found at the optimum level for O. niloticus distribution. In agreement with the present study, several studies conducted in Lake Langeno (Temesgen et al., Citation2016), Tekeze Reservoir (Gebru et al., Citation2019; Teame et al., Citation2016; Tewabe et al., Citation2009), Gilgel Gibe I Reservoir (Wakjira, Citation2013), and Lake Ziway (Abera et al., Citation2018) also reported the dominance of O. niloticus at all sampling sites. The second abundant fish species (L. intermedius) was also reported in Tekeze Reservoir (Gebru et al., Citation2019; Tewabe et al., Citation2009). Clarias gariepinus was the third abundant species numerically contributed to (23.3%). Almost comparable studies were conducted in Lake Langeno (Temesgen et al., Citation2016) and Lake Ziway (Abera et al., Citation2018). However, a very less abundance of this species was reported in Tekeze Reservoir (Gebru et al., Citation2019; Teame et al., Citation2016). In the case of (%IRI), O. niloticus and C. gariepinus were the most important fish species in the reservoir (Table ). The high relative importance of O. niloticus was also reported in Lake Zeway (Abera et al., Citation2018), Tekeze Reservoir (Gebru et al., Citation2019), and Lake Langeno (Temesgen et al., Citation2016). However, the lowest contribution of C. gariepinus was reported in the Tekeze Reservoir (Gebru et al., Citation2019; Tewabe et al., Citation2009).
In the present study, similar fish species were recorded during the sampling period with the highest number of catches in the dry season. However, the highest number of catches in the wet season in Lake Langeno (Temesgen et al., Citation2016), in Tekeze Reservoir (Gebru et al., Citation2019; Tewabe et al., Citation2009), in Lake Zeway (Abera et al., Citation2018), in Lake Tana (Tewabe, Citation2014) and Omo Turkana River System (Wakjira & Getahun, Citation2017).
5. Conclusions
The physico-chemical parameters of the reservoir were found in the optimum condition for survival, growth, and production of freshwater fishes. Oreochromis niloticus followed by C. gariepinus dominated the catch composition of the reservoir. The middle of the reservoir contributed the highest catch composition of the fishes. Oreochromis niloticus was the most important fish species during the dry season, while C. gariepinus was important in the wet season. All fishes were positively and strongly correlated with dissolved oxygen. In conclusion, the fish diversity of the reservoir is very poor due to the dam effect followed by anthropogenic activities such as high sedimentation load, excessive irrigation practices, poor land management and illegal fishing. In the case of fish catch, temporal and spatial variations were observed might be due to human-induced and natural factors. Therefore, an appropriate dam design with a fish ladder and effective watershed management setup is needed to sustain fish resources utilization in the country for food security.
Author’s contribution
Agumassie Tesfahun and Sale Alebachew: Conceptualization, Methodology, Software: Sale Alebachew and Agumassie Tesfahun: Data curation, Writing – Original draft preparation. Negese Kebtieneh: Visualization, Investigation, Supervision. Agumassie Tesfahun: Software, Validation. Sale Alebachew and Agumassie Tesfahun: Writing – Reviewing and Editing.
Data availability
All data sets and the materials used in this manuscript are confidentially available.
Statement of ethical approval
Ethics approval and consent to participate permits for this research were issued by the Ethiopian Wildlife Conservation Authority (EWCA), Debre Tabor University, and Farta District Livestock and Fisheries Office. Fish sampling was conducted in accordance with the Ethiopian Wildlife Conservation Authority proclamation number 41/1993.
Acknowledgements
We would like to extend our gratitude to Guna Tana Integrated Field Research and Development Center, Debre Tabor University that provided funds and logistics. We also duly acknowledged Natural and Computational Sciences; Debre Tabor University provided logistics separately. We also acknowledged the fishermen from Ribb Reservoir during data collection. Moreover, we are happy to extend our gratitude to all individuals who contributed their effort to us during data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Sale Alebachew
Sale Alebachew pursued his MSc dDegree in Animal production from Debre Markos University, Ethiopia. Presently, he is a lecturer and researcher at the Department of Animal Sciences, Debre Tabor University, Ethiopia.
Agumassie Tesfahun has an MSc degree in Fisheries, Limnology, and Aquatic-ecotoxicology from Hawassa University, Ethiopia. Currently, he s the Assistant Professor at the Department of Biology, Debre Tabor University, Ethiopia.

Negesse Kebtieneh
Negesse Kebtieneh has an MSc degree in Fisheries, Limnology, and Aquatic-ecotoxicology from Hawassa University, Ethiopia. Currently, he is an assistant professor at the Department of Biology, Debre Tabor University, Ethiopia.
