Abstract
The development of native perennial seed crops is an area of increasing interest in Australia. Key reasons for this include potential production of high-value seed for land restoration and emerging niche food markets, the cultural significance that native plants hold for Aboriginal people, reduced farm input costs in comparison to annual seed crop production and potential environmental benefits from diversified agricultural systems. One species of interest is Themeda triandra, a C4 perennial tussock grass that dominated many grasslands and grassy woodlands across Australia prior to European invasion. This review aims to inform those seeking to grow T. triandra as a seed crop in south-eastern Australia of the genetic, environmental and management factors that influence plant growth, development, and seed yield. By doing so, the review highlights the agronomic possibilities and constraints relating to production of a T. triandra as a seed crop and identifies future research needs. Key agronomic possibilities include the requirement to establish a crop once that can then persist for years; its adaptation to a range of environments and its ability to produce high-value seed every year. Key agronomic constraints include low and variable seed yields, limited knowledge of important aspects of crop management and difficulties in broadacre crop establishment associated with its seed diaspore morphology, low seed quality, germination requirements and weed competition. Future research should investigate ways to maximise seed production through effective crop management, improve upon current sowing and harvest techniques and identify ecotypes with agronomic traits preferable for seed crop production.
1. Introduction
Themeda triandra (Forssk.), commonly known as kangaroo grass in Australia, is a warm season perennial tussock grass. It is one of the world’s most widely distributed C4 grasses (Dunning et al., Citation2017) and is found in every state and territory of Australia (Hovendon & Morris, Citation2002; Snyman et al., Citation2013). Since European invasion of Australia, commercial agricultural practices and land disturbances more broadly, has led to T. triandra being replaced by both native and introduced annual and perennial grass and broadleaf species (Lunt, Citation2003; McIntyre et al., Citation1995; Prober & Thiele, Citation1995). Today, native grassland communities where T. triandra was once prominent are some of the most poorly conserved and degraded vegetation communities (BI Cole & Lunt, Citation2005) and exist mainly in remnants restricted to undisturbed areas such as railway corridors, roadways and cemeteries; with even these populations being impacted by introduced species (Godfree et al., Citation2017; Groves, Citation1965; McDougall & Kirkpatrick, Citation1994).
Past studies have focused on the species’ ecology and its role in land restoration, with knowledge on its use in agricultural systems being centred around its grazing potential as a pasture species and not its seed production (BI Cole & Lunt, Citation2005; Hill, Citation1985; Hutton, Citation1971; Mason, Citation2005; Snyman et al., Citation2013). In Australia, the lack of historic research into the production of native perennial seed crops is reflective of the perception that they are not well suited for seed production, for reasons such as their low and variable seed yields (IA Cole & Johnston, Citation2006; CL Davies et al., Citation2005a). There has been little attempt to establish broadacre areas of T. triandra, with areas established in past land restoration studies typically being small (<1 ha) (Stafford, Citation1991).
The development of native perennial seed crops is an area of increasing research interest in Australia. This is in line with other parts of the world, particularly North America, where native perennial species have been the subject of extensive research aimed at evaluating whether they can offer a more sustainable alternative to current annual crop production systems. Desirable characteristics of perennial seed crops include their ability to mimic natural systems, to be drought tolerant, to maintain longevity under variable climatic conditions and the ability to restore and improve the productivity of degraded land (Cassman & Connor, Citation2022; Johnston et al., Citation1999; Mitchell et al., Citation2015; Sindel et al., Citation1993; Whalley & Jones, Citation1996). The use of perennial grasses as niche grain-producing crops in diversified monocultural agricultural systems is a possibility being explored in Australia (Canning, Citation2022; Newell et al., Citation2022; The University of Sydney, Institute of Agriculture, Citation2020), but these species have minimal potential to replace annual grain crops such as Triticum aestivum (wheat) as they fail to provide sufficient yields to satisfy global food demand (Cassman & Connor, Citation2022; Loomis, Citation2022).
Other than grain for human consumption, the key reason driving interest in the development of T. triandra is Aboriginal traditional owner groups (TOs) seeking to return culturally significant plants to the landscape for social, cultural and economic purposes (The University of Sydney, Institute of Agriculture, Citation2020). Developing agronomic knowledge on indigenous plants can help TOs in their pursuit for food sovereignty (Canning, Citation2022) and self-determination, of which Australia is committed to through adoption of the United Nations Declaration on the Rights of Indigenous Peoples (UN Declaration) (UN General Assembly, Citation2007). Djaara, the TO group representing the Dja Dja Wurrung people of central Victoria, recognises that T. triandra was historically distributed across their landscape and may have provided a source of food for their marti guli (ancestors). As such, Djaara seeks to return the species to the landscape so to provide cultural, social and economic empowerment to their people. More broadly, incorporation of native perennial grasses into modern-day cropping systems has the potential to be profitable due to comparative ease of management, low crop inputs and high market value for native grass seed. Other income sources include future government ecosystem service and carbon credit schemes, fodder for grazing during drought and restoration of degraded farmland (Canning, Citation2022; Farmers for Climate Action, Citation2021).
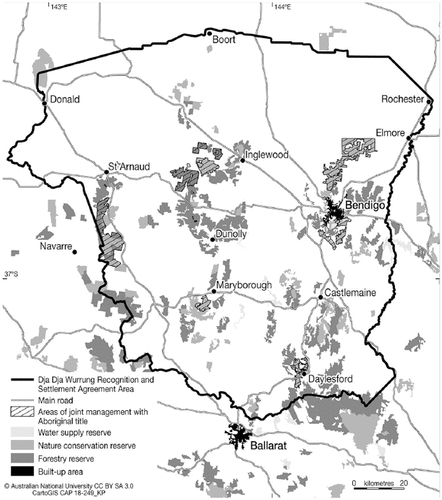
This review aims to inform those who seek to grow T. triandra as a native perennial seed crop in south-eastern Australia by summarising the genetic, environmental and management factors that affect plant growth, development, and seed yield. As Djaara is one TO group seeking to return T. triandra to the landscape for reasons previously stated, this review is targeted towards temperate grasslands of south-eastern Australia where Djaara (Dja Dja Wurrung) country is located (Figure ). Literature from around the world was reviewed, predominantly reporting on the responses of T. triandra in Africa and Australia, with the assumption that T. triandra has similar responses across different regions and climates.
2. Fundamental biology
2.1. Taxonomy
Themeda triandra (Forssk.) is 1 of 27 recognised species in the Themeda genus and belongs to the Poaceae family (Clayton et al., Citation2016). In the past, the species has been classified as Themeda australis (R. Br.) Stapf. (Snyman et al., Citation2013). It is commonly known as kangaroo grass in Australia (Snape et al., Citation2021). In Australia, the species is also known by indigenous names, such as the Djaara name “Buwatji”.
2.2. Distribution
Themeda triandra first emerged in Asia ~1.5 million years ago before spreading via coastal corridors to Australia and the south-west Pacific ~1.3 million years ago and mainland Africa ~0.5 million years ago (Dunning et al., Citation2017; Hayman, Citation1960). Its broad global distribution is largely attributed to its ability to grow in a wide range of climatic and edaphic conditions, including annual rainfall (300–6250 mm), altitude (sea level to >3300 m), and soil types (sands through to heavy clays) (Snyman et al., Citation2013). In Australia, T. triandra naturally dominates temperate grasslands and woodlands, subtropical and tropical savannahs (Hayman, Citation1960; Lunt, Citation2003; Morgan & Lunt, Citation1999) and is a component of grasslands in the arid interior (Snyman et al., Citation2013; Stevens et al., Citation2020) and sub-alpine regions (McDougall & Walsh, Citation2007; Sindel et al., Citation1993; Stevens et al., Citation2020). Themeda triandra is ecologically considered a keystone species in its inhabited grasslands, due to its role in maintaining herbivore populations and determining the structure, composition, and function of its associated plant community (Godfree et al., Citation2017; Snyman et al., Citation2013).
2.3. Morphology
Themeda triandra forms an herbaceous tussock that consists of active and senesced tillers, leaves and reproductive culms that support seed-producing inflorescences (Figure ). A summarised morphological description of T. triandra based on historical and contemporary sources is provided in Table . Figure shows plant morphology as a line diagram and Figure shows the seed diaspore morphology.
Figure 2. A young ~16-month-old) T. triandra tussock in April 2022 on Dja Dja Wurrung country near Dean, Victoria (37°27ʹ S 144°00ʹ E). Photo contributed by Dylan Male.

Figure 3. A line diagram of a T. triandra tussock, panicle, spikelet, and seed diaspore morphology. Source:Gardner (Citation1952).
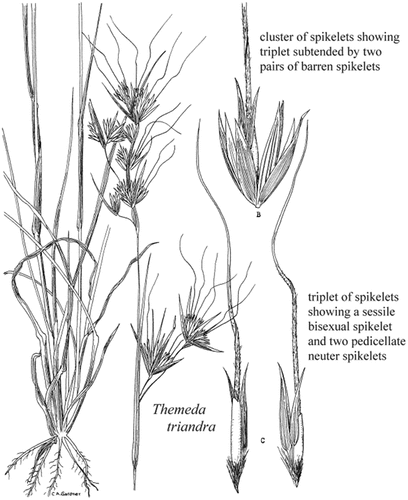
Figure 4. T. triandra seed diaspore (left); T. triandra naked caryopsis (right).Note: Seed length varies, generally <5 mm. Figure contributed by Dylan Male.

Table 1. A summarised morphological description of T. triandra (Martin, Citation1975; Mitchell & Wilcox, Citation1994; Wheaton, 1994; Tainton & Booysen, Citation1965; Morgan, Citation1999; Mitchell, Citation2001; Snyman et al., Citation2013; Australian National Botanic Gardens. Centre for Australian National Biodiversity Research, Citation2016; Godfree et al., Citation2017; Ghebrehiwot et al., Citation2006; Ibrahim et al., Citation2018; Cavanagh et al., Citation2019)
2.4. Growth and development
Throughout the growing season (September–April across Dja Dja Wurrung country), primary tillers elongate and eventually cease production of new leaves, signalling the start of floral initiation and subsequent seed production. Secondary tillers then develop until their own subsequent floral development. Not all tillers transition from vegetative to reproductive, and these are known as blind tillers (Tainton & Booysen, Citation1963). The proportion of reproductive tillers depends on the amount of assimilate available (Tomlinson et al., Citation2007), influenced by genetic (Stevens et al., Citation2020), environmental (Booysen et al., Citation1963) and management factors (Everson et al., Citation1985; Snyman, Citation2004; Tainton & Booysen, Citation1963). Under optimal growth conditions, a greater ratio of reproductive tillers to blind tillers can be expected (Everson et al., Citation1985; Tainton & Booysen, Citation1963; Tomlinson et al., Citation2007).
Once initiated, new leaves take approximately 10 days to reach their maximum size depending on day length and temperature (Danckwerts & Stuart‐Hill, Citation1988; Tainton & Booysen, Citation1965). New leaves can appear every 3–24 days and leaf emergence can continue for 50–140 days (Danckwerts & Aucamp, Citation1985; Hodgkinson et al., Citation1989). Active leaves can remain attached to tillers for 5–6 months (Danckwerts & Aucamp, Citation1985; Hodgkinson et al., Citation1989; Scott & Rabie, Citation1956). The rate of leaf senescence generally equals the rate of leaf emergence (Danckwerts & Aucamp, Citation1985) and green leaves begin to senesce following reproductive development (Scott & Rabie, Citation1956).
Both flowering and seed shedding are non-synchronous at a population level, an individual plant level and a spikelet level (Woodland, Citation1964). Each spikelet matures at different times, with the outer spikelets generally reaching maturity first and the inner spikelets last (Woodland, Citation1964). Development from germination to seed production can occur over as little as 2–3 months (Arndt & Norman, Citation1959), although the asynchronous flowering period can be broad at 46–210 days (Snyman et al., Citation2013). The proportion of active vs senesced biomass varies depending on time of year, environmental conditions, and plant age (McDougall, Citation1989).
There is limited information available on the root systems of T. triandra (Snyman et al., Citation2013). Approximately 25% of all photosynthates are partitioned towards root structural compounds (Danckwerts & Gordon, Citation1990), leading to thickened roots that may contribute to energy storage that supports resprouting after fire or drought.
