 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study evaluated the antifungal and insecticidal properties of soybean extract on Marasmiellus scandens (causal pathogen of white thread blight disease on cocoa), Erythricium salmonicolor (causal pathogen of pink disease of cocoa) and termite (Microtermes subhyalinus). Agar plate test was used to determine the inhibitory activity of the extract on mycelial growth of the pathogens while a contact toxicity bioassay was conducted on the termites. Extract of the soybean reduced mycelial growth in a dose-dependent pattern, with 20% of the extract attaining the highest inhibition. Percentage inhibition of 80.4 and 86.8 were recorded for E. salmonicolor and M. scandens, respectively after 7 days of incubation. The IC50 values ranged between 0.64–2.44% and 0.12–0.45% for E. salmonicolor and M. scandens respectively. However, activity of the extract on the termite was low, with an LC50 of 15.42% after 24 hours of exposure. The effect of the extract on the fungal pathogens indicates that it could be further explored towards the development of a plant-based control method against white thread blight and pink diseases of cocoa in Ghana.
1. Introduction
Ghana’s economy, over decades, has been largely supported by cocoa, the most important cash crop in the country (McKay & Aryeetey, Citation2004). The country continues to be the world’s second largest producer and exporter of cocoa. Insect pests and diseases have been identified as key factors limiting cocoa production worldwide. Diseases such as Phytophthora pod rot (black pod), witches broom, swollen shoot virus, vascular streak dieback, and monilia pod rot have been known to cause 40% of annual global loss of cocoa (Flood et al., Citation2004). Of these diseases, black pod, caused by Phytophthora megakarya and Phytophthora palmivora, is considered as the most economically important fungal disease of cocoa in Ghana (Akrofi et al., Citation2003; Dakwa, Citation1987; Opoku et al., Citation2000). Yield loss due to black pod disease could reach 100% without proper control measures (Dakwa, Citation1987). Apart from black pod, thread blight and pink diseases are emerging as serious diseases of cocoa in Ghana (Akrofi et al., Citation2014; Amoako-Attah et al., Citation2016).
Erythricium salmonicolor (Berk. & Broome), the causal pathogen of pink disease of cocoa, is widely distributed and has been reported to attack many plant species worldwide (Smith, Citation1985). Plants such as coffee, tea, cashew (Hilton, Citation1958; Seth et al., Citation1978), and citrus (Pradhanang, Citation1994) have been reported as economic host plants of the pathogen. Pink disease is widespread in Ghana (Akrofi et al., Citation2008) and cocoa yield loss of 60–100% on experimental plots at Bunso in the Eastern region of Ghana has been reported (Opoku et al., Citation2001). The pathogen girdles around stems and branches, affecting the flow of water and nutrients leading to branch and stem die-back especially in the distal parts of the tree. White thread blight disease, caused by Marasmiellus scandens (Massee) Dennis & Reid is widely distributed and has been found on important economic plants such as apple and tea (Adedeji, Citation2006). Plants such as plantain, banana and coconut which are found in cocoa farms are also susceptible to the disease (Benchimol et al., Citation2001). It is an important disease of cocoa in Ghana and has been reported in all cocoa regions among which the Ashanti Region recorded the highest severity of 13.8% (Amoako-Attah et al., Citation2016). The disease is recognized by thick creamy-white mycelial strands which spread mostly beneath branches and leaves leading to eventual death of whole branches or trees (Opoku et al., Citation2007). High disease incidence and severity is usually recorded in farms with low levels of sanitation (Amoako-Attah et al., Citation2016).
Pruning branches and leaves infected with pink and white thread blight diseases and supplementing with fungicide application help in the control of these diseases (Akrofi et al., Citation2014; Amoako-Attah et al., Citation2016).
Cocoa is also attacked by a diverse range of insect pests (Awudzi et al., Citation2019; Avicor et al., Citation2022) and these are major determinants of productivity. Besides the hemipterans such as mirids, stink bugs, coreid bugs and mealybugs, termites are also important pests of cocoa (Ackonor, Citation1997; Ambele et al., Citation2018; Awudzi et al., Citation2019; Sylvain et al., Citation2015) and other crops (Rouland-Lefèvre, Citation2011), although known for their roles in ecosystem engineering (Evans et al., Citation2011; Jouquet et al., Citation2011; Pringle et al., Citation2010). During a 3-year study in Ghana, Ackonor (Citation1997) reported Microtermes subhyalinus as a pest of cocoa and among the most common termite species in cocoa farms. Microtermes subhyalinus and other Microtermes species are nearly ubiquitous in different habitats and cropping systems (Mora et al., Citation1996; Schyra & Korb, Citation2019; Usher, Citation1975) including cocoa (Kissi et al., Citation2022; Ollo et al., Citation2022; Sylvain et al., Citation2015, Citation2019). Termite attack can cause substantial damage and yield loss. According to Mora et al. (Citation1996), M. subhyalinus infestation of sugarcane resulted in an annual yield loss of 10% in Central African Republic. In cocoa, the activity of termites is most important in the dry season as they chew roots of the crop at establishment (Awudzi et al., Citation2019). Their presence is usually seen by the presence of tunnels or runways on the stems of infested plants. This damage makes establishment difficult, especially in termite-endemic areas. It also makes crop establishment very expensive because of the need for refilling.
