 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Pesticides are routinely used to control pests. For many pesticides, however, information is unavailable concerning dissipation and dietary risk, posing a potential threat to human health. To overcome this shortcoming, we evaluated the dissipation and dietary risk of cyflumetofen and bifenazate as well as their metabolites 2-trifluoromethylbenzoic acid and bifenazate-diazene. A method to detect four components was developed by using ultra-high-performance liquid chromatography, whose precision and linearity were satisfactory. The limit of quantification for these compounds was 0.02 mg/kg, and recovery ranged for four spiked levels from 80% to 100%, with relative standard deviation values between 1.6% and 5.3%. Field trials were carried out in China, and levels of four substances were measured at several time points. A half-life (34.04 days) could be established for bifenazate in whole citrus. The terminal residues in citrus pulp were less than it in whole citrus. Moreover, the dietary risk assessment revealed an acceptable risk of two pesticides, whose risk quotients were 10.65% and 43.86% respectively suggesting 30 days as their preharvest intervals. This work provides a comprehensive evaluation of cyflumetofen, bifenazate and their metabolites in citrus, which can serve as a basis for the rational application of these pesticides in crop fields.
As a common fruit of the Rutaceas family, citrus has been planted all over the world and comprises an important commodity in the fruit industry (Nair et al., Citation2018; Wang et al., Citation2021). Because citrus fruits are rich in certain health-promoting metabolites such as vitamin C, phenolic acids, hesperidin and flavonoids, they can help prevent both infections by SARS-Cov-2 and the damage caused by free radicals (Coelho et al., Citation2013; Laksmiani et al., Citation2020; Singh et al., Citation2020; Zhang et al., Citation2022). However, citrus can be harmed by diverse pests throughout its growth period, resulting in negatively impact entire orchards and hence citrus yield (Gama et al., Citation2020). The citrus red mite, Panonychus citri, is a key pest feeding on leaves and fruit of citrus, which can lead to necrosis, premature leaf fall, shoot dieback and decreased plant vigour (Jamieson et al., Citation2005; Pan et al., Citation2020). How to effectively control this mite in the orchard is essential to keep the quality of citrus. Amongst several target pesticides, cyflumetofen and bifenazate can efficiently inhibit the effects of the mite, which have also been registered respectively and applied for a long period. However, at the same time, they pose some potential health hazards to humans owing to their toxicity.
Cyflumetofen [2-methoxyethyl (RS)-2-(4-tert-butylphenyl)-2-cyano-3-oxo-3-(α, α, α-trifluoro-o-tolyl) propionate] is a kind of benzoyl acetonitrile acaricides. It can be degraded primarily to 2-trifluoromethylbenzoic acid (Li et al., Citation2020). In agricultural production, the pesticide and the metabolite can kill several strains of mites by inhibiting the activity of mitochondria complex II (Sun et al., Citation2016). For bifenazate (isopropyl 2-(4-methoxybiphenyl-3-yl) hydrazino-formate), it belongs to the group of hydrazine derivatives. And bifenazate-diazene is the primary metabolite of bifenazate. Both the pesticide and the metabolite can enhance the GABA effect in mites, which can act quickly to inhibit the mite growth (Hiragaki et al., Citation2012; Li et al., Citation2017; Satheshkumar et al., Citation2014; Xue et al., Citation2021; Zhao et al., Citation2021). After the long-term exposure, the resistance of the citrus red mite has evolved rapidly,(Pan et al., Citation2020) which may lead to the high application dose of pesticides. Therefore, it is crucial to combine cyflumetofen and bifenazate so that the mixture pesticides can achieve satisfactory inhibitory effects on the mite with a low-dose application. However, the simultaneous application of these two pesticides can lead to different residual levels on citrus compared to the single use of either cyflumetofen or bifenazate. If human is exposed to the residues of cyflumetofen, bifenazate and their metabolites for a long time, the risk of accumulating genotoxicity and developing severe cardiac issues may be raised (Li et al., Citation2013; Ma et al., Citation2021; Wang et al., Citation2021; Yoshida et al., Citation2012). Therefore, it is extremely urgent to evaluate the risk of these two pesticides and their metabolites.
