Abstract
The rate of pennycress residue decomposition and mineralization is critical in determining potential nutrient availability for following crops. To better understand pennycress decomposition, we examined biomass, nitrogen, and carbon loss from wild pennycress, gene edited AOP2 knockout pennycress, annual ryegrass, and cereal rye. Biomass was collected from all crops at the time of cash crop planting, and 20 g of biomass was placed in individual mesh forage bags (50 ± 10 mm). We placed 99 bags from each crop between rows of corn (n = 5) on the soil surface of the dominant soil types (SA and DR) at the ISU farm. Replicate bags were collected from each soil type over the following 84 days, weighed for biomass, and analyzed for carbon and nitrogen in the plant residue. Loss of biomass differed by crop and soil type (F = 3.73,32, p = 0.023) with annual ryegrass losing biomass most rapidly followed by cereal rye, wild-type pennycress, and the domesticated low glucosinolate, AOP2 knockout, pennycress. Nitrogen (F = 8.53,36, p < 0.0001) and carbon (F = 67.53,36, p < 0.0001) losses differed by crop and not soil type following a similar trend as biomass loss. Our results suggest that both wild-type and AOP2 knockout pennycress can be expected to decompose similarly to a rye cover crop but with slower nutrient loss. Pennycress has potential to act as an effective short-term nutrient sink in agroecosystems.
1. Introduction
Winter cover crops are an important in-field tool for providing ecosystem services during agricultural fallow periods. One of the primary benefits of utilizing winter cover crops is soil stabilization and reduced erosion (Banik et al., Citation2020; Lacey & Armstrong, Citation2015). Winter cover crops are also shown to reduce weed pressure and provide pollinator habitat (DeSimini et al., Citation2020; Groeneveld & Klein, Citation2014; Johnson et al., Citation2015). From a conservation perspective, winter cover crops may act as an essential short-term nutrient sink (Hanrahan et al., Citation2018; Weyers et al., Citation2019). However, nutrient uptake and biomass accrual by cover crops during the winter fallow period may influence warm season corn and soy production (Kaspar & Bakker, Citation2015; Thompson et al., Citation2020).
Winter cover crops have the potential to temporarily sequester nutrients that are released over the growing season to summer cash crops as they decompose (Malpassi et al., Citation2000). The rate of cover crop decomposition (k) plays an essential role in determining the availability of nutrients to subsequent cash crops (Adair et al., Citation2010; Malpassi et al., Citation2000). In addition to decomposition rate, carbon-to-nitrogen ratios are influential in determining nutrient mineralization rates and dynamics (USDA NRCS East National Technology Support Center & North Dakota NRCS, Citation2011). Generally, the decomposition of cover crops with a high carbon-to-nitrogen ratio will immobilize nutrients, particularly nitrogen, from soils while cover crops with lower carbon-to-nitrogen ratios mineralize nutrients rapidly (Jahanzad et al., Citation2016; Kuo & Sainju, Citation1998; Ruffo & Bollero, Citation2003; Sievers & Cook, Citation2018; Singh et al., Citation2020). For example, cereal rye and hairy vetch, two common cover crops, demonstrate the importance of carbon-to-nitrogen ratios in nutrient mineralization. With a lower carbon-to-nitrogen ratio than cereal rye, the decomposition of hairy vetch biomass mineralizes more nitrogen than cereal rye resulting in more nitrogen available for subsequent cash crops (Jahanzad et al., Citation2016; Kuo & Sainju, Citation1998; Ruffo & Bollero, Citation2003; Sievers & Cook, Citation2018; Singh et al., Citation2020). Considering a cover crop’s placement in a cropping sequence, the carbon-to-nitrogen ratio of that cover crop may inform its implementation. Microbial decomposition of cover crop residue with high carbon-to-nitrogen ratios will initially cause immobilization and decrease the amount of plant available nutrients in soil available to cash crops, while crops with low carbon-to-nitrogen ratios may provide nutrients to the subsequent cash crop as it mineralizes during the growing season (Malpassi et al., Citation2000; Ruffo & Bollero, Citation2003).
