?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The rising number of individuals diagnosed with type 2 diabetes is a global health concern. This chronic disease can lead to a diverse range of complications. In this study, we assessed the extracorporeal hypoglycemic mechanism as determined by the inhibitory activity of TGE (Fagopyrum tataricum grain crude extract) and TBE (Fagopyrum tataricum bran crude extract) on elevated blood glucose, oxidative stress and inflammatory response. In addition, to characterize hypoglycemic activity in vivo, we investigated the anti-diabetic effects of water—ethanol extracts of Fagopyrum tataricum grain and bran with flavonoid-rich and D-CI fractions in type 2 diabetic mice induced by streptozotocin and a high-fat/high-sugar diet. Moreover, compared with the TBE group through kit detection and in vitro test analysis, treatment of mice with TGE has a better ability of antioxidant activity, Reduce inflammatory overreaction, hypoglycemia, improving symptoms of type 2 diabetic mice and reducing organ damage. In conclusion, these findings yet indicate that TGE could be used to manage the disease, for the improvement of blood glucose level and exhibits antioxidant properties for type 2 diabetes. By providing a comprehensive review of the molecular mechanisms underlying glucose metabolism, oxidative stress, inflammation, organ structural damage, and insulin resistance in individuals with type 2 diabetes mellitus. Our findings not only offer a deeper understanding of these processes but also provide novel insights and theoretical guidance for the treatment of diabetes mellitus.
1. Introduction
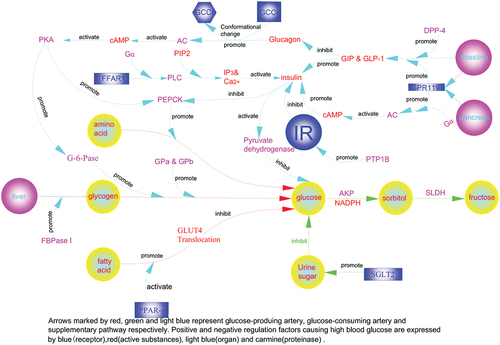
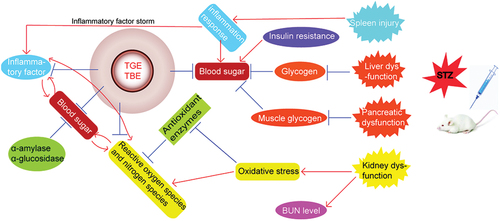
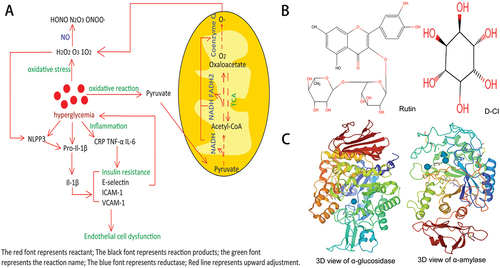
Mathematical method estimated the global diabetes prevalence in 2021 was to be 536.6 million people, rising to 783.2 million in 2045, most of these cases associated with T2D(type 2 diabetes) based on insulin resistance, β-cell dysfunction and elevated hepatic glucose output symptoms (Sun et al., Citation2022; T. Zhou et al., Citation2018) compared with type 1 diabetes patients who cannot secrete insulin. Insulin resistance is mainly manifested as a decrease in the sensitivity of the body to normal levels of insulin. β- Cell dysfunction refers to the insufficient secretion of insulin by cells, which can not compensate for insulin resistance precipitating hyperglycemia and overt diabetes (Savage et al., Citation2007). Hyperglycemia is often accompanied by oxidative stress and inflammatory reactions, and the three factors interact with one another (Uemura et al., Citation2001; Wu et al., Citation2021). Conversely, long-term high glucose levels cause not only chronic disease characterized by metabolic disorder but also irreversible damage to the eyes, nerves, kidneys and heart (Sroor et al., Citation2019). According to reports, approximately 120 million diabetes patients worldwide have been treated with metformin to no significant therapeutic effect (Dujic et al., Citation2014). Therefore, it is imperative to find a drug that can treat type 2 diabetes in a multi-faceted manner that incorporates anti-oxidation, hypoglycemic, and anti-inflammatory effects, as well as their interrelationships. α-Glucosidase inhibitors have been recognized as effective chemotherapy agents for the treatment of diabetes, as they inhibit the ability of the membranes of small intestinal lining cells to bind α-glucosidase (Henrissat, Citation1991). Upon inhibiting α-glucosidase and α-amylase, carbohydrate digestion in the intestine is reduced, minimizing the amount of glucose available for absorption and thereby inhibiting postprandial hyperglycemia (Bhandari et al., Citation2008; Krentz & Bailey, Citation2005). Reactive oxygen species and nitrogen species (H2O2, O3, 1O2, HONO, NO(III), N2O3) that are produced by hyperglycemia and oxidative stress can react with NO in endothelial cells to produce peroxynitrite (ONOO-, HOONO) (H. Cai & Harrison, Citation2000; Lu & Kang, Citation2001). This change in the function of NO is highly reactive and affects protein modification in pro-inflammatory cells. Another oxidative channel is exhibited by diabetic cells with high glucose concentrations, in which glucose-derived pyruvate is oxidized in the TCA cycle, increasing the number of electron donors (NADH and FADH2) entering the electron transport chain. As a result, the voltage gradient across the mitochondrial membrane increases, and electron transfer is blocked. Electrons are returned to coenzyme Q, which supplies electrons to one molecular oxygen at a time to produce superoxide (Konstantinov, Citation1990). Diabetes leads to an abnormal increase in the concentration of inflammatory cytokines (TNF-α, CRP, IL-6, IL-1β and IL-8), which compromises regular cytokine signal transduction channels (Böni-Schnetzler et al., Citation2008; Hu et al., Citation2004). TNF-α, IL-6 and CRP not only directly promote insulin resistance, but also stimulate endothelial cells to produce adhesion molecules (E-selectin, ICAM-1and VCAM-1), causing endothelial cell dysfunction (Slack et al., Citation1993). The NLRP3 inflammatory corpuscle promotes the maturation and secretion of the cytokine pro-IL-1β, which is involved in the early development of T2D and responds to stimulation by mitochondrial ROS (Schroder & Tschopp, Citation2010). Studying the relationship (Figure ) between hyperglycemia, oxidative stress and inflammation response will improve our ability to prevent and treat T2D and its complications. Simultaneously, it is very important to understand the hypoglycemic mechanisms of related receptors and to study damage to related receptors in diabetic patients (Figure ). Many researchers have reported that rutin is the main active compound in F. tataricum grain (TBG) and bran (TBB) (Cho et al., Citation2014; Sedej et al., Citation2012) and rutin accounts for 85–90% of the total antioxidant activity of F. tataricum (Morishita et al., Citation2007). Nevertheless, more and more studies show that D-chiro-Inositol(D-CI) with an insulin-like bioactivity can lower the blood glucose level and is mainly enriched from TBE. Here, we selected flavonoids and D-CI from TGE and TBE to evaluate the differences of their anti-diabetic activity and screen out suitable anti-diabetes natural products.
Figure 1. (A). Relationship of molecular mechanism between blood glucose levels, superoxide ions and inflammatory factors;(B). Structure of Rutin T. Zhou et al. (Citation2018) and D-CI Sun et al. (Citation2022); (C) the three-dimensional structure of α-glucosidase and α-amylase were retrieved from RCSB Protein Data Bank (www.Rcsb.org/pdb) (PDB ID code 3A4A and PDB ID code 1B2Y).
