Abstract
Kenaf (Hibiscus cannabinus L.) is an annual herbaceous dicotyledonous plant native to Asia and Africa belonging to the Malvaceae family. Due to its many applications, it is a valuable multifunctional crop. This is an essential food, fibre and medicinal plant that can tolerate a variety of environmental conditions. This plant, along with other food alternatives, has potential to be used in feed resource-based inventories. It is an alternative crop that can be used as a protein source in both human and animal feed production. Kenaf contains a great number of bioactive compounds which can be of great importance in human and animal health. Furthermore, kenaf meal can replace soyabean meal and fish meal as protein supplements. Kenaf is greatly adaptable and available therefore, its fast growth and nutritional qualities give it a great potential to solve food insecurity in future. This paper gives an overview of kenaf’s future potential in the food and feed industries.
1. Introduction
Mitigating the combined impact of human population growth and global climate shift patterns has become a primary concern for agricultural industries. The emphasis on global sustainable development goals has shifted towards addressing hunger and malnutrition (S. Lin et al., Citation2002; Okello et al., Citation2017) because climate change already impacts negatively on food and feed production (Masipa, Citation2017), leading to food insecurity and declines in human health (Intergovernmental Panel on Climate Change (IPCC), Citation2007; Liliana, Citation2005; Vogel, Citation2005; World Bank, Citation2016). The negative climate impact is rising food and feed cost, which has resulted in the contraction of the global economy (FAO, IFAD, UNICEF, WFP and WHO, Citation2021), which is exacerbated by the recent Russian invasion of Ukraine that destroyed agricultural infrastructure and disrupted commodity exports. The sudden loss in commodity exports due to disruption and imposed sanctions have resulted in a global economic shock (Organisation for Economic Co-operation and development (OECD), Citation2022), which further underscores the importance of investigating underutilised crops.
Notably, kenaf is one of the fodder plants that have potential in the feed resource-based inventory (Oh et al., Citation2018). This plant stands as a potential protein source in kenaf-based diets for optimal animal productivity and in overcoming malnutrition among humans (W.Y. Cheng et al., Citation2016). Along with other food alternatives, this plant is regarded as a vegetable for human consumption. It is mostly preferred due to its availability of nutritional and phytochemical compounds found in the seeds and the leaves (Obouayeba et al., Citation2015; Ryu et al., Citation2017). Moreover, it has been reported that kenaf has various bioactive compounds, such as phenolics, saponins, tocopherols, phytosterols, crude proteins, fatty acids, alkaloids and phospholipids (Hossain et al., Citation2011). Also, it contains high amounts of vitamin C, phosphorus, iron, calcium and nitrogen (Chan & Ismail, Citation2009). With the availability of bioactive compounds, kenaf has various medicinal properties which are of great significance to human and animal health, such as anticancer, antioxidants, analgesic, anti-inflammatory, aphrodisiacs and hepatoprotective activities (Kubmarawa et al., Citation2009; Monti & Alexopoulou, Citation2013).
Kenaf can be used as a potential ingredient in food products, such as appetizers, food fortificants, antioxidant factors, aphrodisiacs, nutraceuticals and other technological applications (Alexopoulou et al., Citation2013; Khare, Citation2007; Kubmarawa et al., Citation2009). Therefore, constant production of high nutritious crops such as kenaf, which can survive in varying climatic conditions, should be maintained globally (Saba et al., Citation2015). However, kenaf was often overlooked as a food and feed source for animal and human consumption. Thus, the purpose of this review is to (1) summarise the availability and adaptability of the kenaf fodder plant; (2) outline the utilisation of kenaf leaves and seeds as potential source of food and feed; (3) describe in detail the available bioactive compounds in each kenaf tissues and their importance (seeds, leaves, stalks, flowers, and stem bark); and (4) discuss the nutritional and health benefits of kenaf plants both in humans and animals.
2. Kenaf morphology and adaptability description
Kenaf is an annual herbaceous dicotyledonous plant that belongs to the Malvaceae family and is widely distributed in Asia and Africa. It grows primarily in temperate to tropical regions (Zhao et al., Citation2014). The Malvaceae family is well known for its economic and horticultural significance (Akinrotimi & Okocha, Citation2018). Kenaf belongs to the genus Hibiscus, which is widespread and comprises more than 200 species that are annual or perennial (Hassan et al., Citation2018). This plant is a non-wood fibre crop native to tropical Africa and the East Indies, and it is cultivated in Southern China, Java, West Africa, Central America and other tropical countries. It is therefore related to cotton (Gossypium hirsutum L.), jute (Corchorus Spp.), and okra (Abelmoschus esculentus L.; Webber et al., Citation2002). Kenaf is an important crop as a new annually renewable source of industrial fibre in many developing countries, including China, India, Thailand and Malaysia (Alexopoulou et al., Citation2013). It has wide applications in the manufacturing industry (Danalatos & Archontoulis, Citation2010) and that consequently prompted its adoption under various geographical and climatic conditions throughout the world. The nutrient content available for animals, particularly crude protein content, is known to vary depending on the region of inhabitation, climate, harvest time and the portion of the plant used (Chantiratikul et al., Citation2009). Moreover, the growth, utilisation and potentiality of kenaf in some of the countries such as South Africa are less often discussed. Therefore, there is a need to perform experiments on this plant as to uproot its use and potential, especially to the feed and food production industries to take advantage of its availability and nutritional quality. Because of this current climate change, some annual fodder plants tend not to survive the conditions.
Kenaf is more adaptable to climatic conditions and soils than other commercially grown fibre crops (LeMahieu et al., Citation2003). Under favourable conditions, kenaf can grow to a height of 5 m in 6 to 8 months and produce up to 30 t/ha of dry stem material (Coetzee et al., Citation2008; Wood, Citation2003). It is ecologically adaptable, growing in tropical and temperate climates and flourishing in areas with abundant solar radiation and high rainfall (Liu, Citation2005; Liu & Labuschagne, Citation2009). The high temperature and moisture are conducive to growth; kenaf can respond to soil moisture variations due to its prolific root system, which includes a long taproot and wide-ranging lateral roots (Lauriault & Puppala, Citation2009). As a result, kenaf tolerates drought and salinity to varying degrees (Banuelos et al., Citation2002). Despite being adapted to temperate climates for production, it cannot withstand frost. As a result, to maximise yield, the temperature must remain above 10 °C throughout the growing season, because it affects biomass and fibre yield as well as seed production (Jin et al., Citation2012). Kenaf typically remains vegetative until the daylight hour falls below 12.5 hours, at which point flowering occurs and aside from temperature, photoperiod influences floral induction (Paul & Smith, Citation2003). The origin and composition of the soil have no effect on kenaf culture, which grows in a variety of soil types. In fact, kenaf is better adapted to poor soils than most commercial crops and can be planted on marginal land; however, the main requirement is that soils have good drainage yet with the absence of nematodes (Liu, Citation2005). As a result, nematodes such as Meloidogyne spp. has been identified as a major problem affecting kenaf production, causing stunted growth and seedling death (Agbaje et al., Citation2008). Many scientific studies focused on the production of kenaf as a medicinal plant and its production of seed oils; as a result, information on kenaf being a source of human food and animal feed is limited.