The main method used by farmers in controlling cocoa pests such as insect pests and diseases is the use of synthetic pesticides. Application of synthetic pesticides remains expensive and there is the risk of environmental pollution, development of resistance and high residues in crops (Balakumar et al., Citation2011; Sande et al., Citation2011). It is therefore necessary to search for safer and effective alternatives which will not have adverse effects on the environment and reduce health risk to farmers. Studies have shown that plant extracts including soybean (Glycine max) contain vital secondary metabolites which provide protective effects against fungal and insect attack (Igboabuchi & Llodibia, Citation2018; Kikuta, Citation2020; Villalobos et al., Citation2016). Soybean is rich in protein and contains several secondary metabolites such as isoflavones, phenolic acids, alkaloids and saponins (Lisanti & Arwin, Citation2019; Villalobos et al., Citation2016). Aqueous phenolic extract from soy flour possessed antimicrobial activity against several medically important microbes such as Listeria monocytogenes, Listeria innocua, Staphylococcus aureus, Bacillus cereus, Enterococcus faecalis, Yersinia enterocolitica, Escherichia coli and Salmonella enterica and moderate activity on 5 yeast species; Torulaspora spp, Cryptococcus spp, Rhodotorula spp, Aureobasidium pullulans and Debaryomyces spp (Villalobos et al., Citation2016). Soy flour and soybean extract containing the major soybean isoflavone, genistein, adversely affected the survivorship of Tribolium castaneum (Kikuta, Citation2020) and reduced larval and pupal weight of Anticarsia gemmatalis (Piubelli et al., Citation2005), respectively. Several authors have also reported on the inhibition of fungi (Candida albicans and Aspergillus niger) and bacteria (S. aureus and L. monocytogenes) growth by soybean extracts (Hosseini Chaleshtori et al., Citation2017; Igboabuchi & Llodibia, Citation2018; Wang et al., Citation2010). Although several studies indicate the activity of soybean extracts against insects and microbes, knowledge of their activity on cocoa pests for potential integration into pest management strategies for cocoa production is scant. Therefore, this study assessed the in vitro fungicidal and insecticidal activity of soybean extract in controlling the causative pathogens of white thread blight and pink diseases of cocoa as well as the termite Microtermes subhyalinus.
2. Material and methods
2.1. Soybean extract
Soybean seeds were obtained from Tamale, the capital city of the Northern region of Ghana and the crude aqueous extract was obtained following the description in Gagman et al. (Citation2022) with some modifications. The seeds were washed first with tap water and then distilled water before being shade-dried for 3 weeks. They were then pulverised into powder. Water (5 L) was added to the powdered seeds (1 kg) and incubated at room temperature for 1 week. This was then filtered with a muslin mesh and concentrated to drying. The concentrated powder (60 g) was dissolved in 100 ml of water to obtain a stock solution of 60% v/v. Water was used for the extraction because it is a non-toxic and cheap solvent (Cater et al., Citation1974; González & Muñoz, Citation2017). It is also abundant and has a wider application (Cater et al., Citation1974; González & Muñoz, Citation2017) and water-based extracts can easily be prepared by resource-poor farmers.
2.2. Isolation of pathogens
Isolation techniques described in Amoako-Attah et al. (Citation2020) were used to obtain pure cultures of Marasmiellus scandens and Erythricium salmonicolor from cocoa trees infected with white thread blight and pink diseases, respectively. Pink pustular growth forms and hyphal strands from infected branches and leaves were directly plated on water agar and subsequently cultured on Potato Dextrose Agar (PDA) media and incubated at 28°C for seven days. Sub-culturing was done until pure cultures of M. scandens and E. salmonicolor were obtained. Three isolates each of M. scandens (MS 1, MS 2, MS 3) and E. salmonicolor (ES 1, ES 2, ES 3) were used in all experiments. Pure cultures were identified microscopically using morphological characteristics as described by Humber (Citation2005) and Kirk et al. (Citation2008). Erythricium salmonicolor isolates on agar plates were pinkish and later turned creamy whiles M. scandens isolates formed white thread-like mycelia with feathering margins (Akrofi et al., Citation2014; Amoako-Attah et al., Citation2016). The isolates have previously been differentiated using molecular techniques (Amoako-Attah et al., Citation2020; Kwarteng et al., Citation2018), coded and added to the pathogen library at the Cocoa Research Institute of Ghana.
3. In vitro antifungal activity
The antifungal activity of the soybean extract was performed using the agar plate test as described by Nwosu and Okafor (Citation1995). Molten PDA was amended with different concentrations (20, 10, 1, 0.1, 0.01 and 0.001% v/v) of the soybean extract to a volume of 100 ml and poured into Petri dishes to set overnight. The plates were inoculated with 5 mm disc plugs taken from the edge of pure cultures of M. scandens and E. salmonicolor. Non-amended PDA plates inoculated with the test pathogens were included as controls. There were three replicated plates for each treatment per pathogen and incubated at 28 ± 2°C for 7 days. Colony diameters were recorded each day and percentage inhibition of radial growth was calculated as:
where PIRG = Percentage Inhibition of Radial Growth; C = average radial growth in control plates; T = average radial growth in test plates
4. Termiticidal activity
Termites (Microtermes subhyalinus) collected from experimental plots of the Cocoa Research Institute of Ghana (CRIG) at New Tafo-Akim were used for the test. Concentrations (50%, 40%, 30%, 20% and 10% v/v) of the soybean extract were prepared with distilled water and used for the residual toxicity test (Ackonor & Adu-Acheampong, Citation2007) as follows. Whatman No. 10 filter paper was used to line the base of a Petri dish and 1 ml of the extract concentration was used to moisten it. Ten worker termites were placed on the filter paper in each Petri dish and the mortality of termites after 24 hours was noted. Distilled water was used to moisten filter paper for the control. Five replicates each were done for the extract concentrations and the control. The experiment was conducted at a temperature of 25 ± 4°C, relative humidity of 80 ± 7% and a photoperiod of 12 h: 12 h Light: Dark.
5. Data analysis
The PIRG of each fungus in response to the extract concentrations was computed. These were used to obtain the Minimum Inhibition Concentration (MIC) and then used in probit analysis to obtain the concentration inhibiting 50% of radial growth (IC50), slope, intercept, standard error and 95% confidence interval (CI) with IC50 values with overlapping 95% CIs not considered as significantly different. Analysis of variance (ANOVA) was also performed on the PIRG data using Genstat 10th edition and at a significance level of 5%, Tukey post hoc test was done. The number of dead termites compared to the total number of test termites was used to compute the percent mortality per concentration. Mortality data was arcsine-transformed and used for ANOVA with Tukey post hoc test done at 5% significance level. The mortalities for the various concentrations were analysed using probit to obtain the median lethal concentration (LC50) and associated parameters as indicated for the IC50. Probit analysis was done using the QCal software by Lozano-Fuentes et al. (Citation2012).