2. Methods and materials
2.1. Materials and chemicals
Human normal red blood cells were obtained from the Harbin Institute of Technology Hospital (Harbin, Heilongjiang Province, China). All commercial kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All reagents were at least of analytical grade purchased from Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China) and Tianjin Jindong Tianzheng Fine Chemical Reagent Factory (Tianjin, China). Microplate reader is Model 550 of Japan BIO-RAD. Blood glucose meter is purchased from Sinocare Inc. Changsha, Hunan, China.
2.2. Determination of flavonoids and D-CI from F. tataricum
TBG and TBB, from Liangshan Yi Autonomous Prefecture of Sichuan Province, were crushed into powder through a crusher and sieved through a 100 mesh sieve and were dissolved in 75% ethanol. The crude extracts were filtered and ethanol was subsequently evaporated. We use AB-8 resin for purification and get TGE and TBE according to the method of Cai et al (W. Cai et al., Citation2010). Rutin(Figure ) reacts with aluminum trichloride to form a yellow complex with a large absorption peak at 421 nm (Moghaddasian et al., Citation2012). The standard curve equation we obtained is as follows: y = 0.0571×+0.0042, R2 = 0.999. Determine the content of rutin in TGE and TBE samples through this standard curve. D-CI is difficult to be directly detected by ultraviolet spectrophotometer or HPLC because of its cyclytol structure (Figure ). It was derived as previously described by Iwasaki et al (Fc et al., Citation2019; Iwasaki et al., Citation2009). In brief, the concentration range of DCI standard working solution was set to 0.025, 0.05, 0.1, 0.25, 0.5, 1, and 2 mg/mL. Standard working solution or sample solution with benzoyl chloride, and NaOH solution were allowed to react at 25°C for 10 min. We used 6 mol/L phosphoric acid solution to stop the reaction. An equal volume of ethylacetate and vortexed the mixture were used to extract the supernatant fluid 10,000 g, 10 min. Determination of DCI by the HPLC (Agilent 1100, California, USA; SDSHYPERSIL 250 mm × 4.6 mm C18 column, Shanghai, China). The mobile phase is acetonitrile and water with a gradient elution(0–16 min: 80% acetonitrile, 20% water, flow rate 1 mL/min; 16.5–21 min: 100% acetonitrile, flow rate 1.5 mL/min) which consisted of 30°C column temperature and 20 min analytical time. After filtered through 0.45 μm micropore film, 2 μL of sample was transferred to a chromatographic bottle. The scanning wavelength is 230 nm.
2.3. In vitro analysis of hypoglycemic activity
α-glucosidase and ɑ-amylase(Figure ) inhibitory activity were evaluated using a modified version of the method described by Apostolidis et al (Ademiluyi et al., Citation2014; Apostolidis et al., Citation2007). For this reaction, 200 μL phosphate buffer solution (pH 6.8, 0.1 mol/L), 100 μL of different concentrations of TGE and TBE samples with different concentrations of acarbose, 100 μL reduced glutathione (0.5 mg/mL), and 200 μL α-glucosidase (2.5 U/mL), were combined and incubated at 37°C for 10 min. After the addition of 200 μL pNPG (0.5 mg/mL), samples were mixed and incubated in a water bath at 37°C for 15 min. The reaction was stopped by the addition of 200 μL sodium carbonate solution (0.1 mol/L). Acarbose was used as a positive control. Solution absorbance was assessed at 405 nm.
Where A1 is the OD value of the inhibition rate of α-glucosidase in the sample group; A2 is the OD value of phosphate buffer instead of the sample group; A3 is the phosphate buffer instead of the enzyme group OD value.
The α-amylase inhibitory activities of TGE and TBE were evaluated using a modified method. For this assay, 200 μL of TGE and TBE at different concentrations were combined with 500 μL porcine pancreatic α-amylase (0.5 mg/mL), dissolved in 0.02 M sodium phosphate buffer (pH = 6.9, containing 0.006 mol/L NaCl) and incubated at 25°C for 10 min. After the incubation, 500 μL 1% starch solution in 0.02 mol/L sodium phosphate buffer (pH 6.9, containing 0.006 mol/L NaCl) was added to the reaction mixture. Subsequently, the reaction mixture was incubated at 25°C for 10 min, after which 1.0 mL DNS was added. Finally, the reaction was stopped by a 5 min incubation in boiling water, after which samples were cooled to room temperature. The reaction mixture was then diluted with 10 mL of distilled water, and absorbance was measured at 540 nm. Acarbose was used as a positive control.
where A1 is the OD value of the inhibition rate of the sample in the α-amylase measurement group; A2 is the OD value of phosphate buffer instead of the sample group; A0 is the OD value of distilled water instead of the DNS group.
2.4. Determination of free radical scavenging activity
Evaluation of TGE and TBE antioxidant activity was performed using an improved method (Huang et al., Citation2005; Na et al., Citation2017; Romanet et al., Citation2021). Ascorbic acid was used as a positive control.
where A1 is the OD value of the hydroxyl radical scavenging activity of the sample group; A2 is the OD value of an equal volume of distilled water instead of the H2O2 group/DPPH ethanol solution group; A3 is the OD value of an equal volume of water instead of the F. tataricum sample group.
Under room temperature conditions, 125 μL 50 mmol/L Tris-HCl buffer solution (containing 1 mmol/L EDTA, pH = 8.2) was first combined with 100 μL of different concentrations of sample solution and mixed thoroughly. Next, 25 μL of 3 mmol/L pyrogallol (configured with 1 mmol/L HCl) was added and mixed evenly. The timing was started upon the second mixing, and absorbance was measured every 30 s at 325 nm until 5 min30. Ascorbic acid was used as a positive control.
where k1 is the slope of the line of the absorbance value plotted against time for the sample superoxide radical scavenging active group; K2 is the slope of the line of absorbance value versus time plotted for an equal volume of distilled water instead of sample.
2.5. In vitro analysis of anti-inflammatory activity
In vitro, anti-inflammatory activity studies were performed based on the similar stability of HRBC (human erythrocyte) membranes and lysosomal membranes. Assessing the anti-inflammatory activity of TGE and TBEs was performed using an improved version of Kumar’s method. The haemoglobin content of the supernatant was estimated at 560 nm using a UV-Vis spectrophotometer (Kumar et al., Citation2019). Diclofenac was used as a standard.
Where A1 is the OD value of the sample against the human erythrocyte membrane protection rate; A2 represents volumetric distilled water instead of the OD value of the sample.
2.6. Streptozotocin-induced diabetic mice
Forty clean Kunming male mice (Heilongjiang University of Traditional Chinese Medicine Experimental Animal Center, SCXK Hei 200,800,4) weighing 20 ± 5 g and 4–6 weeks old were selected. The temperature of the animal-specific breeding room was maintained at 22 ± 2°C with humidity of 55% −60%. Eight mice as normal control group were fed with normal feed and 32 mice were fed with a high-fat, high-sugar diet (containing 23.5% protein, 33.2% total fat, and 10.7% carbohydrates) for one week. Streptozotocin (STZ) was dissolved in citrate buffer (pH 4.38), and the 32 mice were injected with STZ solution at a dose of 25 mg/kg · BW twice (with an interval of 24 h). After the last injection, mice with blood glucose values >10 mmol/L were considered as diabetic mice (Zhong et al., Citation2022). Forty diabetic mice were divided into five groups: NC (Normal control group) and HG (the hyperglycemic mice) is fed with normal saline, MET (positive control group) with metformin hydrochloride (100 mg/kg), TGE group (400 mg/kg) with TGE, and TBE group (400 mg/kg) with TBE (Belmouhoub et al., Citation2018; Murad et al., Citation2015). Mice were treated twice a day for five weeks. Fasting blood glucose was measured every week (Shao et al., Citation2018). At the end of the experiment, mice were individually housed in metabolic cages for 24 h to perform intake/output assessment.
