Abstract
The large production of broilers and fruits in Brazil demands the exploration of new sources of nutrients for the birds and of sustainable disposal of the fruit industrialization wastes. The effects of feeding broiler chickens with residues from acerola industrialization (AM) on meat composition, fatty acids profile, oxidative stability and sensory characteristics were evaluated. Four soybean-corn based diets were tested: negative control, with no antioxidant nor AM; positive control, with butyl hydroxytoluene (BHT) and no AM; and two diets containing either 5 or 7.5% AM and no antioxidant. Official validated methods were used for the chemical and sensorial analyses of the meat. On breast, 7.5% AM increased protein (14.13%) and ash (10.6%) concentrations. On both cuts, AM reduced around 30% the cholesterol levels, decreased saturated fatty acids, increased polyunsaturated fatty acids and reduced n-6:n-3. AM at 5% delayed the onset of lipid peroxidation in frozen stored breasts more than did the antioxidant BHT (36 x 22.5 days). Breasts from treatment containing BHT had the worst sensorial acceptance by consumers. The addition of acerola meal to broiler chickens’ diets revealed a good option to provide healthier meat with longer shelf life, besides representing a way of reducing fruit waste in the environment.
1. Introduction
Favourable climate, water and soil availability, diversity of renewable energy matrices, high grain production and strict sanitary control are some factors that led Brazil to be the largest chicken meat exporter in the world. Moreover, the attention to animal welfare and the use of antimicrobials are firmly regulated in the country by the national public authorities, following the regulatory updates of international organizations (Brazilian Association of Animal Protein, Citation2021). The poultry industry also plays a social role in Brazil, generating employment and income and providing food security, because chicken is the most consumed meat in the country, due to sensory, health and economical aspects (Brazilian Association of Animal Protein, Citation2020).
The commitment of the productive sector with sustainability raises interests on the use of ingredients for animal diets that do not compete with human feeding, as recommended by the Food and Agriculture Organization of the United Nations—FAO (Wadhwa & Bakshi, Citation2013). For instance, the processing of acerola fruit for juice production originates peel, seed and pomace residues that do not fit human nutrition but, instead, could be used for poultry feeding since it contains valuable nutrients such as proteins, fibres and antioxidants like vitamin C, carotenoids and phenolic compounds (Sagar et al., Citation2018; Silva et al., Citation2014).
Some researchers investigated the use of acerola residues, also named acerola meal (AM), in chicken production and found no adverse effects to the birds` health and productive performance when it was used up to 10% (Barros et al. Citation2017a; Barros et al. Citation2017b; Pinto et al., Citation2014; Zanetti et al., Citation2014). Moreover, a lower final cost per broiler was reported when AM replaced dietary corn at 10% (Zanetti et al., Citation2014). Furthermore, the use of these residues in poultry diets might avoid improper discard, thus contributing to environmental sustainability.
Ingredients like corn and soy used in broiler chicken feed determine the content of polyunsaturated fatty acids in the meat, which, despite their nutritional value, makes it highly susceptible to lipid peroxidation, even when stored under refrigeration or freezing (Del Puerto et al., Citation2017; Falowo et al., Citation2014). Several harmful effects derive from this reaction, starting with changes in the sensory properties, loss of nutrients like essential fatty acids and vitamins, and ending with the production of toxic compounds (Domínguez et al., Citation2019).
These events can be minimized on the meat by adding natural or synthetic antioxidants to chickens` feeds because the living animals can absorb and distribute these compounds throughout their tissues (Bou et al., Citation2009). However, some synthetic antioxidants may cause toxic effects to human health and are undesirable by consumers (Karre et al., Citation2013; Loetscher et al., Citation2013). Therefore, the possibility of using natural products with antioxidant activity in the diets of broiler chickens, such as acerola meal, is welcome and may be beneficial to the people and industry.