6. Results
6.1. In vitro antifungal activity
The PIRGs of E. salmonicolor and M. scandens induced by the soybean extract are presented in Figures , respectively. Generally, the highest concentration (20%) of the soybean extract highly inhibited all the isolates of E. salmonicolor and M. scandens. The 20% concentration of the extract inhibited ES 2 by 80.4% after 7 days of incubation and this percentage inhibition (PI) was the highest among the isolates of E. salmonicolor (Figure ). Generally ES 1 had the lowest PI among the isolates of E. salmonicolor across all the tested concentrations of the soybean extract. There was no significant difference in PI of isolate ES 1 between 20% and 10% extract concentrations after 5 and 7 days of incubation. However, there was a significant difference between the PI of both concentrations after 5 and 7 days of incubation for the other isolates of E. salmonicolor (ES 2 and ES 3). The highest PI of 86.8% was recorded at 20% concentration of the soybean extract after 7 days of incubation on plates inoculated with MS 1. This value was however not significantly different from the PI obtained at 20% concentration for the other M. scandens isolates after 7 days of incubation. There was no significant difference between the PI by the 10% and 20% soybean extract for all the isolates of M. scandens after 5 and 7 days of incubation except for MS 2 where there was a significant difference in PI between the 10% and 20% concentrations after 5 days of incubation.
Figure 1. Effect of different concentrations of soybean extract on the mycelial growth of E. salmonicolor (ES) at 5 and 7 days of incubation (28 ± 2°C). Values of percentage inhibition bearing the same letter within the same incubation day are not significantly different at p < 0.05 using Tukey’s post hoc test.
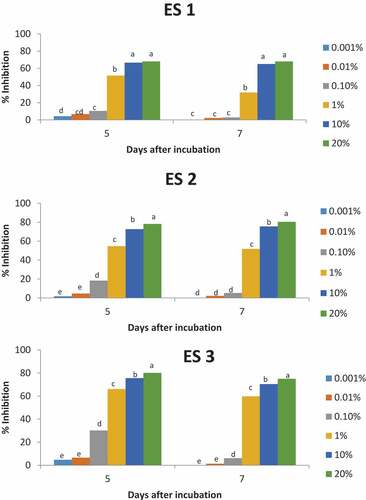
Figure 2. Effect of different concentrations of soybean extract on the mycelial growth of Marasmiellus scandens (MS) isolates at 5 and 7 days of incubation (28 ± 2°C). Values of percentage inhibition bearing the same letter within the same incubation day are not significantly different at p < 0.05 using Tukey’s post hoc test.
Figures show the effect of the soybean extract on E. salmonicolor and M. scandens after 5 and 7 days of incubation. Higher inhibitions of the isolates were obtained as the concentration of the soybean extract increased.
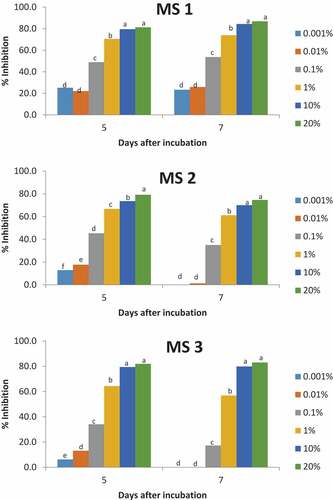
Figure 3. Binary logistic model of soybean extract on the mycelial growth of isolates of Erythricium salmonicolor (ES) after different incubation days. A: ES 1 after 5 days of incubation B: ES 1 after 7 days of incubation C: ES 2 after 5 days of incubation D: ES 2 after 7 days of incubation E: ES 3 after 5 days of incubation F: ES 3 after 7 days of incubation.
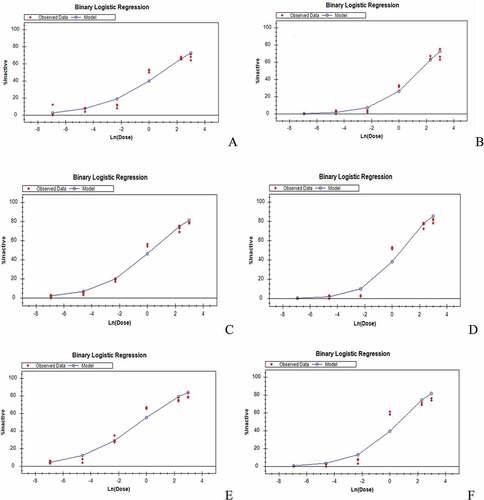
Figure 4. Binary logistic model of soybean extract on the mycelial growth of isolates of Marasmiellus scandens (MS) after different incubation days. A: MS 1 after 5 days of incubation B: MS 1 after 7 days of incubation C: MS 2 after 5 days of incubation D: MS 2 after 7 days of incubation E: MS 3 after 5 days of incubation F: MS 3 after 7 days of incubation.
The E. salmonicolor isolates had higher IC50 values than the isolates of M. scandens after 5 days of incubation. The IC50 of E. salmonicolor was from 0.6428 to 2.4370 %v/v while that of M. scandens was 0.1193 to 0.4500 %v/v (Table ). The E. salmonicolor isolates had significantly different IC50 values. Two isolates (MS 2 and MS 3) of M. scandens had IC50 values that were not significantly different from each other but they were significantly different from MS 1. However, MS 3 and ES 3 had IC50 values that were not significantly different (Table ).
Table 1. Inhibition activity of soybean extract on isolates of Erythricium salmonicolor and Marasmiellus scandens after 5 days of incubation
After 7 days of incubation, ES 2 and ES 3 had the same IC50 value and were significantly different from all the other fungal isolates while MS 2 and MS 3 also had similar IC50 values and were significantly different from the other fungal isolates (Table ).
Table 2. Inhibition activity of soybean extract on isolates of Erythricium salmonicolor and Marasmiellus scandens after 7 days of incubation
7. Termiticidal activity
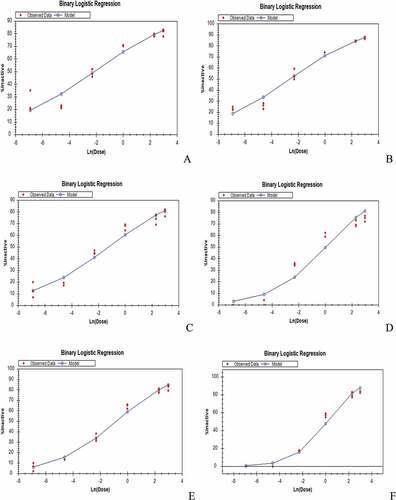
Termite mortality due to the various extract concentrations was significantly different except mortality caused by 30% and 40% extract concentrations and also the 30% and 20% extract concentrations. The control treatment recorded 2% termite mortality. The binary logistic regression of the effect of the soybean extract on termite workers indicates an increase in activity with increase in extract concentration and significantly different LC50 values among the termite species (Figure , Table ).