2.7. Measurement of organ index of diabetic mice
At the end of the experimental period, animals were sacrificed after a 12 h overnight fast following animal ethics guidelines. Fresh liver, pancreas, spleen, kidney, and thigh bicep samples were obtained, rinsed with sterile saline and weighed.
Where m1 is the mass of organs (g); m2 is the body weight (g).
2.8. Biochemical parameter determination
Serum insulin was determined using ELISA kits. Serum levels of BUN, SOD, CAT, GSH-Px, IL-6, TNF-α were measured using commercial kits. Mouse liver and bicep samples were used to determine levels of mouse liver glycogen and muscle glycogen according to the reagent instructions.
2.9. Statistical analysis
All experiments were performed in triplicate at least. The data were presented as the mean ± SD and conducted using SPSS 19 software. One-way ANOVA methods were used for testing differences and p < 0.05 was considered statistically significant. The animal experiments part of this research was approved by the experimental animal welfare ethics committee of Harbin Institute of Technology (IACUC-2019021).
3. Results
3.1. Preparation of F. tataricum flavonoids and D-CI
F. tataricum is rich in flavonoids and is a major source of rutin in the human diet. Our data showed that flavonoid content can account for up to 50.31% in TGE, while D-CI was only 2%. In TBE, flavonoid content can account for up to 47.15% and D-CI content accounts for 30.91%. Although there was a significant difference in crude extract content, we observed no significant difference in the proportion of flavonoid content in the crude extract. It has a similar flavonoid content (39.43 ± 0.68 mg/g in TBG 13.15 ± 0.39 mg/g in TBB) to F. tataricum according to previous descriptions (X.-L. Zhou et al., Citation2019) although our results differ from those reported by Lee et al (Lee et al., Citation2016; Li et al., Citation2019). The content of the D-CI is 1.62 ± 0.21 in TBG mg/g, 8.65 ± 0.21 mg/g in TBB. Nan et al and Steadman et al detected a higher content of D-CI from the bran milling fractions by HPLC-ELSD and GC-MS (Nan et al., Citation2008; Steadman et al., Citation2000).
3.2. Inhibition against α-glucosidase and α-amylase by TGE and TBE
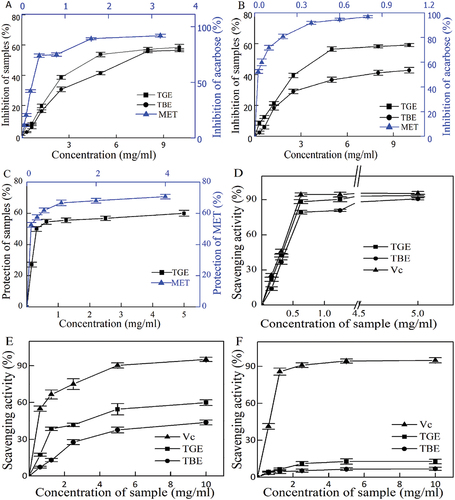
Assessment of α-glucosidase and α-amylase inhibition was carried out to determine the anti-diabetic properties of TGE and TBE. At a concentration of 10.00 mg/mL, the maximum inhibition activity of TGE was 58.5 ± 1.89%, and the inhibition activity of TBE was 56.63 ± 1.04% (Figure ). The maximum α-amylase inhibitory activities of TGE and TBE were 59.12 ± 1.02% and 42.78 ± 1.75%, respectively (Figure ). By comparing the α-glucosidase inhibition activity and α-amylase inhibition activity of individual samples, we observed a certain correlation between the inhibition activities of different enzymes; that is, a drug with a strong inhibitory activity against one enzyme often showed strong inhibitory activity against other enzymes.
Figure 3. (A). α-glucosidase inhibitory activity of TGE and TBE, (B). α-amylase inhibitory activity of TGE and TBE, (C). Anti-inflammatory protective effects of TGE and TBE on human erythrocytes. (D). Scavenging ability of TGE and TBE for DPPH radicals, (E). Scavenging ability of TGE and TBE for Hydroxyl radicals, (F). Scavenging ability of TGE and TBE for Superoxide radicals.
3.3. Analysis of anti-inflammatory protective effects
The destruction of lysosomal membranes promotes inflammatory reactions through the release of lysosomal enzymes (proteases and bacterial enzymes). In vitro anti-inflammatory activity studies were performed based on the similar stability of HRBC (human erythrocyte) membranes and lysosomal membranes. Figure shows the maximum membrane protection rate of TGE at 5 mg/mL was 60.02 ± 1.85%. TGE exhibits a higher lysosomal membrane stability and reduces the risk of elevated blood glucose caused by inflammation.
3.4. Antioxidant activity of TGE and TBE
The antioxidant activities of TGE and TBE were evaluated using DPPH, hydroxyl and superoxide radical scavenging activity assays. Both extracts exhibited a certain scavenging activity, and DPPH radical scavenging activity could be ranked as Vc>TGE>TBE (Figure ). IC50 is a good measure of antioxidant capacity. Not only is it used to qualitatively measure the antioxidant strengths of two substances, but it can also be used to quantitatively compare the active electron bases of two antioxidants or multiple relationships relevant to antioxidant performance. According to the regression formula, the IC50 of TGE for DPPH is 0.588 mg/mL, while the IC50 of TBE is 0.903 mg/mL (Figure ). Therefore, according to IC50, both TGE and TBE have antioxidant activity, and the antioxidant performance of TGE is 1.54 times that of TBE.
Figure 4. (A) Peak area of D-CI standard, (B) Flavonoid and D-CI yields in TBG and TBB, (C) IC50 values of TGE and TBE inhibiting α-glucosidase/α-amylase activity and anti-inflammatory protective effects, (D) IC50 value of buckwheat flavonoid’s ability to remove DPPH·, OH· and O2·-.
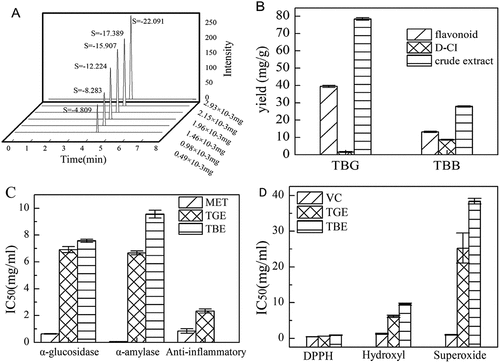
At high sample concentrations, according to our results, the hydroxyl radical scavenging rate of TGE is 59.88 ± 2.26%, and the hydroxyl radical scavenging rate of TBE is 43.73 ± 1.90% (Figure ). According to the regression formula, the IC50 of TGE for Hydroxyl is 6.239 mg/mL; the IC50 of TBE is 9.543 mg/mL (Figure ). The above results show that both TGE and TBE have a certain hydroxyl radical scavenging activity, although TGE scavenging activity is higher than that of TBE, consistent with the previous DPPH scavenging results.
