Abstract
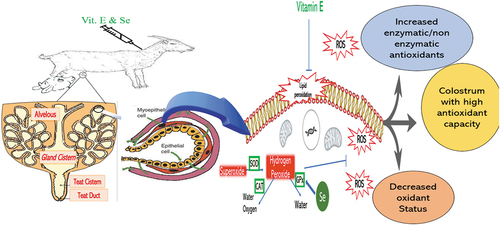
This study was aimed to explore whether vitamin E (Vit-E) and selenium (Se) injections improve colostrum quality in goats. Twenty-four, healthy pregnant Beetal goats were randomly allocated into control (n = 12) and treated (n = 12) groups respectively. The injectable form of Vit-E 2.5 mg/kg BW and Se 2.2 mg/kg BW was administered fortnightly, while normal saline (0.9% NaCl) was used and served as a control group. Colostrum samples were collected immediately after parturition and subjected to biochemical analysis. Colostrum enzymatic and non-enzymatic antioxidants and total soluble proteins were investigated using a spectrophotometer. Antioxidants with enzymatic activities including superoxide dismutase (SOD) and peroxidases (POD) and ascorbic acid remained significantly higher in treated animals (P <0.05) in contrast to the control group. The Total antioxidant capacity (TAC) and total soluble proteins (TSP) levels were noted higher (P<0.05), however; the level of MDA existed significantly lesser in the treated group (P <0.05) in comparison with the control group. Overall, results showed that parental exposure to antioxidants (Vit-E and Se) improved antioxidant status in colostrum and upgrade its quality.
1. Introduction
Colostrum is a nutritionally important component which is consumed by the offspring to improve their health status and survival (Lérias et al., Citation2013). It has a different nutritional value than mature milk and is a complete diet for infants. Insufficient supply of colostrum to neonates increased morbidity and mortality in newborn animals. Previous studies have reported that colostrum also contains enzymatic and non-enzymatic antioxidants that protect against neonatal oxidative stress (Gökçe et al., Citation2022; Kirikci & Çam, Citation2020). The enzymatic antioxidants include superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and lactoperoxidase (Lérias et al., Citation2014b) while, non-enzymatic antioxidants consist of vitamin A, C, E, cysteine, lactoferrin, selenium, and zinc (Albera & Kankofer, Citation2009). Due to the presence of different antioxidants in colostrum, it may serve as a free radical scavenger in neonatal oxidative stress (Gong & Xiao, Citation2016). A study conducted by Albera and Kankofer (Citation2011) has explored that first milk contains a high amount of oxidants but fewer antioxidants than mature milk. Hence, calves are highly vulnerable to oxidative stress after consuming colostrum (Albera & Kankofer, Citation2011).
It has been reported that dairy cows provided with antioxidant-supplemented feed produced more milk with improved quality (Lipko-Przybylska & Kankofer, Citation2012). The presence of SOD in colostrum and milk reduces metal ions and dismutate superoxide (O−) into hydrogen peroxide and molecular oxygen. Then hydrogen peroxide degrades into the water by the antioxidant enzyme, G-Px. The transfer of enzymatic and non-enzymatic antioxidants towards the fetus takes place during late gestation, but it is still insufficient to scavenge the overproduction of ROS after birth (Lelyon et al., Citation2012).
Immunoglobulins and different lipid-soluble vitamins are found in colostrum in high amounts. Therefore, it is presumed that before intake of colostrum, Vit-E level of neonates cannot be manipulated through maternal diet (Lérias et al., Citation2014a; Morsy et al., Citation2018). Se demand increases throughout the gestation and lactation period due to the increased transfer from the dam to the growing fetus in order to synthesis of proteins in colostrum and milk (EHernandez-Castellano et al., Citation2014). A large supply of Se takes place in late gestation through placental and mammary tissues. The level of Se in colostrum decreases as the lactation advances (Gong & Xiao, Citation2016). Normally, selenium in colostrum is found in the form of selenomethionine, selenocysteine, selenocysteine, and glutathione-peroxidase which are easily absorbed by newborns. Antioxidant activity of the colostrum and milk indicates the presence of selenium-containing amino acids in proteins or in the enzymes while Vit-E is also utilized as the mainline of antioxidant defense (Prince et al., Citation2017). The depletion of antioxidants induces lipid peroxidation which may lead to the formation of intolerable changes in nutritive value (Alyaqoubi et al., Citation2014). The concentration of Se in colostrum depends on its availability in natural food and its supply to the animal in the form of diet. Supplementation of Vit-E in combination with Se in late gestation enhanced the level of Se in colostrum and offering such a diet before and after birth improved antioxidant status in neonates and thus protecting from birth oxidative stress. Another study showed that parental administration of Vit-E and Se during late gestation and early lactation strengthen the antioxidant status of colostrum and increased the viability of newborns (Franco & Martínez-Pinilla, Citation2017). The current study was designed to exploit the effect of injectable form of Vit-E and Se in late gestation on antioxidant potential or oxidant status and protein content in the goat colostrum.
