?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The contamination of heavy metals in the Largescale Yellowfish (Labeobarbus marequensis) from the Solwezi River in North-western Zambia was investigated. The fish were captured from three different sampling sites on the Solwezi River. Site I was located before the mining effluents could join the river, site II was located immediately after the entry point of the mining effluent into the river, while site III was located downstream of where the effluents from the mining company had joined the river. The water and fish organs (muscle, gills, and liver) were checked for contamination with heavy metals. The values were compared with the established permissive levels considered safe for human consumption by the World Health Organization (WHO), and the Food and Agriculture Organization. In decreasing order, the contamination levels in fish organs were: Fe > Zn > Cu (gills), Fe > Zn > Cu (liver) and Zn > Fe > Cu (muscle). Co, Mn, and Pb were not detected in all the parts of the fish investigated. In water, the concentrations were in the order Zn > Fe > CU. The Metal Pollution Index (MPI) showed that site II was the most concentrated with heavy metals, while the gills were the most concentrated part of the fish. These results showed that the levels of the heavy metals investigated in the water and fish were within permissive levels by WHO and FAO. However, future expansion of existing companies and the introduction of new ones should strictly be monitored and their risks studied to safeguard fish and other aquatic products.
PUBLIC INTEREST STATEMENT
Fish plays a critical role in food and nutrition security globally besides its contribution to household income and economic development. Fish is an important source of proteins and relatively cheaper when compared to other animal sources. Furthermore, fish is easily accessible even in rural communities where it might be difficult to access other animal protein sources such as beef, chicken and pork. However, due to rising urbanization and industrialization that pose a threat to aquatic ecosystems, especially water and sediment quality, fish is at an increased risk of being contaminated with heavy metals. Heavy metals threaten human health and can lead to significant heavy-related complications particularly if the contamination levels are above established permissible ranges. Therefore, assessing and monitoring the contamination levels of heavy metals in water and food products is an urgent need to safeguard human health.
1. Introduction
Heavy metals are among the major environmental pollutants resulting from both natural and anthropogenic activities. However, the increased accumulation of heavy metals in the environment is often caused by anthropogenic activities, such as mining and smelting, industrial activities, agrochemicals, atmospheric deposition, and natural denudation of bedrock and ore deposits (Ali et al., Citation2019; Amin et al., Citation2021; Chowdhury et al., Citation2021; Kumari et al., Citation2018; Muhammad & Usman, Citation2021). This has not only raised environmental issues but also public health concerns. In aquatic ecosystems, rapid urbanization coupled with heavy industrialization has been recognized as the major driver of heavy metal accumulation (Ahmad et al., Citation2020; Amin et al., Citation2021; Awasthi et al., Citation2022; Parihar et al., Citation2021). This is worsened when industries release effluents that could contain heavy metals above permissive levels into rivers, streams, or lakes (Chowdhury et al., Citation2021; Kumari et al., Citation2018; Naz et al., Citation2018; Velma et al., Citation2009). Heavy metals are chemical elements which are metallic with a comparatively higher density and are poisonous above a tolerable range. These metals and metalloids are associated with pollution of the environment, and toxicity and they cause adverse effects on all living organisms both aquatic and terrestrial (Ali and Khan, Citation2017; Das Sarkar et al., Citation2022; Sankhla et al., Citation2016). Although there are numerous heavy metals in literature, the most common ones include Cadmium (Cd), Chromium (Cr), Copper (Cu), lead (Pb), Mercury (Hg), Nickel (Ni), and Zinc (Zn; Ahmad et al., Citation2020; Awasthi et al., Citation2022; Masindi & Muedi, Citation2018; Naz et al., Citation2018). Arsenic is one of the common metalloids that is known to be a persistent toxic element. It is reported to affect a vast population of human beings across the globe (Das Sarkar et al., Citation2022). Just like other toxic heavy metals and metalloids, if it goes beyond the permissible limits in food and drinking water, it is lethal and unsafe for humans (Das Sarkar et al., Citation2022).
Furthermore, it is important to note that some heavy metals such as Co, Cr, Cu, Fe, Mn, and Zn are essential in the formation of the cofactor for many enzymes as well as in metabolic activities (Javed & Usmani, Citation2019; Muhammad et al., Citation2021a). They only become toxic to animal and human life when available at excessive levels above the standards established by the Food and Agriculture Organization (FAO) and other national and international standards. Conversely, some heavy metals, such as Cd, Pb, and Hg are known to be toxic even in their trace levels and they have no clear role in fish’s metabolism (Das Sarkar et al., Citation2022; Demirezen & Uruc, Citation2006; Mehana et al., Citation2020). According to Huseen and Mohammed (Citation2019) however, all heavy metals could affect fish by interfering with their metabolic processes and mutagenesis. Besides, several studies have reported bioaccumulation of these heavy metals in fish and plants (Ahmad et al., Citation2020; Awasthi et al., Citation2022; Burger & Gochfeld, Citation2005; Yilmaz et al., Citation2007; Zhang & Wang, Citation2012; Zhao et al., Citation2012) which raises serious public health concerns.
Studies investigating the effect of heavy metals on fish have gained considerable interest in recent years due to the importance of fish as a protein source (Muhammad et al., Citation2021a, Citation2021b; Mwakalapa et al., Citation2019; Simukoko et al., Citation2022; Sonone et al., Citation2021). In Zambia, fish is an important food item as well as a source of direct and indirect employment opportunities (Maulu et al., Citation2020). According to Maulu et al. (Citation2019), capture fisheries remain a major source of fish in Zambia despite aquaculture growing at a faster rate. Rivers and lakes are the most important capture fishery areas as well as an important source of water for aquaculture activities in Zambia. Despite not being classified as a fishery, the Solwezi River is an important source of livelihood, particularly through fishing for the local community in the Solwezi district. It is also a source of water used in aquaculture by some fish farms including Solwezi Aquaculture Research Station (SARS) which is a government-owned fish farm. The river is located in the Solwezi district of North-Western Province of Zambia which is the second major mining area in the country after Copperbelt Province. Close to this river is a major copper and gold mining company whose effluents directly or indirectly enter this river. This presents a potential health risk to the communities that consume the fish from this river. In the Kafue River close to the mining areas, Mbewe et al. (Citation2016) reported high sequestration of heavy metals in liver tissues compared with other body parts of tilapia species. This is because the liver of fishes plays a critical role in the detoxification of pollutants (Alijani et al., Citation2017; Parihar et al., Citation2021; Sankhla et al., Citation2021, Citation2016) that may have been taken in feed via digestion or absorbed through the gills and the skin. These toxicants if they go beyond the threshold the liver can handle, can affect the health of fish and they can also be passed to humans (Alijani et al., Citation2017; Mehana et al., Citation2020; Muhammad et al., Citation2021a; Mwakalapa et al., Citation2019; Simukoko et al., Citation2022). Mwase et al. (Citation1998) indicated that heavy metal contamination could affect the hatchability of eggs and survival of tilapia fry cultured around the mining areas.