The oilseed-producing brassica, pennycress (Thlaspi arvense L.) is being commercially domesticated and planted (in test trials) as a winter cash crop that may provide both economic and environmental benefits during the winter fallow period of the upper Midwest (Liu et al., Citation2020; Zanetti et al., Citation2019). As a winter cash crop, pennycress will be planted and harvested at similar times as common cover crops, e.g., cereal rye and annual ryegrass. Due to the different life cycles and growth forms of pennycress, cereal rye, and annual ryegrass, at harvest pennycress will have senesced and cereal rye and annual ryegrass may still be in a vegetative state. Genetic editing with ethylmethane sulfonate and Clustered Regularly Interspaced Short Palindromic Repeat techniques have been utilized to knock out the AOP2 gene, which is responsible for producing glucosinolates in pennycress (Chopra et al., Citation2020). Knocking out the AOP2 gene from wild-type pennycress has resulted in a low-glucosinolate variety of pennycress (AOP2 knockout pennycress) which is more suited to biofuel, livestock feed, and human food purposes (Chopra et al., Citation2020). Pennycress, with a life cycle similar to canola or rapeseed, is ideally planted as corn or soy is harvested in late September or early October. Shortly after planting, pennycress forms a rosette that overwinters and in late March pennycress will bolt, flower, and produce seeds. When temperatures begin to exceed 32°C Pennycress becomes carbon sterile and self terminates after which it is harvested in late May.
Pennycress has been shown to have many of the ecosystem benefits of cereal rye and annual ryegrass including reductions in early season weed pressure and immobilization of nitrate nitrogen held within soil water (Johnson et al., Citation2015; Perry et al., unpublished data; Weyers et al., Citation2019). Additionally, pennycress may also act as a novel cool season forage source for pollinators (Eberle et al., Citation2015; Groeneveld & Klein, Citation2014). The environmental benefit of pennycress as a cover crop coupled with the economic benefit of pennycress as an oilseed may support large-scale implementation.
The purpose of this study is to investigate how wild type and AOP2 knockout pennycress decompose relative to cereal rye and annual ryegrass. Our primary objective was to quantify biomass, nitrogen, and carbon loss from wild-type pennycress, AOP2 knockout pennycress, cereal rye, and annual ryegrass. AOP2 knockout pennycress represents a more commercially viable crop relative to wild-type pennycress as its oil and seed can be used for biofuel production and animal feed. Thus, we are interested in examining the differences between these two strains of pennycress. The secondary objective of this study was to determine if biomass, carbon, and nitrogen loss differed by soil type between all crop varieties. We examined rates of total biomass, nitrogen, and carbon loss and initial carbon-to-nitrogen ratios between two pennycress lines (wild type and AOP2 knockout pennycress), annual ryegrass, and cereal rye for 84 days. We hypothesized that annual ryegrass and cereal rye will decompose similarly to each other, but more rapidly than both pennycress varieties, which would also decompose similarly. The results of the study of pennycress decomposition can inform the efforts to understand the potential for commercialization and implementation by developing a better understanding of how pennycress immobilizes nutrients and the rate and timing over which these nutrients are released.
2. Materials and methods
2.1. Study area
We conducted this study at the Illinois State University (ISU) Research Farm located near Lexington, Illinois (40.67N, −88.77W). A no-till corn (Zea mays L.) and soybean (Glycine max L.) rotation is used at the study site. In the season prior to the start of this study, soybeans were grown at the study site and during the study 115 day no-till corn was growing at the study site. An average of 933 mm of annual rainfall and a mean annual temperature of 11°C were observed for the study site (Northwest Alliance for Computational Science and Engineering, Citation2022). Saybrook silt loam (SA), a fine-silty, mixed super active, mesic Oxyaquatic Argiudoll, and Drummer and Elpaso silty clay loam (DR), a fine-silty, mixed, super active, mesic Typic Endoaquoll, are the dominant soil types at the study site (USDA, Citation2022).