2. Materials and methods
2.1. Ethical statement
The Ethical Committee of the Nuclear Institute for Agriculture and Biology, Pakistan Institute of Engineering and Applied Sciences (NIAB-C, PIEAS), Faisalabad, has approved the experiment on Beetal goats (NIAB-C-PIEAS-2019/05)
2.2. Animal housing and their management
The experiment was organized at the Animal farm, NIAB, Faisalabad, Pakistan from April-2019 to May 2019. The regular minimum and maximum documented temperatures in this period were 18–23°C and 33–38° C respectively and the normal precipitation level was 13–14 mm. To carry out experiment, twenty-four healthy multiparous pregnant Beetal goats, aged 2–3 years with 45 kg body weight, were selected. Healthy and physically fit animals were used in this study. The pregnancy status was confirmed using ultrasound (Department of Theriogenology, University of Agriculture, Faisalabad, Pakistan). All animals were allowed for two times free grazing with morning and evening schedule on Acacia ampliceps, Leptochloa fusca (Kallar grass) and Atriplex lentiformis and were fenced in a well-ventilated, semi-covered cemented floor shed. Moreover, seasonal green fodder (barseem/rye grass) with wheat straw was also offered and ensured an easy approach to fresh drinking water. The concentrate was given at 200 g/day/animal throughout the entire research period.
2.3. Study design
Twenty-four pregnant goats were randomly allocated into control (n = 12) and treated (n = 12) groups respectively. The treated animals were injected with Vit-E at 2.5 mg/kg, BW in combination with Se at 2.2 mg/kg BW subcutaneously according to the manufacture recommendations. The injections were used fortnightly in treated group while control group received 0.9% NaCl (normal saline) subcutaneously. The total length of the experiment was nearly 30 days, starting from last month of pregnancy till kidding period after getting colostrum samples.
2.4. Colostrum sampling
Within 30 minutes of parturition, a 12 ml colostrum sample from each animal was collected employing hand milking using hygienic measures. Goat teats were thoroughly washed with antiseptics before sampling and then poured in sterilized 15 ml falcon tubes. The samples were immediately shifted to the laboratory and kept at −20°C for following biochemical analysis ().
2.5. Biochemical evaluation
Defatting of colostrum was performed by centrifugation 2500×g for 15 minutes. The supernatant (fat layer) was separated and the leftover was utilized for biochemical analysis.
2.5.1. Examination of enzymatic antioxidant activities
2.5.1.1. Superoxide Dismutase (SOD) activity
Obtained colostrum samples were examined for the activity of enzyme superoxide dismutase by detecting its potential to suppress the photochemical reduction of nitroblue tetrazolium (NBT) as per the protocol of Giannopolitis and Ries (Giannopolitis & Ries, Citation1977). A single unit of enzyme SOD activity was described as the volume of enzyme which caused 50 % inhibition of photochemical reduction of NBT.
2.5.1.2. Catalase (CAT) activity
The activity of the catalase enzyme in colostrum was measured by a method described by Beers and Sizer (Beers & Sizer, Citation1952). The variation in absorbance in 0.01 min−1 was designated as 1 U catalase enzyme activity.
2.5.1.3. Peroxidase (POD) activity
The activity of POD in colostrum was determined by the previously said method of Change and Maehly (Change & Maehly, Citation1955) with more or fewer amendments. The one-unit activity of the antioxidant POD enzyme was defined as a change in absorbance in 0.01 min−1.