The structure of the T. triandra seed dispersal unit, or diaspore, is designed to maximise the chance of seed germination by assisting seed burial into the soil. This is achieved when fluctuations in relative humidity (>30%) cause the awn to twist, propelling the seed short distances along the soil surface until it finds a suitable microsite, e.g. a soil crack, where it then enters to depths of up to 10 mm before being secured in place by the diaspore’s barb and hairs (Cavanagh et al., Citation2019; Lock & Milburn, Citation1971; Sindel et al., Citation1993). Here, the temperature and moisture conditions are likely more favourable for germination, seed-soil contact is improved, and the risk of predation is lowered; thereby reducing the likelihood of seed desiccation and improving its survival and subsequent establishment (Cavanagh et al., Citation2019; Peart, Citation1979, Citation1984; Schöning et al., Citation2004; Sindel et al., Citation1993). Awn length varies between populations, with longer awns in lower rainfall environments allowing for more efficient seed transport and burial through faster and farther seed movement (Morris, Citation2020). The seed diaspore’s structure and relatively heavyweight mean that its capacity for other mechanisms of dispersal, such as movement through wind, is poor (TM Everson et al., Citation2009).
3. Genetic, environmental and management factors that influence growth, development, and seed yield
Natural populations of T. triandra in south-eastern Australia vary in their growth, development, and seed yield depending on environmental and genetic differences. These factors will also affect production as a seed crop, in addition to the effects of agronomic management practices. The following sections explore how genetic, environmental and management factors could influence the growth, development, and seed yield of T. triandra.
3.1. Genetic factors
3.1.1. Ploidy
Themeda triandra’s widespread distribution can be attributed to its broad genetic diversity (Snyman et al., Citation2013). Although there are varying levels of ploidy found, in southern Australia >98% of T. triandra populations are either diploid or tetraploid (Hayman, Citation1960). Diploids tend to be found almost exclusively in the higher rainfall zones of eastern Australia, whereas polyploids are most prevalent in the lower rainfall zones of Australia’s interior (Ahrens et al. Citation2020; Godfree et al., Citation2017). Diploids that are found in coastal areas of both Australia and Africa are likely derived from the centre of origin in tropical Asia (Hayman, Citation1960; Liebenberg et al., Citation1993). Polyploids found further inland on both continents are probably the result of hybridisation and polyploidisation of these original diploid populations (Liebenberg et al., Citation1993). Polyploid (tetraploid) plants produce heavier, more viable and more dormant seeds than diploids, along with faster seedling growth (Stevens et al., Citation2020). Non-dormant tetraploids are more sensitive to germination stress compared to non-dormant diploids (Stevens et al., Citation2020). Hexaploids are often sterile (R. Whalley, personal communication, 4 March 2021).
An important first step in establishing a T. triandra seed crop will be to identify and grow ecotypes that have desirable agronomic traits. Polyploid ecotypes have larger seed and greater seedling vigour than diploid ecotypes and this is likely to make polyploids more agronomically desirable but with higher seed dormancy a possible trade-off (Stevens et al., Citation2020). Future plant breeding may allow the development of cultivars of T. triandra with improved agronomic traits.
3.1.2. Seed quality
Establishment of a T. triandra seed crop will be improved by planting high-quality seed. For seed to be high quality, it should have high viability, low dormancy and high purity. Themeda triandra is known for its low seed quality and can exhibit significant intraspecific and inter-population variation, similar to other native grasses (Danckwerts & Stuart‐Hill, Citation1988; Snyman et al., Citation2013; Stevens et al., Citation2020). Its low seed viability may result from competition between multiple embryo sacs or the failure of mature embryos to develop as the result of endosperm failure (Woodland, Citation1964). Caryopsis that completely fill the seed coat are 1.5 times more likely to be viable than those that are almost full, and up to three times more likely than those that fill little of the seedcoat (McDougall, Citation1989). Dark brown/black seeds are ~2.5 times more likely to be viable than those that are light brown, whilst white seeds are seldom viable (McDougall, Citation1989).
Similar to many warm season native Australian grasses (Bell, Citation1999; Silcock et al., Citation1990; Watt, Citation1974), T. triandra exhibits seed dormancy as a survival adaptation. Seeds shed in summer tend to remain ungerminated until the following spring when increasing soil temperatures (>15°C) and high moisture support germination and early seedling growth (Adams, Citation1996; Snyman et al., Citation2013), whilst also preventing young seedlings desiccating from cold weather in winter (Hagon, Citation1976; Hagon & Groves, Citation1977). Some seeds will remain ungerminated despite their exposure to favourable environmental conditions (Nadella et al., Citation2003). This is known ecologically as “bet-hedging”, a mechanism that leads to a delay and staggering in germination which can increase the chance of seedling survival by mechanisms such as reducing competition between siblings (Baskin & Baskin, Citation2014; Clauss & Venable, Citation2000).
Themeda triandra dormancy is likely to be influenced mostly by non-deep physiological dormancy. This is commonly experienced by a broad range of plant families and consists of seeds that have fully developed embryos but have a physiological mechanism to inhibit germination (Baskin & Baskin, Citation2020). Dormancy in T. triandra may also be influenced by the seed’s physical structure, such as glumes causing resistance to imbibition (Martin, Citation1975).
In many instances, grass species found in cooler and wetter environments have higher levels of germination and lower seed dormancy such as Microlaena stipoides (Johnston et al., Citation1998; Whalley & Jones, Citation1996), Rytidosperna (Johnston et al., Citation1998) and Anthosachne (Johnston et al., Citation1998). A similar pattern occurs between T. triandra ecotypes, where low seed dormancy has been recorded in ecotypes from reliable rainfall sites that experience constant temperatures in contrast to higher dormancy in seed from less reliable rainfall locations that experience seasonal temperature changes (Groves et al., Citation1982).
3.2. Environmental factors
Temperature and soil moisture are the two most important factors influencing T. triandra growth, development, and seed yield. Based on historical literature, cardinal temperatures for germination could be approximated as 15–20°C, 30–35°C and 45°C for base temperature (Tbase), optimal temperature (Topt) and maximum temperature (Tmax), respectively (BI Cole & Lunt, Citation2005; Hagon, Citation1976; Hagon & Groves, Citation1977; Mott & Groves, Citation1981; Snyman et al., Citation2013); whereas cardinal temperatures for growth could be approximated as 15°C, 28–33°C and unknown for Tbase, Topt and Tmax, respectively (Groves, Citation1975). These may vary between ecotypes.
Germination, growth, and flowering are all similarly influenced by soil moisture (Groves et al., Citation1982; Hagon & Chan, Citation1977; Nolan, Citation1994; Opperman & Roberts, Citation1978). Germination is highly sensitive to water stress and improves as soil moisture near field capacity (Hagon & Chan, Citation1977; Nolan, Citation1994); although moisture requirements vary geographically, with ecotypes in arid environments being the most sensitive to water stress (Groves et al., Citation1982). Themeda triandra is a characteristic water “spender”, meaning that it opportunistically uses water when it is abundant, and then becomes semi-dormant when soil moisture is low (Williams et al., Citation1998).
Growth and development in temperate climates is generally initiated in spring when soil moisture is high and temperatures are rising, resulting in maximum biomass production in late spring and summer (Danckwerts, Citation1987; Evans & Knox, Citation1969; Groves, Citation1975; McDougall, Citation1989; Snyman et al., Citation2013) Flowering is responsive to soil water more than any other environmental factor and it influences both the timing and frequency of flowering (McDougall, Citation1989; Opperman & Roberts, Citation1978; Snyman et al., Citation2013). If there is little soil moisture available, T. triandra can become dormant and no flowers or seeds are produced (Snyman et al., Citation1997). Little is known about how much soil waterlogging can impact seed production. High air temperatures increase the rate of seed development and shedding and seed production may be negatively impacted in cooler seasons or environments (V Anderson & Hodgkinson, Citation1999; Groves, Citation1975; McDougall, Citation1989). A single day of hot, dry, and windy conditions ahead of harvest can result in significant loss of seed due to seed shedding (IA Cole & Johnston, Citation2006).
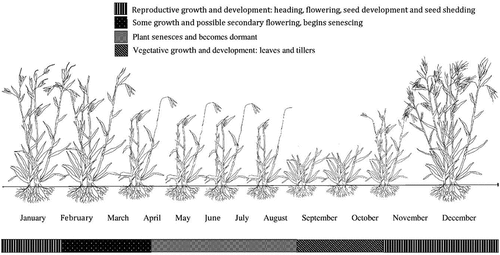
Across Dja Dja Wurrung country in south-eastern Australia, T. triandra will typically germinate during spring (September–November), produce leaves during spring and summer (September–February), flower during late spring and early summer (October–January), fill and shed seed in summer (December–February) and remain dormant from late autumn through winter (May–August). Peak timing of seed shed varies within and between sites and between seasons (McDougall, Citation1989). Figures visually depicts the annual development cycle of a T. triandra stand relative to months of the year on Dja Dja Wurrung country, which would be expected to be similar as an established crop.
Figure 5. The annual development cycle of a T. triandra stand relative to the months of the year on Dja Dja Wurrung country, Victoria, Australia. Figure illustrated by Dylan Male.
Themeda triandra has a highly variable and locally adapted response to vernalisation (the need for cold temperatures to trigger reproductive development) and photoperiod (daylength), e.g. ecotypes from colder areas have been found to respond to vernalisation, whereas those found in warmer areas do not (Evans & Knox, Citation1969; Snyman et al., Citation2013). Growth improves in soils with increasing depth, clay percentage, organic matter, and good drainage and is restricted by shading from surrounding trees, accumulated dead biomass and plant competitors (GD Anderson & Talbot, Citation1965; Hagon & Chan, Citation1977; Snyman et al., Citation2013). Further research is needed to determine the full effect of soil conditions on establishment and seed production (Lunt & Morgan, Citation2000).
3.3. Management factors
3.3.1. Seed cleaning
The structures of the seed diaspore and stem and floret material collected during harvest (Figure ) impair seed flow through conventional sowing equipment (Berto et al., Citation2020), and this presents a major constraint to broadacre establishment of T. triandra seed crops. Additionally, the seed will need to be separated from non-seed structures if the grain is to be used for human consumption. As such, effective seed cleaning will be important for broadacre sowing (if not surface sowing, see below) and emerging niche food markets (Guzzomi et al., Citation2016; Ling et al., Citation2019).
Figure 6. An uncleaned seed lot containing stem, spikelet, and seed diaspore material, harvested from a brush harvester. Photo contributed by Dylan Male.

This can be achieved through manual separation such as beating and rolling, exposure of seed to high air temperatures, the use of sieves and screens and flash flaming (Berto et al., Citation2020; Frischie et al., Citation2020). Currently, these methods are only used on a small scale and future work should look at upscaling these methods. Sowing equipment and machinery custom designed to better handle the poor flowability of native seeds may also be needed. The future development of awnless varieties of T. triandra by genetic means may be another pathway to improve flowability and reduce the burden of seed cleaning. The cleaning of seed is less important when surface sowing seed, such as the broadcasting of seed-bearing hay, as the awn assists with seed burial. Screening of seed prior to sowing is also recommended to ensure seed is pure and free of other crop and weed seeds, pests and disease.
3.4. Overcoming seed dormancy
When establishing T. triandra as a seed crop, it will be preferred that seeds establish uniformly to allow uniform management operations and harvesting. As polyploids have higher seed dormancy than diploids (Stevens et al., Citation2020), diploids might exhibit more uniform germination. However, polyploids are likely to be preferred for seed production due to their larger seed. This poses the question as to how growers of a T. triandra crop can best deal with seed dormancy. Higher seeding rates can be used to ensure increased plant numbers and circumvent high seed dormancy. However, seed is expensive, so techniques aimed at breaking dormancy may be more cost effective.
As mentioned earlier, T. triandra seed likely experiences non-deep physiological dormancy and physical dormancy (Martin, 1974; Baskin & Baskin, Citation2020). An important characteristic of seed with non-deep physiological dormancy is that dormancy-break can occur over time during dry storage (Baskin & Baskin, Citation1985; Favier, Citation1995; Saleem et al., Citation2009). As such, one of the most promising dormancy-breaking techniques for T. triandra is dry storage of seed over an extended period following harvest (>6 months). Known as after-ripening, this process has been reported in seeds of a variety of species that experience a range of conditions, including un-shed seed remaining in the field on their mother plant (Baskin & Baskin, Citation1977), seed stored in closed containers at room temperature (Baskin & Baskin, Citation1977, Citation1983), seed stored in paper bags at room temperature (Moyo et al., Citation2009; Ralowicz & Mancino, Citation1992), seed at room temperature with varying levels of humidity (Kundu & Chaturvedi, Citation2019; Yang et al., Citation2019), and seed in sealed petri dishes at 20°C (Karimmojeni et al., Citation2011). The mechanism for this dormancy breakdown is not fully understood, although it is assumed that the glumes and/or lemma and palea control dormancy by containing inhibitory substances, causing mechanical restriction during germination, reducing oxygen transport to the embryo and reducing imbibition (Hagon, Citation1976). There is some indication that the damage or breakdown of these structures over time leads to an increase in germination (Barton, Citation1965a; Stokes, Citation1965), thus explaining after-ripening.
