?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Bitter melon is a medicinal plant with wide-ranging health benefits. Being complex in chemical constituents, poor evidence is available to establish the exact therapeutic benefits of different phytochemicals present in bitter melon. In the present study, bitter melon fruit skin, flesh, seeds and whole fruit were assessed to estimate alkaloid and saponin contents and subsequently bio-evaluation was performed to ascertain their potential as more potent hyperglycemic and hyperlipidemic moderators for the first time. For the feeding trial, Sprague-Dawley rats were used. Bitter melon seeds have a high amount of total alkaloids, while the flesh part has maximum total saponin contents. The group of rats fed with a diet containing seeds powder showed a more prominent impact in lowering blood glucose levels and increasing serum insulin concentration in hyperglycemic and hyperlipidemic rats. The flesh part with maximum saponin contents exhibited a substantial impact in lowering cholesterol, LDL and triglycerides and increasing HDL levels. The antihyperglycemic and antihyperlipidemic activities have a direct association with the presence of these chemical constituents. Alkaloids were found to be more potent antihyperglycemic while saponins were found to be antihyperlipidemic moderators.
1. Introduction
Bitter melon (Momordica charantia L.) is a subtropical and tropical vine that belongs to Cucurbitaceae, a gourd family of angiosperms (Çiçek, Citation2022). It is recognized as a medicinal plant with different common names such as karela (Urdu or Hindi), balsam pear (English), kugua (Chinese), alqire almra (Arabic), goya (Japanese), gourde amère (French), zucca amara (Italian), kho qua (Vietnamese), labu pahit (Indonesian) and ampalaya (Filipino). The bioactive moieties such as alkaloids, saponins, flavonoids, terpenes, steroids, phenols and glucosinolates present in bitter melon are mainly responsible for wide-ranging pharmaceutical applications (Gayathry & John, Citation2022). The health benefits associated with bitter melon include anti-diabetic (Arshad et al., Citation2017; Gao et al., Citation2021; Sultan et al., Citation2021), anti-hyperlipidemic (Arshad et al., Citation2017; Hussain et al., Citation2019), anti-oxidative (Bortolotti et al., Citation2019; Smail et al., Citation2020; Youn et al., Citation2019), anti-cholesterolemic (Saeed et al., Citation2018), anti-tumour (Fang et al., Citation2019), anti-ulcerogenic (Gill et al., Citation2012), anti-inflammatory (Bortolotti et al., Citation2019; Dwijayanti et al., Citation2019), anti-mutagenic (Li et al., Citation2020), anti-microbial (Mahmood et al., Citation2012) and immune-modulatory activities (Majumda & Debnath, Citation2014; Panda et al., Citation2015). The specific compounds in relation to the proposed therapeutic benefits of bitter melon are still in question (Lucas et al., Citation2010). Hence, understanding bioactive compounds in relation to the medicinal properties of bitter melon is strongly desirable to attain conclusive and molecular-based evidence behind these outcomes.
More than 60 bioactive molecules are present in different parts of this plant, particularly in fruit and seeds that are responsible for reducing complex symptoms incurred during the progression of different diseases including diabetes and hyperlipidemia (Kole et al., Citation2020). Diabetes is becoming a serious health issue for people of all ages and poses a severe challenge and threat to the twenty-first-century global health system. In recent years, the estimated adult population suffering from diabetes was 537 million which is projected to increase by 643 million and 783 million in 2030 and 2045, respectively (International Diabetes Federation, Citation2021). During diabetes, the metabolic pathways related to carbohydrates, lipids and proteins are altered in such a way that leads to other complex symptoms related to neurological, gastrointestinal and renal physiological aspects (Hazarika et al., Citation2012; Karagöz et al., Citation2021). As the disease is more prevalent in developing countries, the use of antidiabetic drugs is restricted due to poor economic conditions (International Diabetes Federation, Citation2021; Liu et al., Citation2021). Similarly, due to alternate underlying etiology, such as uncontrolled diabetes, medications (amiodarone, glucocorticoids), hypothyroidism, unhealthy diet and/or a poor lifestyle regimen may lead to acquired hyperlipidemia. Hyperlipidemia may result in atherosclerosis, which is related to conditions such as peripheral vascular disease, ischemic cerebrovascular disease, coronary heart disease and pancreatitis (Sa’adah & Nurhayati, Citation2020) where all of these diseases represent 32% of global deaths (WHO, Citation2021).
Antihyperglycemic and antihyperlipidemic agents from medicinal plants are gaining importance due to easy availability, prompt absorption in the body and less toxic effects than conventionally used synthetic drugs. Bitter melon is such a plant that possesses the remarkable potential to be used as a phytomedicine to treat pre-diabetic and diabetic conditions, as well as to regulate lipid metabolism and weight management (S. Wang et al., Citation2017). But being complex in the chemical constituents, poor evidence is available to establish the exact therapeutic benefits of different phytochemicals. Many in vitro and in vivo past trials highlighted the anti-glycemic and antilipidemic activities due to the consumption of dietary bitter melons, but the responsible compounds in relation to these beneficial effects on blood glucose and lipid concentrations have not been studied adequately (Çiçek, Citation2022). The alkaloids and saponins in bitter melon might play a role as standard constituents to reduce hyperglycemia and hyperlipidemia due to their insulin-like activities. Hence, the present in vivo study was planned based on innovative concept to assess alkaloids and saponins contents in different parts of bitter melon and subsequent bio-evaluation to assess more potent compound for the treatment of hyperglycemia, hyperlipidemia or both.
2. Materials and methods
2.1. Collection of fruit and parts
The ripe fruits of bitter melon were procured from Vegetable Research Section, Ayub Agriculture Research Institute (AARI), Faisalabad. The bitter melon fruits were thoroughly washed to remove dust, dirt and other foreign debris. Then fruits were peeled, seeds were isolated and pulp was cut into small pieces. Some fruits of each cultivar were cut into small pieces as a whole. In this way, skin, flesh, seeds and whole fruit were obtained.
2.2. Drying and grinding
All the parts were kept at room temperature until dried completely. The dried skin, flesh, seeds and whole fruit were ground by using a small laboratory grinder (National, Japan, Model MT-W166A) to a very fine powder and for further refining, the fine powder was passed through a sieve. After obtaining very fine-quality powder, it was packed separately for further analysis in air-tight plastic jars.
2.3. Reagents
Various reagents of analytical grade were purchased from Merck (Merck KGaA, Darmstadt, Germany) and Sigma-Aldrich (Sigma-Aldrich Tokyo, Japan).
2.4. Estimation of phytochemicals
2.4.1. Total alkaloid contents
To quantify alkaloids in bitter gourd samples, the method of Harborne (Citation2005) was used with slight modifications. One gram of dry powder of the sample was taken, and 100 ml of 10% acetic acid in ethanol was mixed into it. The mixture was covered and kept for 4 h. After that, filtration of the extract was done which was subsequently concentrated to 25 ml of its original volume in a water bath. The ammonium hydroxide in concentrated form was added dropwise to the extract till the settlement of the whole precipitation in the solution. The diluted ammonium hydroxide was used to wash the precipitates and meanwhile, filtration was done using Whatman filter paper. Using an oven, the residues were dried at 40°C and weight was determined. The total alkaloids in each sample were calculated using the following formula:
2.4.2. Total saponin contents
The total saponin contents were determined by the procedure of Obadoni and Ochuko (Citation2001). The 100 ml of 20% ethanol in an aqueous solution was taken, and 1 g of sample was added to it. Stir it thoroughly and then heat with continuous stirring at 45°C for 4-h duration. The Whatman filter paper was used to filter the mixture, and the residue obtained was again extracted with a fresh solution of 100 ml of 25% aqueous ethanol. The rotary evaporator at 40°C was used to concentrate the sample to get 40 ml of extract approximately. The concentrate was extracted twice with 20 ml diethyl ether by transferring it into a separator funnel. Then, the ether layer was removed and the aqueous layer was used to re-extract by adding 30 ml n-butanol. This extract was washed two times using 5% aqueous sodium chloride (10 ml). After that, evaporating of the remaining solution was done and constant weight was obtained after drying the samples in an oven at a temperature of 40°C till constant weight. The following expression was used to assess the saponin contents:
2.4.3. Experimental animals
Sprague-Dawley rats were procured from the National Institutes of Health, Islamabad. These experimental animals were kept in the Animal Room of the College of Pharmacy at Government College University, Faisalabad. To acclimatize to the surrounding environment, the rats were fed a basal diet for a week. The environmental conditions were maintained throughout the experiment including 12 h of light/dark period, 25 ± 3°Ctemperature and 52 ± 5% relative humidity. Some rats were euthanized at the beginning of the trial to get the baseline values for the selected traits. The study was approved (Approval no. FSD-19/M-415) and strictly followed the guidelines provided by the Central Government Ethical Committee.