2.2. Biomass preparation
Annual ryegrass, cereal rye, and wild-type pennycress were harvested with hand shears at the ISU farm on 2 May 2021, with 115 day no-till corn planted at the study site on 7 May 2021. The annual ryegrass and cereal rye were harvested in an early vegetative state to mimic the termination of these crops as cover crops. Prior to cash crop planting, the wild type and AOP2 knockout pennycress were harvested post-senescence as they would be for oil seed harvest and planting of subsequent cash crops. The use of these crops at different life stages reflects their usage prior to planting of summer cash crops. As AOP2 knockout pennycress seeds were not available to ISU, a combine was used to harvest AOP2 knockout pennycress from Western Illinois University for use in this study (40.49N, −90.69W). Western Illinois University and the ISU farm have similar climates and utilize similar agronomic practices reducing differences caused by wild type and AOP2 knockout pennycress originating from different sites. Prior to the start of the study, biomass was dried in a 50°C drying oven for 7 days. Following this, the annual ryegrass and cereal rye biomass was roughly chopped to simulate cash crop planting. The wild-type pennycress biomass was chopped and then processed to simulate passing through a combine. We placed 20 g of the plant biomass into labeled ANKOM© (Macedon, NY) R1020 10 × 20 cm mesh forage bags with 50 ± 10 micron porosity. In total, 396 forage bags were prepared with 99 bags for each crop. Forage bags were closed using loop lock labels and placed on the soil surface on 14 June 2021. The final samples were collected 6 September 2021.
2.3. Litter bag placement, sampling, and experimental design
Forage bags were randomly placed on the soil surface between rows of corn in the two dominant soil types at the ISU farm. Due to cropping practices at the study site, forage bags in SA soil were placed between corn rows oriented north-to-south while forage bags in DR soil were placed in rows oriented from east-to-west. The forage bags were randomly placed in 10 rows of corn (5 in SA soil and 5 in DR soil) with 9 groups of 4 forage bags (one forage bag for each crop) placed in each row roughly 10 meters apart with 5 empty rows of corn in-between each sampled row of corn. At each sampling time, one random group from each row was sampled. At “time 0,” the first set of forage bags was taken to the field and returned to the lab to account for handling loss. Sample dates were chosen such that later dates had more time in between sampling than earlier dates to account for a negative exponential rates of biomass decomposition (Adair et al., Citation2010). Forage bags were sampled at 0, 7, 14, 21, 28, 35, 49, 63, and 84 days from initial placement. Collected forage bags were taken to the laboratory where soil attached to the surface of the forage bags was removed. The forage bags were then dried in a 50°C drying oven for 7 days and weighed for dry mass. The biomass was removed from the forage bags and ground using a Wiley® Mill. Samples from days 0, 14, 35, and 63 were sent to the Morris, Minnesota, ARS facility for analysis of Nitrogen and Carbon content using a LECO© (St. Joseph, MI) combustion analyzer. A limited selection of dates was sent due to financial limitations.
2.4. Statistical analysis
To estimate total biomass decomposition, decomposition rates in grams per day (k) were estimated using the percent mass remaining of biomass fit to a nonlinear regression model for a negative exponential rate of mass loss (M(t) = M(0)e−kt + ε) with M(t) as the mass remaining at time t, M(0) as the initial mass, k as the assumed rate at which all litter constituents decompose, and ε as random error (Adair et al., Citation2010). The data used in this analysis were fitted to both the model above (M(t) = M(0)e−kt + ε) and a two-stage model in which early exponential decay and later linear decay for each crop were analyzed separately. Both models provided the same results, with the full nonlinear regression model (M(t) = M(0)e−kt + ε) used as the final model for analysis as it most succinctly indicated differences in crops and soil types. The resulting decomposition rates (k) are then compared between each crop, soil type, and the interaction between the two using general linear models. Crop, soil type, and the interaction between the two were included in the model as fixed factors. Non-significant factors were dropped from the models at a p-value of 0.15 or higher. Group means were separated with an alpha of 0.05 using a Bonferroni adjustment for multiple comparisons. Analyses of total biomass loss, nitrogen loss, and carbon loss were all conducted with the same methods and models. Statistical analyses were conducted in R (version 4.2.1) using the car, emmeans, lsmeans, and multcompView packages (Fox & Weisberg, Citation2019; Graves et al., Citation2019; Lenth, Citation2022; R Core Team, Citation2022). The additional packages tidyverse, janitor, purrr, broom, and patchwork were used for data preparation and plotting (Firke, Citation2021; Henry & Wickham, Citation2020; Pedersen, Citation2022; Robinson et al., Citation2022; Wickham et al., Citation2019).
