Abstract
To evaluate the impact of intervention strategies on helminthiasis, the nutritional and cognitive status of School-Age Children (SAC), we conducted a community trial with 4 intervention arms; “Nutrition Education Only” (NutEd), “Supplementation Only” (Suppl), “Nutrition Education+Supplementation” (NutEd+Suppl) versus a ‘Non-intervention‘(Control) group. The intervention was conducted on 358 SAC from 8 randomly selected schools (4 schools from fishing and 4 schools from farming communities) in the Kwahu Afram Plains South District of Ghana. Data were collected at baseline, third, and sixth-month post-intervention. Data were obtained through questionnaire, anthropometry, parasitology (faecal and urine analysis), hemoglobin levels (Hb) and whole blood zinc levels. The Ravens Colored Progressive Matrices (RCPM) were used for the cognitive assessment. At baseline, the overall helminthiasis prevalence was 29.6%, which decreased to 6.3% at the 6th-month post-intervention. About 7.5% of all the children were underweight, 11.2% were stunted, 21.5% were acutely malnourished, 46.1% were anemic and 31.4% were zinc deficient. These decreased to 5.3% (underweight), 8.5% (stunting), 9.8% (acute malnutrition), 16.1% (anemia) and 9.3% (zinc deficiency), respectively, at the 6th-month post-intervention. At baseline, 15.6% of the children passed the cognitive test which increased to 32.4% at the 6th-month post-intervention. The “NutEd” treatment recorded the most significantly improved of bmi-for-age z-score (0.27 ± 0.88, p = 0.002), height-for-age z-score (0.16 ± 0.38, p < 0.0001) and anemia (Hb levels; 1.22 ± 1.13 g/dL, p < 0.0001), The “NutEd+Suppl” group recorded the highest improvement in zinc levels (46.39 ± 22.30 µmol/L, p < 0.0001) and the “Suppl” group the highest improvement in cognitive performance (3.08 ± 6.07, p < 0.0001) between the baseline and the 6th-month post-intervention.
1. Introduction
Helminthiasis is a parasitic infection caused by either soil-transmitted helminths (STH) and/or fresh water-transmitted schistosomes (schistosomiasis), and it remains one of the most common infections globally, affecting mostly deprived communities, where poor sanitation and hygiene exist (Gizaw et al., Citation2019). Poor sanitation is implicated in several disease conditions, such as STH infections, trachoma, lymphatic filariasis and schistosomiasis (Clasen et al., Citation2012). Also, because schistosomiasis is transmitted in infested freshwater, streams and lakes, school‐aged children (SAC) are mostly at greater risk of exposure to these infections (Uneke, Citation2010).
Ghana has a high burden of helminthiasis, with schistosomiasis being the most prevalent type (70.9%) (Rollinson et al., Citation2013). Moreover, the burden of under-nutrition among children globally is still high, especially in Low-Middle-Income Countries (LMICs), with Ghana among the 36 countries with the highest prevalence of stunting (Black et al., Citation2008). Childhood undernutrition is a significant public health burden with recent rates of stunting, wasting and underweight in Ghana being 19%, 5% and 11%, respectively (GSS & Demographic, Citation2015).
Infections with helminths tend to increase the nutritional burden of their host since their prevailing relationship with the host is primarily parasitic in nature. For instance, infections by Schistosoma haematobium, causes blood loss, and also have a strong association with iron-deficiency anemia among children (M. A. Tandoh et al., Citation2015, Citation2020). Impairment of physical growth (Wolde et al., Citation2015) and poor cognitive performance (Ezeamama et al., Citation2005) have also been implicated with an increased burden of helminthiasis. Hookworm infections have also been implicated in iron-deficiency anemia due to intestinal blood losses (Crompton & Nesheim, Citation2002). Factors such as community of residence (Danquah et al., Citation2012; Fentiman et al., Citation2001), schistosomiasis infection (Mahgoub et al., Citation2010), and pica behavior (ingestion of non-food items) (Geissler et al., Citation1998; M. A. Tandoh et al., Citation2020), have been implicated in poor nutritional outcomes of children. Similarly, Humphries et al. (Citation2011) also reported some behavioral factors such as lack of latrine, walking barefooted, engaging in farming as an occupation and poor nutritional status as risk factors for hookworm infection.
To reduce the public health burden of helminth infection and its associated morbidities, the World Health Organization (WHO) has developed intervention strategies to address the burden of helminthiasis, especially among school-age children since they are considered the most at-risk group (Montresor et al., Citation2002). The primary aim of their proposed intervention strategy is to ameliorate the load of parasitic infections and also keep prevalence rates at low levels, which will have the potential of improving growth and learning abilities among SAC (Montresor et al., Citation2002). The proposed strategies by WHO targeted at helminth control have focused on four key areas which include the use of, 1. mass drug administration (also known as preventive chemotherapy/periodic drug/deworming treatment) with the primary aim of controlling morbidity, 2. clean water and sanitation through the prevention of helminth infection of humans by avoidance of freshwater contact, 3. health and hygiene education, which focus on improved hygiene behavior and sanitation habits leading to reduced contamination of the environment and water bodies; and 4. biological/chemical control of vector snails which transmits the parasite in fresh water bodies (in the case of schistosomiasis) (Montresor et al., Citation2002; Rollinson et al., Citation2013).
The main drug for schistosomiasis in sub-Saharan Africa is praziquantel (Utzinger et al., Citation2011) and is reported to be a safe and effective drug. The reported cure rate of praziquantel in helminthiasis treatment is 85–90% (Olliaro etal., Citation2011). However, reinfection rates can be high and is estimated to be 94% by the 12th month post treatment (Gass et al., Citation2014). Hence, there is a need for additional interventional strategies to control the disease (Urbani et al., Citation2002).
Nutritional interventions have been suggested as part of the strategies that can be integrated with a deworming program targeted at SAC to improve nutrition status and nutrient deficiencies due to chronic helminthiasis (Montresor et al., Citation2002; Solon et al., Citation2003). This is particularly important as the global prevalence of micronutrient undernutrition is over 2 billion (Chakrabarty et al., Citation2010), and infections with helminth place an extra burden on the nutritional status of children (Hailegebriel, Citation2018; M. A. Tandoh et al., Citation2015, Citation2020). The use of micronutrient supplementation, for instance, has been suggested for areas where iron deficiency anemia is a public health problem (Montresor et al., Citation2002). For instance, supplementation with iron (60 mg elemental iron) and folate (0.40 mg) weekly has been suggested in such areas (Risonar et al., Citation2008).
Micronutrient supplementation has also been found to improve cognitive performance of children (Solon et al., Citation2003), and also to decrease helminth infection rates (Nchito et al., Citation2009; Nga et al., Citation2011). Thus, together with periodic deworming and micronutrient supplementation, health/nutrition education and behavioral change may be additional important intervention strategies that could be employed in the control of helminth infections. The inclusion of health education in school-based deworming program has been found to reduce the cost of deworming, delay re-infection and improve overall knowledge and acceptability of deworming interventions (Gyorkos et al., Citation2013; Mascarini-Serra, Citation2011).
In spite of all the advantages associated with the above-mentioned strategies for the reduction of helminth infections and improvement of nutritional and cognitive deficits in children, there is a lack of evidence-based “nutrition education” intervention that is sustainable in LMICs. A few studies have focused on “nutrition education” as a long-term sustainable approach to provide nutritional knowledge of locally available foods that would improve the nutritional status and resultant cognitive performance of children in helminth-endemic areas (Bhutta et al., Citation2013; Headey, Citation2013). Thus, the aim of this study was to examine the effectiveness of different interventions to improve on helminth infections, nutritional status and cognitive performance of school-age children living in helminth endemic fishing and farming communities in rural Ghana.
2. Methodology
2.1. Study design
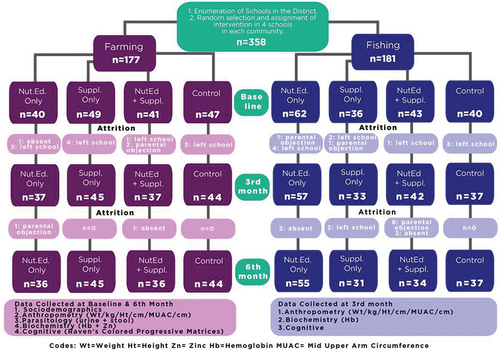
This study was a community level trial which was a follow-up to a previous study conducted to assess helminth and undernutrition burden of school-age children (M. A. Tandoh et al., Citation2020). This intervention study was conducted among 358 (9–12-year-old) school-age children in fishing and farming communities of the Kwahu Afram Plains South District of Ghana.