2.4.4. Study design for bio-evaluation
For bio-evaluation, skin, flesh, seeds and whole fruit powder of bitter melon with pre-determined alkaloids and saponins contents were inspected for their anti-hyperglycemic and anti-hyperlipidemic perspectives in 60-day rats feeding trial. For this purpose, three different studies were designed. In each study, five groups of rats were formed with 10 rats in each group to determine the effect of bitter melon parts against selected maladies. Feed intake was monitored at 10 days intervals throughout the trial period. Body weight was measured at the start (baseline), day 30 and day 60 for each group of rats. On days 30 and 60, blood from the retro-orbital plexus was obtained for each group of rats by keeping fasting conditions for the night. EDTA-coated tubes were used for blood collection. Centrifugation of the blood was performed using a centrifuge machine (Centrifugal Machine, Rotrofix 32-A Hettich, Germany) @ 4000 rpm for 6 min to separate the serum. Microlab (Rendox Toerauta RX-Monza, Republic of Ireland) was used to collect sera for biochemical evaluation.
2.4.5. Study I: rats fed with a normal diet
In this study, a group of rats was given a normal diet. The control group of rats was fed with standard chow while in feeds of an experimental group of rats, bitter melon fruit parts were incorporated.
2.4.6. Study II: hyperglycemic rats
In this trial, a group of rats was given a 40% sucrose-rich diet on ad libitum basis throughout experimental period to induce diabetes in normal rats. To assess its impact on serum glucose and insulin level, the control group of rats was fed standard chow and compared with a group of rats fed a diet containing bitter melon fruit parts.
2.4.7. Study III: hyperlipidemic rats
In this trial, a group of rats was given 1.5% cholesterol to induce hyperlipidemia. The impact of an induced trait in the control group of rats was compared with an experimental group of rats.
2.4.8. Feed plans
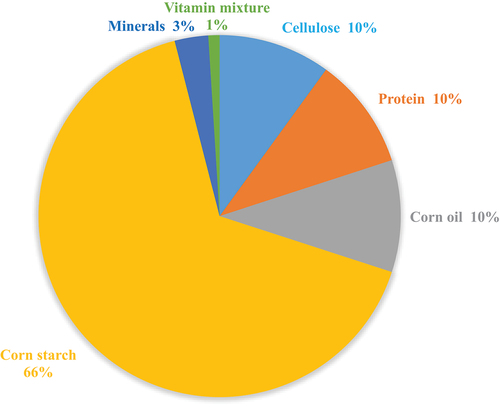
The standard chow containing all major components of food (Figure ) was given to the control group of rats (G0), whereas, bitter melon skin, flesh, seeds and whole fruit (500 mg/kg body weight) were added to the aforementioned diet formulation for G1, G2, G3 and G4, respectively.
2.4.9. Testing of blood samples
The concentration of glucose was determined by GOD-PAP method (Katz et al., Citation2000). Similarly, the level of insulin in each group of rats was observed as described by Ahn et al. (Citation2011). The procedure of J. I. Kim et al. (Citation2011) was used to observe the level of cholesterol in the collected sera of different rat groups using the liquid cholesterol CHOD—PAP method. The procedure of J. I. Kim et al. (Citation2011) was used to determine serum low-density lipoproteins (LDL). Accordingly, to assess high-density lipoproteins (HDL), the cholesterol precipitant method was used (Alshatwi et al., Citation2010). Similarly, to determine serum triglycerides, GPO—PAP method was used by following the procedure of J. I. Kim et al. (Citation2011).
2.4.10. Statistical analysis
The statistical analysis of data was performed using Statistical Packages (SPSS v20 and Microsoft Excel 2016). The level of significance was determined by ANOVA and LSD for comparison (Steel & Torrie, Citation1986).
3. Results
3.1. Estimation of alkaloids and saponins
The total alkaloids were higher in seeds (0.98 ± 0.02%) followed by whole fruit (0.65 ± 0.03%), flesh (0.53 ± 0.02%) and skin (0.52 ± 0.03%), while the total saponin contents were higher in flesh part (3.09 ± 0.18) followed by whole fruit (2.33 ± 0.09%), skin (1.79 ± 0.08%) and seeds (1.06 ± 0.09%) (Table ).
Table 1. Estimation of Alkaloids and saponins (%) in different parts
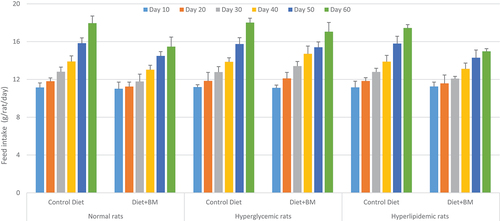
3.2. Feed intake
The diet containing bitter melon imparted significant effects on feed intake (Figure ). In normal rats, the feed intake in rats fed with the control diet was gradually increased from day 10 (11.15 ± 0.48 g/rat/day) to day 60 (17.95 ± 0.76 g/rat/day) but the incorporation of bitter melon in the diet showed remarkable impact in the reduction of feed intake from day 10 (11.01 ± 0.71 g/rat/day) to day 60 (15.47 ± 1.01 g/rat/day) in comparison to control. In hyperglycemic rats, a substantial increase in intake of bitter melon containing diet was observed from the beginning of the trial (11.11 ± 0.29 g/rat/day) to the end of the trial (17.05 ± 0.98 g/rat/day). In hypercholesterolemic rats, consumption of the control diet is remarkably high in comparison to the diet containing bitter melon.
3.3. Impact of bitter melon on blood parameters
3.3.1. Glucose level
In control groups, an increase in the glucose level was observed in normal rats (from 88.88 ± 1.75 to 89.56 ± 1.34 mg/dL), hyperglycemic rats (from 111.17 ± 1.01 to 144.66 ± 1.79 mg/dL) and hyperlipidemia rats (from 96.43 ± 1.21 to 97.12 ± 1.15 mg/dL). However, bitter melon enriched diets substantially maintained blood glucose levels, particularly in hyperglycemic rats. Interestingly, a diet containing seed powder showed a more prominent impact in lowering the blood glucose level in hyperglycemic (98.80 ± 3.36 mg/dL) and hyperlipidemia (90.57 ± 1.94 mg/dL) rats followed by whole fruit powder with values of 102.41 ± 3.01 mg/dL and 92.30 ± 1.38 mg/dL in hyperglycemic and hyperlipidemia rats, respectively (Table ).
