?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Modern breweries are focused on controlling oxidation in beer using natural antioxidants to improve shelf-life stability. The most significant quality issues in the brewing industry are flavor instability and oxidation. In this research, the effects of adding Moringa stenopetala leaf extract to lager beer at 400, 600, and 800 ppm concentrations for 30, 60, and 90 days storage at 25ºC were investigated. The total phenolic and total flavonoid content were measured by Folin-Ciocalteu and aluminum chloride method, respectively. Using the 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity and the phosphomolybdate assay, the antioxidant activity of each treatment was assessed and compared. Addition of the extract in beer showed a linear increment in the total phenolic content from 46.79 up to 88.30 milligram of gallic acid equivalent per liter of beer (mg GAE/L) and total flavonoid content from 123.36 up to 167.09 milligram of catechin equivalent per liter of beer (mg CE/L). Similar increment was observed within the DPPH scavenging potential, from 46.55 up to 67.16% and total antioxidant power from 139.12 up to 216.67 milligram of butylated hydroxytoluene equivalent per liter of beer (mg BHTE/L). The total phenolic and flavonoid contents and antioxidant activities of extract treated beer showed slight reduction as compared to untreated beer with increasing storage time. According to the findings, M. stenopetala leaf extract could be utilized as a component in beer to reduce oxidation and keep it fresh for extended periods of time.
PUBLIC INTEREST STATEMENT
Beer is one of the most widely consumed alcoholic beverages in the world for its fresh taste, low calories, and higher nutritional value than other alcoholic beverages, because of its minerals content. Flavor instability resulting from beer storage and oxidation remains one of the most important quality problems in the brewing industry. Moringa is one of the most powerful sources of natural anti-oxidants by supplying the free atoms and mitigate the effect of free radicals. Furthermore, moringa has antimicrobial properties that extend the shelf life of alcoholic beverages by suppressing lactic acid bacteria. Therefore, this study deals with the possible extension of shelf life of lager beer using moringa leaf extract to replace chemical antioxidants. The incorporation of moringa stenopetala at moderate level in beer reduces oxidation and increase the phenolic content that is typically reduced during the boiling, filtration, bottling, and storage stages of the brewing process. This has a potential promise to improve the stability and shelf-life of commercial beers without the incorporation of artificial or chemical preservatives.
1. Introduction
The fresh taste, low calorie content, and nutritional value of beer make it one of the world’s oldest and most popular alcoholic drinks (Arnold, Citation2005). It is a good source of phenolic compounds (Oñate-Jaén et al., Citation2006) and contains potassium, magnesium, calcium and sodium (Styburski et al., Citation2018). Beer production involves a number of complex chemical and biochemical reactions, so the final beer contains many compounds with antioxidant activity derived mainly from yeast, malt, and hops, or formed during preparation (Quifer-Rada et al., Citation2015). Antioxidant types and concentrations in finished beer are mostly determined by the brewing technology, raw materials, and yeast used throughout the brewing process. Slight variations in the structural composition of these compounds can result in large changes in antioxidant activity, affecting the beer’s overall oxidative or flavor stability. Beer flavor instability is becoming a major concern for most breweries due to the loss of freshness and quality of beer over time as its chemical composition changes. Flavor instability resulting from beer storage and shelf life is becoming a major concern for most breweries due to the loss of freshness and quality of beer over time due to changes in its chemical composition. Flavor stability is now the most critical issue in determining shelf life packaged beer, and reducing flavor staling can help increase shelf life (Aron et al., Citation2011). It is largely determined by oxygen content, brewing processes, and the materials used. As a result, research is currently being focused on improving the antioxidant activity of beer itself, because oxidative staining of beer can occur even at low oxygen levels of 0.1 mg/l. Since oxidative staling of beer is still apparent in low oxygen environments, researchers are now investigating ways to increase the antioxidant activity of beer (Bamforth et al., Citation2018). Scientists are searching different mechanisms to decrease beer oxidation using synthetic antioxidants. However, current research recommends not using synthetic antioxidants in food and beverage industries due to prolonged health effects and the evolution of food laws and regulations which forbids synthetic antioxidants in food (Arnold, Citation2005). Thus, natural antioxidants are increasingly being used in food products both for reasons of quality and potential health benefits. Therefore, replacement of synthetic antioxidants with natural antioxidants is the issue of modern breweries to tackle such antagonists and succeed in their business with customer satisfaction and respect government regulations.