Largescale Yellowfish (Labeobarbus marequensis) belonging to the cyprinids group of fish species is an important species that is found mostly in the rocky bottom and rivers of fast-moving waters. The species is also known to frequent sandy stretches as well as reed-fringed pools in both perennial and seasonal streams. (Burnett et al., Citation2018; Fouché, Citation2009). This species is an omnivore with a wide variety of food items utilized such as algae, aquatic insect larvae, snails, small fish, freshwater mussels, and drifting organisms such as ants and beetles (Fouché, Citation2009; Skelton, Citation2001). Besides the Largescale Yellowfish predominately feeding on plant and algal matter, and insects, the species is known to be a habitat generalist (Fouché, Citation2009). It is among the major fish species captured by fishers in the Solwezi River, especially in spring and summer as it is known to migrate upstream in rain-swollen rivers to spawn in rapids. Therefore, it is an important species for food and nutrition security of the local community, besides income generation. However, the presence of mining companies along the river is a threat to the quality and safety of fish products and consequently human health through heavy contamination. Unfortunately, the levels of heavy metals contamination in fish found in this river is not well known. Therefore, the present study aimed at assessing the levels of heavy metal contamination in water and tissues (i.e. the muscle, gills, and liver) of L. marequensis collected from the Solwezi River in North-Western Zambia. The muscle of fish is the most edible part, while the gills and the liver are considered the major indicators of heavy metal contamination in fish (Javed & Usmani, Citation2019).
2. Materials and methods
2.1. Collection and preparation of fish samples
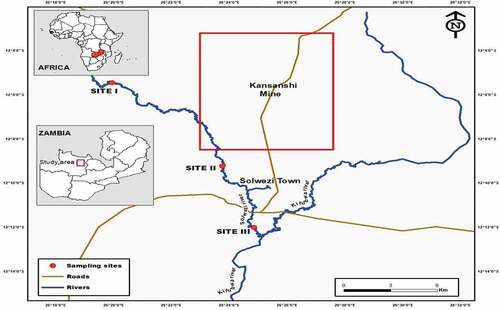
A fleet of gillnets was used to catch Largescale Yellowfish (Labeobarbus marequensis) from the Solwezi River in the North-Western Province of Zambia (). The nets were set across the river and left overnight. The setting of nets was done in the evening (18:00 hrs.) and the hauling of fishing nets was done the following day in the morning (06:00 hrs.). The fish which was found entangled on the gills was then removed from the fishing gear one at a time. The fish samples were collected from three different sites (I, II, and III).
These sites were chosen based on their location with respect to potential mining effluents’ entry points. Site I was located before the mining area, Site II was located just opposite the mining area, and Site III was located after the mining area. Around each of the three sites, a total of thirty (30) L. marequensis specimens were collected making a total of ninety (90) fish. The average concentration per site was obtained. The species were identified following Skelton (Citation2001). The morphometric measures for the sampled fish specimens were recorded () and thereafter the collected samples were put in well-labelled polythene plastic bags and quickly taken to Solwezi Aquaculture Research Station (SARS) for refrigeration at −20 oC to prevent spoilage (Simukoko et al., Citation2022). At the end of sampling, all samples were taken to the Copperbelt University, Environmental Engineering Department laboratory where they were kept in a refrigerator at −20 oC until needed for analysis.
Table 1. Location and characteristics of Labeobarbus marequensis: sampling time (month), length in terms of mean and range of individual fish sampled
2.2. Water sampling and analysis
Water samples from three sites were collected in triplicates (i.e. n = 9) using polythene bottles (500 mL) following the modified method of Khan et al. (Citation2018) and Cornell et al. (Citation2016). The samples were acidified with nitric acid to prevent organic matter alteration by bacterial activity and transferred in an ice-box to the laboratory of the Environmental Engineering Department, School of Mines and Mineral Sciences of the Copperbelt University. The concentration of heavy metals in the collected water samples was analyzed using the Flame Atomic Absorption Spectrophotometer (AAS: model PinAAcle 500, PerkinElmer Inc., Shelton, USA) according to the American Public Health Association (APHA) method and standards (APHA, Citation2012).
2.3. Laboratory analysis of the fish samples
The fish samples were removed from the refrigerator and thawed and washed in ultrapure deionized water to remove any soil particles and debris on them. The ultrapure deionized water was used to ensure no cross-contamination from charged hydrogen ions and any other undesirable compounds from the water used (APHA, Citation2012). Thereafter, the fish were dissected to remove the key organs including the liver, gills, and muscle for investigation of heavy metal contamination. These organs were then dried in an oven at 105 °C for 24 hours to remove all the moisture. After removal from the oven, 1 g of each sample was put in a clean dry, and well-labelled conical flask for digestion. All the samples were prepared in triplicates. Fifty millilitres (50 mL) of concentrated HNO3 (Nitric acid) was added to each sample and the contents were heated on a hot plate in a fume hood until 90 % of the solution evaporated. The conical flasks were then removed from the hot plate and the solution was allowed to cool after which 10 mL of perchloric acids was added followed by additional heating on a hot plate for 10 minutes. After this, about 25 mL of ultrapure deionized water was added to the solution in each flask, and after additional heating for 5–10 minutes, the solution was cooled. After cooling, about 40 mL of ultrapure deionized water was added to the solution and gently shaken to reduce the concentration. Then the solution was filtered through a WhatmanTM filter paper (No. 91, 12.5 cm) held by a funnel into clean dry volumetric flasks to a mark of 100 mL. The solution was transferred into 100 ml clean and well-labelled plastic beakers awaiting the determination of heavy metal concentrations.
2.4. Concentration levels of heavy metals
The concentrations of heavy metals in the sample solutions were determined using the flame Atomic Absorption Spectrophotometer (AAS: model PinAAcle 500, PerkinElmer Inc., Shelton, USA). Before running samples for heavy metal analysis, the AAS was subjected to calibration and standardization using appropriate standards (Hasimuna et al., Citation2021; Manoj & Padhy, Citation2014). The results given for each sediment sample represent an average of the three replicate readings. The calibration curves of absorbance versus concentration were then plotted for each element and the concentration of each of the three metals was determined from the calibration plot by interpolation as reported in Hasimuna et al. (Citation2021). Throughout this study, all the reagents used were of analytical grade and were certified to be pure by the Chemical and Mineral Laboratory of the Copperbelt University, School of Mines and Mineral Sciences, Metallurgy Department. This was necessary for obtaining a calibration curve after diluting a stock standard solution. After obtaining the values for each solution, the actual concentrations of heavy metals in each fish sample were determined.
2.5. Quality control and assurance
The standard solutions for each of the metals were prepared by diluting the standard stock solution of 1000 mg/L Co, Cu, Fe, Mn, Pb and Zn with ultrapure water (Kumari et al., Citation2018). A linear calibration curve for all six heavy metals was obtained. The following were the correlation coefficients (r) obtained: Co (0.9996), Cu (0.9998), Fe (0.9997), Mn (0.9997), Pb (0.9994) and Zn (0.9998). The reagent blanks for each batch of the analyzed samples were used simultaneously for validating and offering baseline correction for the analytical results following the modified description made by Kwaansa-Ansah et al. (Citation2019) and Kumari et al. (Citation2018). The analytical precision and accuracy were evaluated by way of using fish protein and fish muscle Certified Reference Material for Trace Metals (CRM- National Research Council of Canada, Institute for National Measurement Standards, M-12, Ottawa, ON K1A 0R6, Canada). The reference material (DORM-3) is used in both the calibration procedure as well as in the development of the procedure for the analysis of heavy metals in aquatic species. This reference material was prepared from the homogenate of fish protein and muscle (Kwaansa-Ansah et al., Citation2019). The fish from the three sites were analyzed for heavy metals and the average concentrations of the selected metals were recorded in mg/kg dry weight. The percentage of recovery of each heavy metal was calculated by using the following equation:
2.5.1. Metal pollution index (MPI)
To calculate the estimated accumulation of total heavy metals at each site, the MPI was employed using the formula below:
Where, Cfi is the mean concentration for the metal i in the sample, while n is the number of heavy metal elements. In simpler words, the MPI is the geometric mean of the concentration of the metals that have been used in coming up with the index. In this study, since the concentrations of three metals are reported, the MPI will be: (metal 1 (Cu) x metal 2 (Fe) x metal 3 (Zn)) ^ (1/3) (Usero etal., Citation1997).