An alternate seed dormancy-breaking technique for T. triandra seed is the use of fire and smoke. Exposure to high temperatures and the compounds found within smoke have been found to significantly increase seed germination and emergence (Baxter et al., Citation1994; Ghebrehiwot et al., Citation2012). These findings warrant future research into the use of smoke as a mechanism to break dormancy of T. triandra and as a possible plant growth stimulant (Ghebrehiwot et al., Citation2012), which may have added cultural value in an Aboriginal food plant system.
Germination is controlled by the relative levels of growth inhibitors (such as abscisic acid) and promoters (such as gibberellins and cytokinins) that occur in the seed (Amen, Citation1968; Cresswell & Nelson, Citation1972; Khan, Citation1971; Wareing et al., Citation1972). Dormancy can be reduced by lowering the levels of growth inhibitors or increasing the levels of promoters, e.g. the application of gibberellic acid (Hagon, Citation1976; Martin, Citation1975).
Other physical dormancy-breaking techniques include mechanical scarification (Kildisheva et al., Citation2013a), chemical scarification (e.g. with concentrated sulfuric acid) (Maid et al., Citation2013) and dipping in hot or boiling water (Kildisheva et al., Citation2013b; Kimura & Islam, Citation2012). Selective breeding may also lead to eventual breakdown in seed dormancy, as seen in other seed crops (Harlan et al., Citation1973). More research is needed to identify which techniques are most successful and cost efficient for large-scale dormancy-breaking in T. triandra.
3.5. Time of sowing
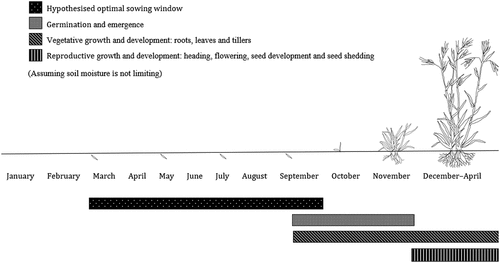
In south-eastern Australia, high temperatures frequently coincide with low soil moisture. This presents a challenge to T. triandra establishment as seed requires both high temperatures and high soil moisture for germination. As such, it is important to identify a window of time where viable seed will be in the soil during times of both high soil moisture and warm temperatures (Hagon, Citation1976). This is most likely to occur in spring, meaning that sowing in the lead up to spring is likely to maximise opportunities for seed germination. Furthermore, early seedling growth and development is supported in spring by increasing soil temperatures and solar radiation (Hagon & Groves, Citation1977; Sindel et al., Citation1993). It has been argued that autumn is more optimal than spring as soil surfaces tend not to dry out as quickly (NSW DPI, Citation2021a); however, autumn-emerged seedlings may be vulnerable to winter conditions, i.e. frosts. Figure shows the hypothesised first-year development of a dryland T. triandra seed crop relative to months of the year on Dja Dja Wurrung country.
Figure 7. The hypothesised first-year development of a dryland T. triandra seed crop relative to months of the year on Dja Dja Wurrung country, Victoria, Australia. Figure illustrated by Dylan Male.
The time when suitable temperature and moisture requirements coincide will be site-specific and vary between regions (Hagon & Groves, Citation1977; Lodge, Citation1981; Lodge & Whalley, Citation1981). Caution must be taken if awaiting until rain before sowing, as the seedbed may dry out by the time paddocks are trafficable (Fletcher et al., Citation2015). Sowing time will influence what weeds are present and have implications for weed management, e.g. spring sowing would enable control of cool season weeds (IA Cole & Johnston, Citation2006) but increased competition from any emerging summer weeds.
3.6. Sowing methodology
Restoration of T. triandra stands has historically been achieved by surface-spreading awned seeds in seed-bearing hay over cleared land (BI Cole & Lunt, Citation2005; McDougall, Citation1989; NSW DPI, Citation2021a). For example, harvesting seed still attached to the inflorescence and broadcasting it over an area cleared by herbicides or fire (McDougall, Citation1989). Other methods include mixing the seed material with water and then spraying onto the soil surface in what is known as “aqua-seeding” (Sindel et al., Citation1993), planting tube stock (IA Cole et al., Citation2000), transplanting mature plants in intact sods from remnant populations (Carbutt & Kirkman, Citation2022; McDougall, Citation1989) or by sowing awned seed that is incorporated with fibre mulch in what is known as hydro mulching (Cole, Citation2003; NSW DPI, Citation2021a). Despite these methods being successful at establishing T. triandra in small areas and avoiding the expenses associated with cleaning seed (NSW DPI, Citation2021a), they remain expensive, laborious and time consuming and thus unlikely to be viable for establishing broadacre areas. For example, planting seedling tube stock has a high economic cost as seed must typically be transported to a nursery for rearing and then seedlings transplanted into the soil once it has reached a suitable size.
If seed flowability challenges are effectively overcome, the most desirable method to establish the species as a broadacre crop is through the direct sowing of seed by conventional machinery (Merritt & Dixon, Citation2011; Baskin & Baskin, Citation2020; Sampaio et al., Citation2019). In this case, it will be important that seed is planted into seedbed conditions that allow good soil-seed contact and high moisture retention (DS Loch & Ferguson, Citation1999; Rolston et al., Citation1998). As seed size largely determines energy reserves and seedling vigour (Huxtable, Citation1999; DS Loch & Ferguson, Citation1999; Southwood et al., Citation1981), small seeds tend to achieve optimal emergence at shallow sowing depths whilst larger seeds can be buried at greater depth. Themeda triandra seed varies in size and weight (Stevens et al., Citation2020); therefore, different ecotypes might have different optimal sowing depths. Generally, the optimal depth will be 1 cm in both sandy and clay soils and no deeper than 2 cm (BI Cole & Lunt, Citation2005; NSW DPI, Citation2021a; Sindel et al., Citation1993). This can be achieved using shallow tillage with tined implements to produce a lightly disturbed seedbed, where seed can be broadcast and fall into crevices and then be covered by surrounding loose soil and litter through use of press wheels or light harrows (Cole, Citation2003; NSW DPI, Citation2021a; Sr & Smith, Citation1997). Alternatively, custom-made native grass planting machinery with larger tubes and more aggressive agitators in the seed box may allow placement of seed into drill rows, such as the US manufactured “Great Plains Ag Native Grass Drills” (Great Plains Ag, Citation2022). Tillage should be minimised to prevent exposing weed seed stored in the soil to conditions that promote their germination.
Planting seeds in rows will enable easier determination of sowing rates and provide greater control of seed placement, along with allowing weed control with inter-row cultivation or herbicide application using shielded applicators (IA Cole & Johnston, Citation2006; DS Loch & Ferguson, Citation1999; Sr & Smith, Citation1997). Additionally, the use of row spacings can improve crop management and soil protection by enabling controlled traffic corridors that have been achieved in many cereal cropping systems (Yule, Citation1998). Wide row spacing (0.5–1.0 m) has been recommended for large and robust native grass species (IA Cole & Johnston, Citation2006). These guidelines are likely to be highly variable and will become better informed by future research.
3.7. Sowing rates and target plant densities
As T. triandra is yet to be planted as a broadacre crop, it remains unknown what sowing rates and target plant densities will optimise seed production. Cole (Citation2003) suggested that to ensure rapid coverage, T. triandra seed should be placed approximately 20 mm apart when sown in rows using 50 g of cleaned seed per 100 m of drill row. Generally, higher sowing rates will result in a higher number of seedlings emerging. The advantage of this would be more rapid canopy coverage and enhanced competitiveness against weeds. A disadvantage would be competition between seedlings themselves, leading to smaller individual plants and potential loss of smothered plants.
The number of viable seeds in the seed lot must be known to inform sowing rate (Hall et al., Citation2006). Cole (Citation2003) argues that it would be reasonable to expect a mere 5% of spring sown viable seed to develop into established plants by the end of summer during a dry season, and up to 50% in a season with above average rainfall. As a perennial grass that persists well and can proliferate over time, seedling densities of 1–2 plants/m2 can develop into thick stands within 3–5 years if favourable growing and recruitment conditions prevail (Cole, Citation2003).
As high sowing rates represent a major cost, more work is warranted into the relationships between sowing rates and implications for establishment, crop yield and crop management. This will enable the most efficient and cost-effective establishment target and sowing rate to be used. Until more is known about these factors, it could be fair to assume that higher seeding rates will result in more competitive crops. However, lower seeding rates could lead to larger plants and therefore higher seed yield per plant, along with allowing expensive seed to be spread over larger areas. These trade-offs need to be resolved through future research.
3.8. Weeds
Weeds compete with a crop for resources such as light, water and nutrients, and can cause contamination of harvested seed (DSL Loch & Harvey, Citation1997). Additionally, there is cultural significance to having a native crop free of exotic species as it is a sign of healed country (Rodney Carter, personal communication, 2021). As such, the effective management of weeds will be important in a T. triandra crop, which will be most susceptible to weeds during the emergence and early seedling stages when competition from broadleaf annual weeds and faster growing annual and perennial grasses can cause establishment failures (Cole et al., Citation2003; DSL Loch & Harvey, Citation1997; NSW DPI, Citation2021b). Reducing weed competition will lead to more productive T. triandra plants that produce more inflorescences and seed (McDougall, Citation1989). Once well-established, native perennial grasses tend to compete well with annual weeds (DSL Loch & Harvey, Citation1997). This is true for established T. triandra populations, which are known to resist invasion from competitive weed species such as Nassella neesiana (Morgan & Lunt, Citation1999). This strong competitiveness can be attributed to sustained growth over warmer months and persistence under dry conditions, allowing T. triandra to dominate annual competitors (Stafford, Citation1991). It may also be attributed to the species ability to cycle nitrogen in forms unavailable to other plants (Farrell & Prober, Citation2021).
Reducing the weed burden pre and post sowing can be achieved through integrated weed management that uses a diverse range of techniques to suppress weeds (IA Cole & Johnston, Citation2006; Semple et al., Citation1999). Integrated weed management can be categorised into three key areas based on techniques used: chemical, mechanical, and cultural.
Chemical control involves using herbicides to kill and supress weeds. In a grass crop such as T. triandra, grass weeds and annual weeds of a similar growth pattern are the most difficult to control using herbicides (IA Cole & Johnston, Citation2006). The control of these weeds often relies heavily on the use of pre and early post emergent herbicides (DSL Loch & Harvey, Citation1997). Broadleaf weeds are more easily controlled using selective herbicides applied post-germination (DSL Loch & Harvey, Citation1997; Rolston et al., Citation1998; Stafford, Citation1991). The level of tolerance of T. triandra to selective herbicides has been found to vary, with good tolerance to some at all development stages from pre-emergence to maturity, including atrazine, chlorsulfuron, bromoxynil, dicamba and fluazifop, and low tolerance to others such as metsulfuron methyl and simazine (Cole et al., Citation2003; McDougall, Citation1989; Stafford, Citation1991; Whalley & Jones, Citation1996). Crop safety of T. triandra to herbicides may vary depending on its physiological status and environmental conditions, as seen in perennial species such as lucerne (Medicago sativa) (SL Davies et al., Citation2005b). It is possible that non-selective herbicides could be used to control winter growing annuals when T. triandra is dormant. The use of non-selective herbicides using spray shields, roller or wick applicators, or by applying after sowing but prior to seedling emergence, may also become useful in an integrated weed management program (IA Cole & Johnston, Citation2006). More research is needed into the crop safety of herbicides on T. triandra.
Careful consideration of paddock selection and paddock preparation ahead of establishment will help reduce weed competition (NSW DPI, Citation2021b). Selecting areas free of noxious and competitive weed species, particularly vigorous annual weeds such as annual ryegrass (Lolium rigidum Gaudin) is important, otherwise costly pre and post sowing management will be required to eliminate weeds to a level that supports good establishment. If there is no identified area with a low weed burden, then a paddock can be prepared for establishment in the time leading up to sowing through timely herbicide applications, hay production, tillage and the growing of competitive crops in the seasons prior to sowing (IA Cole & Johnston, Citation2006; NSW DPI, Citation2021b).
Choosing a sowing methodology that minimises soil disturbance is another method that can reduce the burden of weeds (IA Cole & Johnston, Citation2006). Inter-row cultivation can help remove weeds between the rows of an establishing crop, although this relies on accurately sown rows (IA Cole & Johnston, Citation2006). High sowing rates and stand densities improve competitiveness of T. triandra to weeds (Semple et al., Citation1999). Timely biomass management may also suppress weeds, such as grazing by livestock, burning, or slashing (BI Cole & Lunt, Citation2005; Stuwe, Citation1986).
To inform the cost-effectiveness of ongoing weed management, further studies are needed to determine the extent that T. triandra can resist competition from weeds and the subsequent impact on seed production.