3. The properties and uses of kenaf as food and feed
Kenaf has previously been reported to be of great importance globally as a fibre crop where it has been used to produce paper, cordage, rope, burlap cloth and fish nets (Akinrotimi & Okocha, Citation2018; Cook, Citation1960). This plant presents multiple options in terms of its potential nutritional contribution towards animal production and human nutrition. All above ground plant organs, including leaves, stems, flowers, and seed have potential to contribute significantly towards food and feed production. Kenaf leaves and stems, when harvested while still tender during early growth stages, are suitable for fodder with nutritional properties comparable with lucerne (Kim et al., Citation2018; Webber et al., Citation2002). As a result, kenaf seed components can be used to create functional food products, livestock feed and medicinal products (Ibrahim et al., Citation2019). In addition, the seed has numerous potential applications in both domestic and industrial settings. As a result, this seed is further produced as a by-product of the kenaf industry and is used to produce an alternative and economic source of edible oil, (Chewet al., Citation2018), which is then processed into cosmetics, high-quality cooking oil, and margarine due to its detectable levels of monounsaturated and polyunsaturated fatty acids, specifically oleic acid and linoleic acid which offers a good source of essential fatty acids (Ayadi et al., Citation2017: Chew et al., Citation2016). Moreover, refined kenaf seed oil can be used to make gum Arabic and coffee creamer utilizing the spray drying technology method for oil encapsulation (Chew et al., Citation2018). In addition, oil microencapsulation could also be used to lessen the undesirable smells, volatility, and reactivity of the oil, boosting its stability under difficult conditions and extending its shelf life (Chew & Nyam, Citation2016). As a result, the overall products are subsequently used for human consumption (Chew et al., Citation2020). This oil has unique compositions and can be considered for human consumption (Chan & Ismail, Citation2009; Mariod et al., Citation2010; Cheng et al., Citation2016). Furthermore, due to the seed’s high crude protein levels, it could be utilised in replacing meat and solves the problems for consumers purchasing high-priced meat and its by-products for nutritional requirements. As result, this will act as a solution for malnutrition problems in most developing countries such as South Africa (Chu et al., Citation2021). Kenaf seed could be used in production of flour, which when mixed with other plant-based flours, meat-like products such as texturised soy protein and textured mung bean proteins are discovered (Brishti et al., Citation2017; Lin et al., Citation2002). Moreover, this textured protein may be flavoured or unflavoured, then processed into crumbles, flakes, chunks or slices and then consumed (Qammar et al., Citation2010). Raw kenaf seeds are not edible and therefore they were previously discarded unless they were used for animal feed and is easily ensilaged (Odetola & Eruvbetine, Citation2012). These leaves can further be used as a great forage for animals especially with its reasonable crude protein content that ranges from (21.4 and 35%), which places it on par with legume crops and has a potential as livestock feed (Zhang, Citation2003; Ibrahim et al. Citation2019). This percentage increases with each subsequent step in processing, which increases the potential for using (even first stage processed) defatted kenaf seed meal (DKSM) for fortification of existing grain-based products. Kenaf seed protein concentrate (KSPC) which is a further processed (third stage) product has a protein content above 70% along with excellent water retention properties (Ibrahim et al., Citation2019). This renders KSPC perfect as a functional food ingredient to a wide range of products. Additionally, kenaf leaves can be dried off and crushed into a fine powder, then added into several types of food (Alexopoulou et al., Citation2015). Kenaf seed oil (23.7%) is safe for human consumption due to its unique fatty acid composition and antioxidant activity (Ayadi et al., Citation2017). The digestibility of dry matter and crude protein in kenaf feeds ranges from 53% to 58% and 59% to 71%, respectively (Kipriotis et al., Citation2015). Kenaf meal has outperformed an alfalfa meal-containing ration when used as a supplement in a rice ration for sheep production. This can therefore give kenaf a great chance to be utilised a potential source of protein for livestock feeding (Suriyajantratong et al., Citation1973). In addition, chopped kenaf with 29% dry matter, 15.5% crude protein and 25% acid detergent fibre can be a great source of feed in livestock production where Spanish goats fed on it and resulted in great performance (III et al., Citation2016). Also, broiler chickens were fed processed seed meal and they performed very well with improved growth performance. The ones fed raw kenaf seed-based (10% inclusion) meal had higher weight gain and live weight, implying that kenaf seed is a good source of protein and can be included in broiler diets without negatively affecting performance or carcass quality (Kwari, Citation2011). However, there is limited information on the utilisation of kenaf as food and feed and its properties. Therefore, there is a need for further research to be conducted on the kenaf’s properties and its uses as feed source for humans and animals. below presents uses of kenaf plant as a potential food and feed.
Table 1. Uses of kenaf plant as a potential food and feed
4. Nutritional and chemical properties of kenaf
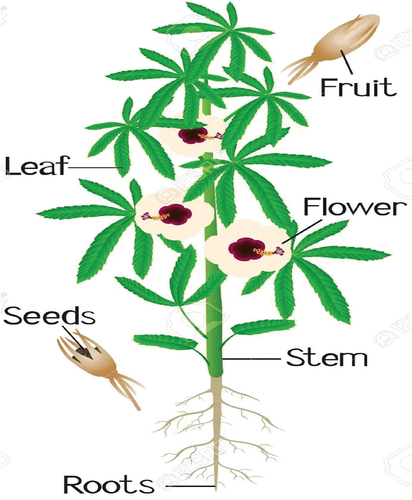
The kenaf plant has been of great importance previously in paper production, whereas looking at its great number of industrial applications, this plant has been characterised as a multipurpose crop (Falasca et al., Citation2014). It has further been described as important fibre crop where its fibrous stem has various industrial applications (paper and pulp, fabrics, textiles, bio-composites, animal bedding etc.; (Ayadi et al., Citation2017) Moreover, in recent times, kenaf has been described as one of the medicinal crops due to its bioactive compounds (Cheng, Citation2001). According to Durgo et al. (Citation2012), this plant contains significant amounts of polyphenols and flavonoids, natural antioxidants that are thought to lower the risk of developing cancer. In addition, phenolic compounds, anti-tumor chemicals, and phytosterols have been pharmacologically studied and have been found to have antioxidant, cardio-protective, anti-inflammatory, anti-hypertensive, and anti-proliferative properties in kenaf, according to Maganha et al. (Citation2010). Kenaf is also a possible source of food and feed for both animals and people due to its high crude protein content (between 16 and 23%; Swingle et al., Citation1978). Therefore, it can further be planted for its fibre and oil production (Alexopoulou & Monti, Citation2013). Kenaf constitutes various parts (), such as seeds, leaves, stalks, flowers and stem bark, which are of great importance especially in fibre, food and feed production for humans, as well as for animal health and consumption in meeting their nutritional requirements.