Figure 5. Binary logistic model of soybean extract on Microtermes subhyalinus after exposure for 24 hours.
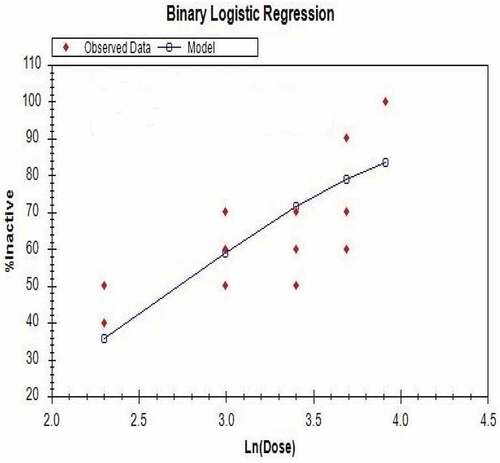
Table 3. Mortality effect of soybean extract on Microtermes subhyalinus after 24 hours of exposure
8. Discussion
The bioactivity of soybean extract was evaluated against isolates of E. salmonicolor and M. scandens. The results show that the soybean extract had inhibitory effect on the pathogens in culture. Various studies on the antimicrobial properties of soybean have been reported (Ponnusha et al., Citation2011; Wang et al., Citation2010) with different inhibition activities. In our study, the highest inhibition of E. salmonicolor and M. scandens by the soybean extract was 80.4% and 86.8%, respectively at the highest concentration of 20% after seven days of incubation. The inhibition percentage values obtained at both 10% and 20% were higher than those obtained by Igboabuchi and Llodibia (Citation2018) who tested the inhibitory activity of 15% ethanol extracts of soybean (seed and leaf) on various bacteria and fungi. The percentage inhibition of the seed extract against A. niger and E. coli was 62.2% and 68.9%. However the leaf extract inhibited A. niger at 49.4% and E. coli at 53.9%. Percentage inhibition of 10.9 to 61.0% were recorded when gyceollins extracted from soybean seeds were tested for their antifungal activities against Fusarium oxysporum, Phytophthora capsici, Sclerotinia sclerotiorum and Botrytis cinera (Hyo et al., Citation2010). Nevertheless, complete inhibition of microbial growth using plant extracts have been reported such as the study of Tao et al. (Citation2014) on Penicillium italicum and Penicillium digitatum using essential oil of Citrus reticulata at 0.00025% and 0.004%, respectively.
Generally, the mycelial inhibition of the test pathogens increased when the concentration of the soybean extract was increased from 0.001% to 20.0% (v/v). This trend is consistent with reports of antifungal activities of plant extracts including soybean (Mohammadi et al., Citation2015; Velazquez-Nuenez et al., Citation2013). Studies by Hosseini Chaleshtori et al. (Citation2017) indicated an increase in the diameter of the zone of inhibition of L. monocytogenes from 11.78 to 22.16 mm and 10.21 to 20.01 mm when the concentration of methanolic extracts of 2 soybean varieties were increased from 25 to 100 mg/ml.
All the test isolates of E. salmonicolor obtained MICs of 0.01% whiles M. scandens 1, M. scandens 2 and M. scandens 3 obtained MICs of <0.001, 0.01 and 0.1%, respectively after seven days of incubation. The MIC values obtained in this present study are similar to the MIC (0.01%) obtained when essential oil of soybean seeds was tested against B. subtilis and E. coli (Ghahari et al., Citation2017). Studies by Hyo et al. (Citation2010) reported MIC values ranging from 0.0025% to 0.0075% when glyceollins isolated from soybean seeds were tested for their antifungal activities against F. oxysporum, P. capsici, S. sclerotiorum and B. cinera. MIC of 0.013% was also reported when isolates of P. digitatum were inhibited in medium amended with Night shade crude extract (Kanan & AL-Najar, Citation2008). However, Sobowale et al. (Citation2013) and Rahman et al. (Citation2016) obtained higher MIC values when extracts of Calotropis procera and Cassia alata were tested against A. niger and Penicillium marneffei, respectively.
The IC50 values obtained for isolates of E. salmonicolor ranged from 0.6428 to 2.437% while values for M. scandens ranged from 0.1193 to 0.45%. Lower values of IC50 obtained for M. scandens indicate that they are more sensitive to the soybean extracts than isolates of E. salmonicolor. Rahman et al. (Citation2016) obtained IC50 values of 2.8% when methanolic extracts of P. sarmentosum were tested against Pseudomonas fuscovaginae. However, lower values of IC50 (0.0168–0.0185%) were recorded when Bello et al. (Citation2019) evaluated the antimicrobial activities of hexane extract of Balanite aegyptiaca and Tapinanthus preussi against Candida albicans.
Antimicrobial activities of plant extracts have been attributed to the presence of bioactive compounds in the extracts (Burt, Citation2004; Chah et al., Citation2006; Daferera et al., Citation2000). Many studies have attributed the antimicrobial properties of soybean extract to the presence of phytochemicals such as phenols, saponins, flavonoids and alkaloids (Cowan, Citation1999; Dahanukar et al., Citation2000; Villalobos et al., Citation2016). Igboabuchi and Llodibia (Citation2018) attributed the inhibition of S. aureus, E. coli and A. niger by the ethanolic extract of the leaves and seeds of soybean to the presence of phytochemicals such as phenols and flavonoids in the extracts. Similarly the inhibition of nucleic acid synthesis in S. aureus by soybean isoflavones has been reported by Wang et al. (Citation2010). The inhibition of E. salmonicolor and M. scandens by soybean extract in this study could be attributed to the presence of phytochemicals in the extract. Bioactive compounds in plants inhibit the growth of microorganisms by the denaturing of vital enzymes, alteration of cell wall and degradation of fungal hyphae (Hyldgaard et al., Citation2012; Nychas, Citation1995; Tian et al., Citation2011). Manso et al. (Citation2013) also reported the disintegration of conidia and mycelia of A. flavus in a medium amended with cinnamon essential oil. Soybean toxin, an antifungal protein isolated from soybean has also been reported to inhibit hyphal development and block nutrient uptake systems in Candida albicans (Morais et al., Citation2013).