2.6. Statistical analysis
The descriptive statistics for Cu, Fe and Zn concentrations in the organs of L. marequensis were determined The means for the three heavy metals were tested for normality and homoscedasticity using the Shapiro-Wilk and Leven tests respectively. Since the data were normally distributed, we employed the independent student’s t-test to contrast the mean concentrations for each of the three heavy metals (Cu, Fe and Zn) from the three sites. The tests were conducted at a 5% level of statistical significance. All statistical calculations were carried out in R Statistical Package version 4.1.2 (R Core Team, Citation2021).
3. Results
3.1. Heavy metal concentration in water samples
The concentrations of the different heavy metal elements investigated in this study are shown in . Copper (Cu) and Zinc (Zn) concentrations were significantly higher at Site I compared with sites II and III. The concentration of Iron (Fe) showed an increasing trend from Site I to Site III (I < II < III) and the differences were significant. Cobalt (Co), Manganese (Mn), and Lead (Pb) concentrations were extremely low and below detection levels, while the MPI exhibited a trend of Site II < III < I and the differences among them were significant.
Table 2. Concentrations of heavy metals (mg/kg) and Metal Pollution Index (MPI) in the water collected from Solwezi River
3.2. Heavy metals concentration in fish samples
3.2.1. Muscle
The concentration of heavy metals in the muscle tissue of L. marequensis obtained from different sites of the Solwezi River is shown in . As can be seen from the table, Cobalt (Co), Manganese (Mn), and Lead (Pb) were not detected in the muscle tissue of the fish from the three sampling sites.
Table 3. Concentrations of heavy metals (mg/kg) and Metal Pollution Index (MPI) in the muscle of the largescale yellowfish (Labeobarbus marequensis) collected from three different sites along Solwezi River, Zambia
The fish caught adjacent to the entry point of mining effluents had the highest concentration of heavy metals. The concentrations of Iron (Fe), Copper (Cu), and Zinc (Zn) were highest in the muscle tissue of the fish obtained from Site II, and the concentration was significantly higher compared with those detected in the fish from Sites I and III. Although the concentrations of the fish muscle from Site I were higher compared with the fish obtained from Site III, the difference was not significant. Also, the MPI was low in the muscle tissue of the fish from all three sites.
3.2.2. Gills
Heavy metal concentration in the gills of the fish collected from the Solwezi River at three different sampling sites is shown in . The concentrations of Iron (Fe) and Zinc (Zn) were highest (P ≤ 0.05) in the gills of the fish caught from Site II compared to the fish from the other two sites, (P < 0.05). The concentration of Fe and Zn was lowest in the fish from Site III. Copper (Cu) concentration was highest (P ≤ 0.05) in the gills of the fish obtained from Site I and the lowest was detected in the fish caught from Site III. The Metal Pollution Index was highest at Site II and lowest at Site III. Compared to the other organs of the fish, the gills had the highest concentration of heavy metals with Fe generally being the most concentrated element in the fish.
Table 4. Concentrations of heavy metals and Metal Pollution Index (MPI) in the gills of the largescale yellowfish (Labeobarbus marequensis) collected from three different sites along the Solwezi River, Zambia
3.2.3. Liver
The different heavy metals and their concentrations in the liver tissue of the fish collected from different sampling sites of the Solwezi River are presented in . Iron (Fe), Copper (Cu), and Zinc (Zn) exhibited an increasing concentration trend for the fish from Site I to Site II and later declined at Site III. The concentrations were highest in the liver of the fish from Site II and lowest in those collected from Site III (P ≤ 0.05). A similar concentration trend was observed for the metal pollution index.
Table 5. Concentrations of heavy metals and Metal Pollution Index (MPI) in the liver tissues of largescale yellowfish (Labeobarbus marequensis) collected from the Solwezi River, Zambia
3.2.4. Metal pollution index (MPI) variations
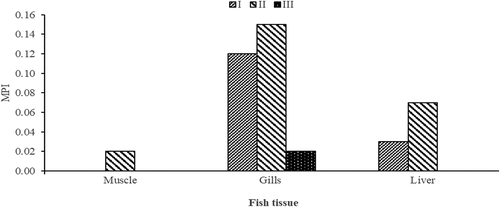
The MPI of the different tissues of the fish from three different sampling sites of the Solwezi River is indicated in . In the muscle tissue, the MPI was only found at Site II with a value of 0.02. In the gills, the MPI values were 0.15, 0.12, and 0.02 at Sites II, I, and III respectively while for the liver only Sites II and I yielded the MPI values. Therefore, only Site II had all the MPI values obtained with the gills recording the highest values.
3.3. Correlation between selected heavy metals and fish length
This study reports a significant positive correlation (Pearson’s correlation) between fish length with Cu, Fe and Zn. A significant positive correlation was observed between the three heavy metals (Cu, Fe, and Zn).
4. Discussion
Globally, studies investigating the impact of heavy metals on aquatic ecosystems have increased in recent years due to the need to sustain production from these resources and maintain high-quality food products (Ekere et al., Citation2018; Matouke & Abdullahi, Citation2020; Mbewe et al., Citation2016; Mehana et al., Citation2020; Rashed, Citation2001; Sankar et al., Citation2010). In fish, some of the major organs or tissues which have been studied to determine the levels of heavy metal pollution include the muscle, gills, liver, kidney, heart and intestines (Alijani et al., Citation2017; Öztürk et al., Citation2009; Vinodhini & Narayanan, Citation2008; Wagner & Boman, Citation2003). These organs accumulate heavy metals and indeed other toxicants differently. This is because each of these organs performs different functions as such the rates of accumulating pollutants differ (Mendis et al., Citation2015; Öztürk et al., Citation2009; Vinodhini & Narayanan, Citation2008). The levels of metals in the gills can represent the immediate levels of heavy metals in the water where the fish lives, while the levels in the muscle and liver reflect the longer-term accumulation of heavy metals in the fish. This makes fish a good biological indicator for monitoring the ecological status of an ecosystem.
In our study, several heavy metal elements including Iron (Fe), Copper (Cu), Lead (Pb), Cobalt (Co), Manganese (Mn), and Zinc (Zn) accumulation were investigated in water samples and the muscle, gills, and liver of L. marequensis collected from the Solwezi River. However, the concentrations of Pb, Co, and Mn in water and fish were extremely low and below detectable levels. Besides, the concentrations of the heavy metals in water samples were within permissible levels set by EC, FAO, FAO/WHO and WHO () and generally lower than in the fish gills and liver. The results of our water samples also suggest that mining was not the only activity polluting the water with heavy metals. For example, some heavy metal elements and the MPI were highest at the site before the mine effluent entry points. A similar finding was reported in the previous study on sediments in the same river (Hasimuna et al., Citation2021).