According to Wallace et al. (Citation2010), the use of plants bioactive compounds with antioxidant properties in poultry feeding may act by i) protecting the feed ingredients from oxidative damage, ii) controlling the oxidative metabolism of the animals, and iii) improving the storage quality of the products. Nevertheless, the effects of these compounds on the organoleptic properties of the final products should be investigated since they influence the food purchasing decisions of consumers. So, considering the high production of chicken and fruit residues in Brazil, the aim of this study was to investigate the effects of the dietary intake of acerola meal on the quality of chicken meat.
2. Materials and methods
2.1. Experimental design, diets, and birds rearing
Two hundred one-day-old Cobb 500 male chicks from a commercial hatchery were randomly assigned to one of four treatment groups with five replicate pens of 10 animals, in a completely randomized design. The dietary treatments were as follows: negative control (NC), with no synthetic antioxidant and no AM; positive control (PC), containing butyl hydroxytoluene (BHT) at 0.01% (except in the finisher feed, as a standard procedure for the elimination of residues in the meat) and no AM; and two diets containing AM at 5% or 7.5% and no synthetic antioxidant (AM 5% and AM 7.5%). AM concentrations adopted in this study were based on previous scientific studies that attested its harmlessness to animal health and performance as well as its economic viability (Barros et al. Citation2017a; Barros et al. Citation2017b; Pinto et al., Citation2014; Zanetti et al., Citation2014).
The basal isoenergetic rations for all phases were formulated to meet the nutritional requirements for broiler chickens, according to Rostagno et al. (Citation2011). The ingredients and chemical composition of the experimental diets are given in Table . AM was donated by a fruit waste processing company and contained 17.5% moisture, 82.5% dry matter, 12.69% crude protein, 1.41% lipids, 24.9% ash and 43,5% crude fibre, determined according to the methods described by AOAC (Citation2006). AM also contained vitamin C (100 mg/100 g), carotenoids (0.28 mg/100 g), and phenolic compounds (776.5 mg/100 g). Vitamin C was determined by the titrimetric assay of Tillman (IAL, Citation2008) and expressed as mg ascorbic acid per 100 g of AM; the concentration of carotenoids was calculated from the absorbance values (at 475 nm) of acetone extracts, using 2500 as the coefficient of extinction (Grassi et al., Citation2018); and the phenolic content was calculated according to the colorimetric method of Folin-Ciocalteu and expressed as mg gallic acid equivalent per 100 g of AM (Tlili et al., Citation2011).
Table 1. Composition and calculated values of experimental diets for broiler chickens
The experiment was conducted in an acclimatized shed using an adiabatic evaporative cooling system with negative pressure ventilation. The rearing pens (0.6 m high x 1.5 m wide x 3.0 m long) were bedded with wood-shavings and had feeders and nipple drinkers inside to provide ad libitum feed intake and free access to water. Heating (400 W) was intermittent during the first 14 days and lighting was constant during the whole experimental period. At day 42, three animals from each pen (60 birds) were randomly selected, kept on eight hours fasting, slaughtered by cutting their jugular veins, defeathered and eviscerated in a school slaughterhouse. All procedures used in this study were approved by the Unesp Ethics Committee on the Use of Animals (Protocol n. 1058–2015).
2.2. Samples preparation and chemical analyses
After removing skin, bones and connective tissue from the 60 carcasses, breasts and thighs were excised and ground separately in a meat grinder. Fifty grams portions of fresh ground breast (60 samples) were packaged in polyethylene bags, covered with aluminium foil and frozen at—20 °C for the determination of the thiobarbituric acid reactive substances (TBARS; expressed as mg malondialdehyde per kg of meat; Tarladgis et al., Citation1960) at days 0, 15, 30, 60 and 90. In this case, a completely randomized design was used in a 4 × 5 factorial scheme with four treatments (four diets) and five storage periods (0, 15, 30, 60, and 90 days).