Natural antioxidants, such as Moringa, are one of the most powerful sources since they provide bioactive compounds and inhibit the effects of free radicals. According to Nadeem et al. (Citation2013), M. stenopetala is rich in phenolic compounds like cryptochlorogenic acid, astragalin, glucosinolates, and isothiocyanates. Also the leaves are rich in flavonoids such as isoquercetin and rutin, and the beta carotene present in M. stenopetala leaves also acts as antioxidants (Engeda & Rupasinghe, Citation2021; Tesfaye & Solomon, Citation2014). It also showed antimicrobial effect for shelf life extension of alcoholic beverages by suppressing lactic acid bacteria (Florence et al., Citation2016). Therefore, the aim of this study was to evaluate the effect of M. stenopetala extract on total phenolic and flavonoid contents and antioxidant activity of Lager beer stored at room temperature.
2. Materials and methods
2.1. Raw materials
M. stenopetala leaves were taken from the Hawassa Teachers Training Center. Malts were obtained from Assela malt factory. From BGI Ethiopia, new Saccharomyces cerevisiae yeast (S-189 type) and brewing liquor were obtained for standard brewing.
2.2. Sample preparation and extraction
M. stenopetala leaves were carefully collected, wrapped in aluminum foil, and delivered to Hawassa University’s Food Science and Post-harvest Technology lab. After that, it was cleaned with deionized water, dried and ground with grinder (Nadeem et al., Citation2013). The fine powder was combined with 80 percent ethanol at a ratio of 1 gm to 10 mL in a duplicate Pyrex beaker. An electrical shaker was used to macerate the beakers for 18 hours after they were tightly wrapped with cover bush. After that, the extracts were separated and filtered before being evaporated to dryness at 40°C under vacuum (Buchi, 3000 series, Switzerland). A stock solution was made and kept at 4°C in the refrigerator (Siddhuraju & Becker, Citation2003).
2.3. Experimental design and treatments
The stock solution (1000 ppm) was prepared by dissolving 50 mg of powder dried leaf extract in distilled water to make 50 mL solution. Using a factorial design, diluted leaf extract solutions with concentrations of 400, 600, and 800 ppm, as well as storage times of 1, 30, 60, and 90 days were investigated on total phenolic and flavonoid content and antioxidant potential of a Lager beer. A beer containing 12 ppm potassium metabisulphite (KMS) was used as a positive control, and a beer without the extract was used as a negative control. Three concentration of treatments (400, 600, and 800 ppm) were chosen based on the previous findings (Gedefaw et al., Citation2022) on sensory evaluation in which trained panelists assessed the maximum amount of M. stenopetala leaf extracts that could be applied without changing the original beer flavor.
2.4. Beer preparation
The beer was made utilizing a dry milling system using Pires and Brányik (Citation2015) method. The mash was prepared with malt and water at 55°C, with water to grain ratio of 2.3 L/kg. It was heated at 64°C for 20 min. and then at 74°C for 15 min. Following saccharification of the mash with temperature increases to 78°C, the mash was filtered. A wort sample was boiled for 60 minutes with 0.12 kg CO2 hop extract per hectoliter, and the hot trub was removed, cooled to 10°C, and aerated to 18 ppm oxygen. It was then fermented at 12°C until the initial gravity dropped to 8ºP, and then at 16°C until the vicinal diketones (VDK) level decrease to less than 0.18 ppm. The matured beer was then purged and filtered after being stored at −2°C for two days to allow for maturation at 0.5 bar counter pressure. The beer was diluted to 11.05°P with de-aerated water, carbonated to 5.8 gm/lit CO2, and packed using 330 mL sanitized amber bottles and crowned with manual crowner after addition of 3.3 mL of extracts at various concentrations. All samples were labeled and pasteurized at 60°C for about 20 min and kept at 25°C. Starting on the first day of sample preparation, the total phenolic, flavonoids and antioxidant activities of each sample were tested every 30 days for three months.