This community trial consisted of 3 months intervention with four intervention arms; nutrition education only (NutEd), micronutrient supplement only (Suppl), nutrition education plus micronutrient supplement (NutEd+Suppl) and a control (no intervention). Data was also collected on sociodemographic, anthropometrics, dietary and cognitive assessment (at baseline, 3rd, and 6th-month post intervention) whilst parasitology (schistosomes, roundworms, whipworms and hookworms infections) by stool and urine and the biochemical parameters (hemoglobin and zinc) were assessed at baseline and the 6th-month post-intervention.
2.2. Ethical considerations
Ethical clearance to conduct the study was obtained from the University of Georgia Human Subjects Institutional Review Board (MOD00005562) and the Kwame Nkrumah University of Science and Technology Committee on Human Research, Publication and Ethics (CHRPE/AP/355/18). Permission was also sought from the Regional and District Directors of the Ghana Health Services and the Ghana Education Service before the commencement of the study. Furthermore, permission was sought from community leaders, headmasters and teachers of the respective schools, which were randomly selected.
2.3. Sampling method
Multistage sampling was employed. A random sample of 4 farming and 4 fishing communities was selected from a list of all schools in the fishing and farming communities in the Kwahu Afram Plains South District obtained from the School Health and Education Program (SHEP) Coordinator in the district. A random selection of four (4) schools from each of the two communities (fishing and farming) were randomly assigned to one-arm of the intervention treatment. Informed consent and assent were obtained from parent and index child, respectively.
2.4. Intervention arms of the study
The participating children in all the treatment arms were dewormed at baseline using either 400 mg of albendazole for soil-transmitted helminth infections or 40 mg of praziquantel/kg body weight for schistosome infections. The “tablet pole” was used to determine the number of praziquantel tablets to be administered to each child (WHO, Citation2011). The choice of deworming drugs used was based on the predominant type of helminth infection observed from the previous study in the district (M. Tandoh et al., Citation2018). From that study, STH infection (Trichuris and Hookworm infections) occurred among children in the farming community (9.6%) with no case in the fishing community (p = 0.007), whereas S. haematobium infection predominantly occurred among children in the fishing community (33.8%) versus 1.2% in their farming community counterparts (p < 0.0001).
The “NutEd” group involved educating children on good nutrition and health practices (hygiene and sanitation), such as the washing of hands with soap and water after toilet-use and before eating, avoiding open defecation, not walking bare footed, avoiding the habit of fingernail biting, not swimming in the river among others (WHO, Citation2011). Whilst the nutrition component focused on avoiding pica behavior, increasing the consumption of a varied diet and providing general information on locally available foods (Aagaard-Hansen, Citation2009, Gyorkos et al., Citation2013, Nock, Citation2006). This intervention was given once a week for three (3) months by a trained teacher in the school using a self-structured nutrition and health education manual for trainers and a nutrition education material for children (Supplemental 1 and 2). To test for their understanding, children were assessed on their nutritional knowledge with a self-structured in-class knowledge test on helminths, and also given homework on food and sanitation records (Supplemental 3).
The “Suppl” group involved the use of two (2) micronutrient supplements (6.5 mg FeSO4) (Stoltzfus et al., Citation1998), and (10 mg ZnSO4) (Hotz and Brown, Citation2004), administered three (3) times a week for three (3) months by trained head-teachers of the school. These two supplements were chosen because zinc is a micronutrient that is essential for optimum growth and immune function of children (Walker & Black, Citation2007). In addition to this, iron supplementation is recommended in places with high iron deficiency (Risonar et al., Citation2008), and where urinary schistosomiasis is endemic, and the most effective strategy is to combine anthelminthic chemotherapy with iron supplementation to treat potential severe anemia (Stoltzfus et al., Citation1998). Commercially available supplements containing these micronutrients were used, Zinc sulphate tablets (Zintab Zinc sulphate monohydrate BP) and ferrous sulphate tablets (Ferrous Sulphate BP). The contents of the two micronutrient supplements were verified at the Food and Drug Authority of Ghana (Certificate number: PCM-18/06/0129). Zinc sulphate has been the reference compound for zinc availability because of its widespread use and low cost (Solomons et al., Citation2011). Iron absorption from ferrous sulphate has also been found to be greater than either forms of iron (iron+Na2EDTA) (Pérez-Expósito et al., Citation2005).
The third group received a combination of Nutrition Education and Supplementation (NutEd+Suppl). This group received nutrition education (i.e. good nutrition, hygiene and sanitation practices) once a week for three (3) months, and the two (2) micronutrient supplementation (6.5 mg FeSO4 and 10 mg ZnSO4) three (3) times a week for three (3) months.
2.5. Socio-demographic data collection
Index children and their parents or primary caregivers were assisted by trained research assistants to complete a questionnaire that collected information on age, sex, ethnicity, sanitation and other demographic information. In this study, a modified questionnaire based on the Child Form-Parasitological Nutrition Survey recommended by the WHO as a guide for managers of helminth control was used (WHO, 2011).
2.6. Anthropometrics
The anthropometric indicators of subjects were determined using a standardized protocol (Gibson, Citation2005). Weight (in kg), height (in cm) and mid-upper arm circumference (MUAC) (in cm) were taken in duplicates by trained personnel at baseline, the 3rd month and the 6th-month post-intervention. The anthropometric indices were achieved by the measurement of the children’s weight (kg) to the nearest 0.1 kg in light clothing using a Seca scale, whilst the height (cm) was measured to the nearest 1 mm without shoes using a Seca stadiometer, and MUAC (cm), to the nearest 1 mm using a non-stretch flexible tape. The mean of the respective anthropometric measure was then estimated at every point to obtain the final anthropometric value. Cut-offs for MUAC used were <13.5 cm for severe acute malnutrition, ≥13.5–14.5 cm for moderate acute malnutrition and ≥ 14.5 cm for well nourished for 9 year-olds, whereas <16.0 cm for severe acute malnutrition, ≥16.0 cm-18.5 cm for moderate acute malnutrition and ≥ 18.5 cm for well nourished, were for the 10–14-year-old children. For the purpose of statistical analysis, moderate acute malnutrition and severe acute malnutrition were merged and reclassified as acute malnutrition.
With the use of a WHO anthropometric calculator (Anthro Plus version 1.0.3, http://who anthroplus.software.informer.com/1.0/), the information obtained for weight and height were used to compute the BMI-for-Age Z score (BAZ), which determines underweight, whilst that of the height was used to compute for Height-Age-Z- score (HAZ), which determines the degree of stunting. Participants who fell below minus two standard deviations (−2SD) from the median of the reference population were classified as moderately malnourished (underweight/stunted), whereas those who fell below minus three standard deviations (−3SD) were classified as severely malnourished, and those above −2SD classified as normal. For the purpose of statistical analysis, severe underweight was merged with underweight. Likewise, severe stunting was merged with stunting.
2.7. Biochemical assessment
At baseline of data collection and 6th-month post-intervention, 5 mL of whole morning random venipuncture uncoagulated blood samples were collected from subjects by trained phlebotomists for micronutrient analysis (zinc and Hb). However, at the 3rd month of the data collection, the finger prick method was used to collect blood samples for Hb analysis only. The hemoglobin was assessed using the HemoCue Hb 201+ analyzer. Cut offs for Hb levels used were; <11.5 g/dL (anemic) for the 5–11 year olds, and <12.0 g/dL (anemic) for the 12–13 year olds (Stoltzfus et al., Citation1998).