To date, there have been some studies singularly focusing on either cyflumetofen or bifenazate in different crops. The dissipation of cyflumetofen has been reported for green bean pod, strawberry, lemon and apple (El-Gammal et al., Citation2022; Guo et al., Citation2018; Ibrahim & El-Hefny, Citation2021). And the relative aspects of bifenazate have also been explored in garlic plant (Bian et al., Citation2022). Nevertheless, two or more pesticides are frequently applied to control pest populations. Different formula of pesticides can produce different residue levels on fruits and hazardous influences on human. But these potential impacts of the combination of cyflumetofen and bifenazate have not been thoroughly examined on any fruit yet. As far as we know, only a few studies have focused on the risk of simultaneous application of two or more pesticides as well as their metabolites in apple, (Zhao et al., Citation2021) strawberry, cucumber and grape (Abdel-Ghany et al., Citation2016; Liu et al., Citation2019; Schusterova et al., Citation2021). Thus, in order to guarantee the safety and quality of citrus, it is urgently needed to determine the terminal residues of cyflumoten, bifenazate and their metabolites, and estimate the potential risk of the fruit harboring those residues and metabolites.
Here we report a quick method for detecting cyflumetofen, bifenazate and their metabolites by using ultra-high-performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS/MS). Field trials were carried out to estimate half-lives of the pesticides and metabolites as well as to determine the terminal residues of target substances in different provinces in China. In addition, we conducted a dietary risk assessment of cyflumetofen and bifenazate evaluated by the risk quotient model. Our results not only guide the rational and safe use of cyflumetofen and bifenazate in agricultural production but also lay the foundation for dietary safety recommendations for these substances.
1. Materials and methods
1.1. Chemicals and reagents
Cyflumetofen (99.2% purity), 2-trifluoromethylbenzoic acid (98.5% purity), bifenazate (99.3% purity), and bifenazate-diazene (98.7% purity) were purchased from LGC Science (Shanghai)] Ltd. (Shanghai, China). A 35% cyflumetofen-bifenazate concentrated suspension (15% cyflumetofen, 20% bifenazate) was provided by Shanghai Shengnong Pesticide Co., Ltd. Analytical grade acetonitrile was purchased from Fisher and Beijing Mairuida Technology Co., Ltd. (MREDA, Beijing, China). Sodium chloride and anhydrous sodium sulfate were also purchased from MREDA. Ultra-pure water was prepared in the laboratory. Syringes (2 mL) were purchased from Jiangsu Zhiyu Medical Equipment Co., Ltd. (Jiangsu, China), and 0.22-µm nylon syringe filters were purchased from Tianjin Jinteng Experimental Equipment Co., Ltd. (Tianjin, China).
1.2. Field trials
Field trials were carried out in 2021 at the following locations in China: Longnan City, Gansu Province; Ningbo City, Zhejiang Province; Yichang City, Hubei Province; Yongzhou City and Changsha City, Hunan Province; Nanning City, Guangxi Province; Qiannan Buyi and Miao Autonomous Prefecture, Guizhou Province; Maoming City and Guangzhou City, Guangdong Province; Danzhou City, Hainan Province; Beibei District, Chongqing City; Hani-Yi Autonomous Prefecture of Honghe, Yunnan Province. The trials were designed based on NY/T 788–2018 issued by the Ministry of Agricultural and Rural Affairs of the People’s Republic of China. Each experimental location was divided into two plots with four orange trees each and three replicates for each treatment, and plots were separated from each other by a buffer zone.
A 1.5-fold amount of the recommended dosage of cyflumetofen and bifenazate was sprayed on experimental trees in the early stage of the growing season, and samples were collected after 2 h and on subsequent days 10, 30, 40, 50. Each fruit sample was collected randomly from each tree in the district, and at least 2 kg of sample was collected. The samples collected on days 30 and 40 were used to quantify the terminal residue. All samples were transported to the lab within 8 h of collection and stored in a chamber at −18°C.
1.3. Preparation of samples
In the field experiments, the citrus pulp and whole citrus samples were collected and chopped via high-speed homogenization and stored at −20°C. A weight of 5.0 ± 0.1 g of the control group and sample groups were placed into 50-mL centrifuge tubes containing 25 mL of 1% formic acid in acetonitrile. Each sample was mixed vigorously for 10 min. To remove the water, 3 g sodium chloride and 3 g anhydrous sodium sulfate were added into each tube. The samples were again mixed for 5 min. After centrifugation for 5 min at 4000 revolutions per min in a Model CL5 centrifuge, each supernatant was passed through a 0.22-µm nylon syringe filter, and each filtrate was transferred into a glass vial for subsequent analysis by UHPLC-MS/MS.