3.9. Irrigation
Rainfall in south-east Australia can be low and variable. In northern Dja Dja Wurrung Country average annual rainfall is ~380 mm and can vary from 128 to 739 mm (Bureau of Meteorology, Citation2022). As T. triandra germination and plant development is susceptible to moisture stress, irrigation may benefit crop establishment and seed production during dry conditions (Danckwerts & Stuart‐Hill, Citation1988; Hagon & Chan, Citation1977; McDougall, Citation1989). Irrigation is used by commercial growers who currently produce T. triandra seed for the revegetation market, with yields of 300–350 kg/ha of cleaned seed and awn product being achieved when irrigated (Darren Vincent, personal communication, 1 July 2022).
3.10. Nutrient input
Natural ecosystems tend to have low rates of nutrient export due to their ability to conserve, concentrate, and recycle nutrients (Groves, Citation1974). This is true of natural T. triandra populations which have modest nutritional requirements, the ability to regulate soil nitrate cycling (Farrell & Prober, Citation2021; Prober & Lunt, Citation2009; Prober et al., Citation2005) and obtain nitrogen from associated nitrogen-fixing bacteria (Ritchie & Raina, Citation2016). Some have found that nitrogen (N) fertiliser applied at low rates or as slow-release improves T. triandra growth (Fynn & Naiken, Citation2009; Grossman & Cresswell, Citation1974), but generally T. triandra responds poorly to the addition of N and phosphorus fertiliser and experiences either no response or a decline in production and/or biomass (Allcock, Citation2002; RW Fynn & O’Connor, Citation2005; Grossman & Cresswell, Citation1974; Hagon & Groves, Citation1977; Mason, Citation2005; Snyman, Citation2002; Snyman et al., Citation2013; Wilson & Haydock, Citation1971). This may be attributed to the enrichment of nutrients leading to an increase in weed competition, particularly nitriphillic annual weeds (BI Cole & Lunt, Citation2005). To overcome this, natural grassland restoration programs have attempted to reduce the amount of plant-available N through nitrogen stripping (Farrell & Prober, Citation2021; Kardol et al., Citation2008; Walker et al., Citation2004). However, as a seed crop, there may be a greater need to replace plant available nutrients in the soil following their removal in harvested seed. More research will be needed into the impact of nutrient input on T. triandra seed production.
3.11. Biomass management
Accumulation of dead plant matter over time reduces T. triandra seed production through plant smothering and shading (Everson et al., Citation1988; McNaughton, Citation1992; Morgan & Lunt, Citation1999), whilst excessive removal of foliage can lead to seed yield reductions through the removal of apical buds, reduced photosynthetic leaf area, a reduction in the plants’ ability to accumulate and store reserve carbohydrates, nutrient losses and a failure to maintain positive carbon balance (McDougall, Citation1989; Danckwerts, Citation1993; Noy-Meir, Citation1993; Ash & McIvor, Citation1998; Snyman et al., Citation2013). For this reason, T. triandra can be described ecologically as a “decreaser” species, meaning it decreases in productivity and abundance when either underutilised (lack of biomass removal) or over utilised (excessive biomass removal) (Mitchell & Wilcox, Citation1994). As such, biomass management in a T. triandra seed crop will be a trade-off between the need to reduce the negative impacts of smothering and shading, and the need to protect apical buds, etc.
The persistence of T. triandra in natural ecosystems is reliant on periodic burning (Bond et al., Citation2003; Morgan & Lunt, Citation1999) and/or grazing. This has been highlighted in the Serengeti region of Tanzania, where T. triandra accounts for approximately 50% of grass cover in areas of light to moderate grazing but is absent from areas where both fire and grazing do not occur (Danckwerts, Citation1993; Dunning et al., Citation2017; Vuorio et al., Citation2014). In pre-European Australia, biomass in T. triandra grasslands would have been reduced by grazing macropods and periodic burning (McDougall, Citation1989).
In a cropping system, the timing, intensity and frequency of biomass removal will all be important considerations influencing seed production. For T. triandra, apical buds of spring-initiated tillers exist above the soil surface in mid-summer but can fail to develop into flowers until the following spring (Tainton & Booysen, Citation1963). These apical buds are susceptible to removal by defoliation for a long period of time prior to inflorescence maturity, including autumn and winter (Rethman & de, Citation1968). When a defoliation does occur, the chance of the apical bud being damaged is lessened when it is located closer to the soil surface (Rethman, Citation1971). For burning, biennial burning, or even burning in four—five-year rotations, are thought to be a suitable compromise that optimises seed production (TM Everson et al., Citation2009; Morgan & Lunt, Citation1999; Snyman et al., Citation2013). Intense burning events result in higher biomass loss and a heightened risk of reduced growth from heat damage (Smith, 1960; Bennett et al., Citation2003; Everson et al., Citation1985; TM Everson et al., Citation2009; Strugnell & Pigott, Citation1978). As such, it is recommended that any burn should occur in cooler times of the year, such as autumn or early spring months. There are few studies on the impact of fire on T. triandra roots, although this will be important to establish as the plant rely on root energy reserves for regrowth (Snyman et al., Citation2013).
Grazing provides the dual benefit of managing biomass and providing a source of feed for livestock. In Australia, T. triandra is naturally adapted to light grazing and can dominate grasslands that are grazed by herbivorous marsupials such as kangaroos and wallabies (Macropus spp.) and introduced pest species such as rabbits and deer (Ash & McIvor, Citation1998; Cole, Citation2003; Snyman et al., Citation2013). Themeda triandra is palatable with moderate nutritive value for animals, more so during young growth (NSW DPI, Citation2022). During periods of active growth, T. triandra should be rested to avoid significant damage to developing reproductive culms (Bailey & Mappledoram, Citation1983; Van der Westhuizen, Citation2003). However, even when grazing occurs outside of the growing period, it can still result in damage (Coetsee, Citation1975; Barnes, 1989). Light grazing is less destructive than heavy grazing and is tolerated better during times of good soil moisture rather than times of drought (Danckwerts & Stuart‐Hill, Citation1988; Fynn & O’Connor, Citation2000; Snyman et al., Citation2013), although frequent defoliation during times of high rainfall can lead to poor growth and higher plant mortality rates (Hodgkinson et al., Citation1989; Mott et al., Citation1992). Animals that graze lower to the soil surface will cause greater damage to apical buds. For example, sheep are more damaging than cattle (Hardy et al., Citation1994; Kirkman, Citation2002a, Citation2002b).
Themeda triandra has been found to tolerate the non-selective defoliation that occurs during mowing (Morris & Tainton, Citation1993); however, frequent mowing can reduce productivity (Moore AC, Citation1989). Mowing is likely to be most effective at increasing seed yield if performed at the beginning of the growing period (early spring in south-eastern Australia), which would allow the removal of biomass before bud elevation and the development of reproductive culms. Ecotypes that have apical buds closer to the soil surface have greater protection against damage during biomass removal (Everson et al., Citation1985; Rethman, Citation1971).
3.12. Pests and disease
Pest and disease can cause significant yield reductions to many agriculturally important crop species. As an emerging crop, little is known about what the pest and disease burden in a T. triandra crop will be and what level of management will be needed. Native grass species currently experience low pest and disease burden, although the risks are likely to increase if their production increases (Australian National Botanic Gardens and Centre for Australian National Biodiversity Research, Canberra, Citation2012; IA Cole & Johnston, Citation2006). The most significant damage is likely to come from either invertebrate pests (insects) or grazing vertebrates. Thrips and the larvae and pupae of Diptera have been previously found in T. triandra spikelets, anthers have suffered attack by micro-coleoptera, and seeds have been destroyed by parasites (McDougall, Citation1989; Woodland, Citation1964).
Ongoing monitoring for signs of pest and disease damage in T. triandra crops will be required to identify the need for management. Biomass management can also serve to reduce the likelihood of pests and disease (IA Cole & Johnston, Citation2006). For example, burning has been used to control blind seed disease, ergot and grass seed nematode in perennial grasses such as creeping red fescue (Festuca rubra L.) (Rolston, Citation1980).
3.13. Mulching
Mulch can support establishment by preventing weed growth and prolonging favourable conditions for seed germination, e.g. high humidity, high soil moisture and soil temperature regulation (BI Cole & Lunt, Citation2005; Hagon & Groves, Citation1977; McDougall, Citation1989; Winkel et al., Citation1991). The effect of mulch on soil moisture and temperature varies with the type of mulch and rate of application (Hagon & Groves, Citation1977; Sindel et al., Citation1993). For example, paper mulch (at 3200 kg/ha) leads to an increase in soil moisture but a decrease in soil temperatures, while soluble bitumen (at 12,000 L/ha) increases soil temperatures but has little impact on soil moisture (Hagon & Groves, Citation1977). Despite the different effects of these mulch types, they have been found to contribute towards improved T. triandra seedling emergence (Hagon & Groves, Citation1977). However, for surface sown awned seeds, mulch will impede seed burial and reduce germination (Sindel et al., Citation1993). High volume mulching may also be detrimental for establishment due to shading (BI Cole & Lunt, Citation2005).
Applying large amounts of mulch will not be feasible for broadacre T. triandra cropping. For this reason, mulch created from the existing, undesired grass biomass or previous crop residue may be the most practical sources (BI Cole & Lunt, Citation2005). Twin sowing may be an effective technique to achieve this. For example, sowing T. triandra seed at the same time as sowing an annual winter seed or cover crop may promote seed dormancy breakdown over winter (after-ripening in the soil) and allow germination under crop residues following seed harvest or cover crop termination (Loi & Yates, Citation2020).
3.14. Harvest timing
Determining optimal harvest time is difficult, particularly for native grass species that experience non-synchronous seed ripening influenced by environmental factors (IA Cole & Johnston, Citation2006; McDougall, Citation1989; PTP & H, Citation1997; Woodland, Citation1964). In natural T. triandra populations, non-uniformity in seed ripening occurs between different sites and within a site. For example, plants shaded by trees or in moister soils shed later than those in dry, exposed parts of the same site (McDougall, Citation1989). On a plant level, seeds ripen at different stages depending on their position on the inflorescence and within a spikelet (Woodland, Citation1964). Further adding to the difficulty of determining optimal harvest timing is that seed retention tends to be poor, and ripening occurs at the same time as seed loss through shedding (Coolbear et al., Citation1997).
In T. triandra, the proportion of viable seed on the plant is low early in the seed shedding period before reaching a peak, meaning that quantities of seed are likely to have already been shed in a crop at the time when the highest number of viable seed is on the plant (McDougall, Citation1989). As such, optimal harvest time will be a trade-off between harvesting seed too early (high amounts of immature seed) and harvesting too late (high loss of seed through shedding).
It will be important to identify what crop signs indicate optimal harvest timing. Current quantitative methods include counting a set number of days or degree days following anthesis, testing seed moisture content and estimating the percentage of seed shed. Current qualitative methods include feeling for seed hardness (milky, doughy or hard) or visually inspecting the colour of the seed, glumes and overall crop (IA Cole & Johnston, Citation2006; McDougall, Citation1989). Based on the currently limited information, the best bet approach would be to harvest at a time when the overall crop colour has yellowed, a proportion of seed in the crop has shed (up to 30–40%), and when most seed is light brown or black in colour and feels hard (McDougall, Citation1989). Future research is needed to better inform optimal harvest timing and better understand the crop indicators that can signal optimal harvest timing.
3.15. Harvest methodology
Themeda triandra seed is harvested at small scales by simple yet labour-intensive seed collection techniques including cutting seed heads and tying into sheaves before hanging them upside down over a plastic sheet, dragging a sheet of hessian material over ripe seed heads or using a line-trimmer or mower to cut stems which are then raked and packed (Cole, Citation2003). These methods produce either a small amount of concentrated seed material or seed-hay which is low in seed content and requires a large volume of product to be dried and stored. For broadacre cropping, these methods will not be practical unless upscaled through commercial hay production techniques.
Future harvest methodologies that mimic those performed in the modern-day harvest of broadacre seed crops will be most desired. These should aim to collect concentrated seed products, such as seed-bearing florets and pure seed, which would allow for easier drying, storage, processing and assessment of seed quality (Cole et al., Citation2005). These methods could include the use of mowers, headers, combines, windrowers, strippers, threshers and balers (McDougall, Citation1989). Currently, the use of a brush harvester (Figure ) is the most efficient method, with the amount of T. triandra seed harvested per gram of flowering material significantly greater when a brush harvester is used in comparison to be mowing and baling with a sickle bar mower (Mason, Citation2005). The “Grasshopper/Grass grabber”, as described by Cole (Citation2003), is one example of this and can harvest up to 100 kg of high-quality seed-floret material per hour. These types of harvesters can collect seed on multiple occasions over a seed shedding period, beneficial for the asynchronous seed ripening of T. triandra (IA Cole & Johnston, Citation2006). A current limitation is that these harvesters are designed to harvest relatively small areas, therefore increasing their capacity to harvest larger areas should be a priority in the production of T. triandra as a seed crop.