The remaining portions of ground breast and thigh were frozen at—40 °C and lyophilized (Liobras L101) for the analyses of proximate composition (AOAC, Citation2006), cholesterol (Bohac et al., Citation1998) and lipid profile. For the latter, the fatty acids (FA) were extracted with hexane + isopropanol, methylated with NaOMe + methanol and separated with ethyl ether + oxalic acid (Christie, Citation1982; Hara & Radin, Citation1970). Hydrogen was used as the carrier gas in the chromatograph (Varian CP-3900 GC) and the FA peaks were identified by matching their retention times with relative standards of the calibration curve Supelco. The FA were quantified by the normalization of the area of the methyl esters and the results were expressed as percentages.
2.3. Sensory analysis
The sensory analysis was performed in a single moment with 60 adult untrained panellists (30 men and 30 women) randomly selected from the gym community, who declared to be regular consumers of chicken and issued their consent to participate in the study. The procedures used for the sensory evaluation were approved by Brazil Platform Ethics Committee on Research (Opinion n. 2.116.615; 13 June 2017).
Unseasoned breast meat samples of each treatment were wrapped in aluminium foil and baked at 180 °C until the inner temperature reached 72 °C. Next, the baked breasts were unwrapped, cut into approximately 1 cm3, and randomly offered to each taster in disposable plates coded with three random digits. Water and crackers were offered as palate cleansers between samples. The acceptability of the sensory attributes colour, flavour, texture and the overall appearance of the samples were measured with 5-point structured scales (1 = disliked very much; 5 = liked very much).
2.4. Statistical analyses
The results of the analyses were tested for normality of error and homogeneity of variances. Data on proximate composition, cholesterol content and fatty acids profile were submitted to analysis of variance and the means were compared by Tukey’s test. Sensorial data were submitted to Friedman’s test and the multiple comparisons between means were performed by Dunn’s test. SAS 9.2 statistical package was used for the analyses (SAS, Citation2008) and the threshold adopted for statistical significance was p < 0.05. The lipid peroxidation during storage was described with regression equations and the derivatives were calculated as f`(x) = 2ax-b.
3. Results and discussion
3.1. Proximate composition, cholesterol content, and fatty acids profile
The proximate composition of breast and thigh meat of chicken fed AM agreed with the Brazilian food composition databases (NEPA, Citation2011; USP & FoRC, Citation2017), which means that the AM used in the diets did not negatively modify the nutritional quality of the meat. In contrast, the use of 7.5% AM in the diet increased breast protein (14.13%) and ash (10.6%) when compared to PC and NC, respectively (Table ). Breasts and thighs had less than 5% fat and were classified as low-fat food (NEPA, Citation2011). For consumers aiming at the prevention of chronic diseases such as obesity and dyslipidaemia caused by excessive fat intake, this kind of food represents a healthy food choice (Lottenberg, Citation2009).
Table 2. Proximate composition and cholesterol content (wet basis) of skinned breast and thigh meat from broiler chickens fed the experimental diets
Dietary AM at 5 or 7.5% decreased around 30% of the cholesterol content on both cuts (Table ), with values below the reported in reference tables for chicken meat (NEPA, Citation2011; Philippi, Citation2017; USDA, Citation2002). According to Abbasi et al. (Citation2015), some components of the diet such as vitamin C, fibres and phenolic compounds may influence the cholesterol content in chicken meat, which seemed to be the case of AM. In contact with water, the soluble fraction of the dietary fibres turns into a gel, reducing the transit time in the gut and the action of enzymes, thus making it difficult for the bolus to meet the intestinal cells. This process reduces the absorption of lipid molecules from the bolus and, consequently, provides lower fat and cholesterol deposition in the carcass (Mateos et al., Citation2012; Ramos et al., Citation2006).
The action of phenolic compounds in decreasing meat cholesterol is associated with the presence of tannins, which bind to bile salts reducing its absorption and increasing its faecal excretion (Ramos et al., Citation2006). Another explanation for the meat cholesterol decrease is the biological effect of the phenolic compounds on the inactivation of digestive enzymes (trypsin, amylase and lipase) and of hydroxy-3-methyl-glutaryl-CoA reductase, an essential enzyme for cholesterol biosynthesis via the mevalonate pathway (Lien et al., Citation2008).