2.5. Model selection
To determine the statistical model used for this analysis we ran each model with all factors and dropped any non-significant interaction or factor at a p-value of 0.15. There was a significant interaction between crop and soil type for decomposition rate in the total biomass analysis (F = 3.73,32, p = 0.023). Due to the significant interaction, estimated marginal means were used for all pairwise comparisons between all crops and soil types. The crop by soil type interaction did not affect nitrogen (F = 1.83,32, p = 0.16) or carbon (F = 1.23,32, p = 0.32) loss. Additionally, soil type did not affect nitrogen (F = 1.41,35, p = 0.24) or carbon (F = 0.0091,35, p = 0.92) loss. The non-significant terms were dropped from the models. The assumptions of homogeneity of variance and normality of residuals were analyzed under the null hypotheses that variance is homogenous, and residuals are normal. Our analyses met the assumptions of a general linear model.
3. Results and discussion
3.1. Biomass decomposition
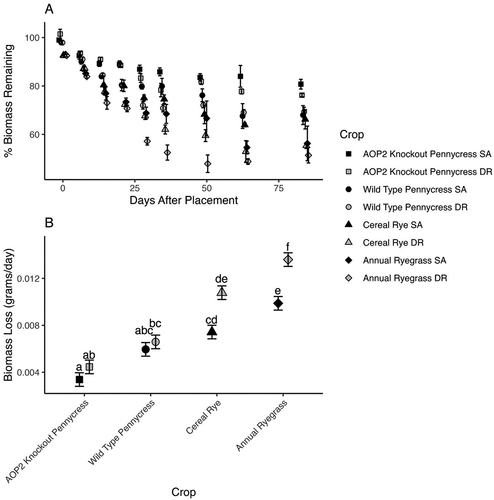
Wild-type and AOP2 knockout pennycress lost biomass at similar rates in both soil types except for wild-type pennycress in DR soils, which lost biomass more rapidly than wild-type pennycress in SA soil and AOP2 knockout pennycress in both soils (Figure ). Analysis of all pairwise comparisons indicate that AOP2 knockout pennycress and wild-type pennycress lose biomass at similar rates, but AOP2 knockout pennycress loses biomass slower than cereal rye and annual ryegrass (Figure ). The slower rates of total biomass loss by AOP2 knockout pennycress and wild-type pennycress are expected as these crops have woody, hollow stems which decompose slower than grasses. Annual ryegrass is the only crop that decomposes differentially by soil type (p = 0.002). While there is a statistically significant interaction between crop and soil type, this interaction may not have much biological significance as shown by only annual ryegrass decomposing differentially by soil type. Saybrook, Drummer, and Elpaso soils are similar suggesting other factors, such as corn row orientation, may be responsible for differences in biomass loss. Thus, the difference in annual ryegrass decomposition by soil type could warrant further investigation.
Figure 1. A. Percent of total biomass remaining over the 84-day study period with best-fit regression line to demonstrate biomass loss. B. Estimated marginal means of biomass (k) by species and soil type for the whole study period. SA refers to Saybrook soils while DR refers to Drummer and Elpaso soils. Letters indicate significant differences at an alpha of 0.05.