References
- Abera, L., Getahun, A., & Lemma, B. (2018). Changes in fish diversity and fisheries in Ziway-Shala basin: The case of Lake Ziway, Ethiopia. International Journal of Advanced Research in Biological Sciences, 5(2), 165–17. http://dx.doi.org/10.22192/ijarbs.2018.05.02.017
- Anteneh, W., Getahun, A., Dejen, E., Sibbin, F. A., Nagelkerke, L. A. J., de Graaf, M., Wudneh, T. V. J., Palstra, A. P., & Palstra, A. P. (2012). Spawning migration of the endemic Labeobarbus (Cyprinidae, Teleostei) species of Lake Tana, Ethiopia: Status and threats. Journal of Fish Biology, 81(2), 750–765. https://doi.org/10.1111/j.1095-8649.2012.03362.x
- Anteneh, W., Getahun, A., & Dejen, E. (2013). Spawning migrations of Lake Tana labeobarbus spp. (Teleostei: Cyprinidae) in the Ribb River, Ethiopia. African Journal of Aquatic Science, 38(1), 1. https://doi.org/10.2989/16085914.2013.776942
- Awoke, T. (2015). Fish species diversity in major river basins of Ethiopia: A review. World Journal Fish Marketing Science, 7(5), 365–374. https://idosi.org/wjfms/wjfms7(5)15/4.pdf
- Ayalew, L. T., & Bharti, R. (2020). Modelling sediment yield of rib watershed, Northwest Ethiopia. ISH Journal of Hydraulic Engineering, 491–502. https://doi.org/10.1080/09715010.2020.1797544
- Basavaraja, D., Narayana, J. K., & Puttaiah, B. (2014). Fish diversity and abundance in relation to water quality of Anjanapura reservoir, Karnataka, India. International Journal of Current. Microbiology Applied Science, 3(3), 747–757. https://www.ijcmas.com/vol-3-3/D.Basavaraja,%20et%20al.pdf
- Bezabih, A. W. (2021). Evaluation of small hydropower plant at Ribb irrigation dam in Amhara regional state, Ethiopia. Environment Systematic Research, 10(1), 1. https://doi.org/10.1186/s40068-020-00196-z
- Bhatnagar, A., & Devi, P. (2013). Water quality guidelines for the management of pond fish culture. Kurukshetra, India. International Journal Environmental Science, 3(6), 61–68. http://www.romanpub.com/resources/ijes-2019-11.pdf
- De Graaf, M., Machiels, M. A. M., Wudneh, T., & Sibbing, F. A. (2004). Declining stocks of Lake Tana’s endemic Barbus species flock (Pisces; Cyprinidae): Natural variation or human impact? Biological Conservation, 116(2), 277–287. https://doi.org/10.1016/S0006-3207(03)00198-8
- de Graaf, E. D., Nentwich, J. W. M. O., Sibbing, F. A., & Sibbing, F. A. (2005). Lacustrine spawning: Is this a new reproductive strategy among ’large’ African cyprinid fishes? Journal of Fish Biology, 66(5), 1214–1236. https://doi.org/10.1111/j.0022-1112.2005.00671.x
- Dejen, E. (2003). Ecology and potential for fishery of the small barbs (Cyprinidae, Teleostei) of Lake Tana, Ethiopia. PhD Thesis, Agricultural University 180 pp. https://edepot.wur.nl/121430
- Fenta, A., & Kidanemariam, A. (2016). Assessment of cyanobacterial blooms associated with water quality status of Lake Chamo, South Ethiopia. Journal Environmental Analytical Toxicology, 6(1), 343. https://doi.org/10.4172/2161-0525.1000343
- Francisca, C., Aguiar Maria, R. F., Maria, J. M., & Maria, T. F. (2019). Effects of a large irrigation reservoir on aquatic and riparian plants: A history of survival and loss. Water, 11(11), 2379. https://doi.org/10.3390/w11112379
- Ganassin, M. J. M., Rafael, M. M., Fagner, J. M., Oliveira, Carolina, M. M., Natália, C. L., Dos Santos Emili, G. B., & Gomes, L. C. (2021). Effects of reservoir cascades on diversity, distribution, and abundance of fish assemblages in three Neotropical basins. Science of the Total EnvironmentVol. 778 14624https://doi.org/10.1016/j.scitotenv.2021.146246