Although used in other crop species that experience asynchronous seed ripening, such as Brassica napus (canola) (Haile et al., Citation2014), windowing would likely not be an effective harvest method for T. triandra as large seed losses would be expected due to seed shedding and unripe seed not maturing once it has been removed from the plant (McDougall, Citation1989). Seed shedding in T. triandra could be overcome through the application of a tacking agent such as polyvinyl acetate (PVA) (McDougall, Citation1989; McWilliam & Wardlaw, Citation1965) or through selective breeding against seed shedding.
3.16. Seed storage
Little is known about the ability of many Australian native grass species to maintain viability in storage (IA Cole & Johnston, Citation2006). As previously discussed, dormancy in T. triandra declines over time under a range of storage conditions such as closed containers or paper bags at room temperature (Baskin & Baskin, Citation1977, Citation1983; Moyo et al., Citation2009; Ralowicz & Mancino, Citation1992). Long storage (>5 years) may decrease seed viability (Mason, Citation2005), although cold storage may enable seed to be stored for longer periods of time. More research is needed to better understand the effects of seed storage on viability.
3.17. Crop persistence and recruitment
The lifespan of individual T. triandra tussocks remains unquantified (Snyman et al., Citation2013), although natural populations endure for decades due the plants’ ability to strongly co-exist or dominate the grasslands where it occurs (Snyman et al., Citation2013). This can similarly be expected for a well-managed T. triandra crop, and high levels of recruitment may not be necessary for crop persistence but rather the established plants’ ability to survive and dominate a space over time. In natural populations, T. triandra seed in the soil contributes little to persistence and is often of low viability (TM Everson et al., Citation2009; Morgan, Citation2001; Snyman et al., Citation2013). This is due to reasons such as predation, inability of the seed to survive for long periods of time, accumulation of litter on the soil and shading by the canopy (TM Everson et al., Citation2009; Lock & Milburn, Citation1971; McDougall, Citation1989; Morgan, Citation2001; Morgan & Lunt, Citation1999; Snyman et al., Citation2013). Additionally, the fact that much of the viable seed in a cropping system would be collected during harvest would reduce the crops’ ability to recruit. With these factors considered, plant recruitment from seed shedding within a crop of T. triandra would be expected to be low once a crop is established, although may play an important role in initial establishment. If viable seed is in the soil, then a spring fire can temporarily reduce weed competition and promote germination and thus recruitment (McDougall, Citation1989).
4. Conclusion: agronomic possibilities and constraints, and future research needs
The aim of this review was to inform those seeking to grow T. triandra as a perennial seed crop in south-eastern Australia of the genetic, environmental and management factors that influence plant growth, development, and seed yield, with a focus on Dja Dja Wurrung country in central Victoria. From this, the agronomic possibilities and constraints in the production of a T. triandra seed crop and future research needs can be identified. In conclusion
Key agronomic possibilities of T. triandra as a perennial seed crop include that it
is relatively low input, requiring a single planting event to establish a persistent crop with little weed management and nutrient inputs
can produce high value seed every year
is widely adapted to environments with different soils and variable climatic conditions
can have improved seed yield through effective crop management strategies
may have improved seed yield through selection of higher yielding polyploid ecotypes
is opportunistic and adapted to variable climates as it experiences rapid growth and development in favourable conditions whilst becoming semi-dormant in non-favourable conditions
can contribute towards land regeneration and protection of biodiversity
Key agronomic constraints of T. triandra as a perennial seed crop include
difficulties in crop establishment, including issues with seed flowability, seed germination requirements, low seed quality and vulnerability to weed competition
limited knowledge of how effective agronomic management can maximise seed production, such as optimal sowing and harvest times and methods, weed control, ecotype selection and biomass management
Based on this review, we have listed below in the order of priority the key future research needs to support the development of T. triandra as a perennial seed crop. Future research and development should aim to
improve upon current sowing methodologies by
i) identifying an optimal sowing window that maximises crop establishment
ii) determining optimal sowing rates and target plant densities for competitive and high yielding crop canopies
iii) developing seed cleaning technologies and processes that improve seed flowability through modern-day broadacre sowing equipment
improve upon current harvest methodologies by
i) identifying the optimal time of harvest to maximise the collection of both viable seed for planting and seed suitable for human consumption
ii) developing more efficient harvesting technology and equipment capable of harvesting large areas
improve weed management
identify ecotypes with agronomic traits preferable for cropping
investigate how biomass management can be used to maximise seed production
better understand how seed storage affects seed viability and dormancy
further investigate the effectiveness of seed dormancy-breaking techniques
determine the influence of genetics on growth and yield and how future breeding may be used to improve seed production
further investigate how environmental and genetic factors influence seed viability
It is hoped that future research along these lines will helpfully inform future production of T. triandra as a perennial seed crop.
Acknowledgements
The authors acknowledge Djaara, the TO group representing the Dja Dja Wurrung people, for their research leadership and vision that has helped guide this review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability Statement
There were no data generated by this review.
Additional information
Funding
References
- Adams, K. M. (1996). Influence of sward defoliation and soil disturbance on seedling emergence and survival in the Southern Tall Grassveld. African Journal of Range and Forage Science, 13(3), 131–26. https://doi.org/10.1080/10220119.1996.9647909
- Ahrens, C. W., James, E. A., Miller, A. D., Scott, F., Aitken, N. C., Jones, A. W., & Rymer, P. D. (2020). Spatial, climate and ploidy factors drive genomic diversity and resilience in the widespread grass Themeda triandra. Molecular Ecology, 29(20), 3872–3888. https://doi.org/10.1111/mec.15614
- Allcock, K. G. (2002). Effects of phosphorus on growth and competitive interactions of native and introduced species found in White Box woodlands. Austral Ecology, 27(6), 638–646. https://doi.org/10.1046/j.1442-9993.2002.01225.x
- Amen, R. D. (1968). A model of seed dormancy. The Botanical Review, 34(1), 1–31. https://doi.org/10.1007/BF02858619
- Anderson, V., & Hodgkinson, K. C. (1999) Synchronous tillering and flowering dynamics of perennial grasses in Australian semi-arid wooded grasslands In: D. Eldridge & D. Freudenberger (eds.), People and rangelands: building the future. Proceedings of the VIth International Rangeland Congress, vol. 1. Aitkenvale: VIth International Rangeland Congress. pp 244–246.
- Anderson, G. D., & Talbot, L. M. (1965). Soil factors affecting the distribution of the grassland types and their utilization by wild animals on the Serengeti Plains, Tanganyika. The Journal of Ecology, 53(1), 33–56. https://doi.org/10.2307/2257564
- Arndt, W., & Norman, M. J. T. (1959) Characteristics of native pasture on Tippera clay loam at Katherine, NT [Australia]. Division of Land Research technical paper.
- Ash, A. J., & McIvor, J. G. (1998). How season of grazing and herbivore selectivity influence monsoon tall‐grass communities of northern Australia. Journal of Vegetation Science, 9(1), 123–132. https://doi.org/10.2307/3237230
- Australian National Botanic Gardens and Centre for Australian National Biodiversity Research, Canberra. (2012). https://www.anbg.gov.au/gnp/interns-2004/themeda-triandra.html#:~:text=This%20plant%20does%20not%20suffer,Territory%20and%20threatened%20in%20Victoria
- Australian National Botanic Gardens. Centre for Australian National Biodiversity Research. (2016). https://www.anbg.gov.au/gnp/interns-2004/themeda-triandra.html
- Bailey, A. W., & Mappledoram, B. D. (1983). Effect of spring grazing on yield of three grasses of the Highland Sourveld of Natal. Proceedings of the Annual Congresses of the Grassland Society of Southern Africa, 18(1), 95–100.
- Barton, L. V. (1965a). Seed dormancy: General survey of dormancy types in seeds, and dormancy imposed by external agents. In Differenzierung und Entwicklung/Differentiation and Development (pp. 2346–2367). Springer.
- Baskin, J. M., & Baskin, C. C. (1977). Germination ecology of Sedum pulchellum Michx.(Crassulaceae). American Journal of Botany, 64(10), 1242–1247. https://doi.org/10.1002/j.1537-2197.1977.tb10817.x
- Baskin, J. M., & Baskin, C. C. (1983). Germination ecology of Veronica arvensis. The Journal of Ecology, 71(1), 57–68. https://doi.org/10.2307/2259963
- Baskin, J. M., & Baskin, C. C. (1985). The annual dormancy cycle in buried weed seeds: A continuum. BioScience, 35(8), 492–498. https://doi.org/10.2307/1309817
- Baskin, C. C., & Baskin, J. M. (2014). Seeds: Ecology, biogeography and evolution of dormancy and germination. (2nd edn ed.). Academic Press.
- Baskin, C. C., & Baskin, J. M. (2020). Breaking seed dormancy during dry storage: A useful tool or major problem for successful restoration via direct seeding? Plants, 9(5), 636. https://doi.org/10.3390/plants9050636
- Baxter, B. J. M., Van Staden, J., Granger, J. E., & Brown, N. A. C. (1994). Plant-derived smoke and smoke extracts stimulate seed germination of the fire-climax grass Themeda triandra. Environmental and Experimental Botany, 34(2), 217–223. https://doi.org/10.1016/0098-8472(94)90042-6
- Bell, D. T. (1999). Australian trees for the rehabilitation of waterlogged and salinity-damaged landscapes. Australian Journal of Botany, 47(5), 697–716. https://doi.org/10.1071/BT96110
- Bennett, L. T., Judd, T. S., & Adams, M. A. (2003). Growth and nutrient content of perennial grasslands following burning in semi-arid, sub-tropical Australia. Plant Ecology, 164(2), 185–199. https://doi.org/10.1023/A:1021253600712
- Berto, B., Erickson, T., & Ritchie, A. (2020). Flash flaming improves flow properties of Mediterranean grasses used for direct seeding. Plants, 9(12), 1699. https://doi.org/10.3390/plants9121699
- Bhadouria, R., Singh, R., Singh, V. K., Borthakur, A., Ahamad, A., Kumar, G., & Singh, P. (2019). Agriculture in the era of climate change: Consequences and effects. In Climate change and agricultural ecosystems (pp. 1–23). Woodhead Publishing.
- Bond, W. J., Midgley, G. F., & Woodward, F. I. (2003). What controls South African vegetation—climate or fire? South African Journal of Botany, 69(1), 79–91. https://doi.org/10.1016/S0254-6299(15)30362-8
- Booysen, P. D. V., & Tainton, N. M. (1965). Growth and development in perennial veld grasses. I. Themeda triandra tillers under various systems of defoliation. South African Journal of Agricultural Science, 8(1), 93–110.
- Booysen, P. D. V., Tainton, N. M., & Scott, J. D. (1963). Shoot apex development in grasses and its importance in grassland management. Herbage Abstracts, 33(4), 209–212.
- Bureau of Meteorology. (2022). http://www.bom.gov.au/climate/change/about/rain_averagemaps.shtml
- Canning, A. D. (2022). Rediscovering wild food to diversify production across Australia’s agricultural landscapes. Frontiers in Sustainable Food Systems, 519.
- Carbutt, C., & Kirkman, K. (2022). Ecological grassland restoration—A South African perspective. Land, 11(4), 575. https://doi.org/10.3390/land11040575
- Cassman, K. G., & Connor, D. J. (2022). Progress towards perennial grains for prairies and plains. Outlook on Agriculture, 51(1), 32–38. https://doi.org/10.1177/00307270211073153
- Cavanagh, A. M., Godfree, R. C., & Morgan, J. W. (2019). An awn typology for Australian native grasses (Poaceae). Australian Journal of Botany, 67(4), 309–334. https://doi.org/10.1071/BT18216
- Clauss, M. J., & Venable, D. L. (2000). Seed germination in desert annuals: An empirical test of adaptive bet hedging. The American Naturalist, 155(2), 168–186. https://doi.org/10.1086/303314
- Clayton, W. D., Vorontsova, M. S., Harman, K. T., & Williamson, H. (2016). GrassBase-the online world grass flora. GrassBase-The Online World Grass Flora. https://www.kew.org/./genindex.htm
- Coetsee, G. (1975). Grazing of Cymbopogon‐Themeda veld in the dormant period. Proceedings of the Annual Congresses of the Grassland Society of Southern Africa 10(1), 147–150.