2.5. Total Phenolic Content (TPC)
The TPC of beer samples were measured by Zhao et al. (Citation2010) using the Folin-Ciocalteu spectrophotometric method. 0.1 mL beer sample (diluted five-folds) was mixed with 1 mL of Folin-Ciocalteu reagent (diluted ten-folds), and left for five minutes. The mixture was then incubated at 25°C for 90 min with 1 mL sodium carbonate (7.5%w/w). The absorbance of the solution was then measured at 765 nm with a UV-visible double beam spectrophotometer. From the gallic acid calibration curve (y = 0.023× + 0.014, R2 = 0.996), the TPC was determined and reported as milligram gallic acid equivalent per liter of beer (mg GAE/L).
Where; y = the absorbance of the sample, x = the concentration established from the calibration curve (mg GAE/L).
2.6. Total Flavonoid Content (TFC)
The TFC of beer samples were determined using Pai et al. (Citation2015) method. One mL of beer samples (diluted five-folds) was diluted with 1.25 mL of deionized water and 75 μL of NaNO2 was added to the mixture. After 6 min, 150 m AlCl3 was added to the reaction liquid, followed by 1 mL 1 M NaOH after 5 min. The absorbance was measured at 510 nm versus prepared water blank. A standard curve was prepared using catechin (5–1000 µg/mL). All of the results were calculated using standard calibration curves (y = 0.011× + 0.132, R2 = 0.973) and presented as milligram catechin equivalents per liter of beer (mg CE/L).
Where; y = the absorbance of the sample, x = the concentration established from the calibration curve (mg GAE/L).
2.7. Determination of antioxidant potential
2.7.1. DPPH radical scavenging activity
The ability beer samples to scavenge DPPH radicals were measured using the method described by Tafulo et al. (Citation2010). The beer samples were diluted to five-fold and 1.0 mL of the sample was mixed with 2.0 mL freshly prepared DPPH solution (0.06%, w/v) in ethanol. After vortexing the reaction mixture, it was left at room temperature in the dark for 30 min. The absorbance was then measured at 520 nm using a double beam UV-visible spectrophotometer (JENWAY-9500, UK). The discoloration of DPPH given as a percentage was then used to calculate free radical scavenging activity using the equation,
where Ac is the absorbance of DPPH in the absence of the extract sample and As is the absorbance of DPPH in the presence of the extracts sample.
2.8. Total antioxidant activity using phosphomolybdate assay
The total antioxidant capacity of beer samples were measured by Huda-Faujan et al. (Citation2009) method using phosphomolybdenum assay. In a capped test tube, 0.3 mL of each sample (diluted five times) was mixed with 3 mL of phosphomolybdenum reagent. The solutions were then incubated in water at 95°C for 90 minutes before being cooled to room temperature. Finally, using a spectrophotometer (JENWAY-6300, UK), the absorbance of each solution was measured at 695 nm against a blank containing 3 mL methanol. The overall antioxidant activities were determined using the equation y = 0.432× + 0.078, R2 = 0.99, and presented as milligrams of butylated hydroxytoluene equivalent per liter of beer (mg BHTE/L).
Where; y = the absorbance of the sample, x = the concentration established from the calibration curve (mg BHTE/L).