Table 2. Impact of dietary supplementation of bitter melon on blood glucose level (mg/dL)
3.3.2. Insulin level
The provision of bitter melon supplemented diets has increased blood insulin concentration in comparison to control groups. At the end of the trial, the increase in insulin level was more pronounced in the group of rats fed with bitter melon seed powder (9.32 ± 0.15, 14.80 ± 0.28 and 10.44 ± 0.40 μIU/mL for normal, hyperglycemic and hyperlipidemic rats, respectively) followed by a group of rats fed with whole fruit, flesh and skin powder (Table ).
Table 3. Impact of dietary supplementation of bitter melon on blood insulin level (μIU/mL)
3.3.3. Cholesterol
In normal rats, the highest cholesterol was observed in the control group (85.26 ± 0.67 and 87.66 ± 1.05 mg/dL at day 30 and 60, respectively, that reduced to 81.27 ± 0.88 and 83.17 ± 2.22 mg/dL (G3), 77.56 ± 0.70 and 81.13 ± 1.25 mg/dL (G1), 76.47 ± 0.45 and 77.02 ± 0.65 mg/dL (G4), 75.34 ± 1.02 and 76.31 mg/dL (G2) at day 30 and 60, respectively. In hyperglycemic rats, a decrease in cholesterol level was noted by dietary supplementation of bitter melon. In the control group of rats, the cholesterol level was high (112.07 ± 1.45 mg/dL) that substantially reduced to 102.41 ± 1.29, 96.41 ± 0.88 91.91 ± 1.54 88.87 ± 0.85 mg/dL in a group of rats fed with diet containing seed powder, skin powder, whole fruit powder and flesh powder, respectively. Similarly, in hyperlipidemic rats, cholesterol level was excessively high in the control group of rats (145.67 ± 3.16 mg/dL), while a significant reduction in cholesterol was observed in all experimental groups of rats with maximum reduction in the case of a diet containing flesh powder (92.55 ± 2.44 mg/dL) and least reduction in a group of rats fed with diet containing seed powder (109.25 ± 3.25 mg/dL) (Table ).
Table 4. Impact of dietary supplementation of bitter melon on cholesterol level (mg/dL)
4. Low-Density Lipoproteins (LDL)
In normal rats, the control group of rats possessed the highest value of LDL (31.69 ± 2.01 mg/dL) than G1 (29.48 ± 1.22 mg/dL), G2 (28.07 ± 2.00 mg/dL), G3 (29.77 ± 2.06 mg/dL) and G4 (28.90 ± 1.92 mg/dL). In hyperglycemic rats and hyperlipidemic rats, a diet containing flesh powder showed a maximum reduction (31.25 ± 4.37 and 38.14 ± 1.44 mg/dL respectively) compared to control (41.85 ± 1.21 and 64.56 ± 1.75 mg/dL respectively). In both studies, a diet containing seed powder was the least effective diet with a reduction of LDL to 36.06 ± 2.40 mg/dL and 43.18 ± 2.08 mg/dL, respectively (Table ).
Table 5. Impact of dietary supplementation of bitter melon on LDL (mg/dL)
5. High-Density Lipoproteins (HDL)
Table reveals the rise in HDL by giving bitter melon dietary supplementation compared to control in different studies. At the end of the trial, the highest increase in HDL level was noted in rats fed with a diet containing flesh powder (43.89 ± 2.17, 46.23 ± 1.17 and 49.03 ± 1.77 mg/dL for normal, hyperglycemic and hyperlipidemic rats, respectively) followed by a diet containing whole fruit powder (42.41 ± 2.41, 44.30 ± 0.98 and 47.30 ± 1.87 mg/dL), skin powder (40.23 ± 1.19, 42.23 ± 1.19 and 44.92 ± 1.04 mg/dL) and seed powder (40.05 ± 1.79, 40.90 ± 1.61 and 42.25 ± 2.34 mg/dL).
Table 6. Impact of dietary supplementation of bitter melon on HDL (mg/dL)
6. Triglycerides
The amounts of triglycerides gradually rise with time. This rise is more noticeable in control groups and supplementation of bitter melon in the diet has resulted in a considerable reduction in triglycerides (Table ). In normal, hyperglycemic and hyperlipidemic rats, a diet containing flesh powder showed maximum reduction in triglycerides level (65.63 ± 1.47, 71.17 ± 0.98 and 81.79 ± 4.21 mg/dL respectively) compared to control (68.34 ± 1.65, 84.28 ± 1.41 and 106.15 ± 1.19 mg/dL respectively). All the diets containing bitter melon parts showed a significant reduction in triglyceride levels, but a diet containing skin and seed powder was comparatively found least effective in normal rats (68.21 ± 2.01 and 68.23 ± 0.71 mg/dL respectively). In the case of hyperglycemic and hyperlipidemic rats, a diet containing seed powder was observed as the least effective treatment with values of 75.24 ± 2.04 and 92.09 ± 2.44 mg/dL, respectively.
Table 7. Impact of dietary supplementation of bitter melon on triglycerides (mg/dL)
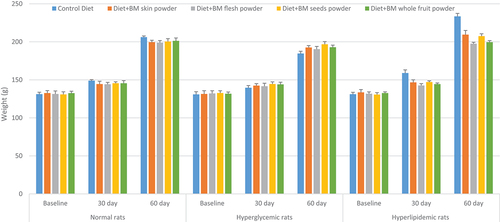
7. Body weight
The present results explicated that diet containing bitter melon imparts substantial changes in body weight (Figure ). In normal rats, a significant increase in body weight was observed in different rat groups with each progressive time episode. At the termination of the trial, in comparison to groups of rats fed with a diet containing skin, flesh, seeds and whole fruit of bitter melon, excessive weight gain was observed in control groups of normal (206.20 ± 1.55 g/rat) and hyperlipidemic rats (233.50 ± 3.87 g/rat) but the weight reduction was noted in hyperglycemic rats (184.63 ± 3.03 g/rat). The diet containing bitter melon flesh powder was found to be more effective in reducing the weight to 198.96 ± 2.69, 190.33 ± 3.58 197.54 ± 1.91 for normal, hyperglycemic and hyperlipidemic rats, respectively, in comparison to dietary incorporation of other parts.
8. Discussion
Bitter melon fruit has a long history of use as a medicinal plant. Due to bevy of complex and diversified bioactive molecules, the actual mechanism behind various pharmacological actions of bitter melon is still in question. Alkaloids, saponins and their derived compounds are widely accepted as medicinal agents. Owing to this reason, alkaloids and saponins in bitter melon fruit are important and are assigned as medicinal plants. The role of these phytochemicals as antihyperglycemic or antihyperlipidemic modulators has not yet been established. Hence, different parts of bitter melon were assessed to estimate alkaloid and saponin contents and subsequently bio-evaluated to relate the potential of these phytochemicals against hyperglycemia and hyperlipidemia.
In the present exploration, the part of the seed is the richest source of alkaloids in comparison to the skin or flesh part. In a previous study, only 0.3% alkaloids were estimated to be present in bitter melon fruit powder without seeds (Krawinkel & Keding, Citation2006). The total saponin contents were higher in the flesh part, which is supported by the work of Li-Dong et al. (Citation2008) who determined saponin contents in pulp and seeds of bitter melon in the range of 1.17% to 4.07% and 0.16% to 1.54%, respectively. In another study, Q. Wang et al. (Citation2011) used ultrasonic-microwave synergistic extraction for 16 min and 2.51% extraction yield of saponins was obtained from bitter melon. Y. Chen et al. (Citation2010) calculated a slightly higher yield (3.2%) and Oishi et al. (Citation2007) found a lower concentration of saponin (1.3%) in bitter melon. A slightly higher amount of saponin in whole fruit (2.9% to 5.16%) and flesh (3.10% to 5.34%) was observed by Habicht et al. (Citation2011) on a dry weight basis. The differences in alkaloids and saponin contents are mainly due to varietal differences, agro-climatic conditions and a large number of environmental factors.