2.8. Digestion of blood samples
Digestion of the whole blood samples preceded the spectroscopic analysis by adding 2 mL of whole blood samples to a 50 mL volumetric digestion flask, and to that, 5 mL of H2SO4 and 2 mL of 1:1 HNO3:HCLO4 (1:1 v/v) were added. The resulting solution was then heated on a hot plate at 200°C ±5°C for 30 min until the sample became colorless. This was then diluted with double distilled water to the 50 mL mark, and allowed to cool and transferred in 50 ml falcon tubes for zinc readings with the flame atomic absorption spectroscopy (Okyere et al., Citation2015).
2.9. Measurement of zinc levels
The flame atomic absorption spectroscopy (FAAS) was used to measure the zinc levels in the blood samples by following standard protocols (Gibson, Citation2005), using an atomic absorption spectrophotometer with a single-beam optical mode (Analytikjena model novAA400P). The cut-offs for zinc deficiency for morning random blood samples were used to classify zinc values as either normal or deficient (<9.9 µmol/L for all children less than 10 years, <10.7 µmol/L for male children≥10-years, and <10.1 µmol/L for female children≥10-years) based on standardized protocols (Gibson, Citation2005).
2.10. Parasitic infections assessment
Trained personnel collected the stools and urine samples on scheduled days in the various schools. The participants who were unable to provide stool or urine samples on a designated day were asked to provide them the following day. During the time of the registration of the study participants (children), they were provided with labelled plastic stool and urine containers, as well as directions on the quantity of urine and stool required to be deposited safely into each container provided. Trained research assistants were assigned to collect the stool and urine samples of the study participants at designated points in the various schools. The samples were kept in a cool ice chest and transported to an on-site laboratory where the presence of intestinal worms, such as Trichuris trichiura, S. mansoni, Ascaris lumbricoides and hookworm (Ancylostoma duodenale and/or Necator americanus) were determined using microscopy. The Kato-Katz (WHO, Citation1994) and the urine filtration methods (WHO, Citation1991) were used, respectively, for STH and urinary schistosomes infections, which were done at both baseline and 6th-month post-intervention.
To estimate for the number of eggs counted per 10 gram of stool (epg), a factor of 24 was used to multiply the total number of helminth eggs counted under the microscope (Williams, Citation1992). Based on the epg obtained, the infections were classified as light (1–999), moderate (1000–9.999) or heavy (≥10,000) intensity for Trichuris; and light (1–1,999), moderate (2,000–3,999) or heavy (≥4000) intensity for Hookworm infections (Montresor et al., Citation2002).
To test for the presence of blood in the urine samples, a urine test strip was used. Furthermore, the urine filtration method was used to filter 10 mL of urine to separate the existing eggs for microscopic analysis and counting. Preceding that, a visual inspection of the urine samples was done to determine visible hematuria, which is usually a clear sign of S. haematobium infection (World Health Organization, Citation1987). The examination of 10 mL of the urine sample for the presence of S. haematobium eggs was done by a trained laboratory technician. Based on the number of eggs counted, the intensity of S. haematobium was then classified based on the following with cut-off point of low (<50 eggs/10 mL urine) and high (>50 eggs/10 mL) (Montresor et al., Citation2002).
2.11. Assessment of cognitive performance
The cognitive performance of all subjects was assessed using the Raven’s Colored Progressive Matrices (RCPM) test scores obtained at baseline, 3rd month and 6th-month (Raven, Citation2003). The RCPM was the choice of cognitive assessment tool because it is a non-verbal assessment tool, and hence overcomes issues of language barrier. It is made up of 36 items in three sets of 12 (A, Ab, and B) and had previously been piloted and used in similar study communities (M. A. Tandoh et al., Citation2020; Whaley et al., Citation2003).
2.12. Statistical analysis
The IBM SPSS Version 24 for Windows (SPSS Inc., Cary, NC, USA) was used for all the data analyses For the categorical variables, such as gender, infection status, and nutritional status, proportions, were calculated. The Chi-squared (χ2) and the Fischer Exact tests were used to perform a bivariate analysis where necessary to determine significant differences between the categorical variables. For the continuous variables, such as age, weight, test scores, the frequencies means, median and standard deviations were determined and an independent t-test was used to determine significant differences. Paired sample T-test was used to compare differences in the nutritional variables and cognitive test scores with the intervention arms at the different time points (baseline and 6 months post-intervention). Furthermore, the univariate logistic regression analysis was used to determine the associations between nutritional status and cognitive test scores and the type of intervention given. The multivariate logistic regression was used to determine the independent predictors of reduction in stunting, underweight, anemia, zinc deficiency and improvement in cognitive performance. The criterion for statistical significance was set at p-value <0.05.
3. Results
3.1. Characteristics of the study participants by intervention
The details of the characteristics of study participants by intervention grouping are given in Table . Most of all the parents interviewed were mothers (46.6%), with the “NutEd+Suppl” group having the highest representative of mothers (57.1%) and the least from the “NutEd” group (36.3%) (p < 0.0001). Furthermore, almost half of all the participants (45.8%) were “Ewes,” with a majority (59.5%) of them falling in the “NutEd+Suppl” group (p < 0.0001). Similarly, about a third of all the parents were farmers, with significant difference between the groups (p < 0.0001).
Table 1. Characteristics of Study Participants by Intervention Type
Over half of the participants obtained their food from their farms, with a majority in the “Control” group (92.0%), compared to the others (p < 0.0001). In addition, 42.7% of all the parents did not have formal education, with the highest proportion in the “Control” group (57.5%) and the least in the “NutEd” group (30.4%).
3.2. Assessment of underweight, stunting, anemia and cognition by intervention
Table also shows that at baseline, 27 (7.5%) of the children were underweight, whilst 40 (11.2%) were stunted, which decreased to 17 (5.3%) and 27 (8.5%), respectively, at post-intervention.
Table also shows that overall, 162 (46.1%) of the participants were anemic and 108 (31.4%) were zinc deficient at baseline, and this decreased to 50 (16.1%) anemia, and 25 (9.3%) zinc deficiency by the 6th-month. Table also shows that overall, at baseline, 56 (15.6%) of the children passed the cognitive test (scored 50% or higher), and this increased to 104 (32.4%) by the 6th-month post-intervention (Table ).
3.2.1. Helminthiasis prevalence and symptoms by community type and study period
Table represents the specific type of helminth infection prevalence and some symptoms based on the community type and the study period. At baseline, visible hematuria (blood in urine) was present in 3.4% of overall urine samples collected, with none occuring in the farming communities, whilst the fishing communities recorded 6.6% (p < 0.0001). This decreased to 0.9% and 1.9%, respectively, by the end of the study (6th month) (p = 0.079). Microhematuria (microscopic blood in urine) was also observed at 15.4% (overall) and 30.4% (fishing communities), with none occuring at the farming communities. Microhematuria also reduced to 3.8% (overall) and 5.7 (fishing) by the 6th month; however, it was observed in the farming communities (1.9%). S. haematuria was also prevalent at 22.3% (overall), 0.6% (farming) and 43.6% (fishing) communities (p < 0.0001). This also reduced to 3.8% (overall), and 7.6% (fishing), with none occurring in the farming communities at the 6th month (p < 0.0001). S. mansoni infection also occurred at 0.6% (overall), 1.1% (fishing), with none occuring in the farming communities (p = 0.161). This also reduced in the 6th month such that none occurred in the subjects. Hookworm infection occurred at 4.7% (overall), 9.0% (farming) and 0.6% (fishing) communities (p < 0.0001). This also reduced to 1.6% (overall), 2.5% (farming) and 0.6% (fishing) communities by the 6th month (p = 0.183). Trichuris infection also occurred at baseline at 1.1% (overall), 2.3% (farming), with none in the fishing communities (p = 0.047). This also reduced to 0.9% (overall) and 1.9% (farming) with none occuring in the fishing communities (p = 0.085). H. nana infection also occurred at 1.45 (overall), 2.3% (farming) and 0.6% (fishing) communities (p = 0.169) at the baseline and decreased to 0.3% (overall) and 0.6% (farming), with none occuring in the fishing communities (p = 0.321). Other helminths (Trichostrongylus spp and Dicrocoellium spp) were also present at 2.0% overall, 1.7% (farming) and 2.2% (fishing) communities at baseline (p = 0.725), but this no longer occurred at the 6th month (Table ).