Finally, the presence of vitamin C in adequate amounts in the adrenal cortex can regulate the synthesis of circulating glucocorticoids and the deleterious responses associated with stress, delaying the depletion of endogenous cholesterol, and allowing it to act as a precursor of steroid hormones (cortisol and aldosterone), thus reducing its biosynthesis and consequent tissue deposition (McDowell, Citation2000).
More than decreasing cholesterol concentration in the meat, the use of AM in the chickens’ diets decreased saturated fatty acids (SFA) and increased polyunsaturated fatty acids (PUFA) on both breast and thigh, so improving the PUFA/SFA ratio (Tables ). As SFA are known as atherogenic and are related to increased risk of cardiovascular disease, it is recommended to replace them with PUFA in human diets aiming at the maintenance of good health (FAO, Citation2008).
Table 3. Fatty acids profile of breast meat from broiler chickens fed the experimental diets
Table 4. Fatty acids profile of thigh meat from broiler chickens fed the experimental diets
Another important finding of the study was the reduction of the n-6:n-3 ratio in breast and thigh due to the dietary AM. This effect may be partly due to the increases in docosahexaenoic acid (DHA, C22:6 n-3) in breast (AM 7.5%) and thigh (AM 5 and 7.5%) and in eicosapentaenoic acid (EPA, C20:5 n-3) in thigh (AM 7.5%). EPA and DHA are essential for the brain functioning and play a role in the prevention of coronary heart disease and other age-related degenerative diseases. These fatty acids bring favourable properties to human health, such as immunomodulatory (reducing inflammatory markers), anti-inflammatory (improving the endothelial function), hypolipidemic (reducing triglyceridemia), and antithrombotic (reducing platelet aggregation; FAO, Citation2008).
According to FAO (Citation2008), the appropriate n-6:n-3 PUFA ratio in human food should range from 5:1 to 10:1. However, in typical Western diets, this ratio ranges from 10:1 to 25:1, mainly due to the modern breeding systems, in which the use of n-6 oils (like soybean oil) as energy source in animal diets reduces the n-3 levels in several final foods such as poultry, eggs and fish (Shin et al., Citation2012). Results obtained in this study confirm the ability of AM to improve the lipid pattern of chicken meat, which represents an attractive factor for consumers looking for healthier food.
3.2. Oxidative stability
Lipid peroxidation increased as a quadratic function of the storage time in all treatments (Figure ) and the derivatives of the equations showed that the appearance of TBARS in breast meat was delayed when AM was added to the broiler diets: 36 (AM 5%) and 33.5 (AM 7.5%) days x 19 (NC) and 22.5 (PC) days of storage. These results sound extraordinary as they resolve the concern about lipid peroxidation on the increased PUFA in the AM fed chicken.
Figure 1. Linear regressions of thiobarbituric acid reactive substances (TBARS) in breast meat of experimental groups NC (negative control), PC (positive control), AM (acerola meal) 5% and AM 7.5%, line equations and coefficients of determination (R2).
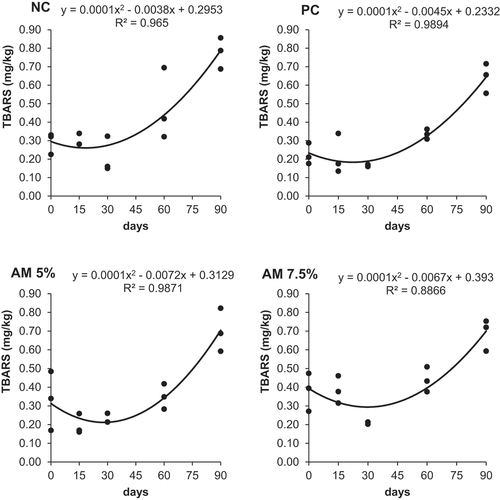
Moreover, the results indicated that both concentrations of AM were able to delay the onset of lipid oxidation more than did the BHT used in PC. This finding was ascribed to the antioxidant properties of the phytochemicals detected in the acerola meal used in the chickens` diets (carotenoids, phenolic compounds, and vitamin C) and is particularly important because it evidences the possibility of using a natural antioxidant in broiler chickens’ diets, meeting the demand of consumers who look for organic or clean label foods, with no or minimum synthetic additives.