2.9. Statistical Analysis
On the experimental data, an analysis of variance (ANOVA) was performed and Duncan’s multiple range tests were employed to find differences (p < 0.05) between the mean values. The data was analyzed using SAS 9.0 and origin 8 program, and the results were expressed as mean and standard deviation.
3. Results and discussion
3.1. Effect of M. Stenopetala leaf extract on TPC and TFC of lager beer
The effect of M. stenopetala leaf extract at different concentrations and storage period on the TPC and TFC of beer is presented in Table . The concentration of TPC in extract-treated beer influenced it considerably. Untreated beer had a total phenolic concentration of 46.79 mg GAE/L, whereas adding 400 ppm extract increased total phenolic content to 70.51 mg GAE/L, a 33% increase. Increasing the extract concentration to 800 ppm resulted in significantly (P < 0.05) increase in TPC to 88.60 mg GAE/L. This finding was supported with a study conducted by Ulloa et al. (Citation2017) that bioactive compounds increased with the increasing concentration of propolis extract. This significant increment of TPC in beer samples enriched with extract might come from the potentially high accumulation of bioactive compounds in M. stenopetala leaf (Engeda & Rupasinghe, Citation2021). A study conducted by Tesfaye and Solomon (Citation2014) on the antioxidant potential of M. stenoptetala found up to 92.8 mg GAE/100 g of dry weight which supports our finding that probably increased phenolic content in beer samples enriched with the extract. The TPC of beer sample treated with commercial beer antioxidant, potassium metabisulphite (66.79 mg GAE/L) was significantly (P < 0.05) lower than the TPC of beer sample treated with 400 ppm of extract.
Table 1. Effect of M. stenopetala leaf extract and storage period on TPC and TFC of lager beer
There was drastic decline of total phenolic content (44% reduction) of the controlled beer up to 26.16 mg GAE/L within 90 storage days. While beer samples enriched with potassium metabisulphite showed only 15% reduction in total phenolic content (66.79–56.78 mg GAE/L). Besides, with storage 90 days, M. stenopetala leaf extract treated beer samples showed slight reduction of total phenolic content (70.51–61.73 mg GAE/L for 400 ppm, 82.75–73.08 mg GAE/L for 600 ppm and 88.60–84.34 mg GAE/L for 800 ppm).
Similarly the TFC of beer samples enhanced with increasing the concentration of the extract. Increasing the concentration of the extract from 400 ppm to 600 ppm significantly (p < 0.05) increased the total flavonoid content from 147.51 up to 159.82 mg CE/L and further increment to 800 ppm significantly (p < 0.05) increased its total flavonoid content to 167.09 mg CE/L. The beer sample enriched with 12 ppm potassium metabisulphite had also raised the concentration to 143.99 mg CE/L. The total flavonoid content for untreated beer sample was significantly affected by storage time with drastic reduction of total flavonoid content. The result disclosed that total flavonoid content had been reduced linearly from 123.36 mgCE/L (at the first date of storage) to 85.71 mg CE/L (after 90 days of storage) which implies 30% reduction. On the contrary, only 15% total flavonoid content reduction was observed for potassium metabisulfite treated beer sample (143.99–120.02 mg CE/L) with in the same storage time.
Beer treated with different concentrations of M. stenopetala leaf extracts, S01: untreated, S02: treated with 12 ppm potassium metabisulphite, S03: treated with 400 ppm extract, S04: treated with 600 ppm extract, S05: treated with 800 ppm extract. Values are means and standard deviations of duplicated determinations; the means with the same letter across the column are not significantly different at P ˂ 0.05.