Table 2. Helminth type and symptoms by community type and intervention period
3.3. Helminth infections and intervention type
Table gives the results of the helminth infection prevalence by the type of intervention at both the baseline and at 6 months post-intervention. At baseline, 106 (29.6%) of the children had at least one type of helminth infection. The highest prevalence of helminth infection at baseline occurred in the “Suppl” group [31 (36.5%)], with the least infection [16 (19.0%)] in the “NutEd+Suppl” group which was not significant (p = 0.079). By the 6th-month post-intervention, the overall prevalence of helminth infection had decreased to 20 (6.3%).
Table 3. Total helminthiasis prevalence by intervention type
Table represents helminthiassis symptoms and the specific helminth infection prevalence at both baseline and 6 months post intervention. At baseline, visible hematuria (or blood in the urine) was present in 12 (3.4%) of urine samples collected, with the highest prevalence 5 (5.9%) occuring in the “Suppl” group, and none in the “NutEd” group (p = 0.130). At the end of the study, only 3 (0.9%) of the children presented with visible hematuria, with 2 children from the “Suppl” group (2.6%), and none occurring in both the “NutEd+Suppl” group and the “Control” group (p = 0.285). Microhematuria was observed in 55 (15.4%) of children at baseline, with the highest prevalence occurring in the “NutEd” group [25 (24.5%)], and the least occurring in the “NutEd+Suppl” group [2 (2.4%)] which was highly significant (p < 0.0001). At the end of the study, microhematuria was observed in 12 (3.8%) of the children, with the highest recorded in the “NutEd” group [6 (6.5%)] and the least in the “Control” group [1 (1.2%)] which was not significant (p = 0.317). The S. haematobium prevalence was 80 (22.3%) at baseline, with the highest recorded prevalence occurring in the “NutEd” group [30 (29.4%)] and the least in the “NutED+Suppl” group [10 (11.9%)] which was significantly different (p = 0.037). Only two (2) children (0.6%) had S. mansoni infection (overall) at baseline, with 1 in the “NutEd” group and the other in the “Control” group (p = 0.610). By the 6th-month post-intervention, there was no S. mansoni infection observed. Hookworm prevalence was 17 (4.7%) at baseline, with the highest occurring in the “Suppl” group 7 (8.2%), and the least occurring in the “NutEd” group [2 (2.0%)] (p = 0.257). Hookworm prevalence at post-intervention was recorded in 5 children (1.6%), with the “Suppl” group having the highest prevalence of 2 (2.6%), and all the other intervention arms recorded one infection each which was also not significant between them (p = 0.858). The overall Trichuris prevalence at baseline was 4 (1.1%), 2 (2.4%) in the ‘NutEd+Suppl group, with none in the “NutEd” group (p = 0.499). Re-infection of this also occurred in 3 (0.9%) children at the 6th-month post-intervention, with none occurring in both the “NutEd+Suppl” and the “Control” group (p = 0.285). H. nana infection was recorded in 5 (1.4%) children at baseline, with the highest 4 (4.7%) occurring in the “Suppl” group and none observed in both the “NutEd+Suppl” group and the “Control” group which was significantly different between the intervention arms (p = 0.025). At the end of the intervention, only 1 (0.3%) infection was observed in the “Suppl” group (1.3%). Other helminths like Dicrocoelium spp and Trichostrongylus spp were also recorded at baseline [7 (2.0%)], with the highest in the “NutEd” group [3 (2.9%)], and none in the “NutEd+Suppl” group (p = 0.509). No infection with these other species were recorded at the end of the study.
Table 4. Helminthiasis symptom and type by intervention period
3.4. Mean anthropometric values and intervention type
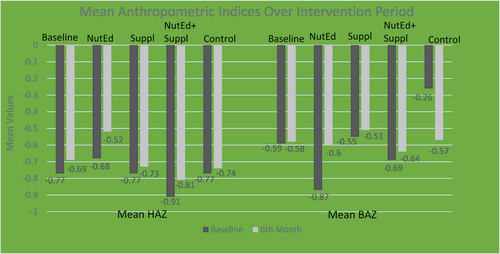
Figure shows the anthropometric indices; Height-for-age z-scores (stunting) and BMI-for-age z-scores [BAZ (underweight)] of the children at baseline and after the intervention. At baseline, the lowest mean HAZ was (−0.91 ± 1.02) among the “NutEd+Suppl” group with the highest occurring in the “NutEd” group (−0.68 ± 1.02). At post-intervention, the highest HAZ occurred in the “NutEd” group’ (−0.52 ± 1.00). Figure also shows that at baseline, the lowest mean BAZ occurred in the “NutEd” group (−0.87 ± 1.16), with the highest occurring in the “Control” group (−0.26 ± 0.65). At the 6th-month post-intervention however, the highest mean BAZ occurred in the “Suppl” group (−0.51 ± 0.87).
3.5. MUAC by intervention
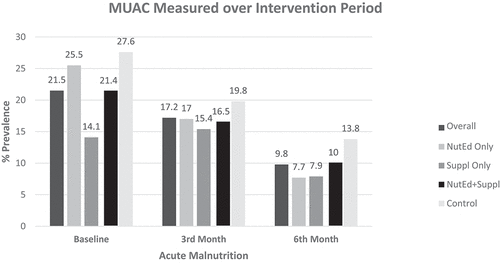
Figure presents the mid-upper arm circumference (MUAC) of the participants based on the different treatment groups. At baseline, 21.5% of all the children in the study had acute malnutrition. There was a general reduction in the rate of acute malnutrition from baseline to the 6th-month for all treatment groups, with the greatest decrease occurring in the “NuteEd” group (25.5% to 7.7%), and the least reduction occurring in the “Suppl” group [(14.1% to 7.9%) (p = 0.534)].
3.6. Anemia and zinc deficiency and intervention
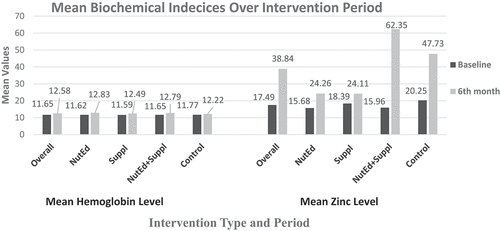
Figure shows the mean biochemical indices (mean Hb and zinc) of the children based on the different intervention arms at baseline and 6th-month post-intervention. The lowest mean Hb at baseline occurred in the “Suppl” group (11.59 ± 0.98 g/dL), with the highest occurring in the “Control” group (11.79 ± 0.83 g/dL). By the 6th-month, the highest Hb was recorded in the “NutEd” group (12.83 ± 0.96 g/dL).
3.7. The mean differences within the baseline and the 6th-month anthropometry and biochemical indices and intervention type
Table represents the mean differences within the baseline and the 6th-month anthropometry and biochemical indices based on the intervention groups. Using Paired sample t-test, Table shows that stunting rates significantly improved in the “NutEd” group and the “NutEd+Suppl” group.
Table 5. Differences in Anthropometric and Biochemical Indices for Intervention Groups
The “NutEd” group had the highest mean difference of 0.16 ± 0.38 (p < 0.0001), followed by the “NutEd+Suppl” group, with a mean difference of 0.10 ± 0.040 (p = 0.04) between the baseline HAZ values and the 6th-month values. Although the “Suppl” group and the “Control” groups also improved in stunting rates, the improvement did not reach statistical significance (Table ).
Table also shows a significant improvement in the underweight rates (BAZ) such that the “NutEd” group had a significant increase in their BAZ between their baseline and the 6th-month values [(0.27 ± 0.81) (p = 0.002)]. However, the other intervention groups did not have a significant increase in their mean differences of BAZ between their baseline and 6th-month post-intervention values.
Table also shows that the “NutEd” group had the highest mean difference in Hb levels between the baseline and 6th-month measures [(1.22 ± 1.13 g/dL) (p < 0.0001)], whereas the “Suppl” group had a mean difference of 0.91 ± 0.99 g/dL (p < 0.0001), with the “Control” group having the least mean difference of 0.46 ± 1.21 g/dL (p < 0.0001).