Botanical extracts can cause toxic effects on pests and as such have been investigated as potential pesticidal agents (Pavela, Citation2015; Žabka et al., Citation2021). In comparison with the findings of plant extracts on diverse insects of medical and agricultural importance in other studies (Pavela, Citation2015; Avicor et al., Citation2021), the extract in this study was less toxic to the test termite species. A study of 41 plant extracts (essential oils) for repellency effect against 3 mosquito species showed that soybean extract was among the most ineffective (Amer & Mehlhorn, Citation2006a). However, it had variable repellency on Culex quinquefasciatus (100%), Anopheles stephensi (76.2%) and Aedes aegypti (54%). It (at 50 ppm) was also among the least effective in terms of mortality, causing no mortality in Ae. aegypti larvae after 24 hours (Amer and Mehlhorn, Citation2006b). The high LC50 of the soybean extract on Microtermes subhyalinus indicates the relative insensitivity of the extract on the insect. This is similar to the reported low efficacy of soybean extract on mosquitoes (Amer & Mehlhorn, Citation2006ab). Naturally, the concentration of the phytochemicals in soybean extract may be less toxic to termites in terms of inducing high mortality. However, as observed in other studies (Kikuta, Citation2020; Amer & Mehlhorn, Citation2006a; Piubelli et al., Citation2005), it possesses other anti-insect properties such as repellent and anti-developmental effects which can contribute to suppress insect pests. Hence, the need for further investigations on the effect of the extract on the behaviour and developmental stages of the pest. Termites are polyphagous and feed on several plant species. Hence, they could have adapted to some of the phytochemicals in plant extracts. As such, the low toxicity of the soybean extract could also be due to the ability of the termites to resist or tolerate biologically active compounds in the extract through several strategies including physiological and metabolic means. Although not observed in this study, extracts from some plants have been shown to be effective termiticidals (Elsayed, Citation2011) on some Amitermes and Microtermes species. Studies by Elango et al. (Citation2012) and Bakaruddin and Ab Majid (Citation2019) also indicate the efficacy of some plant extracts on termites species like Coptotermes formosanus, Coptotermes gestroi and Globitermes sulphureus. It is therefore important to continue the search for effective termiticides from plants to be integrated into a sustainable and environmentally-friendly crop production system.
9. Conclusion
Antifungal effect of soybean extract against M. scandens and E. salmonicolor has been demonstrated in this study. Inhibition of 80.45 and 86.8% were attained by 20% concentration of the extract against E. salmonicolor and M. scandens, respectively after 7 days of incubation. The soybean extract was however ineffective against the termite Microtermes subhyalinus. The bioactive compounds in the soybean extract are yet to be isolated and tested against the pathogens under laboratory and field conditions. This could be part of an Integrated Pest Management (IPM) of these important diseases of cocoa in Ghana.
Data availability
Data used in support of the publication of this manuscript is available upon request from the corresponding author
Acknowledgements
The assistance of the technical staff of the Plant Pathology and Entomology Divisions of the Cocoa Research Institute of Ghana in the laboratory evaluation studies is gratefully acknowledged. The support of the Department of Chemistry of the University of Ghana and the University of Ghana Research Fund are also acknowledged.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ackonor, J. (1997). Preliminary findings on termites (Isoptera) associated with cocoa and coffee farms in Ghana. International Journal of Tropical Insect Science, 17(3–4), 401–14. https://doi.org/10.1017/S1742758400019251
- Ackonor, J. B., & Adu-Acheampong, R. (2007). Standard protocol for screening conventional insecticides at the cocoa research institute of Ghana for mirid control. Journal of the Ghana Science Association, 9(2), 117–121. https://doi.org/10.4314/jgsa.v9i2.18020
- Adedeji, A. R. (2006). Thread blight disease of tea [Camellia sinensis (L.) O. Kuntze] caused by marasmius pulcher (Berk & Br.) petch in the South Western Nigeria. African Scientist, 7(3), 107–109.
- Akrofi, A. Y., Amoako-Atta, I., Assuah, M., & Kumi-Asare, E. (2014). Pink disease caused by erythricium salmonicolor (Berk. & Broome)burdsall: An epidemiological assessment of its potential effect on cocoa production in Ghana. Journal of Plant Pathology and Microbiology, 5(1), 215. https://doi.org/10.4172/2157-7471.1000215
- Akrofi, A. Y., Appiah, A. A., & Opoku, I. Y. (2003). Management of Phytophthora pod rot disease on cocoa farms in Ghana. Crop Protection, 22(3), 469–477. https://doi.org/10.1016/S0261-2194(02)00193-X
- Akrofi, A. Y., Opoku, I. Y., Assuah, M. K., & Sarfo, J. E. (2008). Survey of phytophthora isolates in Ghana. Report of the Cocoa Research Institute of Ghana, 123.
- Ambele, F. C., Daghela, H. B. B., Babalola, O. O., & Ekesi, S. (2018). Soil‐dwelling insect pests of tree crops in Sub‐Saharan Africa, problems and management strategies—A review. Journal of Applied Entomology, 142(6), 539–552. https://doi.org/10.1111/jen.12511
- Amer, A., & Mehlhorn, H. (2006a). Repellency effect of forty-one essential oils against aedes, anopheles, and culex mosquitoes. Parasitology Research, 99(4), 478–490. https://doi.org/10.1007/s00436-006-0184-1
- Amer, A., & Mehlhorn, H. (2006b). Larvicidal effects of various essential oils against aedes, anopheles, and culex larvae (Diptera, Culicidae). Parasitology Research, 99(4), 466–472. https://doi.org/10.1007/s00436-006-0182-3
- Amoako-Attah, I., Akrofi, A. Y., Bin-Hakeem, R., Asamoah, M., & Kumi-Asare, E. (2016). White thread blight disease caused by marasmiellus scandens (Massee) Dennis & Reid on cocoa and its control in Ghana. African Journal of Agricultural Research, 11(50), 5064–5070. https://doi.org/10.5897/AJAR2016.11681
- Amoako-Attah, I., Shahin, A. S., Aime, M. C., Odamtten, G. T., Cornelius, E., Nyaku, S. T., Kumi-Asare, E., Yahaya, H. B., & Bailey, B. A. (2020). Identification and characterization of fungi causing thread blight diseases on cacao in Ghana. Plant Disease, 104(11), 3033–3042. https://doi.org/10.1094/PDIS-03-20-0565-RE
- Avicor, S. W., Adu-Acheampong, R., & Awudzi, G. K. (2022). Outbreak and insecticide susceptibility of pod feeding-larvae on cocoa in Ghana. Pertanika Journal of Tropical Agricultural Science, 45(1), 55–73. https://doi.org/10.47836/pjtas.45.1.04
- Avicor, S. W., Wajidi, M. F. F., Achoribo, E. S., Ong, M. T., & Hamzah, S. N. (2021). Tiger nut (Cyperus esculentus) as a potential phytoinsecticide: Larvicidal activity of crude extracts on aedes aegypti and culex quinquefasciatus. Tropical Biomedicine, 38(2), 186–191. Diptera: Culicidae. https://doi.org/10.47665/tb.38.2.056.