3.3. Sensory analysis
No differences among the treatments were detected for the breasts colour (Table ). As colour is the first sensory attribute perceived by consumers at purchase time, this would not be a reason for rejection, which facilitates the insertion of AM fed chicken in the market. The acceptance for texture, flavour and global appearance was equivalent among NC, AM 5% and AM 7.5% and the differences found for these attributes between PC and AM 7.5% were probably influenced by the presence of BHT in the PC diets.
Table 5. Sensory analysis of breast meat from broiler chickens fed the experimental diets
Joining all these results, we can say that the main quality indices of chicken meat—organoleptic properties, storage stability and nutritive value—were either maintained or improved with the dietary inclusion of acerola meal in the chickens` diets.
4. Conclusion
The use of acerola meal in broiler chickens’ diets did not harm the chemical composition of breast and thigh meats and improved the lipid profile by lowering cholesterol and saturated fatty acids, increasing polyunsaturated fatty acids and reducing n-6:n-3 ratio. Dietary acerola meal delayed the onset of lipid peroxidation on breast meat, more than did the synthetic antioxidant, and did not harm the sensorial properties. In addition, the use of acerola meal in broiler chickens’ diets offers a way to reduce industrialization wastes. So, the meat produced represents an option for consumers concerned about health and environment preservation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data are available upon request from the corresponding author
Additional information
Funding
References
- Abbasi, H., Seidavi, A., Liu, W., & Asadpour, L. (2015). Investigation on the effect of different levels of dried sweet Orange (Citrus sinensis) pulp on performance, carcass characteristics and physiological and biochemical parameters in broiler chicken. Saudi Journal of Biological Sciences, 22(2), 139–12. http://dx.doi.org/10.1016/j.sjbs.2014.09.006
- AOAC. (2006). Official methods of analysis of AOAC International. (18th.). MD. Association of Official Analytical Chemists.
- Barros, T. L., Oliveira, J., Cassiano, R. P., Lima, B. D., Assis, J. V., Nascimento, B. N. T., Garcia-Neto, M., & Ponsano, E. H. G. (2017a April 25–27a). In: Subproduto da acerola na dieta de frangos de corte. Poster presentation. XVI Avesui, Florianópolis:.
- Barros, T. L., Oliveira, J., Cassiano, R. P., Nascimento, B. N. T., Bosco, A. M., Ciarlini, P. C., Garcia-Neto, M., & Ponsano, E. H. G. (2017b April 25–27b). In: Parâmetros bioquímicos de frangos alimentados com subproduto de acerola. Poster presentation. XVI Avesui, Florianópolis:.