4.1. Seeds
Seed (Figure ) produced from the kenaf plant are about 6 mm long and 4 mm in width (Salih, Citation2016) which are contained in a fruit which is about 1.9 to 2.5 cm long and 1.3 to 1.9 cm in diameter (Xu et al., Citation2020). This fruit is hairy and constitutes five segments which carries about 20 to 26 seeds that are brown, glabrous and wedge-shaped (Webber & Bledsoe, Citation2002). Furthermore, kenaf seeds produce oil (16–22%) which is considered of economic importance (Patanè & Sortino, Citation2010). Coetzee (Citation2004) further mentioned this seed has a 98% germination rate. However, the viability of the seeds will decrease with time while stored at high humidity and temperatures; as such, it can happen to any crop-producing seeds that contain high oil percentages (Webber et al., Citation2002). This edible oil can favourably compare to that of cotton seed oil, and thus has the capabilities in the production of margarine and protein stock feed (Berger, Citation1969). Kenaf seeds contain the highest amount of protein as reported by Rajashekher et al. (Citation1993), where it constitutes about 30.88% crude protein, 95.15% organic matter and ether extract content of 18.55% on dry matter basis. Furthermore, milk produced from kenaf seeds contains high amounts of various fatty acids, such as hexadecanoic acid (palmitic acid, 33.21%), 9-octadecenoic acid (oleic acid, 31,26%), stearic acid (50,02%) and 9,12-octadecadienoic acid (linoleic acid, 30,51%; Coetzee et al., Citation2008; Karim et al., Citation2020). Moreover, kenaf seeds further contain different bioactive compounds, namely phenolic acids, phytosterols and tocopherols with many industrial applications (Coetzee et al., Citation2008). However, with these high amounts of nutrients and bioactive compounds, there is limited information on the utilisation of kenaf seeds as a potential source of protein in livestock production. Thus, this necessitates the need to explore more on the inclusion of kenaf seeds in animal feed to improve intake and digestibility.
4.2. Leaves
Kenaf plants are known to produce two types of leaves, divided (Figure ) which is a dominant characteristic, and entire () which is recessive characteristic and thus grows alternatively on the stalk and branches. These leaves’ shapes are affected by age of the plant and various environmental factors (Jones et al., Citation1955; Stricker et al., Citation2001; Webber & Bledsoe, Citation2002). The divided shape leaf looks exactly like that of marijuana (Cannabis sativa) where the entire leaf looks more like that of okra and cotton (Stricker et al., Citation2001). Fodder plants such as kenaf are utilised as fibre crops and further as livestock feed (Dempsey, Citation1975). Leaves of this plant can be utilised as a source of roughage and protein for cattle and sheep due to their high digestibility, and more especially if they are harvested at an early age of growth (González‐Valenzuela et al., Citation2008; Webber III, Citation1993). They are considered edible, and these leaves contain several chemical and biochemical compositions (water—79,0 g, sugar—280 KJ, protein—5.5 g, fat—1.2 g, starch—12.2 g, fibre—2.3 g, calcium—484 mg, phosphorus—18 mg, iron—12.1 mg and ascorbic acid—75 mg (Garjia, Citation2016). Young leaves are said to be utilised in the production of vegetables (Garjia, Citation2016). Furthermore, these leaves constitutes high amounts of calcium and vitamin C, which is highly beneficial to human and animals (Kobaisy et al., Citation2001). However, most of the studies focus mainly on kenaf’s fibre production, rather than its potential as a food and feed source; hence, there are fewer studies addressing its utilisation as food and feed and also its nutritional and chemical composition. Therefore, there is a need do more research based on the uses of kenaf leaves as food and its nutritional and chemical composition.
4.3. Stalks
Kenaf contains a high fibre yield, and it is described as a dicotyledonous herbaceous annual plant (Kuroda et al., Citation2005). As result, this plant’s stalk (Figure ) is made up of various fibres (bast, core and pith), where bast is described as an outer part of the fibre which constitutes 30% of the dry weight of the stalk, and the pith, which is build up by exclusive parenchymatous cells which are polygonal in shape (Tahir et al., Citation2011). The inner core fibre (75–60% dry weight of the stalk) as described by Abdul Khalil et al (Citation2010) produces pulp of a poor quality, and the outer bast fibre (25–40% dry weight of the stalk) produces pulp of a high quality. For a kenaf plant to reach a suitable size, it needs less than 6 months. Therefore, its unbranched prickly stems can grow up to 4.5 m tall (Coetzee, Citation2004). With high-fibre quantities, stalks are used in the production of twine yam, coarse textiles for sacking and packaging cloth (Garjia, Citation2016). Since there is a huge demand for paper and paperboard materials, there has been an increment in kenaf as a wood substitute. Moreover, due to the pulp’s high quality, the plant cell wall composed of cellulose, hemicellulose, pectin, proteins and lignin provides mechanical support to the cells individually followed by the whole plant (Chowdhury et al., Citation2012; Vanholme et al., Citation2010). Abdul Khalil et al. (Citation2010) further described high α-cellulose content in kenaf bast fibres (55%) to be higher than in core fibre (49%) to provide enough strength in paper production and other fibre products. Also, the stalks protein ranges from 16 to 23% (Swingle et al., Citation1978). However, there are less studies addressing kenaf’s stalks’ nutritional and chemical compositions. Hence, it is necessary to conduct studies analysing this stalks’ nutritional and chemical compositions and their importance in animal reproductivity and production.
4.4. Flowers
The kenaf is a fodder plant which produces flowers () which are large, showy, light yellow and creamy coloured and are bell shaped and widely open (Webber & Bledsoe, Citation2002). Some contain a red or maroon-coloured centre; this colour variation depends on the type of environment they grow in (Kozlowski et al., Citation2005; Olanipekun, Citation2019). Furthermore, kenaf produces acidic flowers (Rajashekher et al., Citation1993). Therefore, this flower makes up the edible vegetable (Garjia, Citation2016). However, research on the nutritional and chemical properties of kenaf flowers is not enough. As a result, further studies need to be conducted looking into nutritional and chemical properties of these flowers.
4.5. Stem bark
Kenaf is a type of fodder plant that has a stem bark with an outer cortical tissue layer which is called a phloem (Ashori et al., Citation2005). It constitutes 40% of the stem’s dry weight (Chiaise et al., Citation2011; Sullivan, Citation2003). This part of the plant is processed into a paper pulp of high quality because of the low pectin and woody impurities contents (Webber & Bledsoe, Citation2002). The fibres in the stem bark are further utilised for speciality papers, tea bags and grass mats (Stricker et al., Citation2001), newsprint and multiple tissue paper grades (Bowyer, Citation1999). The stem of kenaf plant can be used as a livestock feed, bulk agent for sewage sludge composting, as potting soil amendment and anti-erosion mats. This part of kenaf is where a leaf develops and grows from (Stricker et al., Citation2001). The bark is characterised through its anatomical structure, chemical composition and chemo-physical properties together with the core where they are said to be the two distinct types of raw material (Edeerozey et al., Citation2007; Kuroda et al., Citation2005). Lignin in the bark varies from that of core lignin and as far as its chemical structure is concerned (Neto et al., Citation1996). Fibres produced from the bark portion of the stem are 2.5 m long like that of softwood fibres (P. Lin et al., Citation2004). However, there is no information addressing kenaf’s stem nutritional composition and its utilisation as feed and food for animal and human consumption. Therefore, this necessitates the need to conduct more studies exploring mostly the nutritional qualities of kenaf’s stem and its potentiality as animal feed.