- Awudzi, G. K., Adu-Acheampong, R., Ahadzi, S. K., & Avicor, S. W. (2019). Field guide for cocoa insect pests identification, damage symptoms and management. Cocoa Research Institute of Ghana Technical Bulletin No, 28, 27.
- Bakaruddin, N. H., & Ab Majid, A. H. (2019). Efficacy of several plants extracts on the tunneling activity and survival of subterranean termites (Coptotermes gestroi and Globitermes sulphureus). Tropical Life Science Research, 30(1), 33–56. https://doi.org/10.21315/tlsr2019.30.1.3
- Balakumar, S., Rajan, S., Thirunalasundari, T., & Jeeva, S. (2011). Antifungal activity of aegle marmelos (L) Correa (Rutaceae) leaf extract on dermatophytes. Asian Pacific Journal of Tropical Medicine, 1(4), 309–312. https://doi.org/10.1016/S2221-1691(11)60049-X
- Bello, O. M., Ibitoye, T., & Adetunji, C. (2019). Assessing antimicrobial agents of Nigeria flora. Journal of King Saud University-Science, 31(4), 1379–1383. https://doi.org/10.1016/j.jksus.2018.04.017
- Benchimol, R. L., Poltronieri, L. S., Trindade, D. R., & Albuquerque, F. C. (2001). White-thread blight: Five new hosts in the state of pará, Brazil. Fitopatologia Brasileira, 26(4), 778. https://doi.org/10.1590/S0100-41582001000400017
- Burt, S. (2004). Essential oils: Their antibacterial properties and potential applications in foods a review. International Journal of Food Microbiology, 94(3), 223–253. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022
- Cater, C. M., Rhee, K. C., Hagenmaier, R. D., & Mattil, K. F. (1974). Aqueous extraction – An alternative oilseed milling process. Journal of the American Oil Chemists’ Society, 51(4), 137–141. https://doi.org/10.1007/BF02639723
- Chah, K. F., Eze, C. A., Emuelosi, C. E., & Esimone, C. O. (2006). Antibacterial and wound healing properties of methanolic extracts of some Nigerian medicinal plants. Journal of Ethnopharmacology, 104(1–2), 164–167. https://doi.org/10.1016/j.jep.2005.08.070
- Cowan, M. M. (1999). Plant products as anti- microbial agents. Clinical Microbiology Reviews, 12(4), 564–582. https://doi.org/10.1128/CMR.12.4.564
- Daferera, D. J., Ziogas, B. N., & Polissiou, M. G. (2000). GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on penicillium digitatum. Journal of Agricultural and Food Chemistry, 48(6), 2576–2581. https://doi.org/10.1021/jf990835x
- Dahanukar, S. A., Kulkarni, R. A., & Rege, N. N. (2000). Pharmacology of medicinal plants and natural products. Indian Journal of Pharmacology, 32, S81–S118.
- Dakwa, J. T. (1987). A serious outbreak of the black pod disease in marginal areas of Ghana. In: Proceedings of the 10th international cocoa research conference, Santo Domingo, Dominican Republic (pp. 447–451). Cocoa Producers Alliance.
- Elango, G., Abdul Rahuman, A., Kamaraj, C., Bagavan, A., Abduz Zahir, A., Santhoshkumar, T., Marimuthu, S., Velayutham, K., Jayaseelan, C., Vishnu Kirthi, A., & Rajakumar, G. (2012). Efficacy of medicinal plant extracts against Formosan subterranean termite, coptotermes formosanus. Industrial Crops and Products, 36(1), 524–530. https://doi.org/10.1016/j.indcrop.2011.10.032
- Elsayed, G. (2011). Insecticidal effect of plant extracts on two termite species. Archives of Phytopathology and Plant Protection, 44(4), 356–361. https://doi.org/10.1080/03235400903057753
- Evans, T. A., Dawes, T. Z., Ward, P. R., & Lo, N. (2011). Ants and termites increase crop yield in a dry climate. Nature Communications, 2(1), 262. https://doi.org/10.1038/ncomms1257
- Flood, J., Guest, D., Holmes, K. A., Keane, P., Padi, B., & Sulistyowati, E. (2004). Cocoa futures: A source book of some important issues facing the cocoa industry. In J. Flood & R. Murphy (Eds.), Cocoa under attack (pp. 33–53). CABI-FEDERACAFE, USDA.
- Gagman, H. A., Ahmad, H., Him, N. A. I. I. N., & Avicor, S. W. (2022). In vitro assessment of deworming potential of guiera senegalensis in Nigerian ethnoveterinary industry using caenorhabditis elegans. Bulletin of the National Research Centre, 46(1), 3. https://doi.org/10.1186/s42269-021-00689-6
- Ghahari, S., Alinezhad, H., Nematzadeh, G., Tajbakhsh, M., & Baharfar, R. (2017). Chemical composition, antioxidant and biological activities of the essential oil and extract of the seeds of glycine max (soybean) from North Iran. Current Microbiology, 74(4), 522–531. https://doi.org/10.1007/s00284-016-1188-4
- González, H. D., & Muñoz, M. J. G. (2017). Water extraction of bioactive compounds. From plants to drug development. (pp. 530). Elsevier, Amsterdam.
- Hilton, R. N. (1958). Pink disease of hevea caused by corticium salmonicolor. Journal of the Rubber Research Institute, Malaya, 15, 275–292.