- Bohac, C. E., Rhee, K. S., Cross, H. R., & Ono, K. (1998). Assessment of methodologies for calorimetric cholesterol assay of meats. Food Science, 53(6), 1642–1644. https://doi.org/10.1111/j.1365-2621.1988.tb07804.x
- Bou, R., Codony, R., Tres, A., Decker, E. A., & Guardiola, F. (2009). Dietary strategies to improve nutritional value, oxidative stability and sensory properties of poultry products. Critical Reviews in Food Science and Nutrition, 49(9), 800–822. https://doi.org/10.1080/10408390902911108
- Brazilian Association of Animal Protein. (2020). Annual Report 2020. https://abpa-br.org/wp-content/uploads/2020/05/abpa_relatorio_anual_2020_portugues_web.pdf
- Brazilian Association of Animal Protein. (2021). Annual Report 2021. https://abpa-br.org/wp-content/uploads/2021/04/ABPA_Relatorio_Anual_2021_web.pdf
- Christie, W. W. (1982). A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. Journal of Lipid Research, 23(7), 1072–1075. https://doi.org/10.1016/S0022-2275(20)38081-0
- Del Puerto, M., Cabrera, M. C., & Saadoun, A. (2017). A note on fatty acids profile of meat from broiler chickens supplemented with inorganic or organic selenium. International Journal of Food Science, 2017 1–8. Article ID 7613069. https://doi.org/10.1155/2017/7613069
- Domínguez, R., Pateiro, M., Gagaoua, M., Barba, F. J., Zhang, W., & Lorenzo, J. M. (2019). A comprehensive review on lipid oxidation in meat and meat products. Antioxidants, 8(10), 429. https://doi.org/10.3390/antiox8100429
- Falowo, A. B., Fayemi, P. O., & Muchenje, V. (2014). Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Research International, 64, 171–181. https://doi.org/10.1016/j.foodres.2014.06.022
- FAO. (2008). Interim summary of conclusions and dietary recommendations on total fat & fatty acids. Food and Agriculture Organization. http://www.uece.br/wp-content/uploads/sites/82/2021/07/FAO-WHO-Fats-and-Fatty-Acids-in-Human-Nutrition.pdf
- Grassi, T. L. M., Oliveira, D. L., Paiva, N. M., Diniz, J. C. P., Bosco, A. M., Pereira, A. A. F., Menezes, A. R. P., Valadares, T. C., Pastor, R. C. P., Ciarlini, P. C., Gonçalves, G. G., Villarroel, M., & Ponsano, E. H. G. (2018). Microbial biomass as an antioxidant for tilapia feed. Aquaculture Research, 49(8), 2881–2890. https://doi.org/10.1111/are.13753
- Hara, A., & Radin, N. S. (1970). Lipid extraction of tissues with low-toxicity solvent. Analytical Biochemistry, 90(1), 420–426. https://doi.org/10.1016/0003-2697(78)90046-5
- IAL. (2008). Métodos físico-químicos para análise de alimentos. (4th ed., 1st ed. digital). Instituto Adolfo Lutz.
- Karre, L., Lopez, K., & Getty, K. J. K. (2013). Natural antioxidants in meat and poultry products. Meat Science, 94(2), 220–227. https://doi.org/10.1016/j.meatsci.2013.01.007
- Lien, T. F., Yeh, H. S., & Su, W. T. (2008). Effect of adding extracted hesperetin, naringenin and pectin on egg cholesterol, serum traits and antioxidant activity in laying hens. Archives of Animal Nutrition, 62(1), 33–43. https://doi.org/10.1080/17450390701780318
- Loetscher, Y., Kreuzer, M., & Messikommer, R. E. (2013). Oxidative stability of the meat of broilers supplemented with rosemary leaves, rosehip fruits, chokeberry pomace, e entire nettle, e effects on performance and meat quality. Poultry Science, 92(2), 2938–2948. https://doi.org/10.3382/ps.2013-03258
- Lottenberg, A. M. (2009). Importance of dietary fat in the prevention and control of metabolic disorders and cardiovascular disease. Arquivo Brasileiro de Endocrinologia E Metabolismo, 53(5), 595–607. https://doi.org/10.1590/s0004-27302009000500012
- Mateos, G. G., Jiménez-Moreno, E., Serrano, M. P., & Lázaro, R. (2012). Poultry response to high levels of dietary fiber sources varying in physical and chemical characteristics. Journal of Applied Poultry Research, 21(1), 156–174. https://doi.org/10.3382/japr.2011-00477
- McDowell, L. R. (2000). Vitamins in animal and human nutrition: Comparative aspects to human nutrition (2nd) ed.). ISU.
- NEPA. (2011). TACO Brazilian table of food composition (4th) Center for studies and research in food (pp. 161).
- Philippi, S. T. (2017). Table of food composition – Support for nutritional decision (6th) ed.). Manole.
- Pinto, M. F., Moreira, A. J. C., Bossolani, I. L. C., Ponsano, E. H. G., Garcia-Neto, M., & Perri, S. H. V. (2014). Production of slow growing broiler chicken using by-product from fruit juice processing. Archivos Latinoamericanos de Producción Animal, 22(5), 32–35.