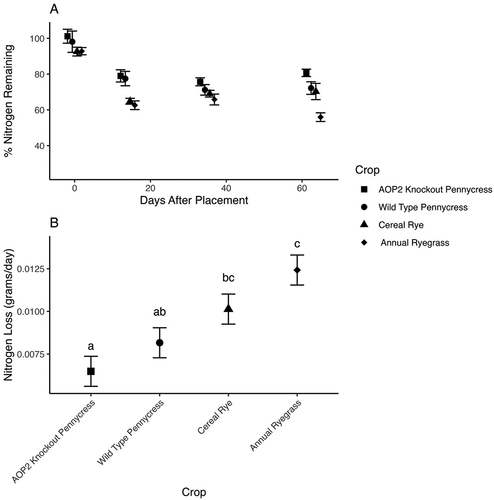
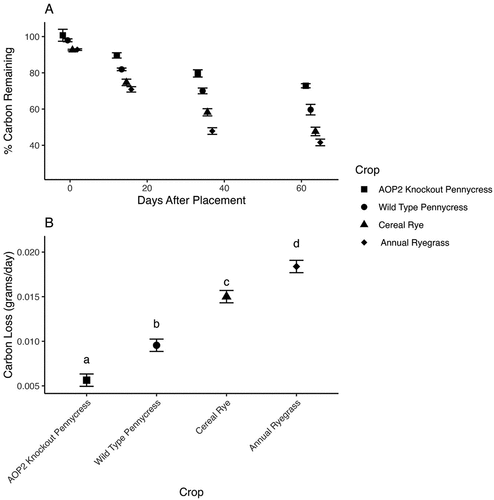
3.2. Nitrogen and carbon loss
Nitrogen loss differed by crop (F = 8.53,36, p < 0.0001). Mean separation using multiple comparisons and a Bonferroni correction indicates that annual ryegrass lost nitrogen most rapidly (k = 0.012) followed by cereal rye (k = 0.010), wild-type pennycress (k = 0.008), and AOP2 knockout pennycress (k = 0.006), respectively (Figure ). Carbon loss also differed by crop (F = 67.5 3,36, p < 0.0001). Mean separation with multiple comparisons and a Bonferroni correction demonstrated that all crops lost carbon at significantly different rates (Figure ). Annual ryegrass loses carbon most rapidly (k = 0.018) followed by cereal rye (k = 0.015), wild-type pennycress (0.009), and AOP2 knockout pennycress (k = 0.006).
3.3. Summary and conclusions
The results of this study indicate that AOP2 knockout pennycress mineralizes nitrogen and carbon less rapidly and loses biomass less rapidly than wild-type pennycress, cereal rye, and annual ryegrass. Decomposition rate differed by crop, and all crops followed a negative exponential rate of decay (Figures , Figures ). These data support our hypothesis that the varieties of pennycress will decompose more slowly than cereal rye and annual ryegrass. The results indicate that the commercial AOP2 knockout pennycress, in particular, will mineralize nutrients at a slower rate than the common cover crops cereal rye and annual ryegrass (Figures , Figures ). Previous studies demonstrate the potential for pennycress to sequester in-field nutrients (Weyers et al., Citation2019). Therefore, with the potential to sequester excess in-field nutrients during the winter period and our demonstrated lower rates of nutrient mineralization, AOP2 knockout pennycress demonstrates a potential to act as a cool season nutrient sink. As a cool season nutrient sink, AOP2 knockout pennycress will mineralize nutrients at times that are beneficial for spring nutrient sequestration and summer cash crop production.
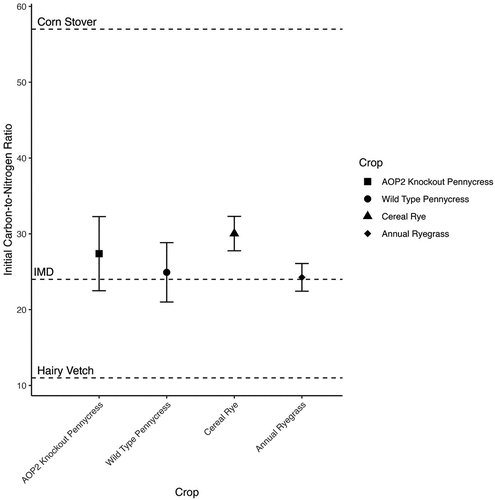
In general, the crops used in this study have initial carbon-to-nitrogen ratios near the ideal microbial diet (24:1), which is the carbon-to-nitrogen ratio where soil microbes most readily mineralize nutrients (Figure ) (USDA NRCS East National Technology Support Center & North Dakota NRCS, Citation2011). In situations where a substrate’s carbon-to-nitrogen ratio is higher than the ideal microbial diet, soil nitrogen may become immobilized too rapidly and become unavailable to subsequent crops. Alternatively, in situations where a substrate’s carbon-to-nitrogen ratio is lower than the ideal microbial diet, excess nitrogen may be available to subsequent crops. The comparable initial carbon-to-nitrogen ratio in the crops used in this study, and how close these carbon-to-nitrogen ratios are to the ideal microbial diet, indicates that the carbon and nitrogen in wild-type pennycress, AOP2 knockout pennycress, cereal rye, and annual ryegrass are readily available to be mineralized by soil microbial communities (Figure ). Despite similar initial carbon-to-nitrogen ratios near the ideal microbial diet, our results indicate that wild-type and AOP2 knockout pennycress still decompose and mineralize nutrients slower than cereal rye and annual ryegrass.