5. Health benefits and antinutritional compounds of kenaf
Besides their valuable macronutrient contents mentioned previously, kenaf plants are regarded as medicinal plants (Wong et al., Citation2014) producing a variety of bioactive compounds, which are biologically active plant chemicals that have a variety of health benefits for humans and animals (Jin et al., Citation2013; Khare, Citation2007). Bioactive compounds include phenolic compounds, anti-tumour compounds and phytosterols performing antioxidant, cardio-protective, anti-inflammatory, anti-hypertensive and anti-proliferative activities (Maganha et al., Citation2010). Furthermore, Durgo et al. (Citation2012) described plants that are high in natural antioxidants such as polyphenols and flavonoids that have the capabilities of reducing the risks of given types of cancers (Chew, Tan, Lai et al., Citation2018), which leads to the revived interest in plant-based foods and drugs. As a result, the kenaf plant has further been described as a traditional medicine in Africa and India, which can treat various diseases such as Guinea worm disease and anaemia, and it can be used as an ayurvedic medicine where it treats diabetes, bilious and throat issues, coughs and blood disorders (Jin et al., Citation2013; Monti & Alexopoulou, Citation2013). Additionally, it can further treat bruises and fever. It can also be used as a supplement improving feed fibre and calcium content (Alexopoulou et al., Citation2013; L.S. Yazan et al., Citation2011). Kenaf plant is composed of different active components, such as tannins, saponins, polyphenolics, alkaloids, essential oils and steroids (G. A. Agbor et al., Citation2005; Kobaisy et al., Citation2001). However, besides high nutritional values, this plant has several anti-nutritional (toxic) factors such as phytates, tannins, oxalates and saponins which are reported to have antioxidant and anticancer activities (Obouayeba et al., Citation2015; Ryu et al., Citation2013; L. S. Yazan et al., Citation2016; Zaini et al., Citation2022). Additionally, antinutritional factors (non-protein amino acids, quinolizidine alkaloids, cyanogenic glycosides, pyrimidine glycosides etc.,) are most common in food and feed derived from pulse, grain and legumes. These are the compounds produced by various mechanisms and the regular metabolism of numerous organisms (Bora, Citation2014). Moreover, kenaf’s various parts, such as the seed, leaf and stem, constitute different bioactive compounds and nutritional values. As a results, these parts can be of great use in food and feed production. Table below present secondary metabolites and their functions and/or health benefits in various tissues of the kenaf plant species.
Table 2. Secondary metabolites and their functions/health benefits in each tissue of kenaf plant species
6. Conclusion
Kenaf is more adaptable to various environmental conditions, and it can grow anywhere and still perform better than many commercial crops. Even though the plant was previously grown primarily for fibre production, it has been reported that its leaves and seeds contain high levels of crude protein as well as other secondary metabolites that can be beneficial to human and animal health, more especially in its early growth stages. Moreover, kenaf leaves and seeds contain about 30% and 30.88% crude protein respectively, which gives them a chance as potential protein supplements replacing soyabean, sunflower and fish meal, as these well-known protein ingredients are currently expensive. In addition, there are various food products resulting from kenaf leaves and seeds for human consumption, such as margarine, mayonnaise, cooking oil and so forth. As a result, it can be concluded that kenaf has a greater potential for use as human and animal feed. However, there is a little information on kenaf’s potentiality as food and feed. Hence, more research on the future of kenaf in feed or food producing companies is required.
Acknowledgements
No financial support was required
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
T. C. Kujoana
Tlou Chritospher Kujoana (MSc. Agriculture in Animal Production) is a Ph.D. candidate in Agriculture (Animal Science) at the University of South Africa (UNISA) in the Department of Agriculture and Animal Health. His main research interest is on Improving Production and Reproductive efficiency of white New Zealand Rabbits through the utilisation on Kenaf (Hibiscus cannabinus) -base diet.

N. A. Sebola
T. C. Kujoana.
References
- Abdel Ghafar, S. A. A., Ismail, M., Yazan, L. S., Fakurazi, S., Ismail, N., Chan, K. W., Tahir, P. (2013). Cytotoxic activity of kenaf seed oils from supercritical carbon dioxide fluid extraction towards human colorectal cancer (HT29) cell lines. Evidence-Based Complementary and Alternative Medicine 2013: 8.Article ID 549705.
- Abdul Khalil, H. P. S., Ireana Yusra, A. F., Bhat, A. H., & Jawaid, M. (2010). Cell wall ultrastructure, anatomy, lignin distribution, and chemical composition of Malaysian cultivated kenaf fiber. Industrial Crops and Products, 31(1), 113. https://doi.org/10.1016/j.indcrop.2009.09.008
- Agbaje, G. O., Saka, J. O., Adegbite, A. A., & Adeyeye, O. O. (2008). Influence of agronomic practices on yield and profitability in kenaf (Hibiscus cannabinus L.) fiber cultivation. African Journal of Biotechnology, 7(5), 565–15. http://www.academicjournals.org/AJB
- Agbor, A. G., Oben, J. E., Brahim, O. B., & Ngogang, Y. J. (2004). Toxicity study of hibiscus cannabinus. Journal of the Cameroon Academy of Science, 4(1), 27–32.
- Agbor, G. A., Oben, J. E., & Ngogang, J. Y. (2005). Haematinic activity of Hibiscus cannabinus. African Journal of Biotechnology, 4(8), 833–837. http://www.academicjournals.org/AJB
- Akinrotimi, C. A., & Okocha, P. I. (2018). Evaluations of genetic divergence in Kenaf (Hibiscus Cannabinus L.) genotypes using agro-morphological characteristics. Journal of Plant Sciences and Agricultural Research, 2(12), 2167–2412.
- Alexopoulou, E., Li, D., Papatheohari, Y., Siqi, H., Scordi, D., & Testa, G. (2015). How kenaf (Hibiscus cannabinus L.) can achieve high yields in Europe and China. Industrial Crops and Products, 68, 131–140. https://doi.org/10.1016/j.indcrop.2014.10.027
- Alexopoulou, E., & Monti, A. (2013). Kenaf: A multi-purpose crop for several industrial applications, 203. New Insights from the Biokenaf Project Springer-Verlag.