- Ramos, L. S. N., Lopes, J. B., Figueiredo, A. V., Freitas, A. C., Farias, L. A., Santos, L. S., & Silva, H. O. (2006). Dietary dehydrated cashew pulp for finishing broilers: Performance and characteristics carcass. Revista Brasileira de Zootecnia, 35(3), 804–810. https://doi.org/10.1590/S1516-35982006000300024
- Rostagno, H. S., Albino, L. F. T., Donzele, J. L., Gomes, P. C., Oliveira, R. F., Lopes, D. C., Ferreira, A. S., Barreto, S. L. T., & Euclides, R. F. (2011). Brazilian tables for poultry and swine: Food composition and nutritional requirements (3rd) ed.). UFV.
- Sagar, N. A., Pareek, S., Sharma, S., Yahia, E. M., & Lobo, M. G. (2018). Fruit and vegetable waste: Bioactive compounds, their extraction and possible utilization. Comprehensive Reviews in Food Science and Food Safety, 17(3), 512–531. https://doi.org/10.1111/1541-4337.12330
- SAS. (2008). SAS/STAT software for PC. Version 9.1.
- Shin, D., Choi, S. H., Go, G., Park, J. G., Narciso-Gayatán, C., Morgan, C. A., Smith, S. B., Sánchez-Plata, M. X., & Ruiz-Feria, C. A. (2012). Effects of dietary combination of n-3 and n-6 fatty acids on the deposition of linoleic and arachidonic acid in broiler chicken meats. Poultry Science, 91(4), 1009–1017. https://doi.org/10.3382/ps.2011-01836
- Silva, L. M. R., Figueiredo, E. A. T., Ricardo, N. M. P. S., Vieira, I. G. P., Figueiredo, R. W., Brasil, I. M., & Gomes, C. L. (2014). Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chemistry, 143, 398–404. https://doi.org/10.1016/j.foodchem.2013.08.001
- Tarladgis, B. G., Watts, B. M., Younathan, M. T., & Dugan, L. R., Jr. (1960). A distillation method for the quantitative determination of malonaldehyde in rancid foods. Journal of the American Oil Chemists’ Society, 37(1), 44–48. https://doi.org/10.1007/BF02630824
- Tlili, I., Hdider, C., Lenucci, M. S., Riadh, I., Jebari, H., & Dalessandro, G. (2011). Bioactive compounds and antioxidant activities of different watermelon (Citrullus lanatus (Thunb.) Mansfeld) cultivars as affected by fruit sampling area. Journal of Food Composition and Analysis, 24(3), 307–314. https://doi.org/10.1016/j.jfca.2010.06.005
- USDA. (2002). Composition of foods - raw, processed, prepared. USDA Nutrient database for standard reference (pp. 40). United States Department of Agriculture.
- USP, & FoRC. (2017). TBCA Brazilian table of food composition. University of São Paulo. Food Research Center. Version 6.0. USP/FoRC
- Wadhwa, M., & Bakshi, M. P. S. (2013). Utilization of fruit and vegetable wastes as livestock feed and as substrates for generation of other value-added products. FAO/WHO. https://www.fao.org/3/i3273e/i3273e.pdf
- Wallace, R. J., Oleszek, W., Franz, C., Hahn, I., Baser, K. H. C., Mathe, A., & Teichmann, K. (2010). Dietary plant bioactives for poultry health and productivity. British Poultry Science, 51(4), 461–487. https://doi.org/10.1080/00071668.2010.506908
- Zanetti, L. H., Polycarpo, G. V., Brich, A. L. C., Barbieri, A., Oliveira, R. F., Sabbag, O. J., Cooke, R. F., & Cruz-Polycarpo, V. C. (2014). Performance and economic analysis of broilers fed diets containing acerola meal in replacement of corn. Brazilian Journal of Veterinary Research and Animal Science, 51(3), 224–232. https://doi.org/10.11606/issn.1678-4456.v51i3p224-232