- Gebre-Mariam, Z., Kebede, E., & Desta, Z. (2002). Long-term changes in chemical features of waters of seven Ethiopian rift-valley lakes. Hydrobiologia, 477(1/3), 81–91. https://doi.org/10.1023/A:1021061015788
- Gebremedhin, S., Getahun, A., Mingist, A., & Anteneh, W. (2014). Some biological aspects of Labeobarbus spp. (Pisces: Cyprinidae) at Arno-Gorno River, Lake Tana Sub-basin, Ethiopia. Journal of Fisheries and Aquatic Science, 9(2), 46–62. https://dx.doi.org/10.3923/jfas.2014.46.62
- Gebremedhin, S. A., Getahun, A., Anteneh, W., Gedif, B. B., Gashu, G. T., Berhanie, B. Z., Alemaw, D., & Alemaw, D. (2017). Effect of large weirs on abundance and diversity of migratory Labeobarbus species in tributaries of Lake Tana, Ethiopia. African Journal of Aquatic Science, 42(4), 367–373. https://doi.org/10.2989/16085914.2017.1411774
- Gebreselassie, T., Asebe, G., & Telahun, K. (2021). Major fish composition of Alwero dam and Baro/Kir River of Gambella, southwest Ethiopia. Asian Journal of Advances in Research, 9(3), 13–22. http://mbimph.com/index.php/AJOAIR/article/view/2315/1998
- Gebru, S., Getahun, A., & Teferi, M. (2019). Diversity and abundance of fishes in Tekeze Reservoir, Tekeze basin, Ethiopia Ethiop. Journal Biological Sciences, 18(1), 55–75. https://www.ajol.info/index.php/ejbs/article/view/201468
- Getahun, A. (2000). Systematic studies of the African species of the genus Garra (Pisces: Cyprinidae). Ph.D. Thesis, City University of New York.
- Getahun, A., Dejen, E., & Anteneh, W. (2008). Fishery Studies of Rib River, Lake Tana Basin, Ethiopia. A Report Submitted to the World Bank, 116 pp. World Bank. http://documents1.worldbank.org
- Habteselassie, R. (2012). Fishes of Ethiopia: annotated checklist with pictorial identification guide (1st ed.). Addis Ababa.
- Haipeng, W. U., Chen, J., Xu, J., Guangming, Z., Lianhai, S., Qiang, L., Zhengjie, Y., Juan, D., Dacong, Y., Liang, J., & Shujing, Y. (2019). Effects of dam construction on biodiversity: A review. Journal of Cleaner Production, 221(3), 480–489. https://doi.org/10.1016/j.jclepro.2019.03.001
- Hilborn, R. (2013). Ocean and dam influences on salmon survival. National Academy Science USA, 110(17), 6618–6619. https://doi.org/10.1073/pnas.1303653110
- Kolding, J. (1989). The Fish Resource of Lake Turkana and Their Environment. M.Sc. Thesis, University of Bergen, Norway, 262 pp. https://www.academia.edu/676514/Fish_resources_of_Turkana_and_their_environment_-_thesis
- Limbu, J. H., & Prasad, A. (2020). Environmental variables and fisheries diversity of the Nuwa River, Panchthar, Nepal. Scientific World, 13(13), 69–74. https://doi.org/10.3126/sw.v13i13.30542
- Limbu, J. H., Jeevan, K. G., Suren, S., Niraj, K., Ashim, A., & Chitra, B. B. (2021). An impact assessment of Betani irrigation dam on fish diversity of damak municipality, Jhapa, Nepal. Egyptian Journal of Aquatic Biology and Fisheries, 25(2), 163–175. https://doi.org/10.21608/ejabf.2021.161363
- Mequanent, D., Mingist, M., Getahun, A., & Anteneh, W. (2021). Impact of irrigation practices on Gilgel Abay, Ribb and Gumara fisheries, Tana Sub-Basin, Ethiopia. Heliyon, 7(3), e06523. https://doi.org/10.1016/j.heliyon.2021.e06523
- Ministry of Water and Energy, MWE. (2010, January). Environmental and social impact assessment of about 20,000 ha irrigation and drainage schemes at Megech Pump (Seraba. Ribb and Anger Dam: Environmental and Social Impact Assessment of the Ribb Irrigation and Drainage Project, 5(1/2), 346.