- Alexopoulou, E., Papatheohari, Y., Christou, M., & Monti, A. (2013). Origin, description, importance, and cultivation area of kenaf. In A. Monti & E. Alexopoulou (Eds.), Kenaf: A multi-purpose crop for several industrial applications (pp.1–15). Springer. https://doi.org/10.1007/978-1-4471-5067-1_1
- Ammar, H., Ismaïl, Y., Lehiani, M. A., Tejido, M. L., Bodas, R., Giráldez, F. J., Salem, A. Z. M., & López, S. (2020). Biomass production and nutritive value of Kenaf (Hibiscus cannabinus) at various stages of growth. Agroforestry Systems, 94(4), 1171–1178. https://doi.org/10.1007/s10457-019-00420-5
- Ashori, A., Raverty, W. D., & Harun, J. (2005). Effect of chitosan addition on the surface properties of kenaf (Hibiscus cannabinus L) paper. Iranian Polymer Journal, 14, 8007–8814.
- Ayadi, R., Hanana, M., Mzid, R., Hamrouni, L., Khouja, M. L., & Salhi Hanachi, A. (2017). kenaf (Hibiscus cannabinus L): A review paper. Journal of Natural Fibres, 14(4), 466–484. https://doi.org/10.1080/15440478.2016.1240639
- Banuelos, G. S., Bryla, D. R., & Cook, C. G. (2002). Vegetative production of kenaf and canola under irrigation in central California. Industrial Crops and Products, 15(3), 237–245. https://doi.org/10.1016/S0926-6690(01)00119-4
- Berger, J. (1969). The world’s major fibre crops- their cultivation and manuring. centre d’ etude de l’azote. Zurich, 227-231.
- Bora, P. (2014). Anti-Nutritional factors in foods and their effects. Journal of Academia and Industrial Research, 3(6), 285–290.
- Bowyer, J. L. (1999). Economic and environmental comparisons of kenaf growth versus plantation grown softwood and hardwood for pulp and paper. In T. Sellers Jr., N. Reichert, E. Columbus, M. Fuller, & K. Williams, Eds. 1999. Chemistry of kenaf: Properties and materials symposium, 323–346. Mississippi State University.
- Brishti, F. H., Zarei, M., Muhammad, S. K. S., Ismail-Fitry, M. R., Shukri, R., & Saari, N. (2017). Evaluation of the functional properties of mung bean protein isolate for development of textured vegetable protein. International Food Research Journal, 24(4), 1595–1605.
- Chan, H. C., Chia, C. H., Zakaria, S., Ahmad, I., & Dufresne, A. (2013). Production and characterisation of cellulose and nano-crystalline cellulose from kenaf core wood. Bio Resources, 8(1), 785–794.
- Chan, K. W., & Ismail, M. (2009). Supercritical carbon dioxide fluid extraction of Hibiscus cannabinus L. seed oil: A potential solvent-free and high antioxidative edible oil. Food Chemistry, 114(3), 970–975. https://doi.org/10.1016/j.foodchem.2008.10.055
- Chantiratikul, A., Chaikong, C., Chinrasri, O., & Kangkun, P. (2009). Evaluation of yield and nutritive value of Kenaf (Hibiscus cannabinus) at various stages of maturity. Pakistan Journal of Nutrition, 8(7), 1055–1058. https://doi.org/10.3923/pjn.2009.1055.1058
- Cheng, Z. (2001). Kenaf research, products and applications in Japan (in Chinese). Plant Fibers Product, 23(3), 16–24.
- Cheng, W. Y., Akanda, J. M. H., & Nyam, K. L. (2016). Kenaf seed oil: A potential new source of edible oil. Trends in Food Science & Technology, 52, 57–65. https://doi.org/10.1016/j.tifs.2016.03.014
- Chew, S. C., & Nyam, K. L. (2016). Microencapsulation of kenaf seed oil by co-extrusion technology. Journal of Food Engineering, 175, 43–50. https://doi.org/10.1016/j.jfoodeng.2015.12.002
- Chew, S. C., Tan, C. P., Lai, O. M., & Nyam, K. L. (2018). Changes in 3-MCPD esters, glycidyl esters, bioactive compounds and oxidation indexes during kenaf seed oil refining. Food Science and Biotechnology, 27(3), 905–914. https://doi.org/10.1007/s10068-017-0295-8
- Chew, S. C., Tan, C. P., Long, K., & Nyam, K. L. (2016). Effect of chemical refining on the quality of kenaf (Hibiscus cannabinus) seed oil. Industrial Crops and Products, 89, 59–65. https://doi.org/10.1016/j.indcrop.2016.05.002
- Chew, S. C., Tran, C. P., Tan, C. H., & Nyam, K. L. (2020). In-vitro bioaccessibility of spray dried refined kenaf (Hibiscus cannabinus) seed oil applied in coffee drink. Journal of Food Science and Technology, 57(7), 2507–2515. https://doi.org/10.1007/s13197-020-04286-9
- Chiaise, P., Ruotolo, G., Dimatteo, A., De Santo Virzo, A., De Marco, A., & Filippone, E. (2011). Cloning and expression analysis of kenaf (Hibiscus cannabinus L.) major lignin and cellulose biosynthesis gene sequences and polymer quantification during plant development. Industrial Crops and Products, 34(1), 1072–1078. https://doi.org/10.1016/j.indcrop.2011.03.019
- Chowdhury, E. M., Choi, B. S., Sang, U. P., Lim, H. S., & Bae, H. (2012). Transcriptional analysis of hydroxycinnamoyl transferase (HCT) in various tissues of Hibiscus cannabinus L. in response to abiotic stress conditions. Plant Omics Journal, 5(3), 305–313. http://www.pomics.com/may2012.html
- Chu, C. C., Chew, S. C., & Nyam, K. L. (2021). Recent advances in encapsulation technologies of kenaf (Hibiscus cannabinus) leaves and seeds for cosmeceutical application. Food and Bioproducts Processing, 127, 99–113. https://doi.org/10.1016/j.fbp.2021.02.009
- Coetzee, R. (2004). Characterization of Kenaf (Hibiscus cannabinus L.) cultivars in South Africa (Masters dissertation, University of the Free State).
- Coetzee, R., Labuschagne, M. T., & Hugo, A. (2008). Fatty acid and oil variation in seed from kenaf (Hibiscus cannabinus L.). Industrial Crops and Products, 27(1), 104–109. https://doi.org/10.1016/j.indcrop.2007.08.005
- Cook, J. G. (1960). Handbook of textile fiber. Merrow publishing.
- Danalatos, N. G., & Archontoulis, S. V. (2010). Growth and biomass productivity of kenaf (Hibiscus cannabinus, L.) under different agricultural inputs and management practices in central Greece. Industrial Crops and Products, 32(3), 231–240. https://doi.org/10.1016/j.indcrop.2010.04.013
- Dempsey, J. M. (1975). Kenaf. Fiber Crops. University of Florida Press. Rose Printing Company.
- Durgo, K., Belščak-Cvitanović, A., Stančić, A., Franekić, J., & Komes, D. (2012). The bioactive potential of red raspberry (Rubus idaeus L.) leaves in exhibiting cytotoxic and cytoprotective activity on human laryngeal carcinoma and colon Adenocarcinoma. Journal of Medicinal Food, 15(3), 258–268. https://doi.org/10.1089/jmf.2011.0087
- Ebije, I., Oladipupo, A., Lawal, A. O. O., & Isiaka, A. O. (2014). Volatile composition of the floral essential oil of Hibiscus sabdariffa L. from Nigeria. Ajeonp, 2, 4–7.
