Abstract
In recent years, there has been an increase in the acceptance of green synthesis as a viable method for producing nanoparticles as active materials that can inhibit bacterial resistance to antibiotics. The current study aimed to evaluate the effect of green synthesized selenium nanoparticles (SeNPs) using Vaccinium arctostaphylos L. fruit extract on Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), and Corynebacterium diphtheriae (C. diphtheriae). The synthesized SeNPs were characterized by Ultraviolet-visible (U-V), Fourier-transform infrared (FTIR), Field Emission Scanning Electron Microscopy (FESEM), Dynamic Light Scattering (DLS), and Zeta Potential (ZP) measurements. The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of the tested bacteria were determined by the resazurin test, and the values were used as a basis to achieve the antibacterial activity test and detection of viability (live/dead cell percentage) using flow cytometry. In this study, the FESEM assay further shows that the Se NPs are precisely spherical and uniform in size (50 ± 1.23 nm) and distribution. The effectiveness of the SeNPs against S. aureus, E. coli, and C. diphtheria ranged from zero when the MIC value was large (0.5 mg/ml) to a significant effect when the MIC value was low (0.015625 mg/ml). The high percentage of bacterial death achieved by the synthesized SeNPs on S. aureus, C. diphtheria, and E. coli, was 44.6%, 29.49%, and 24.4% respectively. In conclusion, the synthesized SeNPs using V. arctostaphylos L. are desirable, safe, and environmentally friendly options that could be used as an efficient antibacterial agent to prevent bacterial infections.
1. Introduction
Nanotechnology is a rapidly growing area of study that investigates manipulating matter at the molecular and atomic levels (Dulta et al., Citation2022). A new industrial revolution was brought about by the development of nanotechnology, especially in the field of biomedicine, where the physicochemical and biological features of NPs have been extensively utilized and exploited (Albukhaty et al., Citation2018, Citation2022). Nanoparticles are an important component of nanomedicine, which is thought to be a new field of medicine. Its main applications include diagnostics, imaging, and smart drug delivery systems that improve therapeutic effectiveness by selectively delivering to specific targets (Mohammed et al., Citation2023) as well as the creation of various nanomaterials against pathogenic microorganisms including pathogenic bacteria (Rudramurthy et al., Citation2016). Among these nanoparticles, Selenium nanoparticles (SeNPs), are known to be an essential trace element for both animal and human health. They are necessary for the immune system to work properly and to stop the transmission of infections (Garza-García et al., Citation2021).
Selenium nanoparticles (SeNPs) are less expensive to produce and can be combined with other biological agents to enhance their biological properties (Y. Huang et al., Citation2021; Nayak et al., Citation2021). As discovered (Vahdati & Tohidi Moghadam, Citation2020), SeNPs and lysozymes work together to kill bacteria. In the nanoscale, selenium activity is notably higher due to the increased surface-to-volume ratio of the particles, which exposes more of the particle’s surface. Since numerous chemical processes used to create nanoparticles have drawbacks such as the consumption of chemicals, materials that can’t be easily recycled, and high energy costs (Bisht et al., Citation2022). Recent studies have focused on the successful use of plant extracts in the green synthesis of nanoparticles due to the plants’ easy availability, safe handling, and abundance of metabolites that can facilitate reduction as well as their biological characteristics (Alyamani et al., Citation2021; Chinnaraj et al., Citation2023; Kalaimurugan et al., Citation2022; Manojkumar et al., Citation2023; Meenambigai et al., Citation2022).
The genus Vaccinium, a member of the Ericaceae family, contains approximately 450 species worldwide. Their fruits are high in antioxidants and contain vitamins, antimicrobial and antitumoral substances, and anthocyanin pigments (Viskelis et al., Citation2009). Vaccinium arctostaphylos contains proanthocyanidins that inhibit bacterial adhesion and subsequent proliferation. Although anthocyanins are found in many other fruits that are similar to V. arctostaphylos, it has been shown that the anthocyanidin chemicals in this fruit can inhibit bacteria (Khodadadi et al., Citation2021). In biological applications, SeNPs exhibit promising potential as antioxidants, cancer therapeutics, and drug carriers. However, few studies indicated green synthesis of Se NPs using V. arctostaphylos (L.) fruit extract for multi-resistant bacterial isolates such as Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), and Corynebacterium diphtheriae (C. diphtheriae). Since nanoparticles are simple to fabricate, they find widespread application in medication delivery systems that employ localized delivery. Nanoparticles’ diminutive size makes them well-suited for transport through narrow blood vessels and uptake by cells, facilitating effective drug accumulation at specific places. Nanoparticles offer superior size control and medication protection when used in drug delivery. The drug clearance time increases if it stays at the active site. The therapeutic efficacy and bioavailability were both improved by the use of nanoparticles. They improved medication stability by decreasing fed/fasted variability. Drugs that are unstable or have unacceptable bioavailability when administered in their non-nanoparticulate dose form can be given in a stable dosage form using nanoparticles (Salem & Fouda, Citation2021).