2.5.2. Determination of non-enzymatic antioxidants
2.5.2.1. Total Phenolic Content (TPC)
The total phenolic contents in the colostrum were measured by the micro colorimetric method as earlier described by Ainsworth and Gillespie (Ainsworth & Gillespie, Citation2007) with slight modifications in Folin-Ciocalteau (F-C) reagent. The phenolic content (gallic acid equivalents) of samples was determined using the linear regression equation.
2.5.2.2. Total flavonoid Content (TFC)
The total flavonoid content was determined according to the aluminum chloride colorimetric method (Lin & Tang, Citation2007). The colostrum sample was homogenized with 0.1 mL of 1 M potassium acetate, 0.1 mL of 10% aluminum chloride hexahydrate, and 2.8 mL of deionized water. Soon after the forty minutes’ incubation at ambient temperature, the absorbance of the reacting solution was examined spectrophotometrically at wavelength 415 nm. Rutin was available as standard (the concentration range: 0.005 to 0.1 mg/mL) and the total flavonoid content was presented as milligram RE per g of colostrum. The absorbance at wavelength 415 nm = 14.171 curtain (mg/mL) + 0.0461, R2 = 0.9991.
2.5.2.3. Ascorbic acid
To evaluate the ascorbic acid level, an easy protocol defined by Hameed et al. (Hameed et al., Citation2005) was applied, which gives the value of only reduced ascorbic acid. A linear regression equation was calculated to find out the ascorbate saturation in unknown samples.
2.5.3. Esterase activity
The α and β-esterase were determined as per the method of Van Asperen (Van Asperen, Citation1962) using α–naphthyl acetate and β–naphthyl acetate as substrates, respectively. Using a standard curve, esterase activity was α or β naphthol made in μM min−1.
2.5.4. Other biochemical parameters
2.5.4.1. Total Oxidant Status (TOS)
TOS was evaluated by adopting the technique of Erel (Erel, Citation2005) in which the oxidation of ferrous-ion into a ferric-ion by oxidants found in colostrum sample in the acidic medium and the evaluation of ferric-ion by xylenol orange (Hameed et al., Citation2005). A standard curve was developed using H2O2. The results were shown in μM H2O2 equivalent per L.
2.5.4.2. Total Antioxidant Capacity (TAC)
The reduction of 2, 2-azino-bis (3-ethylbenzothiazoline-6-sulfonate) radical cation (ABTS•+ that is blue-green) by antioxidants to its original colorless ABTS form is the basis of the ABTS assay. The ABTS•+is discolored by antioxidants based on their antioxidant content (Nenadis et al., Citation2007). The TAC levels were recorded as milli-molar ascorbic acid equivalent to L−1.
2.5.4.3. Malondialdehyde (MDA)
The lipid oxidation in colostrum was measured in the form of malondialdehyde (MDA, a product of lipid peroxidation) content estimated by the thiobarbituric acid (TBA) reaction using a method of Heath and Packer (Heath & Packer, Citation1968) with slight modifications as defined by Dhindsa (Dhindsa et al., Citation1981). The MDA content was calculated by using an extinction coefficient of 155 mM−1 cm−1.
2.5.4.4. Protein content
The evaluation of quantitative protein in colostrum was achieved by the method of Bradford, (Citation1976). Absorbance was calculated at 595 nm by using a spectrophotometer.
2.6. Statistical analysis
All statistical analysis was made using software XL-STAT, Version 2012.1.02, Copyright Addinsoft 1995–2012 (http://www.xlstat.com). Descriptive statistics were adopted to analyze and organize the resulting data. Data were examined using ANOVA with the help of three replications. The significance of data was tested by analysis of variance and Tukey (HSD) Test at P < 0.01. Values presented in the graphs are mean ± S.E.
3. Results
3.1. Enzymatic antioxidants
The oxidant and antioxidant concentration in maternal colostrum was evaluated following Vit-E and Se injection during the prenatal period. The level of enzymatic antioxidants in colostrum is presented in Figure and Table . In treated animals, the SOD and POD activities were significantly greater than the control group while the activity of catalase enzyme was noticed lesser compared with control group (P < 0.01).
Figure 2. The influence of late gestation vitamin E and selenium injection on different enzyme activities such as SOD [A] Catalase [B] and POD [C] in colostrum. Results are expressed as Mean value ± Standard error along with data points having different letters are significantly different at P<0.01.