- Edeerozey, A. M., Akil, H. M., Azar, A. B., & Ariffin, M. I. Z. (2007). Chemical modification of kenaf fibres. Materials Letters, 61(10), 2023–2025. https://doi.org/10.1016/j.matlet.2006.08.006
- Falasca, S. L., Ulberich, A. C., & Pitta-Alvarez, S. (2014). Possibilities for growing kenaf (Hibiscus cannabinus L.) in Argentina as biomass feedstock under dry-subhumid and semiarid climate conditions. Biomass and Bioenergy, 64, 70–80. https://doi.org/10.1016/j.biombioe.2014.03.031
- FAO, IFAD, UNICEF, WFP and WHO. (2021). The state of food security and nutrition in the world 2021.Transforming food systems for food security, improved nutrition and affordable healthy diets for all. Rome, FAO. https://doi.org/10.4060/cb4474en.
- Garjia, Y. A. (2016). A handbook of common vegetables in Taraba State for Schools and colleges. 1stedn. Taraba, Nigeria.
- González‐Valenzuela, E. A., Ávila‐Curiel, J. M., Ortega‐S, J. A., González‐Padrón, M. A., & Muir, J. P. (2008). Harvesting interval changes yield and nutritive value of kenaf in a dry tropical climate. Agronomy Journal, 100(4), 938–941. https://doi.org/10.2134/agronj2007.0147
- Hassan, K. M., Bhuyan, M. I., Islam, M. K., Hoque, M. F., & Monirul, M. (2018). Performance of some jute and allied fiber varieties in the southern part of Bangladesh. International Journal of Advanced Geosciences, 6(1), 117–121. https://doi.org/10.14419/ijag.v6i1.10184
- Hossain, M. D., Hanafi, M. M., & Jol, H. H. (2011). Growth, yield and fibre morphology of kenaf (Hibiscus cannabinus L.) grown on sandy bris soil as influenced by different levels of carbon. African Journal of Biotechnology, 10(50), 10087–10094.
- Ibrahim, G. S. A., Karim, R., Saari, N., Wan Abdullah, W. Z., Zawawi, N., Ab Razak, A. F., Hamim, N. A., & Umar, R. A. (2019). Kenaf (Hibiscus cannabinus L .) Seed and its Potential Food Applications: A Review. Journal of Food Science, 84(8), 2015–2023. https://doi.org/10.1111/1750-3841.14714
- III, C. L. W., White Jr, P. M., Dalley, C., Petrie, E. C., Viator, R. P., & Shrefler, J. W. (2016). Kenaf (Hibiscus cannabinus L.) and cowpea (Vigna unguiculata) as sugarcane cover crops. Journal of Agricultural Science, 8(8). https://doi.org/10.5539/jas.v8n8p13
- Ijinu, T. P., George, V., & Pushpangadan, P. (2022). History of research on medicinal plants in India. Medicinal and Aromatic Plants of India, 1, 35–61.
- Intergovernmental Panel on Climate Change (IPCC). (2007). Intergovernmental panel on climate change fourth assessment report. Cambridge University Press.
- Jin, C. W., Ghimeray, A. K., Wang, L., Xu, M. L., Piao, J. P., & Cho, D. H. (2013). Far infrared assisted kenaf leaf tea preparation and its effect on phenolic compounds, antioxidant and ACE inhibitory activity. 2013. Journal of Medicinal Plants Research, 7, 1121–1128. https://doi.org/10.5897/JMPR11.1431
- Jin, C. W., Sun, Y. L., & Cho, D. H. (2012). Changes in photosynthetic rate, water potential, and proline content in kenaf seedlings under salt stress. Canadian Journal of Plant Science, 92(2), 311–319. https://doi.org/10.4141/cjps2011-144
- Jones, M. D., Puentes, C., & Suarez, R. (1955). Isolation of Kenaf for Seed Increase 1. Agronomy Journal, 47(6), 256–257. https://doi.org/10.2134/agronj1955.00021962004700060005x
- Karim, R., Noh, N. A. M., Ibrahim, S. G., Ibadullah, W. Z. W., Zawawi, N., & Saari, N. (2020 Ed.). Kenaf (Hibiscus cannabinus L.) seed extract as a new plant-based milk alternative and its potential food uses. Milk Substitutes - Selected Aspects (pp. 136). Intech Open. https://doi.org/10.5772/intechopen.94067
- Khare, C. P. (2007). Indian medicinal plants; An illustrated dictionary. Springer-verlag. London, 309.
- Kim, D., Ryu, J., Lee, M., Kim, J. M., Ahn, J., Kim, J., Kang, S., Bae, C., & Kwon, S. (2018). Nutritional properties of various tissues from new Kenaf cultivars. Journal of Science and Biotechnology, 21(3), 229–239.
- Kipriotis, E., Heping, X., Vafeiadakis, T., Kiprioti, M., & Alexopoulou, D. E. (2015). Ramie and kenaf as feed crops. Industrial Crops and Products, 68, 126–130. https://doi.org/10.1016/j.indcrop.2014.10.002
- Kobaisy, M., Tellez, M. R., Webber, C. L., Dayan, F. E., Schrader, K. K., & Wedge, D. E. (2001). Phytotoxic and fungitoxic activities of the essential oil of Kenaf (Hibiscus cannabinus L.) leaves and its composition. Journal of Agricultural Food Chemistry, 49(8), 3768–3771. https://doi.org/10.1021/jf0101455
- Kozlowski, R., Baraniecki, P., & Barriga-Bedoya, J. (2005). Bast fibres (flax, hemp, jute, ramie, kenaf, abaca). Biodegradable and Sustainable Fibres, 36–88.
- Kubmarawa, D., Andenyang, I. F. H., & Magomya, A. M. (2009). Proximate composition and amino acid profile of two non-conventional leafy vegetables (Hibiscus cannabinus L. and Haematostaphis barteri). African Journal of Food Science, 3(9), 233–236. http://www.academicjournals.org/ajfs
- Kuroda, K., Akiko, N., Bibhuti, B. M., Yoshito, O., & Kazuhiko, S. (2005). Evaluation of chemical composition of the core and bast lignins of variety Chinpi-3 kenaf (Hibiscus cannabinus L.) by pyrolysis–gas chromatography/mass spectrometry and cupric oxide oxidation. Industrial Crops and Products, 22(3), 223–232. https://doi.org/10.1016/j.indcrop.2005.01.002
- Kwari, I. D., Igwebuike, J. U., Mohammrd, I. D., & Diarra, S. S. (2011). Growth, hermatology and serum chemistry of broiler chickens raw or differently processed sorrel (Hibiscuss sabdariffa) seed meal in semi-arid environment. International Journal of Science, 2(1), 22–27.