The beer samples enriched with M. stenopetala leaf extracts showed slight reduction of total flavonoid content from starting date to 90 days of storage (147.51–125.69 mg CE/L for 400 ppm, 159.82–141.81 mg CE/L for 600 ppm and 167.09–150.93 mg CE/L for 800 ppm) which is in average of only 10% reduction. According to Vignault et al. (Citation2018), phenolic compounds significantly decrease during storage due to oxidative polymerization and the formation of colloidal hazes. A large amount of phenolic compounds would be liberated by malting and fermentation, and these compounds would undergo reaction with protein, leading to greater declines of phenolic compounds than those bound to proteins in most beers (Szwajgier et al., Citation2005). According to the study conducted by Zhao (Citation2015), extract treated beer samples had a slight reduction in TPC due to the fact that the bioactive phenolic compounds introduced from extract were not equally reduced. In addition, there was no difference in the amount of some phenolic compounds during storage. The slight reduction of total phenolic and flavonoid contents of M. Stenopetala leaf extract treated beer might be the presence of large number of stable phenolic compounds bound with polysaccharides, in leaf cells of M. stenopetala (Dadi et al., Citation2019; Engeda & Rupasinghe, Citation2021).
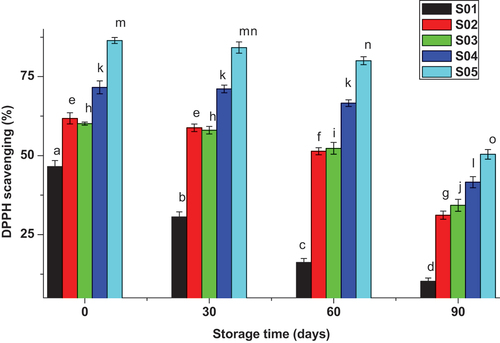
3.2. Effect of M. Stenopetala leaf extract on beer DPPH scavenging activity
The DPPH scavenging activity of M. stenopetala leaf extract-enriched beer over time is demonstrated in Figure . The result for untreated beer was 46.55%, but its potential was decreasing with increasing storage time from 46.55 up to 30.91% which might be due the reduction of endogenous antioxidants happened by reacting with hydroxyl radicals through time (Dennis et al., Citation2004). However, beer enriched with potassium metabisulfite showed 61.80% DPPH scavenging activity, which is significantly much higher than untreated beer, and there was only a minor decrease in DPPH scavenging activity over time from the first date of production to three months of storage. According to Lugasi (Citation2003), metal sulphites had relatively high antioxidant potential which has been used by modern breweries for decades. But due to legislations on health of consumers, many developed countries avoid using this metallic salt.
DPPH scavenging activity for beer samples treated with the extracts revealed linear increment with concentration of 60.14, 71.60 and 86.44% for 400, 600 and 800 ppm, respectively. The abundantly identified bioactive chemicals and antioxidants (Tesfaye & Solomon, Citation2014) in the leaf of M. stenopetala contribute to the increase in DPPH scavenging capability with extract concentration. This finding also showed reduction of DPPH scavenging potential with storage time for beer samples enriched with the extracts. The reduction was from 60.14–34.29% (400 ppm sample), 71.60–41.61% (600 ppm sample) and 86.44–50.40% (800 ppm sample) within three months of storage time (Figure ).
Beer treated with different concentrations of M. stenopetala leaf extracts, S01: untreated, S02: treated with 12 ppm potassium metabisulphite, S03: treated with 400 ppm extract, S04: treated with 600 ppm extract S05: treated with 800 ppm extract. The values are the average of duplicated experiment (mean± SD). At p < 0.05, values of the same concentration in the histogram bar with different letters are significantly different.