Figure 4. Initial carbon-to-nitrogen ratio of the crops in this study. Dashed lines represent data from USDA NRCS East National Technology Support Center & North Dakota NRCS (Citation2011) for comparison. IMD refers to the ideal microbial diet. Cereal rye and annual ryegrass were sampled in their vegetative state explaining their relatively low carbon-to-nitrogen ratios.
With an initial carbon-to-nitrogen ratio similar to the ideal microbial diet, after harvest AOP2 knockout pennycress will begin to decompose and the nutrients in its biomass will be readily utilized by soil microbial communities. Due to its significantly slower rates of biomass, carbon, and nitrogen loss compared to cereal rye and annual ryegrass, wild-type, and AOP2 knockout pennycress may be returning nutrients to agroecosystems later than annual ryegrass and cereal rye. When coupled with the potential for pennycress to sequester in-field nutrients during the winter fallow period, we conclude that pennycress as a winter cash crop can exhibit similar benefits to winter cover crops as a short-term nutrient sink (Hanrahan et al., Citation2018; Weyers et al., Citation2019).
Abbreviations
| DR | = | Drummer and Elpaso soils |
| ISU | = | Illinois State University |
| K | = | decomposition rate |
| SA | = | Saybrook soils |
Acknowledgments
C.M. O’Reilly and J.C. Sedbrook provided comments on early versions of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data supporting the findings of this study are available upon request from the corresponding author, R.M.
Additional information
Funding
Notes on contributors
Ryan T. Meyer
Ryan Meyer is a Master’s Student in the School of Biological Sciences at Illinois State University. He has previously studied environmental science and his current work focuses on the potential for cover crops to improve water quality in the Midwestern United States and in downstream aquatic ecosystems.
Nicholas J. Heller
Dr. Nicholas Heller is an Assistant Professor of Crop Sciences in the Department of Agriculture at Illinois State University. He is actively involved in assessing and analyzing regenerative ag practices with a specific focus on cover crop species and their interactions with preceding and following crops. Dr. Heller teaches breeding, genetics, agronomy, and statistics courses.
Alex W. Hafner
Alex Hafner is a recent graduate of Illinois State University achieving a B.S. in biology with a focus on plant genetics. Hafner currently works as a research assistant in a biometical genetics laboratory.
William L. Perry
Bill Perry is an aquatic ecologist studying the role of watershed management practices on stream water quality in the midwest. Perry has studied the role of eutrophication on lakes in northern Alaska, invasive species in the midwest and now controls of water quality in central Illinois. A major focus of the laboratory is on the potential for existing and new cover crops in helping improve biodiversity and water quality in central IL streams and downstream systems.