![Figure 2. The influence of late gestation vitamin E and selenium injection on different enzyme activities such as SOD [A] Catalase [B] and POD [C] in colostrum. Results are expressed as Mean value ± Standard error along with data points having different letters are significantly different at P<0.01.](/cms/asset/22dda19b-2e54-4324-a157-1f65311dcf9b/oafa_a_2197711_f0002_oc.jpg)
Table 1. SOD, catalase, and POD activity in colostrum. Results are presented as Mean ± Standard error and different alphabets are statistically significantly different at P < 0.01
3.2. Non-enzymatic antioxidants
The non-enzymatic antioxidants such as ascorbic acid and TFC are shown in Table and Figure respectively. The TFC in treated samples was significantly dropped than the non-treated goats (P < 0.01). However, the level of ascorbic acid was noticed significantly high in treated group in contrast to the control group (P < 0.01).
Figure 3. The influence of late gestation vitamin E and selenium injection on total flavonoids content [A] and ascorbic acid [B] level in colostrum. Results are expressed as Mean value ± Standard error along with data points having different letters are significantly different at P<0.01.
![Figure 3. The influence of late gestation vitamin E and selenium injection on total flavonoids content [A] and ascorbic acid [B] level in colostrum. Results are expressed as Mean value ± Standard error along with data points having different letters are significantly different at P<0.01.](/cms/asset/b7457ad5-0054-422d-945f-9f4d1571f9a7/oafa_a_2197711_f0003_oc.jpg)
Table 2. Total flavonoid content and ascorbic acid activity in colostrum. Results are presented as Mean ± Standard error and different alphabets are statistically significantly different at P < 0.01
3.3. Esterase activity
The enzymatic esterase activity in colostrum was evaluated and depicted in Figure and Table . The results revealed that esterase level significantly dropped in treated compared with non-treated animals (P < 0.01).
Figure 4. The influence of late gestation vitamin E and selenium injection on esterase enzyme activity in colostrum. Results are expressed as Mean value ± Standard error along with data points having different letters are significantly different at P<0.01.
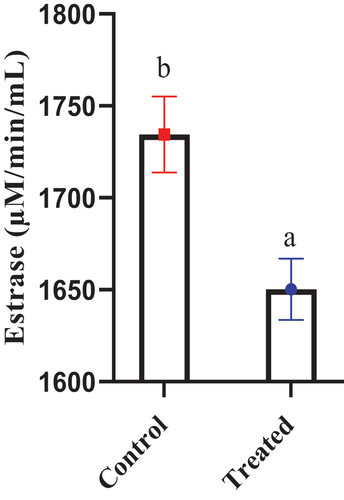
Table 3. Esterase activity in colostrum. Results are presented as Mean ± Standard error and with different alphabets are statistically significantly different at P < 0.01
3.4. Other biochemical parameters in colostrum
The total soluble proteins, total antioxidant status, and MDA concentration are depicted in Table and Figure respectively. The results showed that TAC level was significantly greater in colostrum who received Vit-E and Se as compared to the control group (P < 0.01). The injectable form of Vit-E and Se during late gestation was significantly declined TOS and MDA concentration with opponent group respectively (P < 0.01).
Figure 5. The influence of late gestation vitamin E and selenium injection on TSP [A] TOS [B] TAC [C] MDA [D] level in colostrum. Results are expressed as Mean value ± Standard error along with data points having different letters are significantly different at P<0.01.