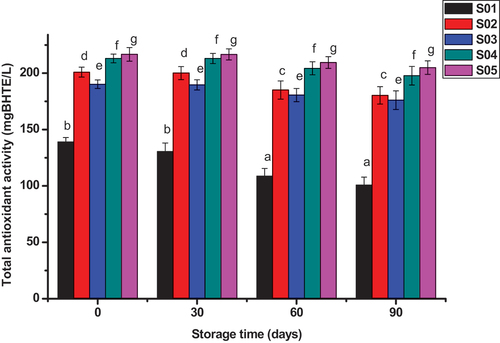
3.3. Effect of M. Stenopetala leaf extract on total antioxidant potential of beer
The total antioxidant potential of beer enriched with M. stenopetala leaf extract with storage time is illustrated in Figure . The total antioxidant potential for untreated beer was found 139.12 mg BHTE/L which is almost similar to study of light lager beers by Zhao et al. (Citation2013) which was 140.23 mg BHTE/L. With increasing concentrations of the extract, the total antioxidant potential of beer sample was significantly (P < 0.05) enhanced. The total antioxidant potential increased by 30% with addition of 400 ppm of the extract and increasing the concentration of extract by 200 ppm raised the beer antioxidant potential by 5%. The increment was linear for all extract treated beer samples. Accordingly, the total antioxidant potential of beer treated with 400, 600 and 800 ppm were 190.16, 213.00 and 216.67 mg BHTE/L, respectively. The beer enriched with potassium metabisulfite also showed similar trained with beer treated with 400 ppm extract. The total antioxidant potential of untreated beer showed linear reduction with increasing storage time by 27% (139.12–101.72 mg BHTE/L) within three months of storage time. The reduction was drastic when compared with extract treated samples. Similar study conducted by Piazzon et al. (Citation2010) that compared the antioxidant capacity of several beer kinds showed that light beer stored without antioxidants would lose 20–30% of its antioxidants within three months of storage. Storage time had slight reduction of total antioxidant potential for treated beer when compared with untreated beer. The slight reduction of total antioxidant for potassium metabisulphite and extract treated beer showed that these additives had strong antiradical potential when compared with untreated beer. The total antioxidant potential of propolis-treated beer samples increased linearly with increasing propolis concentration (Ulloa et al., Citation2017). Similar study conducted by Florence et al. (Citation2016) proved that 600 ppm M. oleifera treated pito improved the total antioxidant potential of beer by 32% and showed slight reduction with storage time, which are consistent with the present findings.
Beer treated with different concentrations of M. stenopetala leaf extracts, S01: untreated, S02: treated with 12 ppm potassium metabisulphite, S03: treated with 400 ppm extract, S04: treated with 600 ppm extract and S05: treated with 800 ppm extract. The values are the average of duplicated experiment (mean± SD). At p < 0.05, values of the same concentration in the histogram bar with different letters are significantly different.
4. Conclusion
The possibility for developing and using M. stenopetala leaf extract as an additional source of bioactive phenolic compounds and increasing antioxidant activity in beer was highlighted in this work. The use of a moderate amount of the leaf extract in beer prevents oxidation and increased phenolic content, decreased slightly during the storage for three months. These findings support that the addition of M. stenopetala leaf extract increased the total phenolic and flavonoid contents and antioxidant activity of the beer samples. However, the effect of other phytochemicals like carotenoids, vitamins and minerals on the quality of Lager beer should be studied. Therefore, without the addition of synthetic preservatives (example, potassium metabisulfite), commercial beers may be able to maintain their flavor stability or reinforce the nutritional property of beer and extend shelf life by incorporating phenolic rich leaf extract of M. stenopetala.
Ethical approval
This study does not require ethical approval.
Acknowledgments
The authors thank BGI Ethiopia, Hawassa Brewery and School of Nutrition, Food Science, and Technology, Hawassa University, for their financial assistance and use of laboratory facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The corresponding author will give the data that supports the findings of this study upon reasonable request.
Additional information
Notes on contributors

Gedefaw Mazengia
Gedefaw Mazengia is a production manager at BGI Ethiopia St. George Brewery in Addis Ababa, Ethiopia. He worked as a polyvalent operator, fabrication shift leader, and production shift leader at BGI Ethiopia Hawassa brewery in Hawassa, Ethiopia.