The alkaloids and saponin contents in skin, flesh, seeds and whole fruit powder of bitter melon were determined before administration as a dietary supplement in experimental rats. In the current research, the slight reduction in feed intake noted in experimental rats might be due to the addition of bitter melon fruit components to the daily diet (Q. Chen et al., Citation2003). In another study, feed intake was reduced in alloxan-induced diabetic rats when treated with bitter melon juice (Reyes et al., Citation2006) or fruit powder (Huang et al., Citation2008). The decrease in feed intake is nominal in most of the cases and even bitter melon provision did not impart any significant differences in some studies (Klomann et al., Citation2010). In hyperglycemic rats, intake of bitter melon containing diet is higher due to excessive loss of glucose in a diabetic state (Shetty et al., Citation2005).
Different components and extracts of bitter gourd are believed to possess hypoglycemic properties through different biochemical, physiological and pharmacological ways (Bhushan et al., Citation2010). The results of different scientific explorations are advocating the hypoglycemic ability of bitter melon (Arshad et al., Citation2017; Clouatre et al., Citation2011; Dwijayanti et al., Citation2020; Jafri et al., Citation2009; Rohajatien et al., Citation2018; Sathishsekar & Subramanian, Citation2005; Singh & Gupta, Citation2007; Yuan et al., Citation2012), but the responsible components regarding glucose lowering effects are still unknown. In the present study, the dietary intake of different bitter melon fruit parts has resulted in a significant reduction in blood glucose levels. Among these parts, a diet containing seeds with high alkaloid contents showed more potential to control blood glucose levels. The ability of alkaloids as hypoglycemic modulators might be related to a large number of factors. The most prominent underlying mechanisms by which these bitter melon bioactive molecules play their role in lowering glucose level include the prevention of glucose uptake from the intestine (Abdollah et al., Citation2010; Jeong et al., Citation2008; Uebanso et al., Citation2007), enhancing the activity of certain enzymes (Poovitha & Parani, Citation2016), repairing islet β cells and enhancing their functional efficiency (Gadang et al., Citation2011), stimulation of peripheral and skeletal muscles to utilize glucose (Akhtar et al., Citation2011; Cummings & Overduin, Citation2007).
The intake of bitter melon dietary components significantly increases the release of insulin in the experimental group of rats compared to the control group. In the present exploration, insulin increase was most obvious in hyperglycemic rats fed with dietary supplementation of bitter melon skin, flesh, seeds and whole fruit powder compared to the hyperglycemic control group. The most prominent increase in insulin level was monitored in rats fed with seed powder. The enriched alkaloid contents in seeds of bitter melon might be responsible for this increase in insulin levels. Mohammady et al. (Citation2012), reported that insulin level was 19.6 µIU/mL in the control group, 19.8 µIU/mL in bitter gourd control, 6.3 µIU/mL in the diabetic control, 7.9 µIU/mL in the diabetic with Avandia (synthetic drug against diabetes type 2) and 12.5 µIU/mL in diabetic with bitter gourd. Similarly, the bitter melon extract showed a positive impact on serum insulin levels by Fernandes et al. (Citation2007). They reported that the serum concentration of insulin in the normal control group was 3.5 µIU/mL and in the diabetic control was 1.6 µIU/mL. The dietary supplementation of bitter melon extract has increased insulin levels of 2.2 µIU/mL and 2.6 µIU/mL by giving oral administration of 150 and 300 mg/kg body weight of the bitter melon extract.
The rise in insulin levels by giving a bitter melon supplemented diet to diabetes-induced rats may be due to the restoration of pancreatic beta cells (Fernandes et al., Citation2007; Singh & Gupta, Citation2007) or an increase in the number of these cells (Mohammady et al., Citation2012). However, Sundaram and Kumar (Citation2002) reported that bitter melon enhances the functional aspects of beta cells and did not possess any role in the restoration of such cells. Bitter melon may decrease oxidative stress by neutralizing the release of free radicals and hence the death of beta cells. In another study, Xiang et al. (Citation2007) proposed that bitter melon plays a role as a growth factor for pancreatic beta cells. In addition, the dietary incorporation of bitter melon stimulates pancreatic islets of Langerhans to release insulin (Biyani et al., Citation2003; Q. Chen et al., Citation2003). It also improves the function of insulin receptors in the liver and the number of beta cells in the pancreas to increase the production of insulin (Shetty et al., Citation2005). Recently, Ketut et al. (Citation2019) revealed that bitter melon has a role in improving damage to beta cells in the pancreas and enhancing the number of Leydig cells in hyperglycemic mice. Among the different bioactive moieties of bitter gourd, alkaloids are more potent hypoglycemic components with the ability to spontaneously increase the production of insulin.
Diabetic patients are at high risk to develop dyslipidemia (Al-Neaimy, Citation2008). The reason behind this lipid accumulation is mainly due to non-enzymatic reactions of the lipoproteins that gradually formed abundant advanced glycosylation end products, which increases hyperlipidemia in a diabetic condition (Zawada et al., Citation2022). The present study established bitter melon efficacy against cholesterol synthesis and metabolism due to its bioactive moieties.
In past reports, the compounds responsible for the lipid-lowering effect of bitter melon are not available. In the present study, bitter melon flesh powder with high saponin contents was found to be more effective in lowering lipid levels. Certainly, saponin fractions must include some molecules that play a pivotal role in modulating lipid metabolism. Senanayake et al. (Citation2004) explicated the role of the dietary methanol fraction of bitter melon in managing cholesterol levels. They observed an inverse relationship between bitter melon consumption and serum cholesterol level. On the other hand, Abas et al. (Citation2015) revealed that in diabetic rats, the amount of total cholesterol was increased significantly in comparison to the control group. In another study, the utilization of bitter melon fruit extract resulted in a significant reduction of cholesterol in Wistar rats (Fernandes et al., Citation2007). Similarly, Bano et al. (Citation2011) observed 21% decrease in cholesterol after oral administration of the bitter melon aqueous extract. Lipid-lowering effect of bitter gourd was also observed by Abo-Ghanema and Saleh (Citation2012) on male Sprague-Dawley rats. Hossain et al. (Citation2012) found that an aqueous extract of bitter melon possessed antihyperglycemic and antihyperlipidemic activities. They observed 12.88%, 14.44% and 17.21% reduction in serum cholesterol level by giving a daily dose of 250, 500 and 750 mg/kg body weight, respectively. Rohajatien et al. (Citation2018) indicated found 49% decline in blood total cholesterol by feeding bitter melon. It was also observed that hepatic lipid accumulation was reduced significantly by feeding ob/ob mice with bitter melon fruit extract (Dwijayanti et al., Citation2020)
Bitter melon has a profound role in reducing serum cholesterol levels, but the actual mechanism behind this reduction is still debatable. It was documented earlier that bitter melon is involved in the breaking down of certain enzymes involved in lipid metabolism and thus responsible for the decline in serum lipid parameters (Saad et al., Citation2017). It was also observed that bitter gourd inhibits the reabsorption of bile acid in the intestine and increases the production of certain enzymes that convert cholesterol into bile acid (Matsui et al., Citation2013). Zhang et al. (Citation2020) demonstrated that the anti-hyperlipidemic effects of bitter melon fruit may be due to the ability of this plant to modulation of gut microbes and the rise in the production of short-chain fatty acids.