![Figure 5. The influence of late gestation vitamin E and selenium injection on TSP [A] TOS [B] TAC [C] MDA [D] level in colostrum. Results are expressed as Mean value ± Standard error along with data points having different letters are significantly different at P<0.01.](/cms/asset/a01746f7-90a1-405e-b5d1-4e512ca81466/oafa_a_2197711_f0005_oc.jpg)
Table 4. TSP, TOS, TAC, and MDA level in colostrum. Results are presented as Mean ± Standard error and different alphabets are statistically significantly different at P < 0.01
4. Discussion
Goats are most commonly used as an income source for poor societies in developing nations; provide meat, milk, and different by-products. Therefore, its importance is considerably increasing (Lérias et al., Citation2013; Morales de la Nuez et al., Citation2014), however, the high mortality ratio of newborns constitutes a big loss for the goat-rearing farmers (Mahboub et al., Citation2011). The newborn gets abrupt exposure to oxygen in an open environment that interrupts the physiological milieu and eventually produces oxidative stress (Torres-Cuevas et al., Citation2017). The first milk produced after parturition is enriched with various antioxidants which hold the competency to deal with oxidative stress. Several studies showed a better survival rate of kids who consumed colostrum instantly afterbirth (EHernandez-Castellano et al., Citation2014). Colostrum also contains some nutritionally essential components which cannot traverse the placental barrier during gestation period. Hence, the composition and quality of colostrum need to be improved by providing different nutrition and supplementation to the dam (Kasapovic et al., Citation2005; Morsy et al., Citation2019). Results are presented as Mean ± Standard error and different alphabets are statistically significantly different at P < 0.01 as superscript in Table .
The saturation of enzymatic antioxidants in colostrum ensures protection against the harmful effects of ROS (Kasapovic et al., Citation2005). The negative effect of ROS becomes evident once the antioxidants have failed to neutralize ROS which ultimately damage biomolecules for such as, proteins, DNA and lipids. The basal level of ROS is maintained by antioxidant enzymatic system including SOD, catalase, and G-Px. SOD plays a key role in the transformation of O− towards O2 and then to H2O2, whereas, catalase and GPx make the conversion of H2O2 into H2O and molecular oxygen (Abou-Zeina et al., Citation2015). In current experiment, the injectable practice of Vit-E and Se in prenatal period has improved antioxidant levels of SOD and POD. This increased concentration of enzymatic antioxidants is to neutralize the toxic oxidant products from the colostrum. Vit-E and Se enhanced the transcription of the genes encoding the amino acid sequence that produces enzymatic antioxidant proteins in colostrum. According to previous studies, Vit-E and Se boost up action of cellular primary enzymatic antioxidants for instance SOD and GPx, (Horky et al., Citation2013; Lelyon et al., Citation2012).
Goat colostrum is a good source of various lipid and water-soluble antioxidants like flavonoids with potent antioxidant activity and considered as the exogenous antioxidant. The presence of different flavonoids in colostrum or milk mainly depends upon the feed intake of animals from different green fodders or grasses hence, the injectable form of Vit-E and Se doesn’t have any effect on its concentration (De Feo et al., Citation2006). In our results, antioxidant supplementation had no effect on flavonoid concentration in colostrum suggesting that treatment was ineffective during prenatal period in goats.
Vitamin C is an important water-soluble antioxidant present in milk and can detoxify alkoxy radicals, superoxide anion, and singlet oxygen by donating its electron (Franco & Martínez-Pinilla, Citation2017). Ascorbic acid and Vit-E collectively improve the photo-oxidative stability and taste in colostrum. Ascorbic acid inhibits the degradation of riboflavin in milk once is it exposed to light (Hagemeier et al., Citation2018). Normally, animals do not consume ascorbic acid as an antioxidant, and its level must be maintained in the maternal blood to secrete in lactation to meet the requirements of newborns. In our experiment, a high level of ascorbic acid was noted in colostrum followed by antioxidant exposure. Animals supplemented with Vit-E and Se strengthen the milk oxidative stability and hindered lipid peroxidation, consequently, improving milk quality (Castillo et al., Citation2013). Our results revealed that combined effect of Vit-E and Se enhanced overall maternal antioxidant potential and improved the antioxidant defense to inhibit the over-production of free radicals.
Proteins are involved in the production of enzymatic antioxidants, immunoglobulins, and other structural and functional biomolecules, hence, cannot be replaced with any other molecule (Langer, Citation2009). Protein transportation in colostrum or milk is mainly based on the nutrition intake and additional supplementation to mother throughout the pregnancy. A deficient supply of required proteins may lead to morbidity or death of newborns in early life (Morsy et al., Citation2019). The present study pointed out that the total protein content in colostrum remained at the upper limit in antioxidant-treated animals during the late pregnancy. It can be assured that parental antioxidant supplementation especially Se provided positive effect on overall protein content. High level of proteins in treated group indicated the sufficient amount of selenium to be involved in protein manufacturing. The results showed that antioxidant-treated pregnant goats may have satisfactory amount of proteins for the supply in colostrum to boost-up antioxidant defense of new born kids.