- Hosseini Chaleshtori, S. A., Ataie Kachouie, M. A., & Hashemi Jazi, S. M. (2017). Antibacterial effects of the methanolic extract of glycine max (soybean). Microbiology Research, 8(2), 7319. https://doi.org/10.4081/mr.2017.7319
- Humber, R. A. (2005). Fungal identification USDA-ARS plant protection research 103 Unit US Plant. Soil & Nutrition Laboratory Tower Road Ithaca.
- Hyldgaard, M., Mygind, T., & Meyer, R. L. (2012). Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Frontiers in Microbiology, 3, 1–24. https://doi.org/10.3389/fmicb.2012.00012
- Hyo, J. K., Hwa-Jin, S., Choong, H. L., Jeong, H. K., Sun, C. K., Sunmin, P., & Jong-Sang, K. (2010). Antifungal activity of glyceollins isolated from soybean elicited with aspergillus sojae. Journal of Agricultural and Food Chemistry, 58(17), 9483–9487. https://doi.org/10.1021/jf101694t
- Igboabuchi, N. A., & Llodibia, C. V. (2018). A study on the antioxidant and antimicrobial activities of seed and leaf extracs of Glycine max (L) Merr. Asian Journal of Research in Botany, 1(1), 1–8.
- Jouquet, P., Traoré, S., Choosai, C., Hartmann, C., & Bignell, D. (2011). Influence of termites on ecosystem functioning. Ecosystem services provided by termites. European Journal of Soil Biology, 47(4), 215–222. https://doi.org/10.1016/j.ejsobi.2011.05.005
- Kanan, G. J., & AL-Najar, A. R. (2008). In vitro antifungal activities of various plant crude extracts and fractions against citrus post-harvest disease agent Penicillium digitatum. Jordan Journal of Biological Sciences, 1(3), 89–99.
- Kikuta, S. (2020). The cytotoxic effect of genistein, a soybean isoflavone, against cultured Tribolium cells. Insects, 11(4), 241. https://doi.org/10.3390/insects11040241
- Kirk, P. M., Cannon, P. F., Minter, D. W., & Stalpers, J. A. (2008). Dictionary of the Fungi (10th) ed., pp. 402). CAB International P.
- Kissi, P., Akpesse, A., Coulibaly, T., & Koua, K. (2022). Influence of cocoa cultivation on the diversity and density of termites in the Azaguié zone (South of Côte d’Ivoire). Faunistic Entomology, 75, 16–27. https://doi.org/10.25518/2030-6318.5798
- Kwarteng, F. G., Cornelius, E., Acquah, K. K., & Asare, E. K. (2018). Morphological and molecular identification of the fungus associated with pink disease of cocoa (Theobroma cacao L) in the Eastern region of Ghana. International Journal of Pathogen Research, 1(1), 1–8. https://doi.org/10.9734/ijpr/2018/v1i11161
- Lisanti, E., & Arwin, A. (2019). Phytochemical screening and proximate analysis of soybeans (Glycine max) variety Gamasugen 1 and Gamasugen 2 derived from gamma rays irradiation. Journal of Physics: Conference series 1402: 055023.
- Lozano-Fuentes, S., Saavedra-Rodriguez, K., Black, W. C., IV, & Eisen, L. (2012). QCal: A software application for the calculation of dose-response curves in insecticide resistance bioassays. Journal of American Mosquito Control Association, 28(1), 59–61. https://doi.org/10.2987/11-6192.1
- Manso, S., Cacho-Nerin, F., Becerril, R., & Nerın, C. (2013). Combined analytical and microbiological tools to study the effect on Aspergillus flavus of cinnamon essential oil contained in food packaging. Food Control, 30(2), 370–378. https://doi.org/10.1016/j.foodcont.2012.07.018
- McKay, A., & Aryeetey, E. (2004). A country case study on ghana. operationalising pro-poor growth work program: A joint initiative of the French development agency (AFD), federal ministry for economic cooperation and development (BMZ): German agency for technical cooperation (GTZ) and KfW development bank. U.K. Department for International Development (DFID), and the World Bank.
- Mohammadi, A., Hashemi, M., & Hosseini, M. S. (2015). Comparison of antifungal activities of various essential oils on the Phytophthora drechsleri, the causal agent of fruit decay. Iranian Journal of Food Microbiology, 7, 31–37.
- Morais, J. K. S., Bader, O., Weig, M., Oliveira, J. T. A., Arantes, M. R., Gomes, V. M., Da Cunha, M., Oliveira, D. H., Sousa, O. B. D., Lourencao, L. A., Vasconcelos, M. I., & Coste, A. T. (2013). Soybean Toxin (SBTX) impairs fungal growth by interfering with molecular transport, carbohydrate/amino acid metabolism and drug/stress responses. Plos One, 8(7), e70425. https://doi.org/10.1371/journal.pone.0070425
- Mora, P., Rouland, C., & Renoux, J. (1996). Foraging, nesting and damage caused by microtermes subhyalinus (Isoptera: Termitidae) in a sugarcane plantation in the Central African republic. Bulletin of Entomological Research, 86(4), 387–395. https://doi.org/10.1017/S0007485300034970
- Nwosu, M. O., & Okafor, J. I. (1995). Preliminary studies of the antifungal activities of some medicinal plants against basidiobolus and some other pathogenic fungi. Mycoses, 38(5–6), 191–195. https://doi.org/10.1111/j.1439-0507.1995.tb00048.x
- Nychas, G. J. E. (1995). Natural antimicrobial from plants. In G. W. Gould (Ed.), New methods of food preservation (pp. 58–89). Blakie Academic and Professional.
- Ollo, S., Hervé, B. D. B., Senan, S., & Sylvain, T. B. C. (2022). Effect of shade on the diversity of termites (Isoptera) in different cocoa agroforestry systems in the Nawa region (Côte d’Ivoire). Journal of Entomology and Zoology Studies, 10(1), 377–387. https://doi.org/10.22271/j.ento.2022.v10.i1e.8957
- Opoku, I. Y., Akrofi, A. Y., Osei-Bonsu, K., & Acheampong, K. (2001) An outbreak of pink disease in Ghana. Proceedings of the 13th international cocoa research conference, Kota Kinabalu, Sabah, Malaysia (pp. 761–767). Cocoa Producers Alliance.