Engeda Dessalegn
Engeda Dessalegn is an Associate Professor at the Chemistry Department, Hawassa College of Education, and also working at School of Nutrition and Food Technology, Hawassa University, Ethiopia. Research interests: Food analysis, Food preservation, and Food safety (aflatoxins and acrylamide mitigation in foods). Under his research group so far 32 Msc students have completed their research thesis and four PhD students conducting their dissertation.
Tilku Dessalegn
Tilku Dessalegn teaches Food Science and Postharvest Technology at the School of Nutrition, Food Science and Technology of the Hawassa University, Ethiopia. Among his research interests are food safety and quality, food processing and preservation, product development, and postharvest handling and technology.
References
- Arnold, J. P. (2005). Origin and history of beer and brewing from prehistoric times to the beginning of brewing science and technology (Reprint Ed.) (p. 411). Alumni Assn. of the Wahl-Henius Institute.
- Aron, P. M., Shellhammer, T. H., & Brew, J. I. (2011). A discussion of polyphenols in beer physical and flavour stability. Journal of the Institute of Brewing, 116(4), 369–9. https://doi.org/10.1002/j.2050-0416.2010.tb00788.x
- Bamforth, C. W., Muller, R. E., & Walker, M. D. (2018). Oxygen and oxygen radicals in malting and brewing: A review. Journal of the American Society of Brewing Chemists, 51(3), 79–88. https://doi.org/10.1094/ASBCJ-51-0079
- Dadi, D. W., Emire, S. A., Hagos, A. D., & Eun, J. B. (2019). Effect of ultrasound-assisted extraction of Moringa stenopetala leaves on bioactive compounds and their antioxidant activity. Food Technology and Biotechnology, 57(1), 77–86. https://doi.org/10.17113/ftb.57.01.19.5877
- Dennis, E. B., Chris, A. B., Peter, A. B., & Roger, S. (2004). Brewing science and practice (1st ed.). Woodhead Publishing.
- Engeda, D., & Rupasinghe, V. H. P. (2021). Phenolic compounds and in vitro antioxidant activity of Moringa stenopetala grown in South Ethiopia. International Journal of Food Properties, 24(1), 1681–1692. https://doi.org/10.1080/10942912.2021.1990943
- Florence, A. A., Courage, K. S. S., Francis, K. A., & Hellie, G. (2016). Shelf life improvement of sorghum beer (pito) through the addition of Moringa oleifera and pasteurization. African Journal of Biotechnology, 15(46), 2627–2636. https://doi.org/10.5897/AJB2016.15581
- Gedefaw, M., Engeda, D., & Tilku, D. (2022). Effect of Moringa stenopetala leaf extracts on the physicochemical characteristics and sensory properties of lager beer. Food and Nutrition, 10(2), 507–514. https://doi.org/10.1002/fsn3.2672
- Huda-Faujan, N., Noriham, A., Norrakiah, A. S., & Babji, A. S. (2009). Antioxidant activity of plants methanolic extracts containing phenolic compounds. African Journal of Biotechnology, 8(3), 484–489.