The fabrication of selenium nanoparticles can be done in a variety of ways. Green biosynthesis is one such method to produce selenium nanoparticles (Elakraa et al., Citation2022; Salem, Citation2022), thus, the present study aimed to investigate the antimicrobial activity of green-synthesized SeNPs derived from V. arctostaphylos (L.) fruit extract against E. coli, S. aureus, and C. diphtheria, which are considered to be serious health issues in poultry, endangering animal health and productivity and leading to the inappropriate use of antimicrobial drugs.
2. Materials and methods
2.1. Materials and reagents
Ciprofloxacin (Sigma-Aldrich), Muller-Hinton agar and broth and agar (Oxoid), Resazurin (Sigma-Aldrich), Sodium selenite (Na2SeO3) (Sigma-Aldrich), Double-distilled water (Sigma-Aldrich), 1 M hydrochloric acid (HCl) (Merck), 1 M sodium hydroxide (NaOH) (Sigma-Aldrich), Muller Hinton broth (Millipore, Merck), eBioscience™ Flow Cytometry Staining Buffer (Invitrogen, Thermofisher), Bioscience™PI Staining Solution (10 g/mL PI in PBS) (Invitrogen, Thermo Fisher), and Hank’s Balanced Salt Solution (HBSS; 1X) (Sigma-Aldrich).
2.2. Collection of plant and obtaining the extract
In October 2022, fresh, ripe berries of V. arctostaphylos were taken from the local market of Al-Diwaniyah City in southern Iraq and confirmed by the Pharmacology Department of the College of Veterinary Medicine at the University of Al-Qadisiyah in Al-Diwaniyah, Iraq. Fresh fruits of V. arctostaphylos L. were collected and washed thoroughly with double-distilled water to remove any impurities or dust particles. The fruits were then diced into small fragments and mixed with DDW (1:10 w/v) using a blender grinder (JEMG 102, India). The resulting mixture was filtered through a muslin cloth to obtain clear fruit extract (Soltani et al., Citation2014).
2.3. Synthesis of SeNPs from plant extract
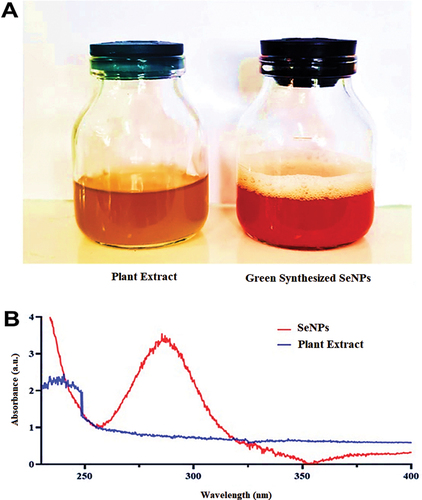
First, a stock solution of sodium selenite (SS) (Na2SeO3) was prepared by dissolving 0.1 g of SS in 100 mL of DDW. Then, to synthesize SeNPs, 1 mL of V. arctostaphylos (L.) fruit extract was added to 9 mL of SS solution by continuous stirring at room temperature for 24 h. The development of SeNPs is indicated by a shift in colour from clear to reddish brown. Impurities and unreacted components were separated from the produced SeNPs by centrifugation at 10,000 rpm for 10 minutes. After two days in a freeze-dryer, the red pellet was ready to be employed in experiments (Gharbavi et al., Citation2022).