The main carrier of cholesterol in the blood is low-density lipoproteins (Tymoczko et al., Citation2002). The amount of this “bad cholesterol” is effectively reduced in the blood by regular consumption of bitter melon (Abo-Ghanema & Saleh, Citation2012). In the present study, LDL levels reduced substantially in all experimental groups and flesh powder with high saponin contents has shown more promising results. Temitope et al. (Citation2013) observed a significant decline in LDL in experimental groups after oral administration of the bitter melon aqueous extract at a dosage of 80, 100, 120, and 140 mg/kg body weight for 14 days. In another study on Sprague-Dawley rats, LDL level was significantly reduced from 141.51 mg/dL for the diabetic group fed with a control diet to 31.18 for the group of rats fed with a bitter melon supplemented diet (Abo-Ghanema & Saleh, Citation2012). J. Kim et al. (Citation2012) also noted that diabetic control groups had high LDL than experimental groups fed with bitter melon. They proposed that reduction in LDL is due to the release of apolipoprotein-B by the liver after intake of a diet containing bitter melon. Rohajatien et al. (Citation2018) found 42% decrease in LDL cholesterol in a group of rats fed with bitter melon.
HDL is designated as “good cholesterol” because its sufficiently high quantity is related to better health outcomes. The low levels of blood HDL level may cause serious cardiovascular complications. The lower level of HDL is particularly involved in the development of atherosclerosis. In the current study, a diet containing bitter melon parts showed a subtle increase in blood HDL levels. Among different parts, flesh powder was noted as a more potent treatment option with maximum saponin contents to reduce hyperlipidemia. Bano et al. (Citation2011) reported administration of an aqueous extract of bitter melon for 5 weeks increased HDL level to 45%. In another study, bitter melon supplementation increased the HDL level to 41.48 mg/dL in comparison to 14.03 mg/dL for the diabetic control group (Abo-Ghanema & Saleh, Citation2012). Temitope et al. (Citation2013) examined that diets containing 100 mg/kg and 140 mg/kg body weight of bitter melon significantly increase HDL levels. Rohajatien et al. (Citation2018) found a 133% increase in HDL after feeding bitter melon as compared to initial levels. In the present study, saponins were found to be the main responsible agents to raise this HDL level.
Hypertriglyceridemia is a risk factor associated with various heart complications, hence reduction of triglyceride may attenuate the progression of cholesterol-dependent diseases. In the present investigation, a diet containing bitter melon has resulted in the reduction of triglyceride synthesis in experimental rats. The diet containing flesh part with a high amount of saponins is more effective in reducing triglyceride level. Senanayake et al. (Citation2004) investigated hamsters fed with a diet containing bitter melon methanol extract. The hamsters were given a cholesterol-enriched and cholesterol-free diet. They found that bitter gourd supplementation at 0.5% and 1.0% reduced the amount of triglycerides to an extent of 26.1% and 36.2%, respectively. Later, Sato et al. (Citation2011) reported that some members of genus Momordica at 3% in the diet significantly lowered the amount of serum triglyceride. According to them, fatty acids lymphatic transport and fecal lipid excretion are responsible for the decline in triglycerides. In another study, dietary intake of bitter melon reduced the serum triglyceride level to 20% in diabetic rats (Bano et al., Citation2011). Hossain et al. (Citation2012) observed 47.02% increase in triglycerides level in diabetic control groups and a decline in experimental groups in a dose-dependent manner. Abas et al. (Citation2015) reported that the level of triglyceride reduced from 0.57 mmol/L after bitter melon consumption in rats compared to a diabetic control group (0.95 mmol/L) following 4 weeks of treatment. Rohajatien et al. (Citation2018) revealed 35% reduction in triglyceride levels after giving a diet containing bitter melon. In a nutshell, bitter melon preparations proved beneficial in managing the serum lipid profile thereby having the potential to address lipid-related abnormalities. Saponins are mainly responsible entity behind these lipid-lowering effects.
Bioactive moieties in bitter melon have a significant impact on managing body weight. The current results regarding body weight are fairly corroborated by the earlier findings of Jafri et al. (Citation2009) who pinpointed that body weight was increased in normal control rats, but in diabetic control rats that increase was lesser as compared to other diabetic groups fed with bitter melon. In a similar antihyperglycemic study, bitter melon seeds aqueous extract significantly increased the body weight in diabetic rats (Sathishsekar & Subramanian, Citation2005). Likewise, Shetty et al. (Citation2005) reported that body weight is marginally increased in diabetic rats fed with a bitter melon supplemented diet. The current findings are also supported by the results of Hossain et al. (Citation2012), Arshad et al. (Citation2017), Rohajatien et al. (Citation2018) and Sultan et al. (Citation2021) demonstrated that body weight was higher in bitter melon-treated diabetic groups than in untreated diabetic groups.
It is deduced from the above discussion that skin, flesh, seeds and whole fruit powder of bitter melon are significantly effective against hyperglycemia and hyperlipidemia. Although alkaloids and saponins in bitter melon fruit play a role as an antihyperglycemic and antihyperlipidemic agent, high antihyperglycemic and low antihyperlipidemic activities were observed in alkaloids and low antihyperglycemic and high antihyperlipidemic activities were observed in saponins. These phytochemicals might play a role in regulating glucose and lipid metabolic pathways and can be used to treat hyperglycemia and hyperlipidemia.
9. Conclusions
The total alkaloids were higher in bitter melon seeds, while the flesh part has a high amount of total saponins. The antihyperglycemic and antihyperlipidemic activities of this plant are directly associated with the presence of these chemical moieties. Alkaloids were found to be more potent antihyperglycemic agents, while saponins were found to be antihyperlipidemic moderators. It has been suggested that these phytochemicals may play a role in regulating glucose and lipid metabolic pathways and can help treat hyperglycemia and hyperlipidemia.
Acknowledgments
The authors acknowledge the Nutritional Labs of Government College Women University, Faisalabad, Pakistan, for facilitation of collecting data in this study. We are also thankful to the Food Analysis Lab of Government College University Faisalabad, Pakistan, and the Agricultural Extension Directorate, MAAR, Damascus, Syria, for providing lab facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
Additional information
Funding
References
- Abas, R., Das, S., & Thent, Z. C. (2015). Momordica charantia fruit extract improve subcellular changes in cardiovascular tissues of diabetic rats. Journal of Medical & Bioengineering, 4(5), 357–16. https://doi.org/10.12720/jomb.4.5.357-362
- Abdollah, M., Zuki, A. B. Z., Goh, Y. M., Rezaeizadeh, A., & Noordin, M. M. (2010). The effects of Momordica charantia on the liver in streptozotocin-induced diabetes in neonatal rats. African Journal of Biotechnology, 9(31), 5004–5012.
- Abo-Ghanema, I., & Saleh, R. (2012). Some physiological effects of Momordica charantia and trigonella foenum-graecum extracts in diabetic rats as compared with Cidophage. International Journal of Animal and Veterinary Sciences, 6(4), 222–230. https://doi.org/10.5281/zenodo.1086261
- Ahn, J., Choi, W., Kim, S., & Ha, T. (2011). Anti-diabetic effect of watermelon (Citrullus vulgaris Schrad) on streptozotocin-induced diabetic mice. Food Science & Biotechnology, 20(1), 251–254. https://doi.org/10.1007/s10068-011-0034-5
- Akhtar, N., Khan, B. A., Majid, A., Khan, S., Mahmood, T., Gulfishan, G., & Saeed, T. (2011). Pharmaceutical and biopharmaceutical evaluation of extracts from different plant parts of indigenous origin for their hypoglycemic responses in rabbits. Acta poloniae pharmaceutica, 68(6), 919–925.