Table 6. Maximum Permissible Limit (MPL) of heavy metals in fish (mg/kg dry wt.) according to international standards
The continued exposure of fish to heavy metal elements may lead to bioaccumulation in some sensitive organs such as the liver, gills, muscle and kidney. In our previous study, Hasimuna et al. (Citation2021), reported higher concentrations of these heavy metals in the sediments of the Solwezi River, we suggest that the accumulation in the fish gills and liver was coming from the sediments. This is because heavy metals don’t dissolve in water but settle into the sediments such that once the sediments are disturbed they will be made available for absorption into the organism. This is in agreement with the report of Farag et al. (Citation1998) who suggested that heavy metals in sediments were responsible for their bioaccumulation in invertebrates. Similarly, Khan et al. (Citation2019) reported a higher accumulation of heavy metals in mussel soft tissues than in the water samples. However, other studies have shown that different species of aquatic animals could accumulate heavy metals differently (Gorur et al., Citation2012; Petrovic et al., Citation2013) which necessitates further investigations into other species in the river.
Iron (Fe) is an important element that facilitates the transportation of oxygen in the red blood cells for metabolic processes in animals. However, excessive levels could harm the liver of the animal (e.g., fish) resulting in metabolic disturbances and structural damage (Alijani et al., Citation2017). In this study, Fe was more accumulated at the site (II) closest to the mine although the levels were within the permissible levels of ≤100 mg/kg set by the World Health and Organization (WHO, Citation1989). Besides, the concentration was highest in the gills compared to the liver and the muscle. The levels of heavy metals in L. marequensis from the Solwezi River were within permissible limits for human consumption but the results may vary between species.
The results also reveal that fish caught far from the entry points of mine effluents had lower levels compared to those caught closer to the entry points which entails that fish from such areas could be safer for human consumption. This is because heavy metals tend to be more concentrated close to point sources such as mines, industries and other anthropogenic activities. This observation is consistent with the findings of Cui et al. (Citation2019) who reported that heavy metals (Cr and Ni) were higher closer to industrialized parts of the Majiagou River catchment compared to rural sections (p < 0.05). Additionally, Varol and Şen (Citation2012) found elevated levels of heavy metals downstream after the copper mine plant discharge points. Similarly, the Fe concentration in the African catfish (Clarias gariepinus) collected from Jabi Lake in Nigeria did not exceed the permissible levels (Matouke & Abdullahi, Citation2020).
The results of this study indicate that the liver was the most concentrated organ with Fe compared to other organs which could be attributed to its physiological function of detoxification besides glucose, lipids and protein metabolism (Alijani et al., Citation2017; Mehana et al., Citation2020). Furthermore, the concentration in the organs depends on both the specific organ and species under study. In terms of organs, for example, the liver in fish just like in humans is the principal organ for metabolism, nutrient distribution and detoxification of toxins (Alijani et al., Citation2017; Chiang, Citation2014; Mehana et al., Citation2020), as such it could be one of the reasons why it had higher levels. Our findings are similar to those of Foley et al. (Citation2019) who reported that higher levels of Cu, Fe and a range of other minerals were high. This could be due to the fact that the liver needs some of these essential elements for it to perform its functions normally (Foley et al., Citation2019).
In terms of species, research has revealed that different fish species owing to their different feeding habits, besides them occupying different ecological niches, tend to differ in accumulating pollutants. This observation is in agreement with other researchers who reported that heavy metal accumulation in fishes depends on feeding behaviour, location (proximity to pollution source), species’ trophic level, age and size, duration of exposure to metals and homeostatic regulation activities of the fish (Gorur et al., Citation2012; Petrovic et al., Citation2013).
Copper (Cu) is an essential element in all living organisms as it is required by a variety of enzymes and cell components (Rajeshkumar & Li, Citation2018). Excessive levels, however, could be deleterious to humans and are reported to be poisonous, causing nausea, acute stomach pains, diarrhoea, and fever (Rajeshkumar & Li, Citation2018). In the present study, Cu concentrations were highest in the muscle and liver of the fish captured closest to the mining area, while in the gills, the highest concentrations were detected in the fish from Site I, which is before the mining area. Largescale Yellowfish is also known to move long distances and undertake spawning migrations during certain periods of the year, this could be the reason why levels of Cu could have been slightly high even downstream. However, the concentrations in all the organs at all sites were within the permissible levels () indicating that the fish may be safe for human consumption. These results suggest that the accumulation of Cu in the fish from the Solwezi River cannot be blamed on the mines alone as higher levels were detected in the fish before the mining area. This is also true as fish are mobile organisms and can move from one end of the river to the other, especially since these are small headwaters (Desai et al., Citation2019; Ramesh et al., Citation2018). A study by Hasimuna et al. (Citation2021) also reported that other factors such as nearby residential areas, agricultural activities, and wastewater could be responsible for Cu accumulation in aquatic ecosystems. On the other hand, more contaminated fish from the highly contaminated area may have moved to the less contaminated area. This is especially possible since L. marequensis are known to migrate (Burnett et al., Citation2018; Desai et al., Citation2019; Fouché, Citation2009; Ramesh et al., Citation2018). In yellow catfish (P. fluvidraco), the highest concentration of Cu was detected in the kidney suggesting variations in concentration depending on the species (Rajeshkumar & Li, Citation2018). The concentration of Cu detected in this study was similar to those reported in other species, for example, 0.02–0.510 mg/kg, and 0.36–1.01 mg/kg in C. gariepinus (Ekere et al., Citation2018; Matouke & Abdullahi, Citation2020).
Zinc (Zn) plays an important role in cellular growth, differentiation, and metabolism of nutrients in humans (Brown et al., Citation2001). However, excessive intake of the element especially for extended periods could have adverse effects on the immune functions, and absorption of other trace elements, such as Cu and Fe especially in children (Chandra, Citation1984; Fischer et al., Citation1984; Maulu et al., Citation2021). According to FAO (Citation1983), the permissible limit of Zn consumption is 30 mg/kg and any levels above this could be considered excessive. In this study, the concentrations of Zn were 0.15–0.40, 0.28–0.43, and 0.03–0.36 mg/kg, in the muscle, gills, and liver, respectively. These results indicated that the concentrations of Zn in the fish from the Solwezi River were within permissive levels and could, therefore, be considered safe for human consumption as far as Zn is concerned. Zn was most concentrated in the gills compared to the muscle and the liver probably because the gills are the primary entry site for contaminants in fish as they are always in contact with water (Vinodhini & Narayanan, Citation2008).