1.4. UHPLC–MS/MS analysis
Pesticide residues in the samples and working solutions were analyzed using an UHPLC-MS/MS system (Agilent 1290–6470, USA) equipped with an electrospray ionization source. The analysis was performed with an analytical column (ZORBAX Eclipse C18; 3.0 × 50 mm, 1.8 μm; Agilent) that was maintained at 40°C. The injection volume was 1 µL. Analytes in samples were separated via gradient elution with 0.1% formic acid in water (solvent A) and acetonitrile (solvent B). The flow rate was 0.4 mL/min, and the chromatographic run time was 5 min, including equilibration of the system. The gradient started with 10% solvent B, increased to 90% B over 2.5 min, was maintained at 90% for 1 min, and then ramped to the original condition (10% B) from 3.5 to 3.6 min.
For 2-trifluoromethylbenzoic acid, MS was performed in the negative electrospray ionization mode with multiple reaction monitoring. For the other three analytes, MS was performed in the positive electrospray ionization mode. All ion-source and ion-optic parameters were optimized as follows: gas temperature 300°C; gas flow 7.0 L/min; sheath gas temperature 150°C; sheath gas flow 11.0 L/min; capillary 3500 V. The optimized MS conditions for cyflumetofen and its main metabolite were as follows: for cyflumetofen, a quantitative transition of 456.0 → 173 m/z (collision energy, 25 eV) and a qualitative transition of 456.0 → 249.1 m/z (collision energy, 11 eV) were applied with a fragmentor energy of 86 eV; for 2-trifluoromethybenzoic acid, a quantitative transition of 189 → 145.1 m/z (collision energy, 10 eV) and a qualitative transition of 189 → 69.2 m/z (collision energy, 43 eV) were applied with a fragmentor energy of 56 eV. For bifenazate, the optimization parameters were as follows: fragmentor energy, 80 eV; mass/charge ratio, 301.1 m/z, 198.1 m/z quantizer (collision energy, 10 eV) and 170 m/z qualifier (collision energy, 20 eV). For bifenazate-diazene, the parameters were optimized as follows: fragmentor energy, 70 eV; mass/charge ratio, 299.3 m/z, 213 m/z quantizer (collision energy, 10 eV) and 197 m/z qualifier (collision energy, 20 eV). All data were processed with Agilent Mass Hunter Quantitative Analysis 10.0. And the representative chromatograms were shown in Figure .
Figure 1. UHPLC-MS/MS chromatograms of cyflumetofen (A), 2-trifluoromethylbenzoic acid (B), bifenazate (C) and bifenazate-diazene (D) of citrus pulp analyzed at 0.002 mg/L.
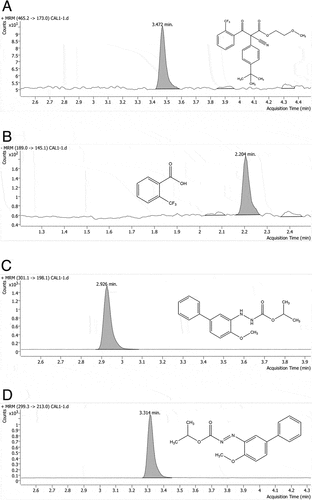
As for the residue determination of each batch of field samples, quality control samples were set up. The concentrations of cyflumetofen and 2-trifluoromethylbenzoic acid were 0.02 mg/kg and 5 mg/kg, and bifenazate and bifenazate-diazene were added at the concentrations of 0.02 mg/kg and 0.7 mg/kg. Two parallels were added for each concentration as quality control samples. Quantification was accomplished by external standard single-point comparison method.