With zinc deficiency, Table shows that the lowest mean zinc levels at baseline occurred in the “NutEd” group (15.68 ± 10.02 µmol/L), with the highest occurring in the “Control” group (20.25 ± 10.30 µmol/L). At the 6th-month, the highest mean zinc level occurred in the “NutEd+Suppl” group (62.35 ± 19.5 µmol/L). Table also shows that a significant difference was observed in all the intervention arms, with the highest mean difference recorded in the “NutEd+Suppl” group [(46.39 ± 22.3 µmol/L) (p < 0.0001)] and the least recorded in the “NutEd” group [(8.58 ± 16.38 µmol/L) (p < 0.0001)].
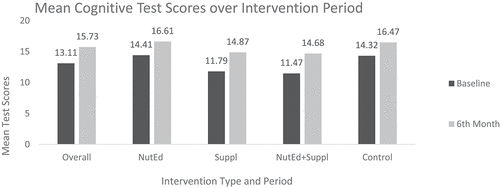
3.8. Cognitive test scores by intervention at different time points
Figure shows the mean cognitive test scores of the children based on the different interventions arms at baseline and 6th-month post-intervention. At baseline, the lowest mean test scores occurred in the “NutEd+Suppl” group (11.47 ± 6.13), with the highest occurring in the “NutEd” group (14.41 ± 4.98). Similarly, at post-intervention, the highest mean test score was recorded in the “NutEd” group (16.61 ± 6.55). Table further presents results of analysis with the Paired sample T- test. It shows that the overall mean cognitive test scores increased significantly by 2.62 ± 5.77 (p < 0.0001) at post intervention. However, the highest mean difference in cognitive test scores occurred among the “Suppl” group [(3.08 ± 6.07) (p < 0.0001)], whereas the least mean difference in cognitive test scores was recorded among the “Control” group [(2.15 ± 5.39) (p = 0.001)].
Table 6. Differences in cognitive indices within intervention groups
3.9. Predictors of anthropometric, biochemical measures of undernutrition and cognitive performance based on intervention type
Table shows the anthropometric and biochemical indicators of undernutrition at baseline and at the end of the study. The results showed a significant decrease in stunting among the “NutEd” group compared to the “Control” group, such that they were 79% less likely to be stunted (OR = 0.21, CI; 0.04–0.99, p = 0.049) by the intervention. No significant improvement was observed among all the other intervention groups. A multivariate analysis adjusting for child age and community type further confirmed that the “NutEd” group was an independent predictor of decreased stunting (AOR = 0.20, CI; 0.04–0.98, p = 0.048), such that they were 80% less likely to be stunted compared to the “Control” group (Table ). No significant associations were observed between the different intervention arms and underweight (p > 0.05) (Table ). However, with anemia, there were significant associations with the “NutEd” group and the “NutEd+Suppl” group compared to the “Control” group (OR = 0.29, CI; 0.13–0.69, p = 0.005) and (OR = 0.34, CI; 0.13–0.85, p = 0.021) respectively. The “NutEd” group were 71% less likely to be anemic, whereas the “NutEd+Suppl” group were also 66% less likely to be anemic compared to the “Control” group. A multivariate analysis adjusting for child age and community type further revealed that the “NutEd” group (AOR = 0.33, CI; 0.14–0.78, p = 0.012) and the “NutEd +Suppl” group (AOR = 0.33, CI; 0.13–0.85, p = 0.022) were independent predictors of anemia, such that they were both 67% less likely to be anemic as a result of the interventions.
Table 7. Predictors of anthropometric, biochemical measures of undernutrition and cognitive performance based on intervention type
Furthermore, an increased risk for zinc deficiency was observed among the “NutEd” group’ and “Suppl” group’. “NutEd” group’ was found to be 6.4 times more likely to be zinc deficient compared to the “Control” group (OR = 6.40, CI; 1.39–29.50, p = 0.02), with the “Suppl” group also being 6 times more likely to be zinc deficient compared to the “Controls” (OR = 6.04, CI = 1.27–28.77, p = 0.02). However, adjusting for child age and community using multivariate statistical analysis further revealed that the “NutEd” group (AOR = 7.08, CI = 1.52–33.05, p = 0.022) and the “Suppl” group (AOR = 5.98, CI = 1.25–25.59, p = 0.025) were independent predictors of increased risk of zinc deficiency, being 7 times and 6 times, respectively, more likely to be zinc deficient (Table ). No significant associations were observed between the different intervention arms and the cognitive test scores (p > 0.05).
4. Discussion
In this study, a majority of the parents/caregivers of the participants were farmers (70.4%). At baseline, helminth infection prevalence was 106 (29.6%), with the most common parasite being S. haematobium (22.3%), but no Ascaris infection was observed. S. haematobium infection was mostly prevalent among the fishing communities (22.3%) compared to just (0.6%) in the farming community. This is not surprising as schistosomiasis is transmitted in infested freshwater streams and lakes, thus children living close to such water bodies are mostly at greater risk of exposure to the infection (Uneke, Citation2010). Furthermore, certain factors such as poor hygienic practices and play habits, which involve direct contact with infested freshwater bodies through swimming are considered to propagate the spread of helminth infections (Gryseels et al. Citation2006, Knopp et al., Citation2013). This is similar to our previous study on the disparities of sanitary conditions/habits and helminth prevalence in farming and fishing communities in Ghana, which showed that a majority of the children (62.8%) swam in the Afram River, with a significantly higher proportion of them being in the fishing communities (78.8%) compared to the farming communities (47.6%) (p < 0.001). Similarly, S. haematobium infection was also found to be significantly higher among children in the fishing communities (33.8%) compared to those in the farming communities (1.2%) (p < 0.001) (M. Tandoh et al., Citation2018). Other helminths observed in this study were Trichostrongylus spp and Dicrocoelium spp (2%), with 2.2% in the fishing and 1.7% in the farming communities, which was surprising given that Trichostrongylus spp is known to mainly parasitize the small intestines of mammals and birds, but some species have been reported to have affected about 70% of the humans mainly in African and Asian countries (Roberts & Janovy, Citation2000). Dicrocoelium spp is also reported to be mainly found in ruminants, and rarely found in humans, with a lot of reported cases of human infections being false parasitism (Roberts & Janovy, Citation2000).
Based on the type of interventions given in our study, the prevalence of helminth infections was highest in the ‘Suppl’group [31 (36.5%)] compared to the other intervention arms which was not significant (p = 0.079), specifically, S. haematobium infection was more in the “NutEd” group 30 (29.4%) compared to the other groups (p = 0.037). Since urinary schistosomiasis is acquired through contact with freshwater bodies, it is plausible that the “NutEd” group had more contact with a fresh water body, since about a third of them (62 out of 102) lived in the fishing community close to a fresh water body (Appendix 1).
Our study also showed that the overall prevalence of helminths re-infection at the 6th-month was 20 (6.3%) compared to the 106 (29.6%) rate of infection at baseline. This overall lower helminth reinfection rates is laudable since it has been reported that helminth reinfection and intensity of infection can reach pre-treatment levels in as few as 6 months, with 94% reinfection after 12 months (Gass et al., Citation2014). This is consistent with another community directed intervention study conducted in Kenya, in which after 6 months post-treatment with praziquantel and albendazole as an integrative treatment for schistosomiasis and STH, the prevalence rates of S. mansoni, hookworm and Trichuris decreased by 33.2%, 69.4% and 42.6%, respectively (Mwinzi et al., Citation2012). Our finding is however in contrast to a randomized double-blind placebo study conducted to ascertain the efficacy of a fortified biscuit (FB) on the growth, cognition and parasitic infections among Vietnamese SAC between 6 to 8 years. Children who were on both the fortified biscuit (FB) and albendazole had the lowest level of helminthiasis prevalence after 4 months of intervention (Nga et al., Citation2011). It was also observed in our study that all the intervention arms had a lower helminth infection rate at the 6th-month post-intervention compared to their baseline prevalence. The lower infection of helminthiasis observed among the “NutEd” group (from 32.4% to 7.6%) in our study, is consistent with another paired-match cluster-randomized trial by Gyorkos et al. (Citation2013) that employed a school-based health hygiene education intervention, and found it to decrease the prevalence of Ascaris and improve helminthiasis knowledge of SAC in the Peruvian Amazon (Gyorkos et al., Citation2013).