- Al-Neaimy, K. S. A. (2008). Comparative effect of metformin and glibenclamide on lipid profile in type 2 diabetic patients. Tikrit Journal of Pharmaceutical Sciences, 1(1), 46–50. https://www.iasj.net/iasj/download/d4a9c649dbfac3c3
- Alshatwi, A. A., Al Obaaid, M. A., Al Sedairy, S. A., Al-Assaf, A. H., Zhang, J. J., & Lei, K. Y. (2010). Tomato powder is more protective than lycopene supplement against lipid peroxidation in rats. Nutrition Research, 30(1), 66–73. https://doi.org/10.1016/j.nutres.2009.12.002
- Arshad, F., Saeed, M. S., Nadeem, M. T., Arshad, M. U., Nadeem, M. T., & Arshad, M. U. (2017). Hypoglycemic and hypolipidemic effects of different parts and formulations of bitter gourd (Momordica Charantia). Lipids in Health and Disease, 16(1), 1–11. https://doi.org/10.1186/s12944-017-0602-7
- Bano, F., Akthar, N., & Naz, H. (2011). Effect of the aqueous extract of Momordica charantia on body weight of rats. Journal of Basic & Applied Sciences, 7(1), 1–5.
- Bhushan, M. S., Rao, C. H. V., Ojha, S. K., Vijayakumar, M., & Verma, A. (2010). An analytical review of plants for anti-diabetic activity with their phytoconstituent and mechanism of action. International Journal of Pharmaceutical Sciences and Research, 1(1), 29–46.
- Biyani, M.K., Banavalikar, M.M., Suthar, A.C., Shahani, S., Sivakami, S. and Vidri, J. (2003). Antihyperglycemic effects of three extracts from Momordica charantia. Journal of Ethanopharmocology, 88(1), 107–111. https://doi.org/10.1016/S0378-8741(03)00184-3
- Bortolotti, M., Mercatelli, D., & Polito, L. (2019). Momordica charantia, a nutraceutical approach for inflammatory related diseases. Frontiers in Pharmacology, 10, 486. https://doi.org/10.3389/fphar.2019.00486
- Chen, Q., Chan, L. L., & Li, E. T. (2003). Bitter melon (Momordica charantia) reduces adiposity, lowers serum insulin and normalizes glucose tolerance in rats fed a high fat diet. The Journal of Nutrition, 133(4), 1088–1093. https://doi.org/10.1093/jn/133.4.1088
- Chen, Y., Liu, Z. X., & Wu, C. S. (2010). Study on extracting technology of saponins from bitter melon. Guangzhou Chemical Industries, 38(1), 59–61.
- Çiçek, S. S. (2022). Momordica charantia L.— diabetes-related bioactivities, quality control, and safety considerations. Frontiers in Pharmacology, 1801. https://doi.org/10.3389/fphar.2022.904643
- Clouatre, D. L., Rao, S. N., & Preuss, H. G. (2011). Bitter melon extracts in diabetic and normal rats favorably influence blood glucose and blood pressure regulation. Journal of Medicinal Food, 14(12), 1496–1504. https://doi.org/10.1089/jmf.2010.0276
- Cummings, D. E., & Overduin, J. (2007). Gastrointestinal regulation of food intake. Journal of Clinical Investigation, 117(1), 13–23. https://doi.org/10.1172/JCI30227
- Dwijayanti, D. R., Okuyama, T., Okumura, T., Ikeya, Y., & Nishizawa, M. (2019). The anti-inflammatory effects of Indonesian and Japanese bitter melon (Momordica charantia L.) fruit extracts on interleukin-1β-treated hepatocytes. Functional Foods in Health and Disease, 9(1), 16–33. https://doi.org/10.31989/ffhd.v9i1.560
- Dwijayanti, D. R., Shimada, T., Ishii, T., Okuyama, T., Ikeya, Y., Mukai, E., & Nishizawa, M. (2020). Bitter melon fruit extract has a hypoglycemic effect and reduces hepatic lipid accumulation in ob/ob mice. Phytotherapy Research, 34(6), 1338–1346. https://doi.org/10.1002/ptr.6600
- Fang, E. F., Froetscher, L., Scheibye-Knudsen, M., Bohr, V. A., Wong, J. H., & Ng, T. B. (2019). Emerging antitumor activities of the bitter melon (Momordica charantia). Current Protein & Peptide Science, 20(3), 296–301. https://doi.org/10.2174/1389203719666180622095800
- Fernandes, N. P., Lagishetty, C. V., Panda, V. S., & Naik, S. R. (2007). An experimental evaluation of the antidiabetic and antilipidemic properties of a standardized Momordica charantia fruit extract. BMC Complementary and Alternative Medicine, 7(1), 29. https://doi.org/10.1186/1472-6882-7-29
- Gadang, V., Gilbert, W., Hettiararchchy, N., Horax, R., Katwa, L., & Devareddy, L. (2011). Dietary bitter melon seed increases peroxisome proliferator-activated receptor-γ gene expression in adipose tissue, down-regulates the nuclear factor-κB expression, and alleviates the symptoms associated with metabolic syndrome. Journal of Medicinal Food, 14(1–2), 86–93. https://doi.org/10.1089/jmf.2010.0010
- Gao, Y., Li, X., Huang, Y., Chen, J., & Qiu, M. (2021). Bitter melon and diabetes mellitus. Food Reviews International, 39(1), 1–21. https://doi.org/10.1080/87559129.2021.1923733
- Gayathry, K. S., & John, J. A. (2022). A comprehensive review on bitter gourd (Momordica charantia L.) as a gold mine of functional bioactive components for therapeutic foods. Food Production, Processing and Nutrition, 4(1), 10. https://doi.org/10.1186/s43014-022-00089-x
- Gill, N. S., Rani, P., Arora, R., Dhawan, V., & Bali, M. (2012). Evaluation of antioxidant, antiinflammatory and antiulcer potential of Momordica charantia methanolic seed extract. Research Journal of Phytochemistry, 6(4), 1–9. https://doi.org/10.3923/rjphyto.2012.96.104
- Habicht, S. D., Kind, V., Rudloff, S., Borsch, C., Mueller, A. S., Pallauf, J., Yang, R.-Y., & Krawinkel, M. B. (2011). Quantification of antidiabetic extracts and compounds in bitter gourd varieties. Food Chemistry, 126(1), 172–176. https://doi.org/10.1016/j.foodchem.2010.10.094
- Harborne, J. B. (2005). Phytochemical methods. A guide to modern techniques of plant analysis (3rd ed.). Springer Pvt. Ltd.
- Hazarika, R., Parida, P., Neog, B., & Yadav, R. N. S. (2012). Binding energy calculation of GSK-3 protein of human against some anti-diabetic compounds of Momordica charantia linn (bitter melon). Bioinformation, 8(6), 251. https://doi.org/10.6026/97320630008251
- Hossain, M. A., Mostofa, M., Debnath, D., Alam, A. K. M. R., Yasmin, Z., & Moitry, N. F. (2012). Antihyperglycemic and antihyperlipidemic of Karala (Momordica charantia) fruits in streptozotocin induced diabetic rats. Journal of Environmental Science & Natural Resources, 5(1), 29–37. https://doi.org/10.3329/jesnr.v5i1.11550
- Huang, H. L., Hong, Y. W., Wong, Y. H., Chen, Y. N., Chyuan, J. H., Huang, C. J., & Chao, P. M. (2008). Bitter melon (Momordica charantia L.) inhibits adipocyte hypertrophy and down regulates lipogenic gene expression in adipose tissue of diet-induced obese rats. The British Journal of Nutrition, 99(2), 230–239. https://doi.org/10.1017/S0007114507793947
- Hussain, M. S., Jahan, N., Rashid, M. M. O., Hossain, M. S., Chen, U., & Rahman, N. (2019). Antihyperlipidemic screening and plasma uric acid reducing potential of Momordica charantia seeds on Swiss albino mice model. Heliyon, 5(5), e01739. https://doi.org/10.1016/j.heliyon.2019.e01739
- International Diabetes Federation. (2021). International diabetes federation. IDF diabetes atlas (10thed.). www.diabetesatlas.org
- Jafri, S. A., Ismail, M. S., & Zaman, G. (2009). Effect of Momordica charantia (karela) in alloxan induced diabetic rats. Pakistan Journal of Science, 61(4), 220–222.