2.4. Characterization of SeNPs
UV-Visible spectroscopy was used to characterize the green synthesized SeNPs (Japan) (Gharbavi et al., Citation2020). The functional group was analyzed using FT-IR (Perkin-Elmer spectrum), with the spectrum recorded at a resolution of 4.0 cm−1 in the 4000–400 cm−1 middle region (Baran et al., Citation2023; Gharbavi et al., Citation2022). FE-SEM analysis was used to identify the particle size and shape (SEM 208S Philips). Furthermore, the size distribution and surface charge of synthesized SeNPs were determined using a DLS and ZP analyzer (Horiba SZ-100, Japan) (Gharbavi et al., Citation2022).
2.5. Determination of MIC and MBC using the Resazurin test
2.5.1. Bacterial strains
Gram-positive bacteria, including S. aureus ATCC 29,213 and C. diphtheria ATCC 13,812, and Gram-negative E. coli ATCC 25,922 were evaluated against green fabricated SeNPs, V. arctostaphylos (L.) extract, and ciprofloxacin in the current investigation. The MIC was then determined by monitoring the observed colour shift.
2.5.2. Preparation of the Resazurin reagent
Resazurin was made at a concentration of 0.015% by dissolving 0.015 g, spinning, and filtering (0.22 µm), and then storing it at 4ºC for no more than 2 weeks (Sowjanya et al., Citation2015).
2.5.3. Preparation of standardized inoculum
The inocula were generated in line with the CSLI guideline, with the OD600 value adjusted to correspond to 108 CFU ml−1 for each bacterium as measured by a calibration curve. 96 well plates were prepared for testing green synthesized SeNPs, V. arctostaphylos (L.) extract, and standard antibiotics. The green synthesized SeNPs were transferred and mixed from columns 1–10 using a multichannel pipette, yielding 50 µl per well. Double serial dilutions from columns 1–10 yielded test values of 0.5–0.0156 mg ml−1 for the various green-produced SeNPs and V. arctostaphylos (L.) extract. The suspension of standard bacteria was then diluted one-hundred-fold in MHB broth. Fifty microliters of titrated bacterial suspension (equal to about 5 × 105 CFU ml−1) were applied to all SeNPs and control wells. The entire preparation and administration of the OD-adjusted bacteria took less than 15 minutes. Resazurin (0.015%) was added to all wells (30 µl per well) after 24 hours of incubation at 37ºC, and the plates were left to sit for another 2–4 hours before being examined for colour change. After the incubation period was over, the MIC value was applied to the columns where the blue resazurin colour had not changed (Figure ). The MBC could be estimated by plating wells at concentrations greater than the MIC. By plating the well contents with no colony development, the MBC was calculated. The data also demonstrate that successive dilutions were performed on the contents of the wells exhibiting growth inhibition symptoms in order to quantify the final concentration at which the bacteria died.
Figure 1. Determination of the minimum inhibitory concentration (MIC) for green synthesized SeNPs, extract, and ciprofloxacin against S. aureus, E. coli, and C. diphtheria. Following the incubation period, resazurin dye was added. Column 1: a negative control (the natural blue/purple color of resazurin reduced to red-colorless). The plate contains concentrations ranging from 0.5 mg/ml at the highest point to 0.03125 mg ml−1 at the lowest point, both of which were achieved using double serial dilution. The MIC value was calculated using the concentration of combinations in Column 8, which did not change color significantly. Wells content varied in concentration from 0.05 to 0.03125 mg/ml.

2.6. Antibacterial activity
The effectiveness of a fruit extract of V. arctostaphylos (L.) and green synthesized SeNPs against S. aureus, E. coli, and C. diphtheria was tested using the agar well diffusion method (Boroumand et al., Citation2019). Muller Hinton broth was used to cultivate the collected clinical isolates at 37ºC for 24 hours while being shaken constantly. Aseptically, using sterile cotton swabs, cultures were transferred from the broth to plates of Muller Hinton agar. A sterile borer was used to punch 6 mm wells in the inoculation plates. In labelled wells, different concentrations of biosynthesized SeNPs and fruit extract of V. arctostaphylos (L.) (0.5, 0.25, 0.125, 0.0625, 0.03125 mg/ml) were transferred from a (1/2) diluted stock solution. The inoculum volume was 25 ul. Each well also received 25 ul of a positive control (ciprofloxacin, 10 mg ml−1 in dimethyl sulphoxide) and a negative control (dimethyl sulphoxide only). Plates were incubated for 24 hours at 37ºC in an upright position and inhibition zones were measured. The assays for the activity were completed in triplicate.