- Opoku, I. Y., Appiah, A. A., Akrofi, A. Y., & Owusu, G. K. (2000). Phytophthora megakarya: A potential threat to the cocoa industry in Ghana. Ghana Journal of Agricultural Science, 33(2), 237–248. https://doi.org/10.4314/gjas.v33i2.1876
- Opoku, I. Y., Assuah, M. K., & Domfeh, O. (2007) CRIG Technical Bulletin No. 16. Manual for the identification and control of diseases of cocoa. Akim-Tafo.
- Pavela, R. (2015). Essential oils for the development of eco-friendly mosquito larvicides: Review. Industrial Crops and Products, 76, 174–187. https://doi.org/10.1016/j.indcrop.2015.06.050
- Piubelli, G. C., Hoffmann-Campo, C. B., Moscardi, F., Miyakubo, S. H., & Neves De Oliveira, M. C. (2005). Are chemical compounds important for soybean resistance to anticarsia gemmatalis? Journal of Chemical Ecology, 31(7), 1509–1525. https://doi.org/10.1007/s10886-005-5794-z
- Ponnusha, B. S., Subramaniyam, S., & Pasupathi, P. (2011). Antioxidant and antimicrobial properties of glycine max-A review. International Journal of Current Biological and Medical Science, 192, 49–62.
- Pradhanang, P. M. (1994). Pink disease (Corticium salmonicolor Berks & Broome) control in mandarin Orange (Citrus reticulata Blanco) by application of bordeauz mixture. Crop Protection, 13(7), 550–552. https://doi.org/10.1016/0261–2194(94)90109-0
- Pringle, R. M., Doak, D. F., Brody, A. K., Jocque, R., Palmer, T. M., & Loreau, M. (2010). Spatial pattern enhances ecosystem functioning in an African Savanna. PLoS Biology, 8(5), e1000377. https://doi.org/10.1371/journal.pbio.1000377
- Rahman, S. F. S., Sijam, K., & Omar, D. (2016). Antibacterial activity of the crude extract of Piper sarmentosum against Pseudomonas fuscovaginae. International Journal of Applied and Pharmaceutical Technology, 7, 67–72.
- Rouland-Lefèvre, C. (2011). Biology of termites: A modern synthesis. In D. E. Bignell, Y. Roisin, & N. Lo (Eds.), Termites as pests of agriculture (pp. 499–517). Springer.
- Sande, D., Mullen, J., Wetzstein, M., & Houston, J. (2011). Environmental impacts from pesticide use: A case study of soil fumigation in Florida tomato production. International Journal of Environmental Research and Public Health, 8(12), 4649–4661. https://doi.org/10.3390/ijerph8124649
- Schyra, J., & Korb, J. (2019). Termite communities along a disturbance gradient in a West African savanna. Insects, 10(1), 17. https://doi.org/10.3390/insects10010017
- Seth, S. K., Bakshi, B. K., Reddy, M. A. R., & Sujan, S. (1978). Pink disease of eucalyptus in India. European Journal of Plant Pathology, 8, 200–216. https://doi.org/10.1111/j.1439-0329.1978.tb00628.x
- Smith, E. S. C. (1985). A review of the relationship between shade types and cocoa pest and disease problems in Papua New Guinea. Papua New Guinea Journal of Agriculture, Forest and Fisheries, 33, 78–88.
- Sobowale, O., Ogbulie, N. J., Itoandon, E. E., Oresegun, M. O., & Olatope, S. O. A. (2013). Phytochemical and antimicrobial evaluation of aqueous and organic extracts of calotropis procera leaf and latex. Nigerian Food Journal, 31(1), 77–82. https://doi.org/10.1016/S0189-7241(15)30059-X
- Sylvain, T. B. C., Senan, S., Lucie, Y. N., Yao, T., & Souleymane, K. (2015). Termites impact on different age of cocoa (Theobroma cacao L.) plantations with different fertilizer treatments in semi-deciduous forest zone (Oume, Ivory Coast). Herald Journal of Agriculture and Food Science Research, 4(4), 021–027.
- Sylvain, T. B. C., Tenon, C., Hortense, B. S., Konaté, S., Philippe, K. K., & Yao, T. (2019). Attacks of termites (Insecta: Isoptera) in cocoa farms (Theobroma cacao L.) in Oumé (Côte d‘Ivoire). International Journal of Current Research, 11(9), 6899–6905. https://doi.org/10.24941/ijcr.36521.09.2019
- Tao, N., Jia, L., & Zhou, H. (2014). Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chemistry, 153, 265–271. https://doi.org/10.1016/j.foodchem.2013.12.070
- Tian, J., Ban, X., Zeng, H., Huang, B., He, J., & Wang, Y. (2011). In vitro and in vivo activity of essential oil from dill (Anethum graveolens L.) against fungal spoilage of cherry tomatoes. Food Control, 22(12), 1992–1999. https://doi.org/10.1016/j.foodcont.2011.05.018
- Usher, M. B. (1975). Studies on a wood-feeding termite community in Ghana. West Africa.Biotropica, 7(4), 217–233. https://doi.org/10.2307/2989735
- Velazquez-Nuenez, M. J., Avila-Sosa, R., Palou, E., & Lo´pez-Malo, A. (2013). Antifungal activity of Orange (Citrus sinensis var. Valencia) peel essential oil applied by direct addition or vapor contact. Food Control, 31(1), 1–4. https://doi.org/10.1016/j.foodcont.2012.09.029
- Villalobos, M. D. C., Serradilla, M. J., Martin, S., Ordiales, E., Ruiz-Moyano, S., & Córdoba, M. D. G. (2016). Antioxidant and antimicrobial activity of natural phenolic extract from defatted soybean flour by-product for stone fruit postharvest application. Journal of the Science and Food Agriculture, 96(6), 2116–2124. https://doi.org/10.1002/jsfa.7327
- Wang, Q., Wang, H., & Xie, M. (2010). Antibacterial mechanism of soybean isoflavone on Staphylococcus aureus. Archives of Microbiology, 192(11), 893–898. https://doi.org/10.1007/s00203-010-0617-1
- Žabka, M., Pavela, R., Kovaˇríková, K., Tˇríska, J., Vrchotová, N., & Bednár, J. (2021). Antifungal and insecticidal potential of the essential oil from ocimum sanctum L. against dangerous fungal and insect species and its safety for non-target useful soil species. Eisenia fetida (Savigny, 1826) Plants, 10(2180). https://doi.org/10.3390/plants10102180