- Jeong, J., Lee, S., Hue, J., Lee, K., Nam, S. Y., & Yun, Y. W. (2008). Effect of bitter melon (Momordica Charantia) on antidiabetic activity in C57BL/6J db/db mice. Korean Journal of Veterinary Research, 48(3), 327–336.
- Karagöz, I. K., Karagöz, A., Özkalaycı, F., Doğan, C., Kocabay, G., & Elbay, A. (2021, August). Relation between platelet reactivity levels and diabetic retinopathy stage in patient with type 2 diabetes mellitus by using multiplate whole blood aggregometry. Seminars in Ophthalmology, 36(5–6), 392–399. Taylor & Francis. https://doi.org/10.1080/08820538.2021.1893759
- Katz, A., Nambi, S. S., Mather, K., Baron, A. D., Follmann, D. A., Sullivan, G., & Quon, M. J. (2000). Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. Journal of Clinical Endocrinology & Metabolism, 85(7), 2402–2410. https://doi.org/10.1210/jcem.85.7.6661
- Ketut, D. M., Adnyana, D. P. A., Rinaldhi, C. P., Octaviani, R. R., & Cempaka, D. K. S. (2019). The antidiabetic effect of bitter melon (Momordica charantia L.) extracts towards glucose concentration, langerhans islets, and leydig cells of hyperglycemic mice (Rattus norvegicus). EurAsian Journal of BioSciences, 13(2), 757–762. https://web.p.ebscohost.com/abstract?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=13079867&AN=149914670&h=ndz0mEnVLAxvNd8ZNN7QRWlI6RNQcxDqWzuTPutqoiJUDOFj4BQ8jXv%2bVdzNLQ2K3Xa%2fvW%2bH5csIDYNW52sFtA%3d%3d&crl=c&resultNs=AdminWebAuth&resultLocal=ErrCrlNotAuth&crlhashurl=login.aspx%3fdirect%3dtrue%26profile%3dehost%26scope%3dsite%26authtype%3dcrawler%26jrnl%3d13079867%26AN%3d149914670
- Kim, J., Cho, H., Moon, S., Kim, K., & Ku, S. (2012). Synergic effects of bitter melon and β-glucan composition on STZ-Induced rat diabetes and its complications. Journal of Microbiology and Biotechnology, 22(1), 147–155. https://doi.org/10.4014/jmb.1106.06037
- Kim, J. I., Paik, J. K., Kim, O. Y., Park, H. W., Lee, J. H., Jang, Y., & Lee, J. H. (2011). Effects of lycopene supplementation on oxidative stress and markers of endothelial function in healthy men. Atherosclerosis, 215(1), 189–195. https://doi.org/10.1016/j.atherosclerosis.2010.11.036
- Klomann, S. D., Mueller, A. S., Pallauf, J., & Krawinkel, M. B. (2010). Antidiabetic effects of bitter gourd extracts in insulin-resistant db/db mice. The British Journal of Nutrition, 104(11), 1613–1620. https://doi.org/10.1017/S0007114510002680
- Kole, C., Matsumura, H., & Behera, T. K. (Eds.). (2020). The bitter gourd genome. Springer. https://doi.org/10.1007/978-3-030-15062-4
- Krawinkel, M. B., & Keding, G. B. (2006). Bitter Gourd (Momordica charantia): A dietary approach to hyperglycemia. Nutrition Reviews, 7(7), 331–337. https://doi.org/10.1111/j.1753-4887.2006.tb00217.x
- Li-Dong, T., Ming-Wei, Z., Si-Yuan, G., Rui-Fen, Z., Jian-Wei, C., Zhen-Cheng, W., Yan, Z., & Xiao-Jun, T. (2008). Comparison of saponin contents of different varieties of Momordica charantia L. and their inhibition on α-glucosidase activity. Scientia Agricultura sinica, 41(10), 3415–3421.
- Liu, Z., Gong, J., Huang, W., Lu, F., Dong, H., & Ullah, R. (2021). The effect of Momordica charantia in the treatment of diabetes mellitus: A review. Evid Based Complement Altern Med, 2021, 1–14. https://doi.org/10.1155/2021/3796265
- Li, Z., Xia, A., Li, S., Yang, G., Jin, W., Zhang, M., & Wang, S. (2020). The pharmacological properties and therapeutic use of bitter melon (Momordica charantia L.). Current Pharmacology Reports, 6(3), 103–109. https://doi.org/10.1007/s40495-020-00219-4
- Lucas, E. A., Dumancas, G. G., Smith, B. J., Clarke, S. L., & Arjmandi, B. H. (2010). In bioactive foods in promoting health ( V. R. Preedy, Ed.). Academic Press. https://doi.org/10.1016/B978-0-12-374628-3.00035-9
- Mahmood, A., Raja, G. K., Mahmood, T., Gulfraz, M., & Khanum, A. (2012). Isolation and characterization of antimicrobial activity conferring component (s) from seeds of bitter gourd (Momordica charantia). Journal of Medicinal Plants Research, 6(4), 566–573. https://doi.org/10.5897/JMPR10.613
- Majumda, B., & Debnath, T. (2014). Immunomodulatory activity of ethanolic extract of bitter gourd (Momordica charantia) in experimental models. Journal of Biomedical and Pharmaceutical Research, 3(3), 59–63.
- Matsui, S., Yamane, T., Takita, T., Oishi, Y., & Kobayashi-Hattori, K. (2013). The hypocholesterolemic activity of Momordica charantia fruit is mediated by the altered cholesterol- and bile acid-regulating gene expression in rat liver. Nutrition Research, 33(7), 580–585. https://doi.org/10.1016/j.nutres.2013.05.002
- Mohammady, I., Elattar, S., Mohammed, S., & Ewais, M. (2012). An evaluation of anti-diabetic and anti-lipidemic properties of Momordica charantia (bitter melon) fruit extract in experimentally induced diabetes. Life Science Journal, 9(2), 363–374.
- Obadoni, B. O., & Ochuko, P. O. (2001). Phytochemical studies and comparative efficacy of the crude extract of some homeostatic plants in Edo and Delta states of Nigeria. Global Journal of Pure and Applied Sciences, 8(2), 203–208. https://doi.org/10.4314/gjpas.v8i2.16033
- Oishi, Y., Sakamoto, T., Udagawa, H., Taniguchi, H., Kobayashi-Hattori, K., Ozawa, Y., & Takita, T. (2007). Inhibition of increases in blood glucose and serum neutral fat by Momordica charantia saponin fraction. Bioscience, Biotechnology, and Biochemistry, 71(3), 735–740. https://doi.org/10.1271/bbb.60570
- Panda, B. C., Mondal, S., Devi, K. S. P., Maiti, T. K., Khatua, S., Acharya, K., & Islam, S. S. (2015). Pectic polysaccharide from the green fruits of Momordica charantia (Karela): Structural characterization and study of immunoenhancing and antioxidant properties. Carbohydrate Research, 401, 24–31. https://doi.org/10.1016/j.carres.2014.10.015
- Poovitha, S., & Parani, M. (2016). In vitro and in vivo α-amylase and αglucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L.). BMC Complementary and Alternative Medicine, 16(Suppl 1), 185. https://doi.org/10.1186/s12906-016-1085-1
- Reyes, B. A. S., Bautista, N. D., Tanquilut, N. C., Anunciado, R. V., Leung, A. B., Sanchez, G. C., Magtoto, R. L., Castronuevo, P., Tsukamura, H., & Maeda, K. I. (2006). Anti-diabetic potentials of Momordica charantia and Andrographis paniculata and their effects on estrous cyclicity of alloxan-induced diabetic rats. Journal of Ethnopharmacology, 105(1–2), 196–200. https://doi.org/10.1016/j.jep.2005.10.018
- Rohajatien, U., Sriwahyuni, T., Estiasih, E., & Sriwahyuni, E. (2018). Bitter melon (Momordica charantia L) fruit decreased blood glucose level and improved lipid profile of streptozotocin induced hyperglycemia rats. Current Research in Nutrition and Food Science Journal, 6(2), 359–370. https://doi.org/10.12944/CRNFSJ.6.2.11
- Sa’adah, N. N., & Nurhayati, A. P. D. (2020). Antihiperlipidemic activity of the methanolic extract of parijoto (medinilla speciosa) on the protein profile of hyperlipidemic rats.