For a range of TGE and TBE concentrations (0.625–10 mg/mL), when the sample concentration was highest, the superoxide radical scavenging rate of TGE was 12.74 ± 1.87%, while the superoxide radical scavenging rate of TBE was 6.73 ± 1.29% (Figure ; Figure ). It can be seen that TGE has higher scavenging activity for superoxide radical than TBE, but significantly less superoxide radical scavenging activity than Vc.
3.5. Diabetic mice weight, food intake, water intake, and urine volume
The weight of mice in the NC group increased normally. The weight of mice in the administration group was unstable over the first few days. The weight gain of mice in the HG group was significantly smaller than that observed in the NC group (P < 0.01), and the TGE and TBE groups had a significant increase compared with the HG group (P < 0.01). Mice in the HG group did not show weight loss. This was presumably because the increase in the weekly age of the mice accompanied the increase in body weight. Although mice in the HG group were not cured, their weight increased slowly. Diabetic mice usually exhibit higher food intake, water intake, and urine output, which were improved in the TGE and TBE groups(Table ).
Table 1. Intake/Output assessment of diabetic mice
3.6. Analysis of organ structural damage
Obvious liver swelling was observed in diabetic mice in this experiment, indicating that STZ effects the mouse liver (Table ). In terms of liver index, the HG group was significantly higher than the NC group (P < 0.01). The liver indexes of both TGE and TBE groups decreased, indicating that the effect of TGE and TBE on liver weight gain in mice was not significantly different from that of NC (P < 0.01). The kidney plays key roles in the regulation of oxygen-free radical production and the scavenging of free radical antioxidant enzymes in the body, consistent with the antioxidant capacity of TGE and TBE described above. The kidney index of the HG group was higher than that of the administration groups (P < 0.01), indicating that non-administered diabetic mice had renal oedema. The kidney index of mice in the TGE group in dropped the most compared with the TGE and MET groups (p < 0.01). Oxidative stress causes an excessive immune response and produces inflammatory factors, and the spleen index reflects immune regulation of the body to a certain extent. The spleen index of mice in the HG group increased significantly compared with the normal group (P < 0.01), and the spleen index of the TGE group of diabetic mice was lower than that of the TBE group (P < 0.01). These results were consistent with the anti-inflammatory capacity of the TGE and TBE groups described above. Obvious swelling of the pancreas was observed in diabetic mice in this experiment, indicating that streptozotocin causes pancreatic disease in mice. The symptoms of pancreatic enlargement in mice were alleviated by drug administration. Although the TGE and TBE groups achieved normal level similar to the NC group, the TGE group exhibited obvious weight loss compared to the MET group (P < 0.01), and the TBE group was not significantly different from the MET group (P < 0.01). Overall, administration had a therapeutic effect on multiple damaged organs in mice.
Table 2. Effects of TGE and TBE on hypoglycemic activity in diabetic mice
3.7. The hypoglycemic active factors of TGE and TBE in vivo
All forms of diabetes are characterized by high blood sugar, disorders of hepatic glycogen and muscle glycogen metabolism, and glomerular and peripheral neuropathy (Giacco et al., Citation2010). It can be seen that the blood sugar levels of mice in the HG/MET/TGE/TBE group remained in the abnormal range at the beginning of the experiment. After one week of administration, the blood glucose values of MET and TGE mice gradually decreased and were maintained in a stable range. The group with the greatest drop was TBE, returning levels to a normal range (3.7–6.9 mmol/L).
Enzyme-linked immunoassay (ELISA) was used to generate the insulin standard regression curve, y = 0.0133× + 0.024, R2 = 0.999. Compared with the NC group, the HG and MET groups showed high serum insulinemia (P < 0.01) and insulin resistance status. Compared with the HG group, TGE and TBE groups reduced serum insulin levels (P < 0.01).
Compared with the HG group (Table ), the liver glycogen reserves of TGE and TBE diabetic mice increased significantly (P < 0.01) and liver glycogen reserves in TBE diabetic mice increased significantly compared to the MET group (P < 0.01). Compared with the NC group, there was no statistical difference in liver glycogen reserve in TBE diabetic mice, indicating that TBE had the best effect. TBE plays an essential role in blood glucose balance of diabetic mice. After mice in all groups were sacrificed by cervical dislocation, thigh biceps of the mice were collected to determine muscle glycogen levels. Compared with the HG group, muscle glycogen content was significantly increased in the TBE group and the TGE group (P < 0.01), while the TAB group was not significantly different from the NC group (P < 0.01).
3.8. Increase of antioxidant enzymes
The most important indicator of renal function is urea nitrogen, and its high and low values reflect the status of renal function-producing antioxidant enzymes. We found a significant difference in urea nitrogen between the HG group and the NC group (P < 0.01), and TBE and TGE groups exhibited a significant improvement to impaired kidney function (P < 0.01). Cells slowdown the rate of oxidation and remove reactive oxygen and nitrogen species through antioxidant enzymes. Compared with the NC group, the antioxidant enzyme content of diabetic mice was decreased in the HG group, disrupting the oxidative balance. In the MET, TGE and TBE groups, levels of oxidase GSH-Px, SOD and CAT increased significantly (P < 0.01).
3.9. Reduce inflammatory factor storm
Oxidative stress causes an inflammatory factor storm due to excessive inflammatory response. It can also be seen that levels of inflammatory factors in the HG group were extremely high compared with the NC group (p < 0.01). To varying degrees, levels of IL-6/TNF-α/CRP in the MET and TGE groups decreased (p < 0.01). The TBE group did not change significantly (p < 0.01), hinting that TBE has little therapeutic effect on inflammatory response in diabetic mice.
4. Discussion
As it is known, active substances of natural products has been widely concerned and studied based on their less toxicity than the synthetic drugs. The main bioactive component of TGE and TBE is rutin which has a variety of health benefits, including anti-diabetic, anti-oxidation, anti-inflammatory and protective impact on organs (B. Liu & Zhu, Citation2007). Although the flavonoid content of TGE is higher than that of TBE, the later still can reduce the level of blood sugar and scavenge-free radicals. Yao et al. have found that TBE contains high mass of D-CI, which may be the reason it has a cost-effective and economical hypoglycemic effect than TGE (Yao et al., Citation2008).
Anti-diabetic activity was assessed by carbohydrate hydrolase inhibition (α- glucosidase and α-amylase) by preventing the absorption of glucose in the digestive tract. α- Glucosidase can hydrolyze polysaccharides into monosaccharides, which participate in the control of postprandial hyperglycemia on the surface of brush cells in the small intestine. α- Amylase catalyzes hydrolysis of α-1,4 glycosidic carbohydrate in the presence of calcium. Two enzymes inhibition will slow and prolong release of glucose into the metabolic route, and thus precluding sudden hyperglycemia after dinner. Small molecular compound of TGE and TBE block enzymes’ normal function through direct physical binding with one or more cell target proteins and exert their activities of inhibiting carbohydrate hydrolase. Findings suggested the interaction mode between flavonoids and α-glucosidase were H-bond formation (acting on bound cleft residuesTyr 158, Asp 307, Tyr 347), arena arena (Phe 303), and arena cation (Arg 442) using molecular docking simulation. Molecular interactions between α- amylase and flavonoids include H-bonds (acting on Asp 197, Gln 63, Trp 59) and aromatic cations (Hua et al., Citation2018). Xiao et al. concluded that the hydroxylation and the glycosylation of flavonoids can improve the inhibitory effect on α-amylase (Xiao et al., Citation2011, Citation2013). Therefore, TGE and TBE displayed different enzyme inhibition depending on the different target proteins.