- Lugasi, A. (2003). Polyphenol content and antioxidant properties of beer. Acta Alimentaria, 32(2), 181–192. https://doi.org/10.1556/AAlim.32.2003.2.7
- Nadeem, M., Abdullah, M., Hussain, I., Inayat, S., Javid, A., & Zahoor, Y. (2013). Antioxidant potential of moringa oleifera leaf extract for the stabilisation of butter at refrigeration temperature. Czech Journal of Food Sciences, 31(4), 332–339. https://doi.org/10.17221/366/2012-CJFS
- Oñate-Jaén, A., Bellido-Milla, D., & Hernández-Artiga, M. P. (2006). Spectrophotometric methods to differentiate beers and evaluate beer ageing. Food Chemistry, 97(2), 361–369. https://doi.org/10.1016/j.foodchem.2005.05.010
- Pai, T. V., Sawant, S. Y., Ghatak, A. A., Chaturvedi, P. A., Gupte, A. M., & Desai, N. S. (2015). Characterization of Indian beers: Chemical composition and antioxidant potential. Journal of Food Science and Technology, 52(3), 1414–1423. https://doi.org/10.1007/s13197-013-1152-2
- Piazzon, A., Forte, M., & Nardini, M. (2010). Characterization of phenolics content and antioxidant activity of different beer types. Journal of Agricultural and Food Chemistry, 58(19), 10677–10683. https://doi.org/10.1021/jf101975q
- Pires, E., & Brányik, T. (2015). Springer briefs in biochemistry and molecular biology: Biochemistry of beer fermentation. Springer International Publishing. https://doi.org/10.1007/978-3-319-15189-2
- Quifer-Rada, P., Vallverdú-Queralt, A., Martínez-Huélamo, M., Chiva-Blanch, G., Jáuregui, O., Estruch, R., & Lamuela-Raventós, R. (2015). A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS). Food Chemistry, 169, 336–343. https://doi.org/10.1016/j.foodchem.2014.07.154
- Siddhuraju, P., & Becker, K. (2003). Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. Journal of Agricultural and Food Chemistry, 51(8), 2144–2155.
- Styburski, D., Janda, K., Baranowska-Bosiacka, I., Łukomska, A., Dec, K., Goschorska, M., Michalkiewicz, B., Ziętek, P., & Gutowska, I. (2018). Beer as a potential source of macroelements in a diet: The analysis of calcium, chlorine, potassium, and phosphorus content in a popular low-alcoholic drink. European Food Research and Technology, 244(10), 1853–1860. https://doi.org/10.1007/s00217-018-3098-0
- Szwajgier, D., Pielecki, J., & Targoñski, Z. (2005). Changes of free ferulic and coumaric acid contents during malting of barley grain. Polonica Journal Food Nutrition Science, 14, 423–429.
- Tafulo, P. A., Queirós, R. B., Delerue-Matos, C. M., & Sales, M. G. (2010). Control and comparison of the antioxidant capacity of beers. Food Research International, 43(6), 1702–1709. https://doi.org/10.1016/j.foodres.2010.05.014
- Tesfaye, T., & Solomon, L. (2014). Assessment of antioxidant potential of Moringa stenopetala leaf extract. Ethiopian Journal of Science and Technology, 7(2), 93–104. https://doi.org/10.4314/ejst.v7i2.3
- Ulloa, P. A., Vidal, J., Ávila, M. I., Labbe, M., Cohen, S., & Salazar, F. N. (2017). Effect of the addition of propolis extract on bioactive compounds and antioxidant activity of craft beer. Journal of Chemistry, 2017, 1–7. https://doi.org/10.1155/2017/6716053
- Vignault, A., González-Centeno, M. R., Pascual, O., Gombau, J., Jourdes, M., Moine, V., Iturmendi, N., Canals, J. M., Zamora, F., & Teissedre, P. L. (2018). Chemical characterization, antioxidant properties and oxygen consumption rate of 36 commercial oenological tannins in a model wine solution. Food Chemistry, 268, 210–219. https://doi.org/10.1016/j.foodchem.2018.06.031
- Zhao, H. (2015). Effects of processing stages on the profile of phenolic compounds in beer. Processing and Impact on Active Components in Food, 2015, 533–539. https://doi.org/10.1016/B978-0-12-404699-3.00064-0
- Zhao, H., Chen, W., Lu, J., & Zhao, M. (2010). Phenolic profiles and antioxidant activities of commercial beers. Food Chemistry, 119(3), 1150–1158. https://doi.org/10.1016/j.foodchem.2009.08.028
- Zhao, H., Li, H., Sun, G., Yang, B., & Shao, M. (2013). Assessment of endogenous antioxidative compounds and antioxidant activities of lager beers. Journal of the Science of Food and Agriculture, 93(4), 910–917. https://doi.org/10.1002/jsfa.5824