- Lauriault, L. M., & Puppala, N. (2009). The influence of rainfed and limited irrigation conditions and early vs. late plantings on kenaf as a potential industrial crop in the southern High Plains, USA. Industrial Crops and Products, 29(2–3), 549–553. https://doi.org/10.1016/j.indcrop.2008.10.006
- LeMahieu, P. J., Oplinger, E. S., & Putnam, D. H. (2003). Kenaf. In alternative field crops manual. University of wisconsin-extension. Cooperative Extension; University of Minnesota, Center for Alternative Plant and Animal Products and the Minnesota Extension Service.
- Liliana, H. (2005). The food gaps: The impacts of climate change on food production: A 2020 perspective, universal ecological fund USA.
- Lin, S., Huff, H. E., & Hsieh, F. (2002). Extrusion process parameters, sensory characteristics, and structural properties of a high moisture soy protein meat analogy. Journal of Food Science, 67(3), 1066–1072. https://doi.org/10.1111/j.1365-2621.2002.tb09454.x
- Lin, P., Lin, L., Wu, J., & Lin, N. (2004). Breeding of Fu Hong 4, a kenaf variety with high yielding and resistance. Plant Fibre Production, 26, 1–4.
- Liu, Y. (2005). Diallel and stability analysis of kenaf (Hibiscus cannabinus L.) in South Africa. Master of Science in Agriculture Faculty of Natural and Agricultural Sciences South Africa (November), 83.
- Liu, Y., & Labuschagne, M. T. (2009). The influence of environment and season on stalk yield in kenaf. Industrial Crops and Products, 29(2–3), 377–380. https://doi.org/10.1016/j.indcrop.2008.07.005
- Maganha, E. G., Halmenschlager, R. C., Rosa, R. M., Henriques, J. A., Ramos, A. L., & Saffi, J. (2010). Pharmacological evidence for the extracts and secondary metabolites from plants of the genus. Hibiscus. Food Chemistry, 118(1), 1–10. https://doi.org/10.1016/j.foodchem.2009.04.005
- Mariod, A. A., Fathy, S. F., & Ismail, M. (2010). Preparation and characterization of protein concentrates from defatted kenaf seed. Food Chemistry, 123,747–752. https://doi.org/10.1016/j.foodchem.2010.05.045
- Masipa, T. S. (2017). The impact of climate change on food security in South Africa: Current realities and challenges ahead. Jàmbá: Journal of Disaster Risk Studies, 9(1),411. https://hdl.handle.net/10520/EJC-a876c549f
- Mohamed, A., Bhardwaj, H., Hamama, A. & Webber, C. (1995). Chemical composition of kenaf (Hibiscus cannabinus L.) seed oil. Industrial Crops and Products, 4, 157–165.
- Monti, A., & Alexopoulou, E. (2013). Kenaf: A multi-purpose crop for several industrial applications; springer. Germany.
- Nandagopalan, V., Gritto, M. J., & Doss, A. (2015). GC-MS analysis of bioactive components of the methanol extract of Hibiscus tiliaceus Linn. Asian Journal of Plant Science and Research, 5(3), 6–10. http://www.pelagiaresearchlibrary.com/
- Neto, C. P., Seca, A., Fradinho, D., Coimbra, M. A., Domingues, F., Evtuguin, D., Silvestre, A., & Cavaleiro, J. A. S. (1996). Chemical composition and structural features of the macromolecular components of Hibiscus cannabinus grown in Portugal. Industrial Crops and Products, 5(3), 189–196. https://doi.org/10.1016/0926-6690(96)89448–9
- Obouayeba, A. P., Diarrassouba, M., Soumahin, E. F., & Kouakou, T. H. (2015). Phytochemical analysis, purification and identification of Hibiscus anthocyanins. Journal of Pharmaceutical Chemistry and Biological Science, 3, 156–168.
- Odetola, O. M., & Eruvbetine, D. (2012). Nutritional evaluation of whole kenaf (Hibiscus cannabinus L.) seed meal in Rats. Journal of Advanced Laboratory Research, 3(3), 215–220. www.sospublication.co.za
- Oh, S., Mbiriri, D. T., Ryu, C., Lee, K., Cho, S., & Choi, N. (2018). In vitro and in vivo evaluation of kenaf (Hibiscus cannabinus L.) as a roughage source for beef cattle. Asian-Australas Journal of Animal Science, 31(10), 1598–1603. https://doi.org/10.5713/ajas.17.0871
- Okello, M., Lamo, J., Ochwo-Ssemakula, M., & Onyilo, F. (2017). Challenges and innovations in achieving zero hunger and environmental sustainability through the lens of sub-Saharan Africa. Outlook on Agriculture, 50(2), 141–147. https://doi.org/10.1177/0030727020975778j
- Olanipekun, S. O. (2019). Growth and yield of kenaf (Hibiscus cannabinus L.) as influenced by fertilisers. spacings and sowing dates in Ibadan, Nigeria (Doctoral dissertation).
- Organisation for Economic Co-operation and development (OECD). (2022). OECD economic outlook, interim report march 2022: Economic and social impacts and policy implications of the War in Ukraine, OECD Publishing.
- Owoade, A. O., Adetutu, A., & Olorunnisola, O. S. (2019). Biosciences and plant biology. International Journal of Current Research in Biosciences and Plant Biology, 6(4), 42–51. https://doi.org/10.20546/ijcrbp.2019.604.006
- Patanè, C., & Sortino, O. (2010). Seed yield in kenaf (Hibiscus cannabinus L.) as affected by sowing time in South Italy. Industrial Crops and Products, 32(3), 381–388. https://doi.org/10.1016/j.indcrop.2010.06.002
- Paul, D. M., & Smith, C. A. (2003). Kenaf seed storage duration on germination, emergence, and yield. Industrial Crops and Products, 17(1), 9–14. https://doi.org/10.1016/S0926-6690(02)00052-3
- Qammar, G., Mohy-ud-Din, G., Huma, N., Sameen, A., & Issa Khan, M. (2010). Textured soy protein (TSP) as pizza topping. Nutrition and Food Science, 40(6), 551–556. https://doi.org/10.1108/00346651011090356
- Rajashekher, A. R., Rao, P. V., & Reddy, V. R. (1993). Chemical composition and nutritive value of ambadi (Hibiscus cannabinus) meal for layers. Animal Feed Science and Technology, 44(1–2), 151–166. https://doi.org/10.1016/0377-8401(93)90044-K
- Rauter, C., Oehme, R., Diterich, I., Engele, M., & Hartung, T. (2002). Distribution of clinically relevant Borrelia genospecies in ticks assessed by a novel, single-run, real-time PCR. Journal of Clinical Microbiology, 40(1), 36–43. https://doi.org/10.1128/JCM.40.1.36-43.2002
- Ryu, J., Ha, B. K., Kim, D. S., Kim, J. B., Kim, S. H., & Kang, S. Y. (2013). Assessment of growth and seed oil composition of kenaf (Hibiscus cannabinus L.) germplasm. Journal of Crop Science and Biotechnology, 16, 297–302.