Insulin signal transduction is triggered by the combination of insulin and insulin receptor that consists of α and β chains. The structure changes of the β chain on cell membrane is caused by a combination of ligand and α-chain, thereby promoting self phosphorylation of intracellular tyrosine residues on the β chain. It activates the phosphorylation of various insulin-dependent kinases (ERK1/2, aPKC, S6K1, (SIK2), AKT, mTOR, and ROCK1) (Kiselyov et al., Citation2009) and creates suitable binding sites on insulin receptor substrate 1 for downstream signal molecule. The triggering mechanism can activate the glucose metabolism process and lead to more glycogen synthesis by promoting utilization of glucose to enter cells through translocation of GLUT-4 and inhibiting glycogen synthase kinase (Ho et al., Citation2016). However, not all insulin receptor substrate phosphorylation is positive. Cell research shows that excessive lipid and inflammatory signaling factors can lead to insulin resistance through the phosphorylation of serine and threonine on the protein residues of insulin receptor substrate (Copps et al., Citation2012). T2D is a chronic disease characterized by metabolic disorder, and its main symptoms are insulin resistance, β cell dysfunction and increased hepatic glucose output. Insulin resistance occurs and insulin levels rise. Both TGE group and TBE group can effectively alleviate the symptoms of insulin resistance. Flavonoids can activate insulin signaling pathway of CaMKK β and AMPK to increase uptake of skeletal muscle glucose. As a sensitizer, it can help target cells make appropriate responses to insulin, thus improving their ability to effectively absorb and metabolize glucose. It can also promote insulin secretion and inhibit α- Glucosidase (Escandón-Rivera et al., Citation2020). The effects of D-CI on body weight, food intake and urine content in mice were not different from those in the diabetes group, but could reduce insulin levels and alleviate insulin resistance. D-CI can up regulate the phosphorylation of PI3K and AKT and activate the insulin-dependent pathway of PI3K/AKT. Quijano et al. found that inositol derivatives can play a hypoglycemic role by stimulating glucose uptake of skeletal muscle cells with insulin (Quijano et al., Citation2016). These support earlier research (Nestler et al., Citation1999) who discovered that D-chiro-inositol improves several metabolic abnormalities related to insulin resistance.
When a person is ravenous, under normal metabolic circumstances, the main energy source is from the glucose produced by disintegration of liver glycogen. Insulin resistance reflects that hyperinsulinemia cannot inhibit gluconeogenesis in the liver of TAD patients, engendering fasting hyperglycemia and the decrease of storage of postprandial liver glycogen. In the early stage of diabetes, the glucose increases especially after the abnormal insulin secretion and insulin resistance in the skeletal muscle (Defronzo et al., Citation2003). The content of liver glycogen in healthy people is generally higher than that of diabetes patients. In our investigation, the hypoglycemic mechanism of TGE and TBE may be related to the significant increase of muscle glycogen storage and inhibition of liver glycogen loss and decomposition in diabetes mice.
The abnormal metabolism of diabetes leads to the excessive production of superoxide, which can activate many proinflammatory pathways in cells. This will drive the continuous expression of proinflammatory genes even when blood glucose is normal (Giacco et al., Citation2010). High concentration of ROS (, OH• and related oxygen species) is harmful to organisms. The use of antioxidants (flavonoids, polyphenols, etc.) and antioxidant enzymes (GSH-Px, SOD and CAT) is a valid method to inhibit the production of ROS. In vivo, we can view the increase of level of GSH-Px, SOD and CAT in the TGE and TBE groups compared with HG group.
Since its introduction in 1958, the DPPH method has been regarded as an important way to measure the antioxidant capacity of biological samples in vitro (Blois, Citation1958; Tizghadam et al., Citation2021). According to the DPPH radical oxidation principle, the number of radical scavenging is proportional to the number of electron-donating groups in the system or the antioxidant performance of the TGE and TBE. Production of hydroxyl radicals in the body has a great destructive effect, as they kill red blood cells and destroy the structure of all biological macromolecules (DNA, proteins, polysaccharides, etc.). In addition, hydroxyl radicals can undergo lipid peroxidation with fatty acid side chains on cell membranes, causing irreversible cell damage (Halliwell, Citation1991). Irena et al. believed that the phenyl of flavonoids played a role in the redox properties, thereby improving the scavenging activity of HO• free radicals (Irena et al., Citation2009). Although superoxide radicals are seen as weaker oxidants, they can induce the generation of hydroxyl radicals and induce lipid peroxidation in the body. Furthermore, they can accelerate bodily aging from the skin to the internal organs, promoting skin lesions, heart vascular diseases, cancer, etc (F. Liu et al., Citation1997). The scavenging activities of these three drugs are different, likely because all chemical reactions, including redox reactions with radicals, are related to the Gibbs free energy change of the reactants and products. Under certain circumstances, the scavenging ability of these three radicals is related to their ability to absorb protons or give electrons.
Studies have shown that myofibrillar peptides without sugar cannot reduce excessive accumulation of inflammatory mediators such as nitric oxide, TNF-α and IL6, in other words, peptides alone do not have anti-inflammatory effects. However, if conjugated with sugars or oligosaccharides, the compounds can work (Kumar et al., Citation2019). Similarly, TBE did not exhibit anti-inflammatory activity, likely due to lower flavonoid levels and the possible existence/absence of some substance (protein) present in TBE. The determination and analysis of inflammatory factors content showed that TGE group was superior to TBE group. In vitro analysis of TGE and TBE also showed that both TGE and TBE have hypoglycemic effects, and TGE has a better effect on hypoglycemic activity than TBE. These differences are related to the flavonoid content and species, consistent with the reports of Ran and Ma (Jiaxin & Tingjun, Citation2016). Many other flavonoids in F. tataricum play roles in antioxidant activity, as noted by Lee (Lee et al., Citation2016).
The hypoglycemic mechanisms and organ effects of TGE and TBE in vivo and in vitro are summarized (Figure ). Severe organ damage affects the body’s ability to resist oxidative stress and inflammation, and liver glycogen levels are related to the degree of liver damage. With regard to kidney, TGE showed a therapeutic effect, potentially through its antioxidant and anti-inflammatory protective effects. In summary, F. tataricum flavones and F. tataricum bran D-CI, as natural products and no toxic side effects, have positive effects on anti-diabetes. This shows that the extraction of two active substances should be emphasized for further study, and combined use of the two will be necessary to improve diabetes relief from multiple aspects.