- Cole, I. (2003). Restoring a kangaroo grass understorey. Virtual Herbarium, Charles Sturt University. https://science-health.csu.edu.au/herbarium
- Cole, I. A., Dawson, I., Mortlock, W., & Winder, S. (2000). A review of establishment requirements. Guideline Number 9: Flora Bank, Canberra
- Cole, I. A., & Johnston, W. H. (2006). Seed production of Australian native grass cultivars: An overview of current information and future research needs. Australian Journal of Experimental Agriculture, 46(3), 361–373. https://doi.org/10.1071/EA04107
- Cole, I., Koen, T., Metcalfe, J., Johnston, W., & Mitchell, M. (2003). Tolerance of Austrodanthonia fulva, Microlaena stipoides and Elymus scaber seedlings to nine herbicides. Plant Protection Quarterly, 18(1), 18–22.
- Cole, B. I., & Lunt, I. D. (2005). Restoring Kangaroo Grass (Themeda triandra) to grassland and woodland understoreys: A review of establishment requirements and restoration exercises in south‐east Australia. Ecological Management and Restoration, 6(1), 28–33. https://doi.org/10.1111/j.1442-8903.2005.00216.x
- Cole, I., Lunt, I. D., & Koen, T. (2005). Effects of sowing treatment and landscape position on establishment of the perennial tussock grass Themeda triandra (Poaceae) in degraded eucalyptus woodlands in southeastern Australia. Restoration Ecology, 13(3), 552–561. https://doi.org/10.1111/j.1526-100X.2005.00069.x
- Coolbear, P., Hill, M. J., & Pe, W. (1997). Maturation of grass and legume seed. Forage Seed Production, Temperate Species, 1, 71–103. https://www.cabdirect.org/cabdirect/abstract/19980702276
- Cresswell, C. F., & Nelson, H. (1972). The effect of micronutrients and gibberellic acid on the germination and metabolism of seedlings of Themeda triandra Forsk. Proceedings of the Annual Congresses of the Grassland Society of Southern Africa, 7(1), 133–137.
- Danckwerts, J. E. (1987). The influence of tiller age and time of year on the growth of Themeda triandra and Sporobolus fimbriatus in semi‐arid grassveld. Journal of the Grassland Society of Southern Africa, 4(3), 89–94. https://doi.org/10.1080/02566702.1987.9648079
- Danckwerts, J. E. (1993). Reserve carbon and photosynthesis: Their role in regrowth of Themeda triandra, a widely distributed subtropical graminaceous species. Functional Ecology, 7(5), 634–641. https://doi.org/10.2307/2390141
- Danckwerts, J. E., & Aucamp, A. J. (1985). The rate of leaf emergence and decay as criteria for optimising the grazing rotation in semi‐arid grassveld. Journal of the Grassland Society of Southern Africa, 2(1), 28–34. https://doi.org/10.1080/02566702.1985.9647995
- Danckwerts, J. E., & Gordon, A. J. (1990). Partitioning, storage and remobilization of 14C assimilated by Themeda triandra Forssk. Journal of the Grassland Society of Southern Africa, 7(2), 97–105. https://doi.org/10.1080/02566702.1990.9648214
- Danckwerts, J. E., & Stuart‐Hill, G. C. (1988). The effect of severe drought and management after drought on the mortality and recovery of semi‐arid grassveld. Journal of the Grassland Society of Southern Africa, 5(4), 218–222. https://doi.org/10.1080/02566702.1988.9648145
- Davies, S. L., Virgona, J. M., McCallum, M. H., Swan, A. D., & Peoples, M. B. (2005b). Effectiveness of grazing and herbicide treatments for Lucerne removal before cropping in southern New South Wales. Australian Journal of Experimental Agriculture, 45(9), 1147–1155. https://doi.org/10.1071/EA04202
- Davies, C. L., Waugh, D. L., & Lefroy, E. C. (2005a). Variation in seed yield and its components in the Australian native grass Microlaena stipoides as a guide to its potential as a perennial grain crop. Australian Journal of Agricultural Research, 56(3), 309–316. https://doi.org/10.1071/AR04204
- Dunning, L. T., Liabot, A. L., Olofsson, J. K., Smith, E. K., Vorontsova, M. S., Besnard, G., & Lehmann, C. E. (2017). The recent and rapid spread of Themeda triandra. Botany Letters, 164(4), 327–337. https://doi.org/10.1080/23818107.2017.1391120
- Evans, L. T., & Knox, R. B. (1969). Environmental control of reproduction in Themeda australis. Australian Journal of Botany, 17(3), 375–389. https://doi.org/10.1071/BT9690375
- Everson, C. S., Everson, T. M., & Tainton, N. M. (1985). The dynamics of Themeda triandra tillers in relation to burning in the Natal Drakensberg. Journal of the Grassland Society of Southern Africa, 2(4), 18–25. https://doi.org/10.1080/02566702.1985.9648014
- Everson, C. S., Everson, T. M., & Tainton, N. M. (1988). Effects of intensity and height of shading on the tiller initiation of six grass species from the Highland sourveld of Natal. South African Journal of Botany, 54(4), 315–318. https://doi.org/10.1016/S0254-6299(16)31297-2
- Everson, T. M., Yeaton, R. I., & Everson, C. S. (2009). Seed dynamics of Themeda triandra in the montane grasslands of South Africa. African Journal of Range and Forage Science, 26(1), 19–26. https://doi.org/10.2989/AJRFS.2009.26.1.3.698
- Farmers for Climate Action. (2021). How can Australia’s agriculture sector realise opportunity in a low emissions future? https://farmersforclimateaction.org.au/wp-content/uploads/2021/09/FCA-EY-FINAL-Report-Low-emissions-future-for-Agriculture.pdf
- Farrell, M., & Prober, S. M. (2021). Keystone perennial grassland species control soil nitrogen flows. Ecosystems, 24(6), 1–16. https://doi.org/10.1007/s10021-020-00597-2
- Favier, J. F. (1995). A model for germination rate during dormancy loss in Hordeum vulgare. Annals of Botany, 76(6), 631–638. https://doi.org/10.1006/anbo.1995.1141
- Fletcher, A. L., Robertson, M. J., Abrecht, D. G., Sharma, D. L., & Holzworth, D. P. (2015). Dry sowing increases farm level wheat yields but not production risks in a Mediterranean environment. Agricultural Systems, 136, 114–124. https://doi.org/10.1016/j.agsy.2015.03.004
- Frischie, S., Miller, A. L., Pedrini, S., & Kildisheva, O. A. (2020). Ensuring seed quality in ecological restoration: Native seed cleaning and testing. Restoration Ecology, 28(S3), S239–S248. https://doi.org/10.1111/rec.13217
- Fynn, R. W. S., & Naiken, J. (2009). Different responses of Eragrostis curvula and Themeda triandra to rapid-and slow-release fertilisers: Insights into their ecology and implications for fertiliser selection in pot experiments. African Journal of Range and Forage Science, 26(1), 43–46. https://doi.org/10.2989/AJRFS.2009.26.1.6.701
- Fynn, R. W. S., & O’Connor, T. G. (2000). Effect of stocking rate and rainfall on rangeland dynamics and cattle performance in a semi‐arid savanna, South Africa. Journal of Applied Ecology, 37(3), 491–507. https://doi.org/10.1046/j.1365-2664.2000.00513.x
- Fynn, R. W., & O’Connor, T. G. (2005). Determinants of community organization of a South African mesic grassland. Journal of Vegetation Science, 16(1), 93–102. https://doi.org/10.1111/j.1654-1103.2005.tb02342.x
- Gardner, C. A. (1952). Flora of Western Australia (Vol. 1). Part 1. In ‘Gramineae’. (Government Printer:Perth).
- Ghebrehiwot, H. M., Fynn, R. W. S., Morris, C. D., & Kirkman, K. P. (2006). Shoot and root biomass allocation and competitive hierarchies of four South African grass species on light, soil resources and cutting gradients. African Journal of Range and Forage Science, 23(2), 113–122. https://doi.org/10.2989/10220110609485894
- Ghebrehiwot, H. M., Kulkarni, M. G., Kirkman, K. P., & Van Staden, J. (2012). Smoke and heat: Influence on seedling emergence from the germinable soil seed bank of mesic grassland in South Africa. Plant Growth Regulation, 66(2), 119–127. https://doi.org/10.1007/s10725-011-9635-5
- Godfree, R. C., Marshall, D. J., Young, A. G., Miller, C. H., & Mathews, S. (2017). Empirical evidence of fixed and homeostatic patterns of polyploid advantage in a keystone grass exposed to drought and heat stress. Royal Society Open Science, 4(11), 170934. https://doi.org/10.1098/rsos.170934
- Great Plains Ag. (2022). https://www.greatplainsag.com/en/products/11078/native-grass-drills
- Grossman, D., & Cresswell, C. F. (1974). The influence of nitrate and ammonia nitrogen on the photosynthetic and photorespiratory activity of selected highveld grasses exhibiting c‐4 photosynthesis. Proceedings of the Annual Congresses of the Grassland Society of Southern Africa, 9(1), 89–94.
- Groves, R. H. (1965). Growth of Themeda australis tussock grassland at St. Albans, Victoria. Australian Journal of Botany, 13(2), 291–302. https://doi.org/10.1071/BT9650291
- Groves, R. H. (1974). Physiology of sclerophyll shrubs in southeastern Australia. Proceedings of the Ecological Society of Australia, 3, 40–45.
- Groves, R. H. (1975). Growth and development of five populations of Themeda australis in response to temperature. Australian Journal of Botany, 23(6), 951–963. https://doi.org/10.1071/BT9750951
- Groves, R. H., Hagon, M. W., & Ramakrishnan, P. S. (1982). Dormancy and germination of seed of eight populations of Themeda australis. Australian Journal of Botany, 30(4), 373–386. https://doi.org/10.1071/BT9820373
- Guzzomi, A. L., Erickson, T. E., Ling, K. Y., Dixon, K. W., & Merritt, D. J. (2016). Flash flaming effectively removes appendages and improves the seed coating potential of grass florets. Restoration Ecology, 24, S98–S105. https://doi.org/10.1111/rec.12386
- Hagon, M. W. (1976). Germination and dormancy of Themeda australis, Danthonia spp., Stipa bigeniculata and Bothriochloa macra. Australian Journal of Botany, 24(3), 319–327. https://doi.org/10.1071/BT9760319
- Hagon, M. W., & Chan, C. W. (1977). The effects of moisture stress on the germination of some Australian native grass seeds. Australian Journal of Experimental Agriculture, 17(84), 86–89. https://doi.org/10.1071/EA9770086
- Hagon, M. W., & Groves, R. H. (1977). Some factors affecting the establishment of four native grasses. Australian Journal of Experimental Agriculture, 17(84), 90–96. https://doi.org/10.1071/EA9770090
- Haile, T. A., Gulden, R. H., & Shirtliffe, S. J. (2014). On-farm seed loss does not differ between windrowed and direct-harvested canola. Canadian Journal of Plant Science, 94(4), 785–789. https://doi.org/10.4141/cjps2013-344
- Hall, M., Delpratt, J., & Roy, P. G. (2006). Viability testing of Victorian Western Plains grasses. Australasian Plant Conservation: Journal of the Australian Network for Plant Conservation, 15(3), 23–25. https://doi.org/10.3316/informit.054973933015845
- Hardy, M. B., Tainton, N. M., & Morris, C. D. (1994). Defoliation patterns of three grass species in sourveld grazed by cattle and sheep. African Journal of Range and Forage Science, 11(2), 37–42. https://doi.org/10.1080/10220119.1994.9638353
- Harlan, J. R., De Wet, J. M. J., & Price, E. G. (1973). Comparative evolution of cereals. Evolution, 27(2), 311–325. https://doi.org/10.1111/j.1558-5646.1973.tb00676.x
- Hayman, D. L. (1960). The distribution and cytology of the chromosome races of Themeda australis in southern Australia. Australian Journal of Botany, 8(1), 58–68. https://doi.org/10.1071/BT9600058
- Hill, B. D. (1985). Persistence of temperate perennial grasses in cutting trials on the central slopes of New South Wales. Australian Journal of Experimental Agriculture, 25(4), 832–839. https://doi.org/10.1071/EA9850832
- Hodgkinson, K. C., Ludlow, M. M., Mott, J. J., & Baruch, Z. (1989). Comparative responses of the savanna grasses Cenchrus ciliaris and Themeda triandra to defoliation. Oecologia, 79(1), 45–52. https://doi.org/10.1007/BF00378238
- Hovendon, M. J., & Morris, D. J. (2002). Occurrence and distribution of native and introduced C4 grasses in Tasmania. Australian Journal of Botany, 50(6), 667–675. https://doi.org/10.1071/BT01093
- Hutton, E. M. (1971). Plant improvement for increased animal production. Journal of Australian Institute of Agricultural Science, 37, 212–225.