References
- Adair, E. C., Hobbie, S. E., & Hobbie, R. K. (2010). Single-pool exponential decomposition models: Potential pitfalls in their use in ecological studies. Ecology, 91(4), 1225–10. https://doi.org/10.1890/09-0430.1
- Banik, C., Bartel, C. A., Laird, D. A., Moore, K. J., & Lenssen, A. W. (2020). Perennial cover crop influences on soil C and N and maize productivity. Nutrient Cycling in Agroecosystems, 116(2), 135–150. https://doi.org/10.1007/s10705-019-10030-3
- Chopra, R., Johnson, E. B., Emenecker, R., Cahoon, E. B., Lyons, J., Kliebenstein, D. J., Daniels, E., Dorn, K. M., Esfahanian, M., Folstad, N., Frels, K., McGinn, M., Ott, M., Gallaher, C., Altendorf, K., Berroyer, A., Ismail, B., Anderson, J. A., Wyse, D. L., David Marks, M. & David Marks, M. Identification and stacking of crucial traits required for the domestication of pennycress. (2020). Nature Food, 1(1), 84–91. Article 1. https://doi.org/10.1038/s43016-019-0007-z
- DeSimini, S. A., Gibson, K. D., Armstrong, S. D., Zimmer, M., Maia, L. O. R., & Johnson, W. G. (2020). Effect of cereal rye and canola on winter and summer annual weed emergence in corn. Weed Technology, 34(6), 787–793. https://doi.org/10.1017/wet.2020.51
- Eberle, C. A., Thom, M. D., Nemec, K. T., Forcella, F., Lundgren, J. G., Gesch, R. W., Riedell, W. E., Papiernik, S. K., Wagner, A., Peterson, D. H., & Eklund, J. J. (2015). Using pennycress, camelina, and canola cash cover crops to provision pollinators. Industrial Crops and Products, 75, 20–25. https://doi.org/10.1016/j.indcrop.2015.06.026
- Firke, S. (2021). janitor: Simple Tools for Examining and Cleaning Dirty Data. https://CRAN.R-project.org/package=janitor
- Fox, J., & Weisberg, S. (2019). An R companion to applied regression, Third Edition. Third). Sage.
- Graves, S., Piepho, H. -P., Selzer, L., & Dorai-Raj, S. (2019). multcompView: Visualizations of Paired Comparisons. https://CRAN.R-project.org/package=multcompView
- Groeneveld, J. H., & Klein, A. -M. (2014). Pollination of two oil-producing plant species: Camelina (Camelina sativa L. Crantz) and pennycress (Thlaspi arvense L.) double-cropping in Germany. Global Change Biology Bioenergy, 6(3), 242–251. https://doi.org/10.1111/gcbb.12122
- Hanrahan, B. R., Tank, J. L., Christopher, S. F., Mahl, U. H., Trentman, M. T., & Royer, T. V. (2018). Winter cover crops reduce nitrate loss in an agricultural watershed in the central U.S. Agriculture, Ecosystems & Environment, 265, 513–523. https://doi.org/10.1016/j.agee.2018.07.004
- Henry, L., & Wickham, H. (2020). purrr: Functional Programming Tools. https://CRAN.R-project.org/package=purrr
- Jahanzad, E., Barker, A. V., Hashemi, M., Eaton, T., Sadeghpour, A., & Weis, S. A. Nitrogen Release dynamics and decomposition of buried and surface cover crop residues. (2016). Agronomy Journal, 108(4), 1735–1741. Article 4. https://doi.org/10.2134/agronj2016.01.0001
- Johnson, G. A., Kantar, M. B., Betts, K. J., & Wyse, D. L. (2015). Field pennycress production and weed control in a double crop system with soybean in Minnesota. Agronomy Journal, 107(2), 532–540. https://doi.org/10.2134/agronj14.0292
- Kaspar, T. C., & Bakker, M. G. Biomass production of 12 winter cereal cover crop cultivars and their effect on subsequent no-till corn yield. (2015). Journal of Soil and Water Conservation, 70(6), 353–364. Article 6. https://doi.org/10.2489/jswc.70.6.353
- Kuo, S., & Sainju, U. M. Nitrogen mineralization and availability of mixed leguminous and non-leguminous cover crop residues in soil. (1998). Biology & Fertility of Soils, 26(4), 346–353. 8. https://doi.org/10.1007/s003740050387