- Mishra, A. R., & Baniya, C. B. (2016). Ichthyofaunal diversity and physico-chemical factors of MelamchiRiver,Sind-upalchok, Nepal. Journal of Institute and Science and Technology, 21(1), 10–18. https://doi.org/10.3126/jist.v21i1.16031
- Nagelkerke, L. A. J., & Sibbing, F. A. (2000). THE LARGE BARBS (BARBUS SPP., CYPRINIDAE, TELEOSTEI) OF LAKE TANA (Ethiopia), WITH A DESCRIPTION OF A NEW SPECIES, Barbus osseensis. Netherlands Journal of Zoology, 50(2), 179–214. https://doi.org/10.1163/156854200505946
- Reyntjens, D., & Wudneh, T. (1998). Fisheries management- a review of the current status and research needs in Ethiopia. SINET: Ethiopian Journal of Science, 21(2), 231–266. https://doi.org/10.4314/sinet.v21i2.18122
- Shannon, C. E., & Weaver, W. (1963). The mathematical theory of communication. University of Illinois Press.
- Shukla, P., & Singh, A. (2013). Distribution and diversity of freshwater fishes in Aami River, Gorakhpur, India. Advanced Biological Research, 7(2), 26–31. https://www.idosi.org/abr/7(2)13/1.pdf
- Shumye, Y. (2016). Species composition, relative abundance, and socio-economic value of fishes in Ribb and Gumara River, eastern Lake Tana. A thesis submitted to the department of zoological sciences. College of Natural and Computational Sciences, Addis Ababa University, in Partial Fulfillment of the Requirement for the Degree of Master of Science in General Biology, 66 http://etd.aau.edu.et/handle/123456789/9896.
- Teame, T., Natarajan, P., & Tesfay, T. (2016). Assessment of fishery activities for enhanced management and improved fish production in Tekeze reservoir, Ethiopia. International Journal of Fauna and Biological Studies, 3(1), 105–113. https://www.faunajournal.com/archives/2016/vol3issue1/PartB/2-6-17.pdf
- Temesgen, M., Getahun, A., & Lemma, B. (2016). Diversity, distribution, and abundance of fish species in Lake Langeno, Ethiopia. Ethiopian Journal Biological Science, 15(2), 109–130. https://www.ajol.info/index.php/ejbs/article/view/188472
- Temesgen, B., Tadesse, Z. T., & Redondo, M. (2021). Diversity, distribution, and abundance of fish species in upper Awash River Basin, West Showa Zone, Ethiopia. Cogent Food and Agriculture, 7(1). https://doi.org/10.1080/23311932.2021.1974181.
- Tewabe, D., Goshu, G., & Aragaw, C. (2009). A survey of a newly constructed Reservoir, Tekeze Hydropower Dam, Ethiopia. In: Proceedings of the first annual conference of Ethiopian fisheries and aquatic sciences association 149–167 pp.
- Tewabe, D., Getahun, A., & Dejen, E. (2010). Diversity and relative abundance of fishes in some rivers of Tekeze and Blue Nile basins, Ethiopia. Ethiopian Journal Biological Science, 8(2), 145–163. https://www.ajol.info/index.php/ejbs/article/view/89679
- Tewabe, D. (2014). Spatial and temporal distributions and some biological aspects of commercially important fish species of Lake Tana, Ethiopia. Journal of Coastal Life Medicine, 2(8), 589–595. https://doi.org/10.12980/JCLM.2.201414J16
- Tilahun, G., & Ahlgren, G. (2010). Seasonal variations in phytoplankton biomass and primary production in lakes Zeway, Hawassa, and Chamo. Limnologica, 40(4), 330–342. https://doi.org/10.1016/j.limno.2009.10.005
- Vijverberg, J., Dejen, E., Getahun, A., & Nagelkerke, L. A. J. (2012). The composition of fish communities of nine Ethiopian lakes along a north-south gradient: Threats and possible solutions. Animal Biology, 62(3), 315–335. https://doi.org/10.1163/157075611X618246
- Wakjira, M. (2013). Feeding habits and some biological aspects of fish species in Gilgel Gibe Reservoir, Ethiopia. International Journal Current Research, 5(12), 4124–4132. http://www.journalcra.com/sites/default/files/issue-pdf/Download%204532_0.pdf
- Wakjira, M., & Getahun, A. (2017). Ichthyofaunal diversity of the Omo-Turkana Basin, East Africa, with specific reference to fish diversity within the limits of Ethiopian waters. The Journal of Biodiversity Data, 13(2), 2059. https://doi.org/10.15560/13.2.2059
- Woldesenbet, A. (2015). Physicochemical and biological water quality assessment of Lake Hawassa for multiple designated waters uses. Journal of Urban Environmental Engineering, 9(2), 146–157. https://www.jstor.org/stable/pdf/26203449.pdf?refreqid=excelsior%3A900cd6e27f1d01e87d5fbd5e3a87ab3c&ab_segments=&origin=