- Huxtable, C. H. A. (1999). Rehabilitation of open-cut coal mines using native grasses: Management guidelines. Department of Land and Water Conservation, Centre for Natural Resources, Environmental Rehabilitation Unit.
- Ibrahim, K. M., Dube, S., Peterson, P. M., & Hosni, H. A. (2018). Grasses of Mali. Smithsonian Institution Scholarly Press.
- Johnston, W. H., Clifton, C. A., Cole, I. A., Koen, T. B., Mitchell, M. L., & Waterhouse, D. B. (1998). Native perennial grasses for productive sustainable pastures in southern Australia. Final report project SCS10 land and water resources research and development resources project. Research and Development Corporation Canberra, NSW Department of Land and Water Conservation, Wagga Wagga, NSW.
- Johnston, W. H., Clifton, C. A., Cole, I. A., Koen, T. B., Mitchell, M. L., & Waterhouse, D. B. (1999). Low input grasses useful in limiting environments (LIGULE). Australian Journal of Agricultural Research, 50(1), 29–54. https://doi.org/10.1071/A97159
- Kardol, P., Van der Wal, A., Bezemer, T. M., de Boer, W., Duyts, H., Holtkamp, R., & Van der Putten, W. H. (2008). Restoration of species-rich grasslands on ex-arable land: Seed addition outweighs soil fertility reduction. Biological Conservation, 141(9), 2208–2217. https://doi.org/10.1016/j.biocon.2008.06.011
- Karimmojeni, H., Rashidi, B., & Behrozi, D. (2011). Effect of different treatments on dormancy-breaking and germination of perennial pepperweed (‘Lepidium latifolium’)(Brassicaceae). Australian Journal of Agricultural Engineering, 2(2), 50. https://doi.org/10.3316/informit.267564762691638
- Khan, A. A. (1971). Cytokinins: Permissive role in seed germination. Science, 171(3974), 853–859. https://doi.org/10.1126/science.171.3974.853
- Kildisheva, O. A., Davis, A. S., & Dumroese, R. K. (2013b). Boiled, tumbled, burned, and heated: Seed scarification techniques for Munro’s globemallow appropriate for large-scale application. Native Plants Journal, 14(1), 42–48. https://doi.org/10.3368/npj.14.1.42
- Kildisheva, O. A., Davis, A. S., & Hamzeh, B. A. (2013a). A hard seed to crack evaluating dormancy-breaking techniques for mamane. Native Plants Journal, 14(3), 243–248. https://doi.org/10.3368/npj.14.3.243
- Kimura, E., & Islam, M. A. (2012). Seed scarification methods and their use in forage legumes. Research Journal of Seed Science, 5(2), 38–50. https://doi.org/10.3923/rjss.2012.38.50
- Kirkman, K. P. (2002a). The influence of various types and frequencies of rest on the production and condition of sourveld grazed by sheep or cattle. 1. Proportional species composition. African Journal of Range and Forage Science, 19(1), 55–62. https://doi.org/10.2989/10220110209485774
- Kirkman, K. P. (2002b). The influence of various types and frequencies of rest on the production and condition of sourveld grazed by sheep or cattle. 2. Vigour. African Journal of Range and Forage Science, 19(2), 93–105. https://doi.org/10.2989/10220110209485780
- Kundu, M., & Chaturvedi, N. (2019). Preliminary studies on seed dormancy of Schleichera oleosa (Lour.) Merr. Tropical Plant Research, 6(1), 133–138. https://doi.org/10.22271/tpr.2019.v6.i1.019
- Liebenberg, H., Lubbinge, J., & Fossey, A. (1993). Cytotaxonomic studies in Themeda triandra Forssk.: IV. A population cytogenetic study of a contact zone between tetraploid and hexaploid populations. South African Journal of Botany, 59(3), 305–310. https://doi.org/10.1016/S0254-6299(16)30733-5
- Ling, E., Guzzomi, A. L., Merritt, D. J., Renton, M., & Erickson, T. E. (2019, September). Flash flaming technology shows promise to improve seed-based rehabilitation outcomes. In Proceedings of the 13th International Conference on Mine Closure (pp. 175–184). Perth. Australian Centre for Geomechanics.
- Loch, D. S., & Ferguson, J. E. (1999). Forage seed production, tropical and subtropical species (Vol. 2, pp. 159–176). CABI Publishing.
- Loch, D. S. L., & Harvey, G. L. (1997). Developing herbicide strategies for tropical herbage seed crops. Australian New Crops Conference, Queensland: Gaton College.
- Lock, J. M., & Milburn, T. R. (1971). Seed biology of Themeda triandra Forsk. in relation to fire. British Ecological Society, 11, 337–349.
- Lodge, G. M. (1981). Establishment of warm-and cool-season native perennial grasses on the north-west slopes of New South Wales. II. Establishment and seedling survival in the field. Australian Journal of Botany, 29(2), 121–133. https://doi.org/10.1071/BT9810121
- Lodge, G. M., & Whalley, R. D. B. (1981). Establishment of warm-and cool-season native perennial grasses on the north-west slopes of New South Wales. I. Dormancy and germination. Australian Journal of Botany, 29(2), 111–119. https://doi.org/10.1071/BT9810111
- Loi, A., & Yates, R. (2020). Twin sowing and summer sowing: Alternative techniques to introduce annual legumes into pastures. Department of Primary Industries and Rural Development. https://www.agric.wa.gov.au/pasture-establishment/twin-sowing-and-summer-sowing-alternative-techniques-introduce-annual-legumes.
- Loomis, R. S. (2022). Perils of production with perennial polycultures. Outlook on Agriculture, 51(1), 22–31. https://doi.org/10.1177/00307270211063910
- Lunt, I. D. (2003). A protocol for integrated management, monitoring, and enhancement of degraded Themeda triandra grasslands based on plantings of indicator species. Restoration Ecology, 11(2), 223–230. https://doi.org/10.1046/j.1526-100X.2003.00201.x
- Lunt, I. D., & Morgan, J. W. (2000). Can competition from Themeda triandra inhibit invasion by the perennial exotic grass Nassella neesiana in native grasslands? Plant Protection Quarterly, 15(3), 92–94. https://www.cabdirect.org/cabdirect/abstract/20002303800
- Maid, R., Aghaie, P., Monfared, E. K., & Alebrahim, M. T. (2013). Evaluating of some treatments on breaking seed dormancy in Mesquite. International Journal of Agronomy and Plant Production, 4(7), 1433–1439. https://www.cabdirect.org/cabdirect/abstract/20133241161
- Martin, C. C. (1975). The role of glumes and gibberellic acid in dormancy of Themeda triandra spikelets. Physiologia Plantarum, 33(2), 171–176. https://doi.org/10.1111/j.1399-3054.1975.tb03787.x
- Mason, B. D. (2005). Directions for best management of Kangaroo grass (Themeda triandra Forssk.) re-establishment in southeastern Australian native grassland remnants. Doctor of Philosophy. Victoria University.
- McDougall, K. L. (1989) The re-establishment of Themeda Triandra (Kangaroo Grass): Implications for the restoration of Grassland. Arthur Rylah Institute for Environmental Research Technical Report Series no. 89.
- McDougall, K. L., & Kirkpatrick, J. B. (1994). Conservation of lowland native grasslands in south-eastern Australia. World Wide Fund for Nature, Sydney.
- McDougall, K. L., & Walsh, N. G. (2007). Treeless vegetation of the Australian Alps. Cunninghamia, 10(1), 1–57. https://core.ac.uk/download/pdf/14529081.pdf
- McIntyre, S., Lavorel, S., & Tremont, R. M. (1995). Plant life-history attributes: Their relationship to disturbance response in herbaceous vegetation. The Journal of Ecology, 83(1), 31–44. https://doi.org/10.2307/2261148
- McNaughton, S. J. (1992). Laboratory‐simulated grazing: Interactive effects of defoliation and canopy closure on Serengeti grasses. Ecology, 73(1), 170–182. https://doi.org/10.2307/1938729
- McWilliam, J. R., & Wardlaw, I. F. (1965). Effect of detaching culms on seed development in Phalaris. Australian Journal of Biological Sciences, 18(2), 283–294. https://doi.org/10.1071/BI9650283
- Merritt, D. J., & Dixon, K. W. (2011). Restoration seed banks—a matter of scale. Science, 332(6028), 424–425. https://doi.org/10.1126/science.1203083
- Mitchell, M. (2001). Grazing management and its influence on rooting depth of native grasses. In Proceedings of the Second National STIPA Conference’University of Melbourne, Institute of Land and Food Resources Dookie College. Stipa Native Grass Association, Gulgong. The Regional Institute Ltd. www.regional.org.au/au/stipa/2001/p-04.htm [accessed: 1 May 2011].
- Mitchell, M. L., Norman, H. C., & Whalley, R. D. B. (2015). Use of functional traits to identify Australian forage grasses, legumes and shrubs for domestication and use in pastoral areas under a changing climate. Crop and Pasture Science, 66(1), 71–89. https://doi.org/10.1071/CP13406
- Mitchell, A. A., & Wilcox, D. G. (1994). Arid shrubland plants of Western Australia. Press release.
- Moore AC (1989) Sekere fenologiese en fisiologiese reaksies van Themada triandra (Forsk.) op verskillende ontblaringskedules [ Doctoral dissertation], Potchefstroom University for Christian Higher Education.
- Morgan, J. W. (1999). Defining grassland fire events and the response of perennial plants to annual fire in temperate grasslands of south-eastern Australia. Plant Ecology, 144(1), 127–124. https://doi.org/10.1023/A:1009731815511
- Morgan, J. W. (2001). Seedling recruitment patterns over 4 years in an Australian perennial grassland community with different fire histories. Journal of Ecology, 89(6), 908–919. https://doi.org/10.1111/j.1365-2745.2001.00617.x
- Morgan, J. W., & Lunt, I. D. (1999). Effects of time-since-fire on the tussock dynamics of a dominant grass (Themeda triandra) in a temperate Australian grassland. Biological Conservation, 88(3), 379–386. https://doi.org/10.1016/S0006-3207(98)00112-8
- Morris, C. (2020). Is a long hygroscopic awn an advantage for Themeda triandra in drier areas? African Journal of Range and Forage Science, 38(2), 179–183. https://doi.org/10.2989/10220119.2020.1783701
- Morris, C. D., & Tainton, N. M. (1993). The effect of defoliation and competition on the regrowth of Themeda triandra and Aristida junciformis subsp. junciformis. African Journal of Range & Forage Science, 10(3), 124–128. https://doi.org/10.1080/10220119.1993.9638338
- Mott, J. J., & Groves, R. H. (1981). Germination strategies. In J. S. Pate & A. J. McComb (Eds.), ‘The biology of Australian plants’ (pp. 307–341). Univ. West. Aust. Press.
- Mott, J. J., Ludlow, M. M., Richards, J. H., & Parsons, A. D. (1992). Effects of moisture supply in the dry season and subsequent defoliation on persistence of the savanna grasses Themeda triandra, Heteropogon contortus and Panicum maximum. Australian Journal of Agricultural Research, 43(2), 241–260. https://doi.org/10.1071/AR9920241
- Moyo, M., Kulkarni, M. G., Finnie, J. F., & Van Staden, J. (2009). After-ripening, light conditions, and cold stratification influence germination of marula [Sclerocarya birrea (A. Rich.) Hochst. subsp. caffra (Sond.) Kokwaro] seeds. HortScience, 44(1), 119–124. https://doi.org/10.21273/HORTSCI.44.1.119
- Nadella, D., Gu, X., & Foley, M. E. (2003) Seed dormancy: Genetics of dormancy. Encyclopedia of Applied Plant Sciences, Three-Volume Set, 1323–1333. https://doi.org/10.1016/B0-12-227050-9/00062-4
- Newell, M., Munday, N., & Hayes, R. (2022). NSW DPI perennial grains factsheet. Riverine Plains Innovation Expo. 27 July 2022.
- Nolan, M. (1994) Harvest, Germination and Establishment Techniques for the Revegetation of Themeda australis. M. Hort, Thesis University of Western Sydney.