1.5. Validation of the method
The method was validated by determining the following five parameters: selectivity, linearity, sensitivity, accuracy and precision, based on Guidance Document on Pesticide Analytical Methods for Risk Assessment and Post-approval Control and Monitoring Purposes (SANTE/2021/12,830) and NY/T 788–2018. Selectivity was examined by analyzing the control samples to confirm whether an interference peak was observed around the retention time of the analyte. To ascertain the linearity, the standard solution of cyflumetofen and 2-trifluoromethylbenzoic acid were analyzed at 0.002, 0.004, 0.04, 0.14, 1.0, 2.0 and 2.5 mg/L with three replicates. For bifenazate and its metabolite, the levels were set as 0.002, 0.004, 0.04, 0.14, 0.2, 0.4 and 0.5 mg/L, respectively. The following parameters were calculated: slope, intercept, and coefficient of determination (R2) for each fitted curve. To determine the average recovery, four concentrations (0.02, 0.7, 5.0, 10.0 mg/kg of cyflumetofen and 2-trifluoromethylbenzoic acid, and 0.02, 0.7, 1.0, 2.0 mg/kg of bifenazate and bifenazate-diazene) were added to the the various citrus samples. The limit of quantification (LOQ) was defined as the lowest concentration validated with an acceptable recovery of ≥70%.
1.6. Data analyses
The matrix effect of each of the four pesticide analytes was calculated as in Equationequation (1)(1)
(1) .
Ideally, when the value is zero, there is no matrix effect. A matrix effect of less than 0 indicates signal suppression, and a value of greater than 0 indicates ionization enhancement (Zhang et al., Citation2022). The dissipation kinetics of cyflumetofen and bifenazate in citrus were modeled by single first-order kinetics. KinGUIIv2.1 was applied to fit the curve and evaluate the degradation rate (k) as indicated in Equationequation (2)(2)
(2) .
Here, C is the residue concentration (mg/kg) of the target pesticide at time point t (day), and C0 is the initial concentration (mg/kg) of the pesticide. At the same time, half-life (T1/2) can be calculated (Zhang et al., Citation2022).
1.7. Dietary risk assessment
Terminal residue data were used to assess the safety of the two pesticides at various time points after application to the fruit. To assess dietary risk for cyflumetofen and bifenazate in citrus, owing to the lack of an acute reference dose value, only the risk quotient (RQ) model was applied for purposes of this study, which was calculated as in Equationequations (3)(3)
(3) and (Equation4
(4)
(4) ).
NEDI refers to the national estimated daily intake, ADI is the acceptable daily intake, STMRi refers to the supervised trials median residue (mg/kg), F is consumption of crop foods (g/d), and bw is body weight (kg). Risk correlates with the RQ value: an RQ of ≤100% implies that a chronic risk to humans is acceptable, whereas an RQ of >100% represents an unacceptable risk (Wang et al., Citation2021).
2. Results and discussion
2.1. Validation of the method
2.1.1. Matrix effect, linearity and LOQ values
During the process of method development, the matrix effect was investigated by comparing the detector response for each standard solution of cyflumetofen, 2-trifluoromethylbenzoic acid, bifenazate and bifenazate-diazene with that for each whole citrus and citrus pulp matrix into which all four analytes were added at various concentrations. Based on these results, corresponding values for peak area were calculated by measuring analyte concentration in the different standard working solutions containing different analytes. According to the peak area, a linear regression equation was obtained. Table shows that the linear relationship between the injection volume and the peak area was acceptable (R2 > 0.99) over the range of concentrations. Also, the matrix effects between matrix and solvent were all lower than 20%. To maximize the accuracy of the method, the standard curves of the four pesticide analytes in whole citrus and citrus pulp were used to determine those contents of samples. A LOQ value of 0.02 mg/kg was calculated for each of the pesticides and metabolites in these matrices.
Table 1. Comparison of matrix-matched and solvent calibration of four substances
2.1.2. Accuracy and precision
Recovery assays were performed to determine the accuracy and precision of the method. Samples of whole citrus and citrus pulp were spiked with four concentrations of each of the four pesticide analytes. These parameters were expressed as mean recovery (%) and relative standard deviation (RSD) for cyflumetofen, 2-trifluoromethylbenzoic acid, bifenazate and bifenazate-diazene for each spiked sample with the use of external matrix-matched standards for quantification. As shown in Table , the mean recovery of cyflumetofen at four different spiked concentrations among whole citrus and citrus pulp ranged from 80% to 95%, and RSD values ranged from 1.6% to 4.4%. The recovery of 2-trifluoromethylbenzoic acid in samples ranged from 86% to 92%, and RSD ranged from 0.4% to 1.9%. For bifenazate in citrus samples, recovery ranged from 88% to 100%, and RSD ranged from 1.9% to 5.3%. For bifenazate-diazene, recovery ranged from 85% to 95%, and RSD ranged from 0.8% to 3.9%. All values for mean recovery and RSD complied with the standards of SANTE/2021/12,830, which demands a recovery range from 70% to 110% and RSD values less than 10%. Thus, our method could be applicable for analysis of cyflumetofen and bifenazate in whole citrus and citrus pulp samples collected from fields, stores and orchards.