- Lacey, C., & Armstrong, S. The efficacy of winter cover crops to stabilize soil inorganic nitrogen after fall-applied anhydrous ammonia. (2015). Journal of Environmental Quality, 44(2), 442–448. Article 2. https://doi.org/10.2134/jeq2013.12.0529
- Lenth, R. (2022). Estimated Marginal Means, aka Least-Squares Means. https://CRAN.R-project.org/package=emmeans
- Liu, R., Wells, M. S., Garcia, Y., & Garcia, A. Relay and sequential cropping corn with winter oilseed crops in northern climates. (2020). Nutrient Cycling in Agroecosystems, 116(2), 195–203. Article 2. https://doi.org/10.1007/s10705-019-10036-x
- Malpassi, R. N., Kaspar, T. C., Parkin, T. B., Cambardella, C. A., & Nubel, N. A. Oat and Rye root decomposition effects on nitrogen mineralization. (2000). Soil Science Society of America Journal, 64(1), 208–215. Article 1. https://doi.org/10.2136/sssaj2000.641208x
- Northwest Alliance for Computational Science and Engineering. (2022). PRISM Climate Group. Oregon State University. https://prism.oregonstate.edu/
- Pedersen, T. L. (2022). patchwork: The Composer of Plots. https://CRAN.R-project.org/package=patchwork
- R Core Team. (2022). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. https://www.R-project.org/
- Robinson, D., Hayes, A., & Couch, S. (2022). broom: Convert Statistical Objects into Tidy Tibbles. https://CRAN.R-project.org/package=broom
- Ruffo, M. L., & Bollero, G. A. Modeling rye and hairy vetch residue decomposition as a function of degree-days and decomposition-days. (2003). Agronomy Journal, 95(4), 900–907. Article 4. https://doi.org/10.2134/agronj2003.9000
- Sievers, T., & Cook, R. L. Aboveground and root decomposition of cereal rye and hairy vetch cover crops. (2018). Soil Science Society of America Journal, 82(1), 147–155. Article 1. https://doi.org/10.2136/sssaj2017.05.0139
- Singh, G., Kaur, G., Williard, K., Schoonover, J., & Nelson, K. A. Managing phosphorus loss from agroecosystems of the Midwestern United States: A review. (2020). Agronomy, 10(4), 561. Article 4. https://doi.org/10.3390/agronomy10040561
- Thompson, N. M., Armstrong, S. D., Roth, R. T., Ruffatti, M. D., & Reeling, C. J. Short‐run net returns to a cereal rye cover crop mix in a midwest corn–soybean rotation. (2020). Agronomy Journal, 112(2), 1068–1083. Article 2. https://doi.org/10.1002/agj2.20132
- USDA. (2022). National Cooperative Soil Survey. https://www.nrcs.usda.gov/wps/portal/nrcs/main/soils/survey/partnership/ncss/
- USDA NRCS East National Technology Support Center & North Dakota NRCS. (2011). Carbon to Nitrogen Ratios in Cropping Systems. USDA Natural Resources Conservation Service. https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcseprd331820.pdf
- Weyers, S., Thom, M., Forcella, F., Eberle, C., Matthees, H., Gesch, R., Ott, M., Feyereisen, G., Strock, J., & Wyse, D. Reduced potential for nitrogen loss in cover crop-soybean relay systems in a cold climate. (2019). Journal of Environmental Quality, 48(3), 660–669. Article 3. https://doi.org/10.2134/jeq2018.09.0350
- Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L. D., François, R., Grolemund, G., Hayes, A., Henry, L., Hester, J., Kuhn, M., Pedersen, T. L., Miller, E., Bache, S. M., Müller, K., Ooms, J., Robinson, D., Seidel, D. P., Spinu, V., Yutani, H. & Yutani, H. (2019). Welcome to the Tidyverse. Journal of Open Source Software, 4(43), 1686. https://doi.org/10.21105/joss.01686
- Zanetti, F., Isbell, T. A., Gesch, R. W., Evangelista, R. L., Alexopoulou, E., Moser, B., & Monti, A. (2019). Turning a burden into an opportunity: Pennycress (Thlaspi arvense L.) a new oilseed crop for biofuel production. Biomass & bioenergy, 130, 1–7. https://doi.org/10.1016/j.biombioe.2019.105354