The results of the present study declared that the amount of total protein in colostrum was higher which reinforced its antioxidant action and presented a positive association with TAC. The TAC is well-define activity of all antioxidants present in the colostrum such as non-enzymatic and enzymatic antioxidants, all vitamins, and unknown scavengers of free radicals (Zarban et al., Citation2009). Our results illustrated that animals that received antioxidants showed a high level of antioxidant capacity and low level of MDA which proposes that there was a strong and sufficient level of antioxidants to defend the initiation or propagation of chain reaction for lipid peroxidation in colostrum. Whereas the non-treated pregnant goats didn’t show such results. Our findings are in line with the previous studies in which improved concentration of TAC and reduced level of lipid peroxidation in colostrum and milk were observed followed by antioxidant treatments during late gestation (Marta & Justyna, Citation2008).
5. Conclusion
The injectable form of vitamin E (2.5 mg/kg, BW) and Se (2.2 mg/kg BW) in late gestation ameliorated the oxidant effect on colostrum by enhancing the antioxidant indices and consequently improving its quality. Further studies should be conducted offering diverse antioxidants approach during late pregnancy to explore the effect on colostrum as well as milk quality and immunoglobulin levels. The molecular approach in this aspect should also be investigated.
Acknowledgments
We are thankful to Mr. Mujahid Hussain, Deputy Chief Scientist for his kind support to conduct this research experiment at Animal Farm, NIAB.
Disclosure statement
Author reported no any conflict of interest.
Additional information
Funding
References
- Abou-Zeina, H. A., Nasr, S. M., Abdel-Aziem, S. H., Nassar, S. A., & Mohamed, A. M. (2015). Effect of different dietary supplementation with antioxidants on gene expression and blood antioxidant markers as well as thyroid hormones status in goat kids. Middle-East Journal of Scientific Research, 23(6), 993–11.
- Ainsworth, E. A., & Gillespie, K. M. (2007). Estimation of total phenolic content and other oxidation substrates in plant tissues using folin–ciocalteu reagent. Nature Protocols, 2(4), 875–877. https://doi.org/10.1038/nprot.2007.102
- Albera, E., & Kankofer, M. (2009). Antioxidants in colostrum and milk of sows and cows. Reproduction in Domestic Animals, 44(4), 606–611. https://doi.org/10.1111/j.1439-0531.2007.01027.x
- Albera, E., & Kankofer, M. (2011). The comparison of antioxidative/oxidative profile in blood, colostrum and milk of early post‐partum cows and their newborns. Reproduction in Domestic Animals, 46(5), 763–769. https://doi.org/10.1111/j.1439-0531.2010.01737.x
- Alyaqoubi, S., Abdullah, A., Samudi, M., Abdullah, N., Addai, Z. R., & Al-Ghazali, M. (2014). Effect of different factors on goat milk antioxidant activity. International Journal of ChemTech Research, 6(5), 3091–3196.
- Beers, R. F., & Sizer, I. W. (1952). A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. The Journal of Biological Chemistry, 195(1), 133–140. https://doi.org/10.1016/S0021-9258(19)50881-X
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254. https://doi.org/10.1016/0003-2697(76)90527-3
- Castillo, C., Pereira, V., Abuelo, Á., & Hernández, J. (2013). Effect of supplementation with antioxidants on the quality of bovine milk and meat production. Scientific World Journal, 2013, 1–8. https://doi.org/10.1155/2013/616098
- Change, B., & Maehly, A. (1955). Assay of catalases and peroxidase. Methods in Enzymology, 2, 764–775. https://doi.org/10.1016/S0076-6879(55)02300-8
- De Feo, V., Quaranta, E., Fedele, V., Claps, S., Rubino, R., & Pizza, C. (2006). Flavonoids and terpenoids in goat milk in relation to forage intake. Italian Journal of Food Science, 18(1), 85–92.