Findings from our study also showed that the “NutEd” group had the highest improvement in mean blood hemoglobin levels from baseline to the 6th-month which was highly significant (p < 0.0001). Subsequent analysis using univariate logistic regression analysis revealed that both “NutEd” group and the “NutEd+Suppl” group were 67% less likely to be anemic (OR = 0.33, CI; 0.14–0.78, p = 0.012) and (OR = 0.33, CI; 0.13–0.85, p = 0.022), respectively. Our findings also revealed that all the various interventions resulted in decreased numbers of underweight (BAZ) among the SAC, but the best amongst them was the “NutEd” group which recorded the most significant improvement (p = 0.002). Thus, the provision of quality nutrition education has the potential to decrease anemia and underweight among SAC in helminth-endemic areas. This finding corroborate the community-based study of a South African study which concluded that education program in combination with the food aid successfully improved the weight status of the children (Walsh et al., Citation2002). Our finding is also consistent with a randomized controlled study in Lushoto district of the Tanga Region of Tanzania, which included 7–15 year old SAC and focused their intervention messages on factors such as personal hygiene, clean water and good nutrition. At the end of their intervention period, the study participants (children) showed an increased knowledge in good health practices, such as handwashing with soap after toilet use, etc. The researchers found improvements in knowledge and behavior that promoted overall health (Lansdown et al., Citation2002).
At the end of our study, the highest improvement in zinc deficiency was observed in the “NutEd+Suppl” group (p < 0.0001) and the least was among the “NutEd” group. Although the highest mean difference in zinc levels was observed in the “NutEd+Suppl” group, we found that contrary to the positive effect of the NutEd’ on the anemia, children who fell into this group and the “Suppl” group, respectively, were 6.4 and 6.0 times more likely to be zinc deficient. This was in contrast to a randomized controlled trial in Peru that administered a daily supplement of 10 mg of zinc or a multiple micronutrient showed a greater improvement in two (2) zinc groups; the zinc only supplementation (27.3 µg/dL) and the zinc+other vitamins and minerals group (6.2 µg/dL), compared to the placebo group (6.1 µg/dL) which was highly significant (p < 0.0001) (Penny et al., Citation2004). This could possibly be attributed to a lower compliance rate of the micronutrient supplementation in our study.
The “NutEd” group also showed a significant decrease in stunting rates by the 6th-month (p < 0.0001), followed by the “NutEd+Suppl” group (p = 0.041). In addition to that, the “NutEd” group was the only intervention group that was significantly associated with decreased stunting rates (p = 0.049) based on the univariate logistic regression analysis, as they were found to be 79.0% less likely to be stunted, and also, the best predictor for decreased stunting based on multivariate analysis such that they were 80% less likely to be stunted (p = 0.048). The results, however, do not corroborate the findings of a South African study that found no significant improvement of stunting rates using nutrition education intervention (Walsh et al., Citation2002).
Our study also found that various interventions resulting in the reduction in the prevalence rate of acute malnutrition in the children, although marginally significant (p = 0.0534). However, at the end of the 6th-month, those in the “NutEd” group recorded the highest reduction rate of acute malnutrition (from 25.5% to 7.7%) compared to the other groups (p = 0.534). This suggests that nutrition education, if planned and implemented well, could be the most beneficial intervention strategy to reduce acute malnutrition in children in helminth-endemic areas. There is therefore the need to complement deworming programs with nutrition education for a better nutritional outcome in SAC.
In terms of cognitive performance, the “Suppl” group recorded the highest mean improvement in cognitive test scores (p < 0.0001). This is consistent with a study conducted in the Philippines using a multi-fruit fortified drink for 16 weeks, in which they found that children who were iron and/or iodine deficient at baseline and received the fortified beverage improved their non-verbal ability test scores (Solon et al., Citation2003). Similarly, another randomized double-blind placebo study conducted to ascertain the efficacy of a fortified biscuit (FB) on the growth, cognition and parasitic infections among Vietnamese SAC between 6 to 8 years showed that, children who received the FB for 4 months scored higher on two cognitive test (RCPM and the Digit Span Forward test) (Nga et al., Citation2011).
5. Limitation of the study
This study had some limitations including some attrition by the end of the study (Appendix 1), which affected the overall sample size. Furthermore, the compliance could not be ascertained because the scope of the study did not factor close monitoring, this is because with the micronutrient supplementation, some children in the intervention arm missed school and did not take the full intervention according to the protocol. However, records of the micronutrient supplement administration were kept by head teachers to ensure a maximum compliance of the intervention. Finally, compliance to the nutrition education could not be guaranteed as we were unable to monitor the home environment, but training was given to selected teachers in the schools (Supplemental 1), who gave reminders of the nutrition education once a week to the children throughout the intervention period using the nutrition education material for children (Supplemental 2). Children were also assessed on the nutrition education given once a month by the trained teachers to ensure the maximum understanding of the contents (Supplemental 3).
6. Conclusion
Overall, the “NutEd” treatment exhibited the most significantly improved mean outcomes in terms of BAZ, HAZ and anemia (Hb levels), whilst the “NutEd+Suppl” group had the highest improvement in blood zinc, with the “Suppl” group exhibiting the highest improvement in the mean difference in baseline and 6th-month outcomes of cognitive performance. The best predictors for improved nutritional indices were the ‘NutEd’group (decreased risk of stunting and anemia) and the “NutEd+Suppl” group (decreased risk of anemia).
Findings from this study have proven to some extent that nutrition education, if well planned and accompanied by deworming, can be effective in decreasing the high prevalence of undernutrition such as underweight, stunting, acute malnutrition, zinc deficiency and anemia among school children in helminth-endemic areas, as well as improve on their cognitive performances. There is therefore a need for the integration of comprehensive nutrition education approaches in school-based interventions for a more effective and sustainable nutritional and cognitive outcomes among SAC in helminth-endemic areas. Thus, similar intervention studies are needed to confirm the effectiveness of the intervention strategies of the present study and subsequent scaling-up.
Availability of data
The data set for this study will be available upon reasonable request from Dr. Marina Aferiba Tandoh ([email protected])
Authors contributions
MAT: Proposal development, data collection, entry and analysis, as well as manuscript writing and review.
FCMR: Study implementation, data interpretation and review of draft manuscript.
RAA: Statistical analysis and interpretation of the data.
MDW: Proposal concept development and reviewing of draft manuscript.
AKA: Overseeing to the proposal development, data collection, data entry and analysis, in addition to the writing and review of the manuscript.
All authors read and approved the final versions of the manuscript.
Supplemental Material
Download Zip (1.6 MB)Acknowledgments
We would like to acknowledge the headmasters, teachers and pupils of Trebu D/A Primary School, Anglican Primary School, Eche D/A Primary School, Kwasi Fante D/A Primary School, Nyamebekyere D/A Primary School, Kwaekese Primary School, Samankwae Primary and Asanyansu Primary School for their assistance with the data collection. As well as the Regional and District Directors of Education and Ghana Health Service, Afram Plains South District, Eastern Region, Ghana. We also acknowledge the support provided by the staff of the Holy Spirit Health Center. We would also like to acknowledge the KNUST Staff, especially Dr. Charles Apprey, Mr. Frank Agyemang Bonsu, Mr. Odeafo Asamoah and Mr. Nat Ato Yawson) and the Laboratory Technicians of the Central and Food Science Lab at KNUST for assisting with the laboratory studies.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/23311932.2023.2201032.
Additional information
Funding
Notes on contributors
M. A. Tandoh
Dr. Marina Aferiba Tandoh is a lecturer at the Department of Biochemistry (Human Nutrition and Dietetics), KNUST, Ghana. She has a BSc. Biochemistry (KNUST, Ghana), an MPhil in Dietetics (University of Ghana) and a PhD in Foods and Nutrition (University of Georgia, U.S.A).
F. C. Mills-Robertson
Prof. Felix Charles Mills-Robertson is an Associate Professor at KNUST, with a PhD degrees in Biochemistry from the University of Ghana.
R. A. Annan
Dr Reginald Adjetey Annan is senior lecturer at KNUST, with a PhD in Human Nutrition and a masters degree in public health nutrition (University of Southampton, UK).