Table 2. Recovery (%) and RSD (%) for cyflumetofen, 2-Trifluoromethylbenzoic acid, bifenazate and bifenazate-diazene from whole citrus and citrus pulp at four spiked levels (n = 5)
2.2. Dissipation dynamics of cyflumetofen and bifenazate in whole citrus
In this study, after applying the two pesticides, the amount of residual pesticide in the citrus pulp samples was below the LOQ for each of the four analytes. Thus, only the dissipation in whole citrus is further discussed. The initial residue levels (at 2 h post-application) ranged from 0.022–0.280 mg/kg for cyflumetofen and were <0.02 mg/kg for 2-trifluoromethylbenzoic acid (Table ). The range was 0.044–0.18 mg/kg for bifenazate and <0.02–0.12 mg/kg for bifenazate-diazene. For samples obtained from Changsha, Honghe and Guangzhou, the level of these residues gradually decreased by more than 90%, which was lower than the LOQ. Finally, the dissipation kinetics of cyflumetofen and bifenazate could be fitted from data obtained for samples from the Beibei District of Chongqing. A dissipation curve could be fitted only for bifenazate, with a relatively high correlation coefficient (R2 = 0.6926) from Equationequation (5)(5)
(5) .
Table 3. The residue levels of four components in citrus pulp and whole citrus in different collecting intervals
Based on the data, the half-life of bifenazate was fitted as 34.04 days. Compared with the half-lives of bifenazate on tea (1.03–1.36 days) and apple (4.3–7.8 days), (Satheshkumar et al., Citation2014; Zhao et al., Citation2021) the value of citrus was bigger, which also showed that the degradation speed of bifenazate can be different on different crops. In addition, according to published data from several sources, the evaluated half-lives of cyflumetofen are 5.33 days in lemon fruit, 22 days in apple, and 1.16–1.81 days in strawberry fruit and green-bean pods, (El-Gammal et al., Citation2022; Guo et al., Citation2018; Ibrahim & El-Hefny, Citation2021) which means the dissipation of cyflumetofen on citrus was quicker. In our study, we found that the residue of cyflumetofen was lower than the LOQ value after 10 days. Thus, the dissipation curve of cyflumetofen cannot be fitted. And the difference of half-lives of these two pesticides might be caused by the diversity of application doses, matrices and regional climates (Zhang et al., Citation2022).
2.3. Terminal residues of cyflumetofen and bifenazate in citrus
Table presents data for terminal residue abundance in samples of whole citrus and citrus pulp. In citrus pulp, residue levels were in the range of <0.02–0.027 mg/kg for cyflumetofen and <0.02 mg/kg for its metabolite. The residual level of bifenazate was in the range of <0.02–0.020 mg/kg. And the residues of bifenazate-diazene were less than 0.02 mg/kg. For whole citrus samples, however, the range of residue level for each of the four analytes had mostly higher values compared with citrus pulp. The terminal residue levels were in the range of <0.02–0.10 mg/kg for cyflumetofen and were <0.02 mg/kg for 2-trifluoromethylbenzoic acid in whole citrus. Residue levels were in the range of 0.02–0.11 mg/kg for bifenazate and 0.02–0.029 mg/kg for bifenazate-diazene in whole citrus.