Abbreviations
| AMPK | = | AMP-activated protein kinase |
| AKR | = | aldehyde ketone reductase |
| AKT | = | Protein kinase B |
| aPKC | = | Atypical protein kinase C |
| CaMKK β | = | Ca2+/Calmodulin-dependentβ protein kinase |
| c-AMP | = | Cyclic adenosine phosphate |
| CRP | = | C-reactive protein; |
| DNS | = | A Reagent for determination of reducing sugar content |
| DPP-4 | = | Dipeptidyl peptidase IV |
| DPPH | = | 1,1-diphenyl-2-picrylhydrazyl |
| ELSD | = | an evaporative light scattering detector |
| ERK | = | Extracellular signal-regulated kinase |
| E-selectin | = | adhesion molecules |
| FBPase | = | Fructose-1,6-bisphosphatase |
| FFAR1 | = | Free fatty acid receptor 1 |
| G-6-Pase | = | glucose-6-phosphatase |
| GCGr | = | glucagon receptor |
| GC-MS | = | gas chromatography—mass spectroscopy |
| GIP | = | Insulin stimulating polypeptide |
| GLP-1 | = | Glucagon like peptide-1 |
| GLUT4 | = | glucose transporter |
| GP | = | Glycogen phosphorylase |
| GPCR | = | the protein-coupled receptor |
| GPR119 | = | G protein coupled receptor 119 |
| Gα | = | A subunit of G protein |
| HPLC | = | The high-performance liquid chromatography |
| HRBC | = | human erythrocyte |
| IL-1β | = | interleukin-1β |
| IL −6 | = | interleukin −6 |
| IL-8 | = | interleukin-8 |
| ICAM-1 | = | intercellular adhesion molecule-1 |
| IP3 | = | inositol triphosphate |
| mTOR | = | mammalian target of rapamycin |
| NADPH | = | the cofactor nicotinamide adenine dinucleotide phosphate |
| PBS | = | Phosphate buffer |
| PEPCK | = | phosphorene Alcohol pyruvate carboxykinase |
| plc | = | phospholipase-C |
| PNPG | = | 4-Nitrophenyl-α-D-Glucopyranoside |
| PPAR-γ | = | Peroxisome proliferator activated receptor |
| PTP1B | = | ProteinTyrosinePhosphatase1B |
| ROCK1 | = | Rho-associated coiled-coil containing protein kinase 1 |
| ROS | = | reactive oxygen species |
| S6K1 | = | Ribosomal S6 protein kinase 1 |
| SGLT2 | = | sodium-glucose transporter |
| SIK2 | = | Salt-inducible kinase 2 |
| STZ | = | Streptozotocin |
| T2D | = | type 2 diabetes |
| TGE | = | Tartary buckwheat grain crude extract |
| TBB | = | Tartary buckwheat bran |
| TBE | = | Tartary buckwheat bran crude extract |
| TBG | = | Tartary buckwheat grain |
| TCA | = | Tricarboxylic acid cycle |
| TNF-α | = | tumor necrosis factor |
| VCAM-1 | = | vascular adhesion molecule-1. |
Acknowledgments
The authors gratefully thank Ying Ma and Haitian Zhao from the School of Food Science and Engineering, Harbin Institute of Technology, for their helpful suggestions and assistance. We also thank thirteen five national key research the thirteen five national key research and development projects and development projects (No. 2016YFC0500307-07) in providing key management information and funding.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Xinxin Si
Xinxin Si is an assistant experimenter, Department of Food Science, Zhaoqing University, Zhaoqing, China.
Yanyan Si
Yanyan Si, master, is a pharmacist-in-charge, The 966th Hospital of the Joint Logistic Support Force of PLA, Dandong city, Liaoning province, China. Her research field is the quality standards of traditional Chinese medicine.
Shuai Zhang
Shuai Zhang, Professor, Department of Food Science, Zhaoqing University, Zhaoqing, China. Mainly engaged in scientific research on food fermentation.
Yong Liu
Yong Liu, Associate professor, Department of Food Science, Zhaoqing University, Zhaoqing, China. His research focusses on food processing and preservation, design and delivery of nutritional factors.
Yanqing Liu
Yanqing Liu, Professor, Department of Food Science, Zhaoqing University, Zhaoqing, China. Her main research interests include extraction, isolation and structural identification of active ingredients of natural drugs.
Hongwu Wang
Hongwu Wang, Professor, Department of Food Science, Zhaoqing University, Zhaoqing, China. He is currently engaged in the research of food nutrition and health, food safety and other aspects.
Zhenyu Wang
Zhenyu Wang, Professor, School of Chemistry and Chemical Engineering, Harbin Institute of Technology. His research interests include new resource food and intelligent equipment research.
References
- Ademiluyi, A. O., Oboh, G., Boligon, A. A., & Athayde, M. L. (2014). Effect of fermented soybean condiment supplemented diet on α-amylase and α-glucosidase activities in streptozotocin-induced diabetic rats. Journal of Functional Foods, 9, 1–17. https://doi.org/10.1016/j.jff.2014.04.003
- Apostolidis, E., Kwon, Y. I., & Shetty, K. (2007). Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innovative Food Science & Emerging Technologies, 8(1), 46–54. https://doi.org/10.1016/j.ifset.2006.1006.1001
- Belmouhoub, M., Chebout, I., & Iguer-Ouada, M. (2018). Antidiabetic and anti-hypercholesterolemic effects of flavonoid-rich fractions of rosmarinus officinalis in streptozotocin-induced diabetes in mice. Phytothérapie, 16(4), 204–210. https://doi.org/10.1007/s10298-10017-11103-10296
- Bhandari, M. R., Nilubon, J. A., Gao, H., & Jun, K. (2008). α-glucosidase and α-amylase inhibitory activities of nepalese medicinal herb pakhanbhed (bergenia ciliata, haw.). Food Chemistry, 106(1), 247–252. https://doi.org/10.1016/j.foodchem.2007.05.077
- Blois, M. S. (1958). Antioxidant determinations by the use of a stable free radical. Nature, 181(4617), 1199–1200. https://doi.org/10.1038/1811199a0
- Böni-Schnetzler, M., Thorne, J., Parnaud, G. R., Marselli, L., Ehses, J. A., Kerr-Conte, J., Pattou, F., Halban, P. A., Weir, G. C., & Donath, M. Y. (2008). Increased Interleukin (IL)-1β messenger ribonucleic acid expression in β-Cells of Individuals with type 2 diabetes and regulation of IL-1β in human islets by glucose and autostimulation. The Journal of Clinical Endocrinology and Metabolism, 93(10), 4065–4074. https://doi.org/10.1210/jc.2008-0396
- Cai, W., Gu, X., & Tang, J. E. (2010). Purification, and characterisation of the flavonoids from opuntia milpa alta skin. Czech Journal of Food Sciences, 28(2), 108–116. https://doi.org/10.17221/122/2009-CJFS
- Cai, H., & Harrison, D. G. (2000). Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circulation Research, 87(10), 840–844. https://doi.org/10.1161/01.RES.87.10.840
- Cho, Y. J., Bae, I. Y., Inglett, G. E., & Lee, S. (2014). Utilization of tartary buckwheat bran as a source of rutin and its effect on the rheological and antioxidant properties of wheat-based products. Industrial Crops and Products, 61, 211–216. https://doi.org/10.1016/j.indcrop.2014.07.003