M. D. Wilson
Prof. Michael David Wilson has over 35 years of experience in diseases prevention and control; including 5 years as a biologist with the research arm of the WHO Onchocerciasis Control Programme in West Africa.
A. K. Anderson
Prof. Alex Kojo Anderson is a professor at the Department of Nutritional Sciences at the College of Family and Consumer Sciences (University of Georgia).
References
- Aagaard-Hansen, J., Mwanga, J., & Bruun, B. (2009). Social science perspectives on schistosomiasis control in Africa: Past trends and future directions. Parasitology, 136, 1747–23.
- Bhutta, Z. A., Das, J. K., Rizvi, A., Gaffey, M. F., Walker, N., Horton, S., Webb, P., Lartey, A., Black, R. E., & Group TLNIR. (2013). Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? The Lancet, 382(9890), 452–477. https://doi.org/10.1016/S0140-6736(13)60996-4
- Black, R. E., Allen, L. H., Bhutta, Z. A., Caulfield, L. E., De Onis, M., Ezzati, M., Mathers, C., Rivera, J., & Maternal & Group CUS. (2008). Maternal and child undernutrition: Global and regional exposures and health consequences. The Lancet, 371(9608), 243–260. https://doi.org/10.1016/S0140-6736(07)61690-0
- Chakrabarty, S., Bharati, P., Berry, J. W., & Grant, J. S. (2010). Verbal and physical abuse experienced by family caregivers of adults with severe disabilities. Italian Journal of Public Health, 7(2), 76–84.
- Christine, H., & Brown, K. H. (2004). ”Assessment of the risk of zinc deficiency in populations and options for its control.”. S91–204.
- Clasen, T., Boisson, S., Routray, P., Cumming, O., Jenkins, M., Ensink, J. H., Bell, M., Freeman, M. C., Peppin, S., & Schmidt, W. -P. (2012). The effect of improved rural sanitation on diarrhoea and helminth infection: Design of a cluster-randomized trial in Orissa, India. Emerging Themes in Epidemiology, 9(1), 7. https://doi.org/10.1186/1742-7622-9-7
- Crompton, D. W. T., & Nesheim, M. (2002). Nutritional impact of intestinal helminthiasis during the human life cycle. Annual Review of Nutrition, 22(1), 35–59. https://doi.org/10.1146/annurev.nutr.22.120501.134539
- Danquah, A., Amoah, A., Steiner-Asiedu, M., & Opare-Obisaw, C. (2012). Nutritional status of participating and non-participating pupils in the Ghana school feeding programme. Journal of Food Research, 1(3), 263. https://doi.org/10.5539/jfr.v1n3p263
- Ezeamama, A. E., Friedman, J. F., Acosta, L. P., Bellinger, D. C., Langdon, G. C., Manalo, D. L., Olveda, R. M., Kurtis, J. D., & Mcgarvey, S. T. (2005). Helminth infection and cognitive impairment among Filipino children. The American Journal of Tropical Medicine and Hygiene, 72(5), 540–548. https://doi.org/10.4269/ajtmh.2005.72.540
- Fentiman, A., Hall, A., & Bundy, D. (2001). Health and cultural factors associated with enrolment in basic education: A study in rural Ghana. Social Science & Medicine, 52(3), 429–439. https://doi.org/10.1016/S0277-9536(00)00152-0
- Gass, K., Addiss, D. G., Freeman, M. C., & Clements, A. C. (2014). Exploring the relationship between access to water, sanitation and hygiene and soil-transmitted helminth infection: A demonstration of two recursive partitioning tools. PLoS Neglected Tropical Diseases, 8(6), e2945. https://doi.org/10.1371/journal.pntd.0002945
- Geissler, P. W., Mwaniki, D. L., Thiong’o, F., Michaelsen, K. F., & Friis, H. (1998). Geophagy, iron status and anaemia among primary school children in Western Kenya. Tropical Medicine & International Health, 3(7), 529–534. https://doi.org/10.1046/j.1365-3156.1998.00272.x
- Gibson, R. S. (2005). Principles of nutritional assessment. Oxford university press.
- Gizaw, Z., Addisu, A., & Dagne, H. (2019). Effects of water, sanitation and hygiene (WASH) education on childhood intestinal parasitic infections in rural Dembiya, northwest Ethiopia: An uncontrolled before-and-after intervention study. Environmental Health and Preventive Medicine, 24(1), 16. https://doi.org/10.1186/s12199-019-0774-z
- Gryseels, B., Polman, K., Clerinx, J., & Kestens, L. (2006). Human schistosomiasis. The Lancet, 368, 1106–1118.
- GSS, G., & Demographic, I. G. (2015). Health Survey 2014. Ghana Statistical Service (GSS), Ghana Health Service (GHS) and ICF International.
- Gyorkos, T. W., Maheu-Giroux, M., Blouin, B., Casapia, M., & de Silva, N. (2013). Impact of health education on soil-transmitted helminth infections in schoolchildren of the Peruvian Amazon: A cluster-randomized controlled trial. PLoS Neglected Tropical Diseases, 7(9), e2397. https://doi.org/10.1371/journal.pntd.0002397
- Hailegebriel, T. (2018). Undernutrition, intestinal parasitic infection and associated risk factors among selected primary school children in Bahir Dar, Ethiopia. BMC Infectious Diseases, 18(1), 394. https://doi.org/10.1186/s12879-018-3306-3
- Headey, D. D. (2013). Developmental drivers of nutritional change: A cross-country analysis. World Development, 42, 76–88. https://doi.org/10.1016/j.worlddev.2012.07.002
- Humphries, D., Mosites, E., Otchere, J., Twum, W. A., Woo, L., Jones-Sanpei, H., Harrison, L. M., Bungiro, R. D., Benham-Pyle, B., & Bimi, L. (2011). Epidemiology of hookworm infection in Kintampo North Municipality, Ghana: Patterns of malaria coinfection, anemia, and albendazole treatment failure. The American Journal of Tropical Medicine and Hygiene, 84, 792–800.
- Knopp, S., Person, B., Ame, S. M., Mohammed, K. A., Ali, S. M., Khamis, I. S., Rabone, M., Allan, F., Gouvras, A., Blair, L., Fenwick, A., Utzinger, J., & Rollinson, D. (2013). Elimination of schistosomiasis transmission in Zanzibar: Baseline findings before the onset of a randomized intervention trial. PLoS Neglected Tropical Diseases, 7(10), e2474. https://doi.org/10.1371/journal.pntd.0002474
- Lansdown, R., Ledward, A., Hall, A., Issae, W., Yona, E., Matulu, J., Mweta, M., Kihamia, C., Nyandindi, U., & Bundy, D. (2002). Schistosomiasis, helminth infection and health education in Tanzania: Achieving behaviour change in primary schools. Health Education Research, 17(4), 425–433. https://doi.org/10.1093/her/17.4.425
- Mahgoub, H., Mohamed, A., Magzoub, M., Gasim, G., Eldein, W., Ahmed, A., & Adam, I. (2010). Schistosoma mansoni infection as a predictor of severe anaemia in schoolchildren in eastern Sudan. Journal of Helminthology, 84(2), 132–135. https://doi.org/10.1017/S0022149X09990368
- Mascarini-Serra, L. (2011). Prevention of soil-transmitted helminth infection. Journal of Global Infectious Diseases, 3(2), 175. https://doi.org/10.4103/0974-777X.81696
- Montresor, A., Crompton, D. W., Gyorkos, T. W., & Savioli, L. (2002). Helminth control in school-age children: A guide for managers of control programmes. World Health Organization.
- Mwinzi, P. N., Montgomery, S. P., Owaga, C. O., Mwanje, M., Muok, E. M., Ayisi, J. G., Laserson, K. F., Muchiri, E. M., Secor, W. E., & Karanja, D. M. (2012). Integrated community-directed intervention for schistosomiasis and soil transmitted helminths in western Kenya–a pilot study. Parasites & Vectors, 5(1), 182. https://doi.org/10.1186/1756-3305-5-182
- Nchito, M., Geissler, P. W., Mubila, L., Friis, H., & Olsen, A. (2009). The effect of iron and multi-micronutrient supplementation on Ascaris lumbricoides reinfection among Zambian schoolchildren. Transactions of the Royal Society of Tropical Medicine and Hygiene, 103(3), 229–236. https://doi.org/10.1016/j.trstmh.2008.08.005
- Nga, T. T., Winichagoon, P., Dijkhuizen, M. A., Khan, N. C., Wasantwisut, E., & Wieringa, F. T. (2011). Decreased parasite load and improved cognitive outcomes caused by deworming and consumption of multi-micronutrient fortified biscuits in rural Vietnamese schoolchildren. The American Journal of Tropical Medicine and Hygiene, 85(2), 333–340. https://doi.org/10.4269/ajtmh.2011.10-0651
- Nock, I. H., T, A., & Galadima, M. (2006). Deworming: Adding public health education to the equation. Trends in Parasitology, 22, 7–8.