Lead (Pb), Co, and Mn were not detected in all the fish organs investigated. As a non-essential element, Pb is considered to be one of the most important heavy metal elements in the environment as it can have adverse effects on living organisms (Wani et al., Citation2015). In humans especially children, exposure to Pb could have several effects including learning deficit, lowering of the Intelligent Quotient (IQ), and severe damage to some organs, such as the brain and kidneys (Maulu et al., Citation2021; Rubin & Strayer, Citation2008). Similarly, Pb was poorly accumulated in fish samples obtained from dam lakes in Turkey (Erdoğrul and Erbilir, Citation2007), some lakes in Ontario (Bradley & Morris, Citation1986), and freshwaters in Vietnam (Wagner & Boman, Citation2003). However, Pb accumulation in aquatic ecosystems or fish samples has been highly associated with anthropogenic activities taking place around water resources (Kargin, Citation1996; Sankar et al., Citation2010). For example, fish captured from waters surrounded by heavy pollution sources, such as large-scale agricultural activities, poultry farms, and chemical and fertilizer industries had elevated levels of Pb in several organs (Rashed, Citation2001; Sankhla et al., Citation2021, Citation2016). Our results, therefore, suggest that the mining effluent around the Kifubwa and Solwezi Rivers had very low Pb levels. Mn is an essential micro-element required for biological processes including catalytic functions in animals (Ghaedi et al., Citation2008). However, excessive Mn intake could be toxic to humans and has been reported to cause dopaminergic dysfunction (O’Neal & Zheng, Citation2015). Cobalt is an essential element and a key component of the B12 vitamin (Fallah et al., Citation2011). It is often highly concentrated in the liver tissues where metabolism takes place (Moiseenko et al., Citation2008). For example, in Oligosarcus spp. and Cyphocharax voga the presence of Cobalt in the liver was associated with the activities of the liver tissues in Co metabolism (Weber et al., Citation2013). When taken at excessive levels, Co could cause serious health problems in humans, including neurological (such as impaired hearing and vision), cardiovascular, and endocrine systems (Leyssens et al., Citation2017). The fact that these elements were not detected in the fish samples investigated in this study suggests that the concentrations of Pb, Co, and Mn were within safe levels for human consumption and could not pose any health risk.
The MPI could be used to compare the pollution status of different sampling sites or samples (Usero et al., Citation1997). Our MPI for the three different sampling sites indicates that the heavy metals investigated in this study were more concentrated in the fish captured close to the mining area compared with those from other sites. This could be justified by the fact that mining effluents usually enter water systems at designated points and later get carried away along and begin to settle at the bottom where they get immobilized in sediments. Therefore, the fish found around the place of entry should have the highest concentration before elements are carried away and even settle on surface sediments. Similar findings were reported in surface sediments obtained from the Solwezi River (Hasimuna et al., Citation2021). Although our results show that the fish from the Solwezi River could safely be consumed as the levels are within permissible limits. However, further expansion of the current mining activities could likely threaten the safety of fish products in this river especially if no proper systems are put in place to prevent direct entry of mine effluents. A comparison of the different organs/tissues showed that the heavy metals were more concentrated in the gills. Besides, site II had the highest concentration due to its location in relation to the mining company. Several other studies have also indicated that the gills are usually the most concentrated with heavy metals compared to other organs/tissues in fish (Erdoğrul & Erbilir, Citation2007; Matouke & Abdullahi, Citation2020; Rajeshkumar & Li, Citation2018). The reasons for the highest concentration of heavy metals in the gills could be due to them being the entry point and the formation of a complexion element with mucus that cannot be removed from the lamellae before analysis of the tissue (Erdoğrul & Erbilir, Citation2007). However, Mbewe et al. (Citation2016) reported higher concentrations of heavy metals in the liver tissues than any other part of tilapia species which suggests the concentration of heavy metals in fish is largely influenced by the target species. Future studies could compare the contamination levels of heavy metals in different parts of different fish species.
5. Conclusion and recommendations
The contamination levels of heavy metals in water and tissues of the Large yellowfish in the Solwezi River have been investigated. The results have established that the contamination levels in fish as well as in water from the Solwezi River were within permissive levels established by the EC, FAO, FAO/WHO and WHO. Therefore, they do not pose a threat to the health and safety of water and fish products, and consequently the people that consume them. However, regular monitoring of these heavy metal contamination levels is imperative to safeguard human health. Future studies should investigate the contamination levels in invertebrates as well as other fish species of nutritional and economic importance to the local communities to see how they vary with our findings. It would also be important to investigate how heavy metal contamination in fish responds to different seasons. We also recommend that studies should be conducted to establish the relationship between heavy metal contamination and physicochemical parameters (e.g conductivity, pH, temperature, total dissolved solids, total suspended solids, total alkalinity, and biological oxygen demand). Future expansion of existing mining companies and introduction of new ones in Solwezi district as well as Northwestern province as a whole should strictly be investigated for the potential increase in the accumulation of heavy metals in the river (s), and therefore cause human health risks to the consumers.
Author contributions
Conceptualization, O.J.H and M.C.; methodology, O.J.H.; software, M.C.; validation, O.J.H., M.C and S.M.; formal analysis, S.M.; investigation, O.J.H and S.M; resources, O.J.H.; data curation, S.M.; writing—original draft preparation, O.J.H and S.M.; writing—review and editing, M.C.; visualization, O.J.H., M.C. and S.M.; supervision, M.C.; funding acquisition, O.J.H.
Acknowledgements
The authors wish to thank Mr. Boniface Nyika the Research Officer at Solwezi Aquaculture Research Station (SARS) for his support in organizing the fishers who helped during the fish collection as well as in locating suitable sampling sites. The fishers are particularly thanked for braving the cold by going in the water to set and haul the nets during the cold months of June and July when the weather is bad. Mr. Jonathan Musonda, Mr. Clyde Manase, and Mr. Happy Chibuye are thanked for the assistance rendered during laboratory analysis.
Data Availability Statement
All materials are within the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors

Oliver J. Hasimuna
Oliver J. Hasimuna is a researcher at the Zambian Department of Fisheries. His research interest includes Aquatic Ecology, ecotoxicology (i.e. heavy metals and their impact on aquatic biota and on humans), fish anaesthetics, fish nutrition, fish breeding and genetics, aquatic animal diseases, fisheries management, and climate change in fisheries.
Sahya Maulu
Sahya Maulu is a co-founder and served as the first board chairperson at the Centre for Innovative Approach Zambia (CIAZ) from 2017 to 2021. His research focuses on agriculture, fisheries, aquaculture, rural development, and climate change. However, his main areas of interest are fish feeds and nutrition, fish health, and diseases.
Moses Chibesa
Moses Chibesa is a senior lecturer and researcher at the Copperbelt University in Zambia. His research interests include developing working knowledge in wildlife ecology, forestry and conservation of vertebrates. He also does research on the ecology of birds, plant-animal interactions, impacts of mining pollution on aquatic life and urban ecology.
References
- Ahmad, K., Muhammad, S., Ali, W., Jadoon, I. A. K., & Rasool, A. (2020). Occurrence, source identification and potential risk evaluation of heavy metals in sediments of the Hunza River and its tributaries, Gilgit-Baltistan. Environmental Technology & Innovation, 18(May), 100700. https://doi.org/10.1016/j.eti.2020.100700
- Alijani, R. A., Movahedinia, A., & Rastgar, S. (2017). Fish liver biomarkers for heavy metal pollution: A review article. American Journals of Toxicology, 2(1), 1–16.
- Ali, H., & Khan, E. (2017). What are heavy metals? the long-standing controversy over the scientific use of the term ‘heavy metals’—proposal of a comprehensive definition. Toxicological and Environmental Chemistry, 100(1), 6–19. https://doi.org/10.1080/02772248.2017.1413652
- Ali, H., Khan, E., & Ilahi, I. (2019). Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. Journal of Chemistry, 2019, 1–14. https://doi.org/10.1155/2019/6730305
- Amin, S., Muhammad, S., & Fatima, H. (2021). Evaluation and risk assessment of potentially toxic elements in water and sediment of the Dor River and its tributaries, Northern Pakistan. Environmental Technology & Innovation, 21, 101333. https://doi.org/10.1016/j.eti.2020.101333
- APHA. (2012). American public health association. standard methods for the examination of water and wastewater. Washington, DC, USA.