- Saad, D. Y., Soliman, M. M., Baiomy, A. A., Yassin, M. H., & El-Sawy, H. B. (2017). Effects of Karela (Bitter Melon; Momordica charantia) on genes of lipids and carbohydrates metabolism in experimental hypercholesterolemia: Biochemical, molecular and histopathological study. BMC Complementary and Alternative Medicine, 17(1), 319. https://doi.org/10.1186/s12906-017-1833-x
- Saeed, F., Afzaal, M., Niaz, B., Arshad, M. U., Tufail, T., Hussain, M. B., & Javed, A. (2018). Bitter melon (Momordica charantia): A natural healthy vegetable. International Journal of Food Properties, 21(1), 1270–1290. https://doi.org/10.1080/10942912.2018.1446023
- Sathishsekar, D., & Subramanian, S. (2005). Antioxidant properties of Momordica Charantia (bitter gourd) seeds on Streptozotocin induced diabetic rats. Asia Pacific Journal of Clinical Nutrition, 14(2), 153–158.
- Sato, M., Ueda, T., Nagata, K., Shiratake, S., Tomoyori, H., Kawakami, M., Ozaki, Y., Okubo, H., Shirouchi, B., & Imaizumi, K. (2011). Dietary kakrol (Momordica dioica Roxb.) flesh inhibits triacylglycerol absorption and lowers the risk for development of fatty liver in rats. Experimental Biology and Medicine, 236(10), 1139–1146. https://doi.org/10.1258/ebm.2011.011037
- Senanayake, G. V., Maruyama, M., Sakono, M., Fukuda, N., Morishita, T., Yukizaki, C., Kawano, M., & Ohta, H. (2004). The effects of bitter melon (Momordica charantia) extracts on serum and liver lipid parameters in hamsters fed cholesterol-free and cholesterol-enriched diets. Journal of Nutritional Science and Vitaminology, 50(4), 253–257. https://doi.org/10.3177/jnsv.50.253
- Shetty, A. K., Kumar, G. S., Sambaiah, K., & Salimath, P. V. (2005). Effect of bitter gourd (Momordica charantia) on glycaemic status in streptozotocin induced diabetic rats. Plant Foods for Human Nutrition, 60(3), 109–112. https://doi.org/10.1007/s11130-005-6837-x
- Singh, N., & Gupta, M. (2007). Regeneration of beta cells in islets of Langerhans of pancreas of alloxan diabetic rats by acetone extract of Momordica charantia (Linn.) (bitter gourd) fruits. Indian Journal of Experimental Biology, 45(12), 1055–1062.
- Smail, M., Howarth, F., Abdulkhalek, S., Ismail, A. M., Singh, R. B., Hanoman, C. & Singh, J. (2020). Medicinal and anti-oxidant effects of Bitter Melon (Momordica charantia) in the treatment of diabetic cardiomyopathy. World Heart Journal, 12(3), 1–9. https://clok.uclan.ac.uk/35904/1/35904%20Proof%20for%20bitter%20melon2020.pdf
- Steel, R. G. D., & Torrie, J. H. (1986). Principles and procedures of statistics: A biometrical approach. McGraw-Hill.
- Sultan, F., Saeed, M. T., Ahmed, A., Riaz, S., Amarowicz, N., Bigiu, R., Manea, R., & Manea, R. (2021). Bitter melon (Momordica charantia L.) fruit bioactives charantin and vicine potential for diabetes prophylaxis and treatment. Plants, 10(4), 730. https://doi.org/10.3390/plants10040730
- Sundaram, E. N., & Kumar, S. (2002). Bitter melon holds hope for treating diabetes. Homeopathic Society, 22, 4.
- Temitope, A. G., Sheriff, O. L., Azeezat, Y. F., Taofik, A., & Fatimah, A. I. (2013). Cardioprotective properties of Momordica charantia in Albino Rats. African Journal of Scientific Research, 11(1), 600–610.
- Tymoczko, J. L., Stryer, B. T., Stryer, L., & Berg, J. M. (2002). Biochemistry ( W. H. Freeman, Ed.). W.H. Freeman.
- Uebanso, T., Arai, H., Taketani, Y., Fukaya, M., Yamamoto, H., Mizuno, A., Uryu, K., Hada, T., & Takeda, E. (2007). Extracts of Momordica charantia suppress postprandial hyperglycemia in rats. Journal of Nutritional Science and Vitaminology (Tokyo), 53(6), 482–486. https://doi.org/10.3177/jnsv.53.482
- Wang, Q., Deng, Y., Zhang, M., Zhang, R., Zhang, Y., Tang, X., Wei, Z., & Chi, J. (2011). Continuous extraction of saponin and polysaccharide from Momordica charantia L. and their inhibitory effect on α-glucosidase. Scientia Agricultura sinica, 44(19), 4058–4065.
- Wang, S., Li, Z., Yang, G., Ho, C. T., & Li, S. (2017). Momordica charantia: A popular health-promoting vegetable with multifunctionality. Food & Function, 8(5), 1749–1762. https://doi.org/10.1039/C6FO01812B
- WHO. (2021). Cardiovascular diseases (CVDs). Retrieved September 15, 2022, from http://www.int/news-room/factsheets/detail/cardiovascular-diseases(cvds)
- Xiang, L., Huang, X., Chen, L., Rao, P., & Ke, L. (2007). The reparative effects of Momordica charantia Linn. extract on HIT-T15 pancreatic beta cells. Asia Pacific journal of clinical nutrition, 16(1), 249–252. https://doi.org/10.5281/zenodo.1086261
- Youn, K. S., Park, E. H., & Yoon, K. Y. (2019). Quality characteristics and antioxidant activity of bitter melon (Momordica charantia L.) dried by different methods. Korean Journal of Food Preservation, 26(2), 185–193. https://doi.org/10.11002/kjfp.2019.26.2.185
- Yuan, X. Q., Yuan, C., & Liu, H. M. (2012). Hypoglycemic effect of Momordica charantia L. Var. abbreviata ser. protein hydrolysate prepared with alcalase. Applied Mechanics & Materials, 195-196, 324–329. https://doi.org/10.4028/www.scientific.net/AMM.195-196.324
- Zawada, A., Machowiak, A., Rychter, A. M., Ratajczak, A. E., Szymczak-Tomczak, A., Dobrowolska, A., & Krela-Ka ´zmierczak, I. (2022). Accumulation of advanced glycation end-products in the body and dietary habits. Nutrients, 14(19), 3982. https://doi.org/10.3390/nu14193982
- Zhang, F., Zhang, X., Yu, J., Tan, Y., Guo, P., & Wu, C. (2020). The gut microbiota confers the lipid-lowering effect of bitter melon (Momordica charantia L.) in high-fat diet (HFD)-Induced hyperlipidemic mice. Biomedicine & Pharmacotherapy, 131, 110667. https://doi.org/10.1016/j.biopha.2020.110667