- Dhindsa, R. S., Plumb-Dhindsa, P., & Thorpe, T. A. (1981). Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany, 32(1), 93–101. https://doi.org/10.1093/jxb/32.1.93
- EHernandez-Castellano, L, M., Almeida, A., Castro, N., & Arguello, A. (2014). The colostrum proteome, ruminant nutrition and immunity: A review. Current Protein & Peptide Science, 15(1), 64–74. https://doi.org/10.2174/1389203715666140221124622
- Erel, O. (2005). A new automated colorimetric method for measuring total oxidant status. Clinical Biochemistry, 38(12), 1103–1111. https://doi.org/10.1016/j.clinbiochem.2005.08.008
- Franco, R., & Martínez-Pinilla, E. (2017). Chemical rules on the assessment of antioxidant potential in food and food additives aimed at reducing oxidative stress and neurodegeneration. Food Chemistry, 235, 318–323. https://doi.org/10.1016/j.foodchem.2017.05.040
- Giannopolitis, C. N., & Ries, S. K. (1977). Superoxide dismutases: I. Occurrence in higher plants. Plant Physiology, 59(2), 309–314. https://doi.org/10.1104/pp.59.2.309
- Gökçe, E., Cihan, P., Atakişi, O., Kirmizigül, A. H., & Erdoğan, H. M. (2022). Oxidative stress in neonatal lambs and its relation to health status and passive colostral immunity. Veterinary Immunology and Immunopathology, 251, 110470. https://doi.org/10.1016/j.vetimm.2022.110470
- Gong, J., & Xiao, M. (2016). Selenium and antioxidant status in dairy cows at different stages of lactation. Biological Trace Element Research, 171(1), 89–93. https://doi.org/10.1007/s12011-015-0513-2
- Hagemeier, J., Ramanathan, M., Schweser, F., Dwyer, M. G., Lin, F., Bergsland, N., Zivadinov, R. … Zivadinov, R. (2018). Iron-related gene variants and brain iron in multiple sclerosis and healthy individuals. NeuroImage: Clinical, 17, 530–540. https://doi.org/10.1016/j.nicl.2017.11.003
- Hameed, A., Iqbal, N., Malik, S. A., Syed, H., & Ahsanul-Haq, M. (2005). Age and organ specific accumulation of ascorbate in wheat (triticum aestivum l.) seedlings grown under etiolation alone and in combination with oxidative stress. https://hdl.handle.net/1807/5384
- Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125(1), 189–198. https://doi.org/10.1016/0003-9861(68)90654-1
- Horky, P., Ruttkay-Nedecky, B., Kremplova, M., Krystofova, O., Kensova, R., Hynek, D., Adam, V., & Adam, V. (2013). Effect of different doses of organically bound selenium on antioxidant status and levels of metal ions in postpartum sows. International Journal of Electrochemical Science, 8, 6162–6179. http://electrochemsci.org/papers/vol8/80506162.pdf
- Kasapovic, J., Pejic, S., Mladenovic, M., Radlovic, N., & Pajovic, S. B. (2005). Superoxide dismutase activity in colostrum, transitional and mature human milk. The Turkish Journal of Pediatrics, 47(4), 343–347.
- Kirikci, K., & Çam, M. A. (2020). The importance of colostrum on neonate development in ruminants. Bahri Dağdaş Hayvancılık Araştırma Dergisi, 9(2), 162–166.