- Noy-Meir, I. (1993). Compensating growth of grazed plants and its relevance to the use of rangelands. Ecological Applications, 3(1), 32–34. https://doi.org/10.2307/1941787
- NSW DPI. (2021a). Themeda triandra (Kangaroo grass) species information. https://www.dpi.nsw.gov.au/agriculture/pastures-and-rangelands/rangelands/publications-and-information/grassedup/species/kangaroo-grass
- NSW DPI. (2021b). Grassed up - General guidelines for seed production. https://www.dpi.nsw.gov.au/agriculture/pastures-and-rangelands/rangelands/publications-and-information/grassedup/seed-production
- NSW DPI. (2022). Themeda triandra (kangaroo grass) species information. https://www.dpi.nsw.gov.au/agriculture/pastures-and-rangelands/rangelands/publications-and-information/grassedup/species/kangaroo-grass#:~:text=Description,to%2012.4%25%20crude%20protein)
- Opperman, D. P. J., & Roberts, B. R. (1978). The phenological development of Themeda triandra, Elyonurus argenteus and Heteropogon contortus under field conditions in the central Orange Free State. African Journal of Range and Forage Science, 13(1), 135–140. https://doi.org/10.1080/00725560.1978.9648848
- Peart, M. H. (1979). Experiments on the biological significance of the morphology of seed-dispersal units in grasses. The Journal of Ecology, 67(3), 843–863. https://doi.org/10.2307/2259218
- Peart, M. H. (1984). The effects of morphology, orientation and position of grass diaspores on seedling survival. The Journal of Ecology, 72(2), 437–453. https://doi.org/10.2307/2260057
- Prober, S. M., & Lunt, I. D. (2009). Restoration of Themeda australis swards suppresses soil nitrate and enhances ecological resistance to invasion by exotic annuals. Biological Invasions, 11(2), 171–181. https://doi.org/10.1007/s10530-008-9222-5
- Prober, S. M., & Thiele, K. R. (1995). Conservation of the grassy white box woodlands: Relative contributions of size and disturbance to floristic composition and diversity of remnants. Australian Journal of Botany, 43(4), 349–366. https://doi.org/10.1071/BT9950349
- Prober, S. M., Thiele, K. R., Lunt, I. D., & Koen, T. B. (2005). Restoring ecological function in temperate grassy woodlands: Manipulating soil nutrients, exotic annuals and native perennial grasses through carbon supplements and spring burns. Journal of Applied Ecology, 42(6), 1073–1085. https://doi.org/10.1111/j.1365-2664.2005.01095.x
- PTP, C., & H, M. J. (1997). Harvest and post-harvest management of forage seed crops. Forage Seed Production. 1. Temperate Species (Fairey DT, Hampton JG Eds.), 181–217.
- Ralowicz, A. E., & Mancino, C. F. (1992). Afterripening in curly Mesquite seeds. Rangeland Ecology and Management/Journal of Range Management Archives, 45(1), 85–87. https://doi.org/10.2307/4002532
- Rethman, N. F. G. (1971). Elevation of shoot‐apices of two ecotypes of Themeda triandra on the Transvaal highveld. Proceedings of the Annual Congresses of the Grassland Society of Southern Africa, 6(1), 86–92.
- Rethman, N. F. G., & de, V. B. P. (1968). The influence of time of defoliation on the vigour of a tall grassveld sward in the next season. Proceedings of the Annual Congresses of the Grassland Society of Southern Africa, 3(1), 91–94.
- Ritchie, M. E., & Raina, R. (2016). Effects of herbivores on nitrogen fixation by grass endophytes, legume symbionts and free-living soil surface bacteria in the Serengeti. Pedobiologia, 59(5–6), 233–241. https://doi.org/10.1016/j.pedobi.2016.09.001
- Rolston, M. P. (1980). Recent developments in seed production in Oregon, USA. In I. A. Lancashire (Ed.), ‘Herbage seed production. Grasslands research and practice series No. 1’ (pp. 15–16). Palmerston North.
- Rolston, M. P., Rowarth, J. S., Young, I. I. I. W. C., & Mueller-Warrant, G. W. (1998). Grass seed crop management. Forage Seed Production, 1, 105–126. https://www.cabdirect.org/cabdirect/abstract/19980702277
- Saleem, A., Hassan, F., Manaf, A., & Ahmedani, M. (2009). Germination of Themeda triandra (Kangaroo grass) as affected by different environmental conditions and storage periods. African Journal of Biotechnology, 8(17). https://doi.org/10.5897/AJB09.510
- Sampaio, A. B., Vieira, D. L., Holl, K. D., Pellizzaro, K. F., Alves, M., Coutinho, A. G., & Schmidt, I. B. (2019). Lessons on direct seeding to restore Neotropical savanna. Ecological Engineering, 138, 148–154. https://doi.org/10.1016/j.ecoleng.2019.07.025
- Schöning, C., Espadaler, X., Hensen, I., & Roces, F. (2004). Seed predation of the tussock-grass Stipa tenacissima L. by ants (Messor spp.) in south-eastern Spain: The adaptive value of trypanocarpy. Journal of Arid Environments, 56(1), 43–61. https://doi.org/10.1016/S0140-1963(03)00024-7
- Scott, J. D., & Rabie, J. W. (1956). Preliminary studies on growth and development of Eragrostis curvula and Themeda triandra. South African Journal of Science, 52, 207–209. https://hdl.handle.net/10520/AJA00382353_2144
- Semple, W. S., Koen, T. B., & Cole, I. A. (1999). Establishing native grasses in degraded pastures of Central Western New South Wales. The Rangeland Journal, 21(2), 153–168. https://doi.org/10.1071/RJ9990153
- Silcock, R. G., Williams, L. M., & Smith, F. (1990). Quality and storage characteristics of the seeds of important native pasture species in south-west Queensland. The Rangeland Journal, 12(1), 14–20. https://doi.org/10.1071/RJ9900014
- Sindel, B. M., Davidson, S. J., Kilby, M. J., & Groves, R. H. (1993). Germination and establishment of Themeda triandra (kangaroo grass) as affected by soil and seed characteristics. Australian Journal of Botany, 41(1), 105–117. https://doi.org/10.1071/BT9930105
- Snape, M. A., Fletcher, D., & Caley, P. (2021). Species composition, herbage mass and grass productivity influence pasture responses to kangaroo grazing in a temperate environment. Ecological Management & Restoration, 22(S1), 16–23. https://doi.org/10.1111/emr.12477
- Snyman, H. A. (2002). Short-term response of rangeland botanical composition and productivity to fertilization (N and P) in a semi-arid climate of South Africa. Journal of Arid Environments, 50(1), 167–183. https://doi.org/10.1006/jare.2001.0858
- Snyman, H. A. (2004). Short-term response in productivity following an unplanned fire in a semi-arid rangeland of South Africa. Journal of Arid Environments, 56(3), 465–485. https://doi.org/10.1016/S0140-1963(03)00069-7
- Snyman, H. A., Ingram, L. J., & Kirkman, K. P. (2013). Themeda triandra: A keystone grass species. African Journal of Range and Forage Science, 30(3), 99–125. https://doi.org/10.2989/10220119.2013.831375
- Snyman, H. A., van Rensburg, W. L. J., & Venter, W. D. (1997). Transpiration and water-use efficiency in response to water stress in Themeda triandra and Eragrostis lehmanniana. South African Journal of Botany, 63(1), 55–59. https://doi.org/10.1016/S0254-6299(15)30693-1
- Southwood, O., Cregan, P. D., Dellow, J. J., & Walker, R. B. (1981) NSW. Pasture establishment. Orange.
- Sr, S., Jr, & Smith, S. (1997). Native grass seed-increase manual. In (US Department of Agriculture, Natural Resources Conservation Service, Ducks Unlimited Canada, Manitoba Forage Seed Association. University of Manitoba.
- Stafford, J. (1991). Techniques for the establishment of kangaroo grass in South Australian conservation reserves. Plant Protection Quarterly, 6(3), 120–122. https://caws.org.nz/PPQ567/PPQ%2006-3%20pp120-122%20Stafford.pdf
- Stevens, A. V., Nicotra, A. B., Godfree, R. C., & Guja, L. K. (2020). Polyploidy affects the seed, dormancy and seedling characteristics of a perennial grass, conferring an advantage in stressful climates. Plant Biology, 22(3), 500–513. https://doi.org/10.1111/plb.13094
- Stokes, P. (1965). Temperature and seed dormancy. In W.RUHLAND (Ed.) Differenzierung und Entwicklung/Differentiation and Development (pp. 2393–2450). Springer. https://doi.org/10.1007/978-3-642-50088-6_60
- Strugnell, R. G., & Pigott, C. D. (1978). Biomass, shoot-production and grazing of two grasslands in the Rwenzori National Park, Uganda. The Journal of Ecology, 66(1), 73–96. https://doi.org/10.2307/2259182
- Stuwe, J. (1986) An assessment of the conservation status of native grasslands on the Western Plains, Victoria and sites of botanical significance. Technical Report Series-Arthur Rylah Institute for Environmental Research (Australia).
- Tainton, N. M., & Booysen, P. D. V. (1963). The effects of management on apical bud development and seeding in Themeda triandra and Tristachya triandra. South African Journal of Agricultural Science, 6(1), 21–30. https://hdl.handle.net/10520/AJA05858860_30
- Tainton, N. M., & Booysen, P. D. V. (1965). Growth and development in perennial veld grasses. I. Themeda triandra tillers under various systems of defoliation. South African Journal of Agricultural Science, 8(1), 93–110. https://hdl.handle.net/10520/AJA05858860_281
- Tomlinson, K. W., Dominy, J. G., Hearne, J. W., & O’Connor, T. G. (2007). A functional-structural model for growth of clonal bunchgrasses. Ecological Modelling, 202(3–4), 243–264. https://doi.org/10.1016/j.ecolmodel.2006.11.002
- UN General Assembly. (2007). United Nations declaration on the rights of indigenous peoples. UN Wash, 12, 1–18. https://waubrafoundation.org.au/wp-content/uploads/2014/06/United-Nations-Declaration-on-the-Rights-of-Indigenous-Peoples.pdf
- The University of Sydney, Institute of Agriculture. (2020). Native grains from Paddock to Plate - Study of the economic, environmental and social sustainability of an ancient system in a modern context. https://www.sydney.edu.au/content/dam/corporate/documents/faculty-of-science/research/life-and-environmental-sciences/sia-native-grains-paddock-to-plate.pdf
- Van der Westhuizen, H. C. (2003). Die gebruik van degradasiegradiënte vir weiveldevaluering in’n semi-ariede gebied. [ Doctoral dissertation], University of the Free State.
- Vuorio, V., Muchiru, A., Reid, R. S., & Ogutu, J. O. (2014). How pastoralism changes savanna vegetation: Impact of old pastoral settlements on plant diversity and abundance in south-western Kenya. Biodiversity and Conservation, 23(13), 3219–3240. https://doi.org/10.1007/s10531-014-0777-4
- Walker, K. J., Stevens, P. A., Stevens, D. P., Mountford, J. O., Manchester, S. J., & Pywell, R. F. (2004). The restoration and re-creation of species-rich lowland grassland on land formerly managed for intensive agriculture in the UK. Biological Conservation, 119(1), 1–18. https://doi.org/10.1016/j.biocon.2003.10.020
- Wareing, P. F., Van Staden, J., & Webb, D. P. (1972). Endogenous hormones in the control of seed dormancy. (S. Ecology’ & W. Heydecker, eds.). London.
- Watt, L. A. (1974). Effect of water potential on the germination behaviour of several warm season grass species, with special reference to cracking black clay soils. Journal of the Soil Conservation Service of New South Wales.
- Whalley, R. D. B., & Jones, C. E. (1996) Commercialisation of the Australian native grass Microlaena stipoides for turf, amenity and forage purposes. Final report project UNEA (pp. 35). University of New England.
- Williams, K. J., Wilsey, B. J., McNaughton, S. J., & Banyikwa, F. F. (1998). Temporally variable rainfall does not limit yields of Serengeti grasses. Oikos, 81(3), 463–470. https://doi.org/10.2307/3546768
- Wilson, J. R., & Haydock, K. P. (1971). The comparative response of tropical and temperate grasses to varying levels of nitrogen and phosphorus nutrition. Australian Journal of Agricultural Research, 22(4), 573–587. https://doi.org/10.1071/AR9710573
- Winkel, V. K., Roundy, B. A., & Cox, J. R. (1991). Influence of seedbed microsite characteristics on grass seedling emergence. Rangeland Ecology and Management/Journal of Range Management Archives, 44(3), 210–214. https://doi.org/10.2307/4002943
- Woodland, P. S. (1964). The floral morphology and embryology of Themeda australis (R. Br.) Stapf. Australian Journal of Botany, 12(2), 157–172. https://doi.org/10.1071/BT9640157
- Yang, W., Liu, S., Yuan, G., Liu, P., Qi, D., Dong, X., & Li, X. (2019). Germination characteristics among different sheepgrass (Leymus chinensis) germplasm during the seed development and after-ripening stages. PeerJ, 7, e6688. https://doi.org/10.7717/peerj.6688
- Yule, D. F. (1998, August). Controlled traffic farming–the future. In Proceedings of the 2nd national controlled traffic conference’, ( EdsJ. N. Tullberg, DF Yule) (pp. 6–12). Gatton, Queensland.