Table 4. The summary of terminal residues of cyflumetofen, bifenazate and their metabolites in citrus at different collecting intervals
Regardless of whether the matrix was citrus pulp or whole citrus, for cyflumetofen, its residue applied after 30 days and 40 days was significantly lower than the maximum residue level (MRL) measured in citrus (0.3 mg/kg in Codex Alimentarius Commission (CAC), the strictest standard among countries). Thus, 30 days is suggested as the preharvest interval for application of cyflumetofen to citrus. For bifenazate, the residues at the interval of two intervals in both whole citrus and citrus pulp samples were much less than the MRL for citrus (0.7 mg/kg in China, the strictest standard among countries). Therefore, 30 days is also recommended as the preharvest interval for application of bifenazate in citrus
2.4. Dietary exposure risk assessment
Based on the primary dietary data for citrus fruits, we estimated the risk of dietary exposure to citrus. Owing to a lack of information on dietary consumption of citrus pulp, only the exposure risk in whole citrus was calculated. As defined in official reports, (CitationJMPR (The Joint FAO/WHO Meeting on Pesticide Residues),CitationJMPR (The Joint FAO/WHO Meeting on Pesticide Residues)) cyflumetofen residue is expressed as the sum of cyflumetofen and 2-trifluoromethylbenzoic acid, and bifenazate residue is defined as the sum of bifenazate and bifenazate-diazene. A few studies have reported that the respective application of these two pesticides on strawberry and garlic plant did not pose a threat to public health (Bian et al., Citation2022; Xuan, Citation2014). For the citrus applied with cyflumetofen and bifenazate, as shown in Table (the maximum residue levels of registered crops in different areas are shown in Table S1 and the calculations are shown in Tables S2 and S3), considering the maximal residue levels at various sampling intervals and ADIs for cyflumetofen and bifenazate, the RQ constitutes the most comprehensive index for measuring the risk of exposure to pesticides, taking the whole diet into account. The RQ values for cyflumetofen and bifenazate were 10.65% and 43.86%, respectively, implying an acceptable risk with respect to human health.
Table 5. The acceptable daily intake (ADI, mg/kg bw/day), the residue (mg/kg), supervised trials median residue (STMR) and risk quotient (RQ, %) of Cyflumetofen and Bifenazate in whole citrus at preharvest intervals (PHIs)
3. Conclusion
We applied a quick method to determine cyflumetofen, bifenazate and their metabolites in citrus. The half-life of bifenazate was fitted as 34.04 days. Besides, the ranges of terminal residue of four analytes were as follows: <0.02–0.10 mg/kg for cyflumetofen, <0.02 mg/kg for 2-trifluoromethylbenzoic acid, <0.02–0.11 mg/kg for bifenazate and <0.02–0.029 mg/kg for bifenazate-diazene. These residual levels suggested a preharvest interval of 30 days for application of cyflumetofen and bifenazate to citrus. According to the safety risk assessment using these residue data, RQ values for cyflumetofen and bifenazate were determined as 10.65% and 43.86%, respectively, which means that the risk caused by cyflumetofen and bifenazate in citrus could be acceptable for consumers. Our study provides fundamental information pertaining to residue dynamics for cyflumetofen and bifenazate and evaluates their dietary risk with respect to the safe application of a mixture of these pesticides to citrus.
Compliance with ethical standards
This is an original research article that has neither been published previously nor considered presently for publication elsewhere.
Informed consent
All authors named in the manuscript are entitled to the authorship and have approved the final version of the submitted manuscript. This article does not contain any studies with human or animal subjects.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Raw data were generated at the project of Institute of Plant Protection in Guizhou Academy of Agricultural Sciences. The data that support the findings of this study are available from the corresponding author [Ying Zhang] on request.
Additional information