- Awasthi, G., Nagar, V., Mandzhieva, S., Minkina, T., Sankhla, M. S., Pandit, P. P., Aseri, V., Awasthi, K. K., Rajput, V. D., Bauer, T., & Srivastava, S. (2022). Sustainable amelioration of heavy metals in soil ecosystem: Existing developments to emerging trends. Minerals, 12(1), 85. https://doi.org/10.3390/min12010085
- Bradley, R. W., & Morris, J. R. (1986). Heavy metals in fish from a series of metal-contaminated lakes near Sudbury, Ontario. Water, Air and Soil Pollution, 22(3–4), 341–354. https://doi.org/10.1007/BF00649416
- Brown, K. H., Wuehler, S. E., & Peerson, J. M. The importance of zinc in human nutrition and estimation of the global prevalence of zinc deficiency. (2001). Food and Nutrition Bulletin, 22(2), 113–125. The United Nations University. https://doi.org/10.1177/156482650102200201
- Burger, J., & Gochfeld, M. (2005). Heavy metals in commercial fish in New Jersey. Environmental Research, 99(3), 403–412. https://doi.org/10.1016/j.envres.2005.02.001
- Burnett, M. J., O’Brien, G., Wepener, V., & Pienaar, D. (2018). The spatial ecology of adult Labeobarbus marequensis and their response to flow and habitat variability in the Crocodile River, Kruger National Park. African Journal of Aquatic Sciences, 43(4), 375–384. https://doi.org/10.2989/16085914.2018.1517077
- Chandra, R. K. (1984). Excessive intake of zinc impairs immune responses. JAMA, 252(11), 1443–1446. https://doi.org/10.1001/jama.1984.03350110043027
- Chiang, J. (2014). Liver physiology: Metabolism and detoxification. In L. M. McManus & R. N. Mitchell (Eds.), Pathobiology of human disease (pp. 1770–1782). Elsevier.
- Chowdhury, A., Naz, A., & Maiti, S. K. (2021). Bioaccumulation of potentially toxic elements in three mangrove species and human health risk due to their ethnobotanical uses. Environmental Science and Pollution Research, 28(25), 33042–33059. https://doi.org/10.1007/s11356-021-12566-w
- Cornell, D., Herman, M., & Ontiveros, F. (2016). Use of a UAV for water sampling to assist remote sensing of bacterial fora in freshwater environments. Undergraduate External Publications. Paper 17. http://fsherpub.sjfc.edu/undergraduate_ext_pub/17.
- Cui, S., Zhang, F., Hu, P., Hough, R., Fu, Q., Zhang, Z., An, L., Li, Y.-F., Li, K., Liu, D., & Chen, P. (2019). Heavy metals in sediment from the Urban and Rural Rivers in Harbin City, Northeast China. International Journal of Environmental Research and Public Health, 16(22), 4313. https://doi.org/10.3390/ijerph16224313
- Das Sarkar, S., Swain, P. R., Manna, S. K., Samanta, S., Majhi, P., Bera, A. K., Das, B. K., & Mohanty, B. P. (2022). Arsenic contamination in the food chain - a menace to food safety, human nutrition and health. J. Environ. Biol, 43(3), 339–349. https://doi.org/10.22438/jeb/43/3/MRN-1973
- Demirezen, D., & Uruc, K. (2006). Comparative study of trace elements in certain fish, meat, and meat products. Meat Science, 74(2), 255–260. https://doi.org/10.1016/j.meatsci.2006.03.012
- Desai, M., Husted, A., Fry, C., Downs, C. T., & O’Brien, G. C. (2019). Spatial shifts and habitat partitioning of ichthyofaunal within the middle-lower region of the Pungwe Basin, Mozambique. Journal of Freshwater Ecology, 34(1), 685–702. https://doi.org/10.1080/02705060.2019.1673221
- Ekere, N., Yakubu, N., & Ihedioha, J. (2018). Assessment of levels and potential health risk of heavy metals in water and selected fish species from the Benue-Niger River Confluence, Lokoja, Nigeria. Journal of Aquatic Food Product Technology, 27(7), 772–782. https://doi.org/10.1080/10498850.2018.1499061
- Erdoğrul, Ö., & Erbilir, F. (2007). Heavy metal and trace elements in various fish samples from Sır Dam Lake, Kahramanmaraş, Turkey. Environmental Monitoring and Assessment, 130(1–3), 373–379. https://doi.org/10.1007/s10661-006-9404-5
- Fallah, A. A., Saei-Dehkordi, S. S., Nematollahi, A., & Jafari, T. (2011). Comparative study of heavy metal and trace element accumulation in edible tissues of farmed and wild rainbow trout (Oncorhynchus mykiss) using ICP-OES technique. Microchemical Journal, 98(2), 275–279. https://doi.org/10.1016/j.microc.2011.02.007
- FAO. (1983). Food and Agriculture Organization. Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fishery Circular No, 464, 5e100.
- Farag, A. M., Woodward, D. F., Goldstein, J. N., Brumbaugh, W., & Meyer, J. S. (1998). Concentrations of metals associated with mining waste in sediments, biofilm, benthic macroinvertebrates, and fish from the Coeur d’Alene River Basin, Idaho Note: Use of trade names does not imply endorsement of a product. -->. Archives of Environmental Contamination and Toxicology, 34(2), 119–127. https://doi.org/10.1007/s002449900295
- Fischer, P. W. F., Giroux, A., & L’Abbe, M. R. (1984). Effect of zinc supplementation on copper status in adult man. American Jounal of Clinical Nutrition, 40(4), 743–746. https://doi.org/10.1093/ajcn/40.4.743
- Foley, M., Askin, N., Belanger, M., & Wittnich, C. (2019). Body condition and heavy metal levels in 2016 Atlantic Herring (Clupea harengus) fish kill stranding event In Nova Scotia, Canada. Journal of Marine Animals and Their Ecology, 11(2), 1–12. https://tspace.library.utoronto.ca/bitstream/1807/109035/1/Foley_Meredith_Louise_202111_MHSc_thesis.pdf
- Fouché, P. S. O. (2009). Aspects of the ecology and biology of the lowveld largescale yellowfish (Labeobarbus marequensis, Smith, 1843) in the luvuvhu river, limpopo river system, South Africa. In Doctorate of philosophy in zoology. University of Limpopo. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.874.1603&rep=rep1&type=pdf
- Ghaedi, M., Shokrollahi, A., Kianfar, A. H., Mirsadeghi, A. S., Pourfarokhi, A., & Soylak, M. (2008). The determination of some heavy metals in food samples by flame atomic absorption spectrometry after their separation-preconcentration on bis salicylaldehyde, 1,3 propane diimine (BSPDI) loaded on activated carbon. Journal of Hazardous Materials, 154(1–3), 128–134. https://doi.org/10.1016/j.jhazmat.2007.10.003
- Gorur, F. K., Keser, R., Akcay, N., & Dizman, S. (2012). Radioactivity and heavy metal concentrations of some commercial fish species consumed in the Black Sea Region of Turkey. Chemosphere, 87(4), 356–361. https://doi.org/10.1016/j.chemosphere.2011.12.022
- Hasimuna, O. J., Chibesa, M., Ellender, B. R., & Maulu, S. (2021). Variability of selected heavy metals in surface sediments and ecological risks in the Solwezi and Kifubwa Rivers, Northwestern Province, Zambia. Scientific African, 12, e00822. https://doi.org/10.1016/j.sciaf.2021.e00822
- Huseen, H. M., & Mohammed, A. J. (2019). Heavy metals causing toxicity in fishes. Journal of Physics: Conference Series 2019(6), 1294. https://doi.org/10.1088/1742-6596/1294/6/062028