- Copps, K., White, D., & M, F. (2012). Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia, 55(10), 2565–2582. https://doi.org/10.1007/s00125-012-2644-8
- Defronzo, R. A.; Mandarino, L.; ferrannini, e. (2003). metabolic and molecular pathogenesis of type 2 diabetes mellitus. International Textbook of Diabetes Mellitus. https://doi.org/10.1002/0470862092.d0310
- Dujic, T., Zhou, K., Donnelly, L. A., Tavendale, R., Palmer, C. N. A., & Pearson, E. R. (2014). Association of organic cation transporter 1 with intolerance to metformin in type 2 diabetes: A GoDARTS Study. Diabetes, 64(5), 1786–1793. https://doi.org/10.2337/db14-1388
- Escandón-Rivera, S., Mata, R., & Andrade Cetto, A. (2020). Molecules isolated from mexican hypoglycemic plants: A review. Molecules, 25(18), 4145. https://doi.org/10.3390/molecules25184145
- Fc, A., Xg, A., Cg, A., Yl, B., & Min, W. A. (2019). The distribution of D-chiro-inositol in buckwheat and its antioxidative effect in HepG2. Journal of Cereal Science, 89, 102808–102808. https://doi.org/10.1016/j.jcs.2019.102808
- Giacco, F., Brownlee, M., & Schmidt, A. M. (2010). Oxidative Stress and Diabetic Complications. Circulation Research, 107(9), 1058–1070. https://doi.org/10.1161/CIRCRESAHA.110.223545
- Halliwell, B. (1991). Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. The American Journal of Medicine, 91(3), S14–S22. https://doi.org/10.1016/0002-9343(91)90279-7
- Henrissat, B. (1991). A classification of glycosyl hydrolases based on amino acid sequence similarities. The Biochemical Journal, 280(2), 309–316. https://doi.org/10.1042/bj2800309
- Ho, C. K., Sriram, G., & Dipple, K. M. (2016). Insulin sensitivity predictions in individuals with obesity and type ii diabetes mellitus using mathematical model of the insulin signal transduction pathway. Molecular Genetics and Metabolism, 119(3), 288–292. https://doi.org/10.1016/j.ymgme.2016.09.007
- Huang, D., Ou, B., & Prior, R. L. (2005). The chemistry behind antioxidant capacity assays. Journal of Agricultural & Food Chemistry, 53(6), 1841–1856. https://doi.org/10.1021/jf030723c
- Hua, F., Zhou, P., Wu, H. Y., Chu, G. X., Xie, Z. W., & Bao, G. H. (2018). Inhibition of α-glucosidase and α-amylase by flavonoid glycosides from Lu’an GuaPian tea: Molecular docking and interaction mechanism. Food & Function, 9(8), 4173–4183. https://doi.org/10.1039/C8FO00562A
- Hu, F. B., Meigs, J. B., Li, T. Y., Rifai, N., & Manson, J. E. (2004). Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes, 53(3), 693–700. https://doi.org/10.2337/diabetes.53.3.693
- Irena, K., Oya, B.-D., Meltem, C.-Ü., Rahmiye, E., & Hassan, Y. (2009). Scavenging capacities of some thiazolyl thiazolidine-2,4-dione compounds on superoxide radical, hydroxyl radical, and DPPH radical. Luminescence, 26(1), 10–16. https://doi.org/10.1002/bio.1105
- Iwasaki, Y., Masayama, A., Mori, A., Ikeda, C., & Nakano, H. (2009). Composition analysis of positional isomers of phosphatidylinositol by high-performance liquid chromatography. Journal of Chromatography A, 1216(32), 6077–6080. https://doi.org/10.1016/j.chroma.2009.06.064
- Jiaxin, R.; Tingjun, M., Effect of total flavonoid and total polyphenol from tartary buckwheat sprouts and their inhibitor activities on a-glycosidase. In Eighth International Conference on Measuring Technology and Mechatronics Automation, Macau, March 11-12, 2016.
- Kiselyov, V. V., Versteyhe, S., Gauguin, L., & Meyts, P. D. (2009). Harmonic oscillator model of the insulin and IGF1 receptors’ allosteric binding and activation. Molecular Systems Biology, 5(1), 243. https://doi.org/10.1038/msb.2008.78
- Konstantinov, A. A. (1990). Vectorial electron and proton transfer steps in the cytochrome bc1 complex. Biochimica et biophysica acta, 1018(2–3), 138–141. https://doi.org/10.1016/0005-2728(90)90234-U
- Krentz, A. J., & Bailey, C. J. (2005). Oral antidiabetic agents: Current role in type 2 diabetes mellitus. Drugs, 65(3), 385–411. https://doi.org/10.2165/00003495-200565030-00005
- Kumar, L. V., Shakila, R. J., & Jeyasekaran, G. (2019). In Vitro anti-cancer, anti-diabetic, anti-inflammation and wound healing properties of collagen peptides derived from unicorn leatherjacket (aluterus monoceros) at different hydrolysis. Turkish Journal of Fisheries and Aquatic Sciences, 19(7), 551–560. https://doi.org/10.4194/1303-2712-v19_7_02
- Lee, L.-S., Choi, E.-J., Kim, C.-H., Sung, J.-M., Kim, Y.-B., Seo, D.-H., Choi, H.-W., Choi, Y.-S., Kum, J.-S., & Park, J.-D. (2016). Contribution of flavonoids to the antioxidant properties of common and tartary buckwheat. Journal of Cereal Science, 68, 181–186. https://doi.org/10.1016/j.jcs.2015.07.005
- Li, H., Lv, Q., Ma, C., Qu, J., Cai, F., Deng, J., Huang, J., Ran, P., Shi, T., & Chen, Q. (2019). Metabolite profiling and transcriptome analyses provide insights into the flavonoid biosynthesis in the developing seed of tartary buckwheat (fagopyrum tataricum). Journal of Agricultural & Food Chemistry, 67(40), 11262–11276. https://doi.org/10.1021/acs.jafc.9b03135
- Liu, F., Ooi, V. E. C., & Chang, S. T. (1997). Free radical scavenging activities of mushroom polysaccharide extracts. Life Sciences, 60(10), 763–771. https://doi.org/10.1016/S0024-3205(97)00004-0
- Liu, B., & Zhu, Y. (2007). Extraction of Flavonoids from Flavonoid-rich parts in tartary buckwheat and identification of the main Flavonoids. Journal of Food Engineering, 78(2), 584–587. https://doi.org/10.1016/j.jfoodeng.2005.11.001
- Lu, C., & Kang, Y. J. (2001). Oxidative stress and diabetic cardiomyopathy: A brief review. Cardiovascular Toxicology, 1(3), 181–193. https://doi.org/10.1385/CT:1:3:181
- Moghaddasian, B., Eradatm, A., & Davood, E. (2012). Anoosh determination of rutin content in caper (capparis spinosa) by three analytical methods. Annals of Biological Research, 3, 4303–4306. http://scholarsresearchlibrary.com/archive.html
- Morishita, T., Yamaguchi, H., & Degi, K. (2007). The contribution of polyphenols to antioxidative activity in common buckwheat and tartary buckwheat grain. Plant Production Science, 10(1), 99–104. https://doi.org/10.1626/pps.10.99