Funding
References
- Abdel-Ghany, M. F., Hussein, L. A., El Azab, N. F., El-Khatib, A. H., & Linscheid, M. W. (2016, September 15). Simultaneous determination of eight neonicotinoid insecticide residues and two primary metabolites in cucumbers and soil by liquid chromatography–tandem mass spectrometry coupled with QuEChERS. Journal of Chromatography B, 1031, 15–14. https://doi.org/10.1016/j.jchromb.2016.06.020
- Bian, Y., Feng, Y., Zhang, A., Qi, X., Pan, J., Han, J., Ma, X., & Liang, L. (2022, June 15). Residue distribution and risk assessment of bifenazate and its metabolite in garlic plant. Food Chemistry, 379, 132013. https://doi.org/10.1016/j.foodchem.2021.132013
- Coelho, R. C., Hermsdorff, H. H., & Bressan, J. (2013, March). Anti-inflammatory properties of Orange juice: Possible favorable molecular and metabolic effects. Plant Foods for Human Nutrition, 68(1), 1. https://doi.org/10.1007/s11130-013-0343-3
- El-Gammal, H. A., Ibrahim, E. D., & Ahmed, M. M. (2022, May 1). Field efficacy of acaricides on citrus red mite and dissipation study for cyflumetofen and pyridabin in lemon using QuEChERS method and LC-ESI-MS. Egyptian Journal of Chemistry, 65(5), 1–2. https://doi.org/10.21608/ejchem.2021.94629.4448
- Gama, A. B., Baggio, J. S., Rebello, C. S., Lourenço, S. D., Gasparoto, M. C., da Silva Junior, G. J., Peres, N. A., & Amorim, L. (2020, June). Sensitivity of Colletotrichum acutatum isolates from citrus to carbendazim, difenoconazole, tebuconazole, and trifloxystrobin. Plant Disease, 104(6), 1621–1628. https://doi.org/10.1094/PDIS-10-19-2195-RE
- Guo, J., Li, M., Liu, Y., Wang, F., Kong, Z., Sun, Y., Lu, J., Jin, N., Huang, Y., Liu, J., Francis, F., & Fan, B. (2018, May). Residue and dietary risk assessment of chiral cyflumetofen in Apple. Molecules, 23(5), 1060. https://doi.org/10.3390/molecules23051060
- Hiragaki, S., Kobayashi, T., Ochiai, N., Toshima, K., Dekeyser, M. A., Matsuda, K., & Takeda, M. (2012, June). A novel action of highly specific acaricide; bifenazate as a synergist for a GABA-gated chloride channel of Tetranychus urticae [Acari: Tetranychidae]. Neurotoxicology, 33(3), 307–313. https://doi.org/10.1016/j.neuro.2012.01.016
- Ibrahim, E. D., & El-Hefny, D. (2021, November 1). Dissipation of cyflumetofen in strawberry fruits and green bean pods under greenhouse conditions using quechers method and Gc/(Μ-Ecd). Egyptian Journal of Chemistry, 64(11), 5–6. https://doi.org/10.21608/ejchem.2021.77606.3787
- Jamieson, L. E., Charles, J. G., Stevens, P. S., McKenna, C. E., & Bawden, R. (2005, August 1). Natural enemies of citrus red mite (Panonychus citri) in citrus orchards. New Zealand Plant Protection, 58, 299–305. https://doi.org/10.30843/nzpp.2005.58.4267
- JMPR (The Joint FAO/WHO Meeting on Pesticide Residues). JMPR Report Cyflumetofen, No. 273; https://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report2014/5.06_CYFLUMETOFEN__273_.pdf
- JMPR (The Joint FAO/WHO Meeting on Pesticide Residues) (2010). JMPR Report Bifenazate, No. 219; https://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report10/bifenazate.pdf
- Laksmiani, N. P., Larasanty, L. P., Santika, A. A., Prayoga, P. A., Dewi, A. A., & Dewi, N. P. (2020, June). Active compounds activity from the medicinal plants against SARS-CoV-2 using in silico assay. Biomedical and Pharmacology Journal, 13(2), 873–881. https://doi.org/10.13005/bpj/1953
- Li, Y. Y., Fan, X., Zhang, G. H., Liu, Y. Q., Chen, H. Q., Liu, H., & Wang, J. J. (2017, January). Sublethal effects of bifenazate on life history and population parameters of Tetranychus urticae (Acari: Tetranychidae). Systematic and Applied Acarology, 22(1), 148–158. https://doi.org/10.11158/saa.22.1.15
- Li, M., Liu, X., Dong, F., Xu, J., Kong, Z., Li, Y., & Zheng, Y. (2013, July 26). Simultaneous determination of cyflumetofen and its main metabolite residues in samples of plant and animal origin using multi-walled carbon nanotubes in dispersive solid-phase extraction and ultrahigh performance liquid chromatography–tandem mass spectrometry. Journal of Chromatography. A, 1300, 95–103. https://doi.org/10.1016/j.chroma.2013.05.052