- Javed, M., & Usmani, N. (2019). An overview of the adverse effects of heavy metal contamination on fish health. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 89(2), 389. https://doi.org/10.1007/s40011-017-0875-7
- Kargin, F. (1996). Seasonal changes in levels of heavy metals in tissues of Mullus barbatus and Sparus aurata collected from Iskenderun Gulf (Turkey). Water, Air and Soil Pollution, 90(3–4), 557–562. https://doi.org/10.1007/BF00282669
- Khan, M. I., Khisroon, M., Khan, A., Gulfam, N., Siraj, M., Zaidi, F., Ahmadullah, Z., Abidullah, M., Fatima, S. H., Noreen, S., Hamidullah, Shah, Z. A., & Qadir, F. (2018). Bioaccumulation of heavy metals in water, sediments, and tissues and their histopathological Effects on Anodonta cygnea (Linea, 1876) in Kabul River, Khyber Pakhtunkhwa, Pakistan. BioMed Research International, 1–10. https://doi.org/10.1155/2018/1910274
- Khan, M. I., Zahoor, M., Khan, A., Gulfam, N., & Khisroon, M. (2019). Bioaccumulation of heavy metals and their genotoxic effect on freshwater mussel. Bulletin of Environmental Contamination and Toxicology, 102(1), 52–58. https://doi.org/10.1007/s00128-018-2492-4
- Kumari, P., Chowdhury, A., & Maiti, K. S. (2018). Assessment of heavy metal in the water, sediment, and two edible fish species of Jamshedpur Urban Agglomeration, India with special emphasis on human health risk, Human and Ecological Risk Assessment. Human and Ecological Risk Assessment, 24(6), 1477–1500. https://doi.org/10.1080/10807039.2017.1415131
- Kwaansa-Ansah, E.E., Nti, S.O., and Opoku, F. (2019). Heavy metals concentration and human health risk assessment in seven commercial fish species from Asafo Market, Ghana. Food Sci Biotechnol, 28(2), 569–579. 10.1007/s10068-018-0485-z
- Leyssens, L., Vinck, B., Van Der Straeten, C., Wuyts, F., & Maes, L. (2017). Cobalt toxicity in humans- A review of the potential sources and systemic health effects. Toxicology, 387, 43–56. https://doi.org/10.1016/j.tox.2017.05.015
- Manoj, K., & Padhy, P. K. (2014). Distribution, enrichment and ecological risk assessment of six elements in bed sediments of a Tropical River, Chottanagpur Plateau: A spatial and temporal appraisal. Journal of Environmental Protection, 5(14), 1419–1434. http://dx.doi.org/10.4236/jep.2014.514136
- Masindi, V., & Muedi, K. L. (2018). Environmental contamination by Heavy Metals. H. Metals, H. E.-D. M. Saleh, & R. F. Aglan (Eds.), Heavy Metals. IntechOpen. https://doi.org/10.5772/intechopen.76082
- Matouke, M., & Abdullahi, K. (2020). Assessment of heavy metals contamination and human health risk in Clarias gariepinus [Burchell, 1822] collected from Jabi Lake, Abuja, Nigeria. Scientific African, 7, e00292. https://doi.org/10.1016/j.sciaf.2020.e00292
- Maulu, S., Hasimuna, O. J., Monde, C., & Mweemba, M. (2020). An assessment of post-harvest fish losses and preservation practices in Siavonga district, Southern Zambia. Fisheries and Aquatic Sciences, 23(1), 25. https://doi.org/10.1186/s41240-020-00170-x
- Maulu, S., Munganga, B. P., Hasimuna, O. J., Haambiya, L. H., & Seemani, B. (2019). A review of the science and technology developments in zambia’s aquaculture industry. Journal of Aquaculture Research and Development, 10, 567.
- Maulu, S., Nawanzi, K., Abdel-Tawwab, M., & Khalil, H. S. (2021). Fish nutritional value as an approach to children’s nutrition. Frontiers in Nutrition, 8, 780844. https://doi.org/10.3389/fnut.2021.780844
- Mbewe, G., Mutondo, M., Maseka, K., & Sichilongo, K. (2016). Assessment of heavy-metal pollution in sediments and tilapia fish species in Kafue River of Zambia. Archives of Environmental Contamination and Toxicology, 71(3), 383–393. https://doi.org/10.1007/s00244-016-0295-3
- Mehana, E. E., Khafaga, A. F., Elblehi, S. S., Abd El-Hack, M. E., Naiel, M. A., Bin-Jumah, M., Othman, S. I., & Allam, A. A. (2020). Biomonitoring of heavy metal pollution using acanthocephalans parasite in the ecosystem: An updated overview. Animals, 10(5), 811. https://doi.org/10.3390/ani10050811
- Mendis, B. R. C., Najim, M. M. M., Kithsiri, H. M. P., & Azmy, S. A. M. (2015). Bioaccumulation of heavy metals in the selected commercially important edible fish species gray mullet (Mugil cephalus) from Negombo Estuary. Journal of Environmental Professionals in Sri Lanka, 4(2), 1–9. https://doi.org/10.4038/jepsl.v4i2.7858
- Moiseenko, T. I., Gashkina, N. A., Sharova, Y. N., & Kudryavstseva, L. P. (2008). Ecotoxicological assessment of water quality and ecosystem health: A case study of the Volga River. Ecotoxicology and Environmental Safety, 71(3), 837–850. https://doi.org/10.1016/j.ecoenv.2008.02.025
- Muhammad, S., Ali, W., & Ur Rehman, I. (2021a). Potentially harmful elements accumulation and health risk assessment of edible fish tissues caught from the Phander Valley, Northern Pakistan. Biological Trace Element Research. https://doi.org/10.1007/s12011-021-03051-z
- Muhammad, S., Ullah, S., Ali, W., Jadoon, I. A. K., & Arif, M. (2021b). Spatial distribution of heavy metal and risk indices of water and sediments in the Kunhar River and its tributaries. Geocarto International. https://doi.org/10.1080/10106049.2021.1926557
- Muhammad, S., & Usman, Q. A. (2021). Heavy metal contamination in the water of Indus River and its tributaries, Northern Pakistan: Evaluation for potential risk and source apportionment. Toxin Reviews, 41(2), 380–388. https://doi.org/10.1080/15569543.2021.1882499
- Mwakalapa, E. B., Simukoko, C. K., Mmochi, A. J., Mdegela, R. H., Berg, V., Müller, M. H. B., Lyche, J. L., & Polder, A. (2019). Heavy metals in farmed and wild milkfish (Chanos chanos) and wild mullet (Mugil cephalus) along the coasts of Tanzania and associated health risks for humans and fish. Chemosphere, 224, 176–186. https://doi.org/10.1016/j.chemosphere.2019.02.063
- Mwase, M., Viktor, T., & Norrgren, L. (1998). Effects on tropical fish of soil sediments from Kafue River, Zambia. Bulletin of Environmental Contamination and Toxicology, 61(1), 96–101. https://doi.org/10.1007/s001289900734
- Naz, A., Chowdhury, A., Mishra, B. K. K., & Karthikeyan, K. (2018). Distribution of heavy metals and associated human health risk in mine, agricultural and roadside soils at the largest chromite mine of India. Environmental Geochemistry and Health, 40(5), 2155–2175. https://doi.org/10.1007/s10653-018-0090-3
- O’Neal, S. L., & Zheng, W. (2015). Manganese toxicity upon overexposure: A decade in review. Current Environmental Health Reports, 2(3), 315–328. https://doi.org/10.1007/s40572-015-0056-x
- Öztürk, M., Özözen, G., Minareci, O., & Minareci, E. (2009). Determination of heavy metals in fish, water and sediments of Avsar Dam Lake in Turkey. Iranian Journal Environmental Health Science and Engineering, 6(2), 73–80.