- Murad, H. A. S., Saleh, H. A., Abdulaziz, G. S., Abdulsattar, M. A., & Ali, S. S. (2015). Effect of metformin and pioglitazone on β-catenin and biochemical markers in sitagliptin-induced pancreatitis in diabetic rats. International Journal of Diabetes in Developing Countries, 35(3), 332–339. https://doi.org/10.1007/s13410-014-0278-8
- Na, L., Jie, S., Wang, X. J., Sun, J. Y., Bing, W., Bing, W., Sun, J. Y., Wang, X. J., Jie, S., & Na, L. (2017). Synthesis and hydroxyl radical scavenging activity of 4-Aryl-3,4-Dihydrocoumarins. Chemistry of Natural Compounds, 53(5), 860–865. https://doi.org/10.1007/s10600-017-2141-x
- Nan, Y., Ren, & Guixing, G. (2008). Determination of d - chiro -inositol in tartary buckwheat using high-performance liquid chromatography with an evaporative light-scattering detector. Journal of Agricultural & Food Chemistry, 56(3), 757–760. https://doi.org/10.1021/jf0717541
- Nestler, J., Jakubowicz, E., Daniela, J., Reamer, P., Allan, & Gunn, G. (1999). Ovulatory and metabolic effects of d- chiro -inositol in the polycystic ovary syndrome. Obstetrical & Gynecological Survey, 340(17), 1314–1320. https://doi.org/10.1097/00006254-199909000-00018
- Quijano, L., David, G.-A., Juan, A.-C., Adolfo, B.-B., Quijano, & Celia, L. (2016). Daniela acute hypoglycemic effect and phytochemical composition of ageratina petiolaris. Journal of Ethnopharmacology, 185, 341–346. https://doi.org/10.1016/j.jep.2016.03.048
- Romanet, R., Sarhane, Z., Bahut, F., Uhl, J., Gougeon, R. D., Nikolantonaki, M., & Gougeon, R. D. (2021). Exploring the chemical space of white wine antioxidant capacity: A combined DPPH, EPR and FT-ICR-MS study. Food Chemistry, 355, 129566. https://doi.org/10.1016/j.foodchem.2021.129566
- Savage, D. B., Petersen, K. F., & Shulman, G. I. (2007). Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiological Reviews, 87(2), 507–520. https://doi.org/10.1152/physrev.00024.2006
- Schroder, K., & Tschopp, J. (2010). The Inflammasomes. Cell, 140(6), 821–832. https://doi.org/10.1016/j.cell.2010.01.040
- Sedej, I., Sakač, M., Mandić, A., Mišan, A., Tumbas, V., & Čanadanović-Brunet, J. (2012). Buckwheat (fagopyrum esculentum moench) grain and fractions: Antioxidant compounds and activities. Journal of Food Science, 77(9), C954–C959. https://doi.org/10.1111/j.1750-3841.2012.02867.x
- Shao, S., Juanjuan, Y., Joe, R., Cuilin, C., Hua, Z., Haitian, Z., & Zhenyu, W. (2018). Protective effects on 60Co-γ radiation damage of pine cone polyphenols from pinus koraiensis-loaded chitosan microspheres in vivo. Mol, 23(6), 1392. https://doi.org/10.3390/molecules23061392
- Slack, J., Mcmahan, C. J., Waugh, S., Schooley, K., Spriggs, M. K., Sims, J. E., & Dower, S. K. (1993). Independent binding of Interleukin-1 Alpha and Interleukin-1 Beta to Type I and Type II Interleukin-1 Receptors. The Journal of Biological Chemistry, 268(4), 2513–2524. https://doi.org/10.1016/s0021-9258(18)53806-0
- Sroor, F. M., Abbas, S. Y., Basyouni, W. M., El-Bayouki, K. A. M., El-Mansy, M. F., Aly, H. F., Ali, S. A., Arafa, A. F., & Haroun, A. A. S. (2019). Structural characterization and in vivo anti-diabetic evaluation of some new sulfonylurea derivatives in normal and silicate coated nanoparticle forms as anti-hyperglycemic agents. Bioorganic Chemistry, 92, 103290. https://doi.org/10.1016/j.bioorg.2019.103290
- Steadman, K., Burgoon, M., Schuster, R., Lewis, B., Edwardson, S., & Obendorf, R. (2000). Fagopyritols, d - chiro -Inositol, and Other Soluble Carbohydrates in Buckwheat Seed Milling Fractions. Journal of Agricultural & Food Chemistry, 48(7), 2843–2847. https://doi.org/10.1021/jf990709t
- Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B. B., Stein, C., Basit, A., Chan, J., Mbanya, J. C., Pavkov, M. E., Ramachandaran, A., Wild, S. H., James, S., Herman, W. H., Zhang, P., Bommer, C., Kuo, S., Boyko, E. J., & Magliano, D. J. (2022). IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice, 183, 109119–. https://doi.org/10.1016/j.diabres.2021.109119
- Tizghadam, P., Roufegari-Nejad, L., Asefi, N., & Jafarian Asl, P. (2021). Physicochemical characteristics and antioxidant capacity of set yogurt fortified with dill (anethume graveolens) extract. Journal of Food Measurement and Characterization, 15(4), 3088–3095. https://doi.org/10.1007/s11694-021-00881-2
- Uemura, S., Matsushita, H., Li, W., Glassford, A. J., Asagami, T., Lee, K. H., Harrison, D. G., & Tsao, P. S. (2001). Diabetes mellitus enhances vascular matrix metalloproteinase activity: role of oxidative stress. Circulation Research, 88, 1291–1298. https://doi.org/10.1161/hh1201.092042
- Wu, X., Huang, L., & Liu, J. (2021). Relationship between oxidative stress and nuclear factor‑erythroid‑2‑related factor 2 signaling in diabetic cardiomyopathy (Review). Experimental and Therapeutic Medicine, 22(1), 678. https://doi.org/10.3892/etm.2021.10110
- Xiao, J., Kai, G., Ni, X., Yang, F., & Chen, X. (2011). Interaction of natural polyphenols with α-amylase in vitro: Molecular property–affinity relationship aspect. Molecular bioSystems, 7(6), 1883–1890. https://doi.org/10.1039/c1mb05008g
- Xiao, J., Ni, X., Kai, G., & Chen, X. (2013). A review on structure–activity relationship of dietary polyphenols inhibiting α-amylase. Critical Reviews in Food Science and Nutrition, 53(5), 497–506. https://doi.org/10.1080/10408398.2010.548108
- Yao, Y., Shan, F., Bian, J., Chen, F., Wang, M., & Ren, G. (2008). D-Chiro-inositol-enriched tartary buckwheat bran extract lowers the blood glucose level in KK-Ay mice. Journal of Agricultural & Food Chemistry, 56(21), 10027–10031. https://doi.org/10.1021/jf801879m
- Zhong, L., Peng, X., Wu, C., Li, Q., Chen, Y., Wang, M., Li, Y., He, K., Shi, Y., & Bie, C. (2022). Polysaccharides and flavonoids from cyclocarya paliurus modulate gut microbiota and attenuate hepatic steatosis, hyperglycemia, and hyperlipidemia in nonalcoholic fatty liver disease rats with type 2 diabetes mellitus. International Journal of Diabetes in Developing Countries. https://doi.org/10.1007/s13410-022-01080-5
- Zhou, X.-L., Chen, Z.-D., Zhou, Y.-M., Shi, R.-H., & Li, Z.-J. (2019). The effect of tartary buckwheat flavonoids in inhibiting the proliferation of MGC80-3 cells during seed germination. Molecules, 24(17), 3092. https://doi.org/10.3390/molecules24173092
- Zhou, T., Xu, X., Du, M., Zhao, T., & Wang, J. (2018). A preclinical overview of metformin for the treatment of type 2 diabetes. Biomedicine & Pharmacotherapy, 106, 1227–1235. https://doi.org/10.1016/j.biopha.2018.07.085