- Liu, S., Kou, H., Mu, B., Wang, J., & Zhang, Z. (2019, August 1). Dietary risk evaluation of tetraconazole and bifenazate residues in fresh strawberry from protected field in North China. Regulatory Toxicology and Pharmacology, 106, 1–6. https://doi.org/10.1016/j.yrtph.2019.04.008
- Li, M., Wang, R., Kong, Z., Gao, T., Wang, F., & Fan, B. (2020, December). Cyflumetofen degradation in different aquatic environments and identification of hydrolytic products. Journal of Environmental Chemical Engineering, 8(6), 104512. https://doi.org/10.1016/j.jece.2020.104512
- Ma, J., Huang, Y., Peng, Y., Xu, Z., Wang, Z., Chen, X., Xie, S., Jiang, P., Zhong, K., & Lu, H. (2021, April 1). Bifenazate exposure induces cardiotoxicity in zebrafish embryos. Environmental Pollution, 274, 116539. https://doi.org/10.1016/j.envpol.2021.116539
- Nair S A, SR R Kurup, Nair A S and Baby S . (2018). Citrus peels prevent cancer. Phytomedicine, 50, 231–237. https://doi.org/10.1016/j.phymed.2017.08.011
- Pan, D., Dou, W., Yuan, G. R., Zhou, Q. H., Wang, J. J., & Anderson, T. (2020, April). Monitoring the resistance of the citrus red mite (Acari: Tetranychidae) to four acaricides in different citrus orchards in China. Journal of Economic Entomology, 113(2), 918–923. https://doi.org/10.1093/jee/toz335
- Satheshkumar, A., Senthurpandian, V. K., & Shanmugaselvan, V. A. (2014, February 15). Dissipation kinetics of bifenazate in tea under tropical conditions. Food Chemistry, 145, 1092–1096. https://doi.org/10.1016/j.foodchem.2013.09.042
- Schusterova, D., Hajslova, J., Kocourek, V., & Pulkrabova, J. (2021, February). Pesticide residues and their metabolites in grapes and wines from conventional and organic farming system. Foods, 10(2), 307. https://doi.org/10.3390/foods10020307
- Singh, B., Singh, J. P., Kaur, A., & Singh, N. (2020, June 1). Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Research International, 132, 109114. https://doi.org/10.1016/j.foodres.2020.109114
- Sun, D., Pang, J., Zhou, Z., & Jiao, B. (2016, October 1). Enantioselective environmental behavior and cytotoxicity of chiral acaricide cyflumetofen. Chemosphere, 161, 167–173. https://doi.org/10.1016/j.chemosphere.2016.06.087
- Wang, Z., Pang, J., Liao, C., Zhang, Q., & Sun, D. (2021, April 1). Determination of etoxazole in different parts of citrus fruit and its potential dietary exposure risk assessment. Chemosphere, 268, 128832. https://doi.org/10.1016/j.chemosphere.2020.128832
- Wang, H., Xin, T., Wang, J., Zou, Z., Zhong, L., Xia, B., Pavelka, K., Vencovský, J., Distler, J. H. W., Šenolt, L., Bečvář, R., & Tomčík, M. (2021, October). Sublethal effects of bifenazate on biological traits and enzymatic properties in the Panonychus citri (Acari: Tetranychidae). Scientific Reports, 11(1), 1. https://doi.org/10.1038/s41598-020-79139-8
- Xuan, H. U. (2014, November 25). Residue dynamics and safely applying technology of cyflumetofen in strawberry. Acta Agriculturae Zhejiangensis, 26(6), 1558. https://www.zjnyxb.cn/EN/Y2014/V26/I6/1558
- Xue, W., Wybouw, N., & Van Leeuwen, T. (2021, December). The G126S substitution in mitochondrially encoded cytochrome b does not confer bifenazate resistance in the spider mite Tetranychus urticae. Experimental & Applied Acarology, 85(2), 161–172. https://doi.org/10.1007/s10493-021-00668-6
- Yoshida, T., Ikemi, N., Takeuchi, Y., Ebino, K., Kojima, S., Chiba, Y., Nakashima, N., Kawakatsu, H., Saka, M., & Harada, T. (2012, February). A repeated dose 90-day oral toxicity study of cyflumetofen, a novel acaricide, in rats. The Journal of Toxicological Sciences, 37(1), 91–104. https://doi.org/10.2131/jts.37.91
- Zhang, Y., Zhou, Y., Duan, T., Kaium, A., & Li, X. (2022, March). Dissipation and dietary risk assessment of carbendazim and epoxiconazole in citrus fruits in China. Journal of the Science of Food and Agriculture, 102(4), 1415–1421. https://doi.org/10.1002/jsfa.11474
- Zhao, P., Chai, Y., Liu, R., & Yuan, L. (2021, November). Dissipation, residue, and dietary risk assessment of bifenthrin, bifenazate, and Its metabolite bifenazate–diazene in apples based on deterministic and probabilistic methods. Journal of Agricultural and Food Chemistry, 69(47), 14302–14310. https://doi.org/10.1021/acs.jafc.1c05847