- Ryu, J., Kwon, S. J., Ahn, J. W., Jo, Y. D., Kim, S. H., Jeong, S. W., Kang, S. Y., Kim, J.-B., & Kang, S.-Y. (2017). Phytochemicals and antioxidant activity in the kenaf plant (Hibiscus cannabinus L.). Journal of Plant Biotechnology, 44(2), 191–202. https://doi.org/10.5010/JPB.2017.44.2.191
- Saba, N., Jawaid, M., Hakeem, K. R., Paridah, M. T., Khalina, A., & Alothman, O. Y. (2015). Potential of bioenergy production from industrial kenaf (Hibiscus cannabinus L.) based on Malaysian perspective. Renewable and Sustainable Energy Reviews, 42, 446–459. https://doi.org/10.1016/j.rser.2014.10.029
- Salem, M. Z. M., Abdel-Megeed, A., & Ali, H. M. (2014). Stem wood and bark extracts of Delonix regia (Boj. Ex. Hook): Chemical analysis and antibacterial, antifungal, and antioxidant properties. Bioresources, 9(2), 2382–2395. https://doi.org/10.15376/biores.9.2.2382-2395
- Salih, R. F. (2016). Influence of potassium, boron and zinc on growth, yield and fiber quality of two kenaf (Hibiscus cannabinus L.) Varieties. Ph.D study.
- Sato, E. (2017). The effect of preparation conditions on the rheological properties of Gomatofu (Sesame Tofu). In I. Kaneda (Ed.), Rheology of biological soft matter (pp. 265–292). Springer.
- Stricker, J. A., Prine, G. M., & Riddle, T. C. (2001). Kenaf-A possible new crop for central Florida. In Agronomy Department, Florida cooperative extension service, institute of food and agricultural sciences. University of Florida. Cooperative Extension Service, Institute of Food and Agriculture Sciences, EDIS.
- Sullivan, P. (2003). Kenaf production. Appropriate Technology Transfer for Rural Areas (ATTRA). National Center for Appropriate Technology.
- Suriyajantratong, W., Tucker, R. E., Sigafus, R. E., & Mitchell Jr, G. E. (1973). Kenaf and rice straw for sheep. Journal of Animal Science, 37(5), 1251–1254. https://doi.org/10.2527/jas1973.3751251x
- Swingle, R. S., Urias, A. R., Doyle, J. C., & Voigt, R. L. (1978). Chemical composition of kenaf forage and its digestibility by lambs and in vitro. Journal of Animal Science, 46(5), 1346–1350. https://doi.org/10.2527/jas1978.4651346x
- Tahir, P. M., Ahmed, B. A., Saiful Azry, S. O. A., & Ahmed, Z. (2011). Retting process of some bast plant fibers and its effect on fiber quality: A review. Bioresources, 6(4), 5260–5281.
- Uddin, Q., Samiulla, L., Singh, V. K., & Jamil, S. S. (2012). Phytochemical and pharmacological profile of Withania somnifera Dunal: A review. Journal of Applied Pharmaceutical Science, 2(1), 170–175. www.japsonline.com
- Vanholme, R., Demedts, B., Morreel, K., Ralph, J., & Boerjan, W. (2010). Lignin biosynthesis and structure. Plant Physiology, 153(3), 895–905. https://doi.org/10.1104/pp.110.155119
- Vogel, C. (2005). Usable Science: An assessment of long-term seasonal forecasts among farmers in rural areas of South Africa. South African Geographical Journal, 82(2), 107–116. https://doi.org/10.1080/03736245.2000.9713700
- Webber, C. L., Bhardwaj, H. L., & Bledsoe, V. K. (2002). Kenaf production: Fiber, feed and seed. In J. Janick & A. Whipkey (Eds.), Trends in new crops and new uses (pp. 327–339). ASHS Press.
- Webber, C. L., & Bledsoe, V. K. (2002). Kenaf yield components and plant composition. In J. Janick & A. Whipkey (Eds.), Trends in new crops and new uses (pp. 348–357). ASHS Press.
- Webber III, C. L. (1993). Crude protein and yield components of six kenaf cultivars as affected by crop maturity. Industrial Crops and Products, 2(1), 27–31. https://doi.org/10.1016/0926-6690(93)90007-V
- Wongsrisiri, S., & Luangsakul, N. (2017). Effect of setting agent on quality of tubed-package sesame tofu. International Journal of Agricultural Technology, 13(7.1), 1517–1526. http://www.ijat-aatsea.com/
- Wong, Y. H., Tan, W. Y., Tan, C. P., Long, K., & Nyam, K. L. (2014). Cytotoxic activity of kenaf (Hibiscus cannabinus L.) seed extract and oil against human cancer cell lines. Asian Pacific Journal of Tropical Biomedicine, 4(1), 510–515. https://doi.org/10.12980/APJTB.4.2014C1090
- Wood, I. (2003). Kenaf: The forgotten fiber crop. The Australian new crops newsletter, 10. http://www.newcrops.uq.edu.au/newslett/ncn10212.htm.
- World Bank. (2016). Arable land (hectares per person), World Bank, viewed 10 April 2022, http://data.worldbank.org/indicator/AG.LND.ARBL.HA.PC
- Xu, J., Tao, A., Qi, J., & Wang, Y. (2020). Bast fibres: Kenaf. Handbook of natural fibres (pp. 71–92).
- Yazan, L. S., Abd Rahman, N., Chan, K. W., Wan Abd Ghani, W. N. H., Tor, Y. S., & Foo, J. B. (2016). Phenolics-saponins rich fraction of defatted kenaf seed meal exhibits cytotoxicity towards cancer cell lines. Asian Pacific Journal of Tropical Biomedicine, 6(5), 404–409. https://doi.org/10.1016/j.apjtb.2016.03.007
- Yazan, L. S., Foo, J. B., Chan, K. W., Tahir, P. M., & Ismail, M. (2011). Kenaf seed oil from supercritical carbon dioxide fluid extraction shows cytotoxic effects towards various cancer cell lines. African Journal of Biotechnology, 10(27), 381–5388. http://www.academicjournals.org/AJB
- Zaini, N. S., Karim, R., Razis, A. F. A., Hamid, N. F. S., & Zawawi, N. (2022). pilot study of toxicological safety evaluation in acute and subacute (28-day) oral assessments of kenaf seed beverage in rats. Food Research International, 162(2022), 111988. https://doi.org/10.1016/j.foodres.2022.111988
- Zhang, T. (2003). Improvement of kenaf yarn for apparel application (Master dissertation of Louisiana State University, US).
- Zhao, S., Li, X., Cho, D. H., Arasu, M. V., Al-Dhabi, N. A., & Park, S. U. (2014). Accumulation of kaempferitrin and expression of phenylpropanoid biosynthetic Genes in Kenaf(Hibiscus cannabinus). Molecules, 19(10), 16987–16997. https://doi.org/10.3390/molecules191016987
- Zuo, F., Chen, Z., Shi, X., Wang, R., & Guo, S. (2016). Yield and textural properties of tofu as affected by soymilk coagulation prepared by a high-temperature pressure cooking process. Food Chemistry, 213, 561–566. https://doi.org/10.1016/j.foodchem.2016.07.008