- Okyere, H., Voegborlo, R., & Agorku, S. (2015). Human exposure to mercury, lead and cadmium through consumption of canned mackerel, tuna, pilchard and sardine. Food Chemistry, 179, 331–335. https://doi.org/10.1016/j.foodchem.2015.01.038
- Olliaro, P. L., Vaillant, M. T., Belizario, V. J., Lwambo, N. J., Ouldabdallahi, M., Pieri, O. S., Amarillo, M. L., Kaatano, G. M., Diaw, M., & Domingues, A. C. (2011). A multicentre randomized controlled trial of the efficacy and safety of single-dose praziquantel at 40 mg/kg vs. 60 mg/kg for treating intestinal schistosomiasis in the Philippines. PLoS neglected tropical diseases, 5(6), e1165.
- Penny, M. E., Marin, R. M., Duran, A., Peerson, J. M., Lanata, C. F., Lönnerdal, B., Black, R. E., & Brown, K. H. (2004). Randomized controlled trial of the effect of daily supplementation with zinc or multiple micronutrients on the morbidity, growth, and micronutrient status of young Peruvian children. The American Journal of Clinical Nutrition, 79(3), 457–465. https://doi.org/10.1093/ajcn/79.3.457
- Pérez-Expósito, A. B., Villalpando, S., Rivera, J. A., Griffin, I. J., & Abrams, S. A. (2005). Ferrous Sulfate is more bioavailable among preschoolers than other forms of iron in a milk-based weaning food distributed by PROGRESA, a national program in Mexico. The Journal of Nutrition, 135(1), 64–69. https://doi.org/10.1093/jn/135.1.64
- Raven, J. (2003). Raven Progressive Matrices. In R. S. McCallum (Ed.), Handbook of Nonverbal Assessment. Boston, MA: Springer. doi:10.1007/978-1-4615-0153-4_11
- Risonar, M., Tengco, L., Rayco-Solon, P., & Solon, F. (2008). The effect of a school-based weekly iron supplementation delivery system among anemic schoolchildren in the Philippines. European Journal of Clinical Nutrition, 62(8), 991. https://doi.org/10.1038/sj.ejcn.1602809
- Roberts, L. S., & Janovy, J., Jr. (2000). Gerald D. Schmidt e Larry S. Roberts' foundations of parasitology. In D. Gerald & L. Schmidt (Eds.), Roberts ' foundations of parasitology (6th ed., pp. xviiii–670). Boston: McGraw-Hill.
- Rollinson, D., Knopp, S., Levitz, S., Stothard, J. R., Tchuenté, L.A., Garba, A., Mohammed, K. A., Schur, N., Person, B., Colley, D. G., & Utzinger, J. (2013). Time to set the agenda for schistosomiasis elimination. Acta tropica, 128(2), 423–440. https://doi.org/10.1016/j.actatropica.2012.04.013
- Solomons, N., Romero-Abal, M., Weiss, G., Michalke, B., & Schűmann, K. (2011). Bioavailability of zinc from NutriSet zinc tablets compared with aqueous zinc sulfate. European Journal of Clinical Nutrition, 65(1), 125–131. https://doi.org/10.1038/ejcn.2010.198
- Solon, F. S., Sarol, J. J., Bernardo, A. B., Solon, J. A. A., Mehansho, H., Sanchez-Fermin, L. E., Wambangco, L. S., & Juhlin, K. D. (2003). Effect of a multiple-micronutrient-fortified fruit powder beverage on the nutrition status, physical fitness, and cognitive performance of schoolchildren in the Philippines. Food and Nutrition Bulletin, 24(4_suppl_1), S129–140. https://doi.org/10.1177/15648265030244S110
- Stoltzfus, R. J., Dreyfuss, M. L., & Organization, W. H. (1998). Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia. Ilsi Press Washington^ eDC DC.
- Tandoh, M., Mills-Robertson, F., Wilson, M., & Anderson, A. (2018). Disparities of sanitary conditions/habits and helminthiasis prevalence between school-age -children living in fishing and farming communities in Ghana: A cross sectional study. Journal of Infectious Diseases Treat, 2018, 4(2), 6.
- Tandoh, M. A., Mills-Robertson, F. C., Wilson, M. D., & Anderson, A. K. (2020). Nutritional and cognitive deficits of school-age children: a study in helminth-endemic fishing and farming communities in Ghana. Nutrition & Food Science, 50(3), 443–462.
- Tandoh, M. A., Steiner-Asiedu, M., Otchere, J., Daisie, L. A., Appawu, M. A., & Wilson, M. D. (2015). Helminthiasis burden and nutritional status of non-enrolled school-aged children in irrigated farmimg communities in Bongo District, Ghana. European Journal of Experimental Biology, 5(1), 8–17.
- Uneke, C. (2010). Soil transmitted helminth infections and schistosomiasis in school age children in sub-Saharan Africa: Efficacy of chemotherapeutic intervention since World Health Assembly Resolution 2001. Tanzania Journal of Health Research, 12(1), 86–99. https://doi.org/10.4314/thrb.v12i1.56366
- Urbani, C., Sinoun, M., Socheat, D., Pholsena, K., Strandgaard, H., Odermatt, P., & Hatz, C. (2002). Epidemiology and control of mekongi schistosomiasis. Acta tropica, 82(2), 157–168. https://doi.org/10.1016/S0001-706X(02)00047-5
- Utzinger, J., N’Goran, E. K., Caffrey, C. R., & Keiser, J. (2011). From innovation to application: Social–ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta tropica, 120, S121–137. https://doi.org/10.1016/j.actatropica.2010.08.020
- Walker, C. L. F., & Black, R. E. (2007). Functional indicators for assessing zinc deficiency. Food and Nutrition Bulletin, 28(3_suppl3), S454–479. https://doi.org/10.1177/15648265070283S305
- Walsh, C., Dannhauser, A., & Joubert, G. (2002). The impact of a nutrition education programme on the anthropometric nutritional status of low-income children in South Africa. Public Health Nutrition, 5(1), 3–9. https://doi.org/10.1079/PHN2001204
- Whaley, S. E., Sigman, M., Neumann, C., Bwibo, N., Guthrie, D., Weiss, R. E., Alber, S., & Murphy, S. P. (2003). The impact of dietary intervention on the cognitive development of Kenyan school children. The Journal of Nutrition, 133(11), 3965S–3971S. https://doi.org/10.1093/jn/133.11.3965S
- WHO. (1991): Basic laboratory methods in medical parasitology.
- WHO. (1994): Bench aids for the diagnosis of intestinal parasites.
- Williams, J. Basic laboratory methods in medical parasitology: Geneva: World Health Organization, 1991. (1992). Transactions of the Royal Society of Tropical Medicine and Hygiene, 86(6), 701. viii+ 114 pp. Price Sw. fr. 21.00, US journal8. 90 (in developing countries Sw. fr. 14.70). ISBN 92-4-1544104. https://doi.org/10.1016/0035-9203(92)90206-R
- Wolde, M., Berhan, Y., & Chala, A. (2015). Determinants of underweight, stunting and wasting among schoolchildren. BMC Public Health, 15(1), 8. https://doi.org/10.1186/s12889-014-1337-2
- World Health Organization. (1987): Prevention and control of intestinal parasitic infections: Report of a WHO expert committee [ meeting held in Geneva from 3 to 7 March 1986].
- World Health Organization. (2011) . Helminth control in school-age children: A guide for managers of control programmes. World Health Organization.
Appendix 1:
A flow chart of the intervention study