- Langer, P. (2009). Differences in the composition of colostrum and milk in eutherians reflect differences in immunoglobulin transfer. Journal of Mammalogy, 90(2), 332–339. https://doi.org/10.1644/08-MAMM-A-071.1
- Lelyon, B., Chatellier, V., & Daniel, K. (2012). Production strategies after the end of milk quotas: An analysis based on bio-economic modeling. INRA Productions Animales, 25(1), 67–76. https://doi.org/10.20870/productions-animales.2012.25.1.3198
- Lérias, J. R., Hernández-Castellano, L. E., Morales DelaNuez, A., Araújo, S. S., Castro, N., Argüello, A., Almeida, A. M., & Almeida, A. M. (2013). Body live weight and milk production parameters in the majorera and palmera goat breeds from the canary islands: Influence of weight loss. Tropical Animal Health and Production, 45(8), 1731–1736. https://doi.org/10.1007/s11250-013-0423-2
- Lérias, J. R., Hernández-Castellano, L. E., Suárez-Trujillo, A., Castro, N., Pourlis, A., & Almeida, A. M. (2014a). The mammary gland in small ruminants: Major morphological and functional events underlying milk production–a review. The Journal of Dairy Research, 81(3), 304–318. https://doi.org/10.1017/S0022029914000235
- Lérias, J. R., Hernández-Castellano, L. E., Suárez-Trujillo, A., Castro, N., Pourlis, A., & Almeida, A. M. (2014b). The mammary gland in small ruminants: Major morphological and functional events underlying milk production-a review. The Journal of Dairy Research, 81(3), 304–318. https://doi.org/10.1017/S0022029914000235/
- Lin, J. -Y., & Tang, C. -Y. (2007). Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chemistry, 101(1), 140–147. https://doi.org/10.1016/j.foodchem.2006.01.014
- Lipko-Przybylska, J., & Kankofer, M. (2012). Antioxidant defence of colostrum and milk in consecutive lactations in sows. Irish Veterinary Journal, 65(1), 4. https://doi.org/10.1186/2046-0481-65-4
- Mahboub, H. D., Darwish, R. A., Ramadan, S. G., Helal, M. A., & Gaafar, K. M. (2011). The effect of protected fat and selenium-vitamin e supplementation during late pregnancy on performance of local egyptian goat breeds and survival of their kids. 11th sci. Cong, Egyptian Society for Cattle Diseases, 5, 37–46.
- Marta, K., & Justyna, L. -P. (2008). Physiological antioxidative/oxidative status in bovine colostrum and mature milk. Acta Veterinaria, 58(2–3), 231–239. https://doi.org/10.2298/AVB0803231K
- Morales de la Nuez, A., Moreno-Indias, I., Sánchez-Macías, D., Hérnandez-Castellano, L., Suárez-Trujillo, A., Assunção, P., Capote, J., & Capote, J. (2014). Effects of crypthecodinium cohnii, chlorela spp. And isochrysis galbana addition to milk replacer on goat kids and lambs growth. Journal of Applied Animal Research, 42(2), 213–216. https://doi.org/10.1080/09712119.2013.827579
- Morsy, A., Eissa, M., Anwer, M., Ghobashy, H., Sallam, S., Soltan, Y., Sadik, W., & Sadik, W. (2018). Colostral immunoglobulin concentration and milk production of ewes fed salt tolerant forages as alternatives to berseem hay. Livestock Science, 210, 125–128. https://doi.org/10.1016/j.livsci.2018.02.012
- Morsy, A., El-Zaiat, H., Saber, A., Anwer, M., & Sallam, S. (2019). Impact of organic selenium and vitamin e on rumen fermentation, milk production, feed digestibility, blood parameters and parasitic response of lactating goats. Journal of Agricultural Science and Technology, 21(7), 1793–1806.
- Nenadis, N., Lazaridou, O., & Tsimidou, M. Z. (2007). Use of reference compounds in antioxidant activity assessment. Journal of Agricultural and Food Chemistry, 55(14), 5452–5460. https://doi.org/10.1021/jf070473q
- Prince, K., Khan, M. S., Ijaz, M., Anjum, A. A., Prince, A., Khan, N. U., & Khan, M. A. (2017). Effect of prepartum vitamin e and selenium on antibody transfer in colostrum and cattle calves. Pakistan Journal of Zoology, 49(6). https://doi.org/10.17582/journal.pjz/2017.49.6.2057.2066
- Torres-Cuevas, I., Parra-Llorca, A., Sánchez-Illana, A., Nuñez-Ramiro, A., Kuligowski, J., Cháfer-Pericás, C., Vento, M., & Vento, M. (2017). Oxygen and oxidative stress in the perinatal period. Redox Biology, 12, 674–681. https://doi.org/10.1016/j.redox.2017.03.011
- Van Asperen, K. (1962). A study of housefly esterases by means of a sensitive colorimetric method. Journal of Insect Physiology, 8(4), 401–416. https://doi.org/10.1016/0022-1910(62)90074-4
- Zarban, A., Taheri, F., Chahkandi, T., Sharifzadeh, G., & Khorashadizadeh, M. (2009). Antioxidant and radical scavenging activity of human colostrum, transitional and mature milk. Journal of Clinical Biochemistry and Nutrition, 45(2), 150–154. https://doi.org/10.3164/jcbn.08-233