- Parihar, K., Sankhla, M. S., Kumar, R., & Singh, A. (2021). Assessment of Copper and Iron Concentration in Water of Yamuna River, Delhi, India. Letters in Applied NanoBioScience. https://doi.org/10.33263/LIANBS102.22512257
- Petrovic, Z., Teodrorovic, V., Dimitrijevic, M., Borozan, S., Beukovic, M., & Milicevic, D. (2013). Environmental Cd and Zn concentration in liver and kidney of European hare from different Serbian regions: Age and tissue difference. Bulletin of Environmental Contamination and Toxicology, 90(2), 203–207. https://doi.org/10.1007/s00128-012-0901-7
- Rajeshkumar, S., & Li, X. (2018). Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicology Reports, 5, 288–295. https://doi.org/10.1016/j.toxrep.2018.01.007
- Ramesh, T., Downs, C. T., & O’Brien, G. C. (2018). Movement response of Orange-Vaal largemouth yellowfish (Labeobarbus kimberleyensis) to water quality and habitat features in the Vaal River, South Africa. Environmental Biology of Fishes, 101(6), 997–1009. https://doi.org/10.1007/s10641-018-0754-y
- Rashed, M. N. (2001). Monitoring of environmental heavy metals in fish from Nasser Lake. Environment International, 2(1), 27–33. https://doi.org/10.1016/S0160-4120(01)00050-2
- R Core Team. (2021). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/
- Rubin, R., & Strayer, D. S. (2008). Rubin’s pathology: Clinicopathologic foundations of medicine. Wolters Kluwer/Lippincott Williams & Wilkins.
- Sankar, R., Ramkumar, L., Rajkumar, M., Sun, J., & Ananthan, G. (2010). Seasonal variations in Physico-chemical parameters and heavy metals in water and sediments of Uppanar estuary, Nagapattinam, India. Journal of Environmental Biology, 31(5), 681–686. https://pubmed.ncbi.nlm.nih.gov/21387922/
- Sankhla, M. S., Kumari, M., Nandan, M., Kumar, R., & Agrawal, P. (2016). Heavy metals contamination in water and their hazardous effect on human health-a review. International Journal of Current Microbiology and Applied Sciences, 5(10), 759–766. http://dx.doi.org/10.20546/ijcmas.2016.510.082
- Sankhla, M. S., Kumar, R., & Prasad, L. (2021). Seasonal variations of lead and chromium concentrations in the water samples from Yamuna River in Delhi, India. Iranian Journal of Toxicology, 15(2), 109–114. https://doi.org/10.32598/IJT.15.2.769.1
- Simukoko, C. K., Mwakalapa, E. B., Bwalya, P., Muzandu, K., Berg, V., Mutoloki, S., Polder, A., & Lyche, J. L. (2022). Assessment of heavy metals in wild and farmed tilapia (Oreochromis niloticus) on Lake Kariba, Zambia: Implications for human and fish health. Food Additives and Contaminants: Part A, 39(1), 74–91. https://doi.org/10.1080/19440049.2021.1975830
- Skelton, P. (2001). A complete guide to freshwater fishes of Southern Africa (Second ed.). Struik Publishers (Pty) Ltd.
- Sonone, S. S., Jadhav, S., Sankhla, M. S., & Kumar, R. (2021). Water contamination by heavy metals and their toxic effect on aquaculture and human health through food chain. Letters in Applied NanoBioScience, 10(2), 2148–2166. https://doi.org/10.33263/LIANBS102.21482166
- Usero, J., Gonzales-Regalado, E., & Gracia, I. (1997). Trace metals in bivalve mollusks, Ruditapes decussatus and Ruditapes from the Atlantic Coast of southern Spain. Environment International Journal, 23(3), 291–298. https://doi.org/10.1016/S0160-4120(97)00030-5
- Varol, M., & Şen, B. (2012). Assessment of nutrient and heavy metal contamination in surface water and sediments of the upper Tigris River, Turkey. Catena, 92, 1–10. https://doi.org/10.1016/j.catena.2011.11.011
- Velma, V., Vutukuru, S. S., & Tchounwou, P. B. (2009). Ecotoxicology of hexavalent chromium in freshwater fish: A critical review. Reviews on Environmental Health, 24(2), 129–145. https://doi.org/10.1515/reveh.2009.24.2.129
- Vinodhini, R., & Narayanan, M. (2008). Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (Common carp). International Journal of Environmental Science and Technology, 5(2), 179–182. https://doi.org/10.1007/BF03326011
- Wagner, A., & Boman, J. (2003). Biomonitoring of trace elements in muscle and liver tissue of freshwater fish. Spectrochimica Acta Part B, 58(12), 2215–2226. https://doi.org/10.1016/j.sab.2003.05.003
- Wani, A. L., Ara, A., & Usmani, J. A. (2015). Lead toxicity: A review. Interdisciplinary Toxicology, 8(2), 55–64. https://doi.org/10.1515/intox-2015-0009
- Weber, P., Behr, E. R., Knorr, C. D. L., Vendruscolo, D. S., Flores, E. M. M., Dressler, V. L., & Baldisserotto, B. (2013). Metals in the water, sediment, and tissues of two fish species from different trophic levels in a subtropical Brazilian river. Microchemical Journal, 106, 61–66. https://doi.org/10.1016/j.microc.2012.05.004
- WHO. (1989). World health organization: Heavy metals-environmental aspects. Environment Health Criteria, (85). Geneva, Switzerland
- Yilmaz, F., Ozdemir, N., Demirak, A., & Tuna, A. L. (2007). Heavy metal levels in two fish species Leuciscus cephalus and Lepomis gibbosus. Food Chemistry, 100(2), 830e5. https://doi.org/10.1016/j.foodchem.2005.09.020
- Zhang, W., & Wang, W. X. (2012). Large-scale spatial and interspecies differences in trace elements and stable isotopes in marine wild fish from Chinese waters. Journal of Hazardous Materials, 215-216, 65–74. https://doi.org/10.1016/j.jhazmat.2012.02.032
- Zhao, S., Feng, C., Quan, W., Chen, X., Niu, J., & Shen, Z. (2012). Role of living environments in the accumulation characteristics of heavy metals in fishes and crabs in the Yangtze River Estuary, China. Marine Pollution Bulletin, 64(6), 1163e71. https://doi.org/10.1016/j.marpolbul.2012.03.023