2.7. Bacterial viability assays
Following the method of Lehtinen et al. (Citation2004) with a minor modification, flow cytometry (EC800 Sony Biotechnology Flow Cytometer) and FlowJo software (Tree Star, Inc., Ashland OR, USA) was used to determine the true percentage of live/dead and cells following implementation with green synthesized SeNPs and V. arctostaphylos (L.). In brief, the bacterial cells were isolated and diluted to a final concentration of 1 × 106 cells/100 µL in FACS tubes. Decant the buffer from the pelleted cells after centrifugation at 300 × g for 5 minutes after duplicate washing of the cells with 2 mL of PBS (or HBSS). The pelleted cells were suspended in 100 µL of Flow Cytometry Staining Buffer for staining (FC001). The flow cytometer was prepared for PI staining by adding 5–10 µl of staining solution to a control tube containing unstained cells. Then, stirred gently and left to sit for a single minute in total darkness. After that, Use FACScanTM equipment to measure PI fluorescence in two or three channels. Each sample should be stained with 5–10 µl of PI solution. A dot plot of forward scatter versus PI was used to determine the viable cell count cut-off.
2.8. Statistical analysis
GraphPad Prism 8 and origin lab software were used to do the statistical analysis. Data were analysed using the unpaired t-test, which compared the studying groups at a significant p-value of p < 0.05. All experiments were performed in triplicate and data were calculated as the mean ± standard deviation.
3. Results
3.1. Green synthesis and UV-Vs analysis of SeNPs using V. arctostaphylos L
In this work, an aqueous solution of fruit extract in 10 ml (10% w/v) with 1 mM sodium selenite used for the green production of SeNPs was analyzed using UV-V spectra. After 45 minutes of dissolving 10 ml of fruit extract (10% w/v), the colour of the SS solution changed from faint yellow to ruby red, indicating the decrease of Se + 4 to Se34 (Figure ). Using a UV-vis absorption spectrophotometer, the optical properties of the colloidal SeNPs and SS solutions were evaluated. () showed the formation of a wider absorption band at 274 ± 4.64 nm. After that, the fabricated SeNPs were kept freeze-dried to be used in further experiments.
3.2. FTIR spectrometer analysis
Figure illustrates the FT-IR spectra of biosynthesized SeNPs using a fruit extract of V. arctostaphylos (L.) as a capping agent. In this context, the detection of the functional group indicates the stability of the synthesized SeNPs is included in Table . Several absorption peaks were seen at 3474.87, 1645.18, 1617.22, 1485.32, and 720.32 cm−1. The biggest peak was 3474.87 cm−1 suggesting formation bound between selenium ions and hydroxyl groups (-OH(R-O-H)) and/or amine groups (−NH2) in the fruit extract of V. arctostaphylos L. The 1617.22 cm−1 peak resembles the connection of alkenes due to the stretching vibration of hydrocarbons (CC), while the involvement of the protein’s amide-I bond (CO) was detected at the 1645.18 cm−1 peak. Furthermore, the bonds that formed between the proteins of the extract and SeNPs via the amine groups resulted in the appearance of amide I vibrations that peaked at 1620.34 cm−1 and 1600.16 cm−1. C-H symmetric vibrations may be responsible for the peak of about 1485 cm−1 in the SeNPs spectra (Gharbavi et al., Citation2022).
Figure 3. FT‐IR spectrum analysis of green synthesized SeNPs by using fruit extract of V. arctostaphylos (L.) as a capping agent.
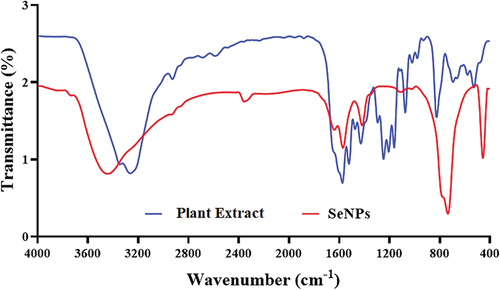
Table 1. Absorption peaks observed in the FTIR spectra, the corresponding functional groups and interpretations
3.3. FE-SEM analysis
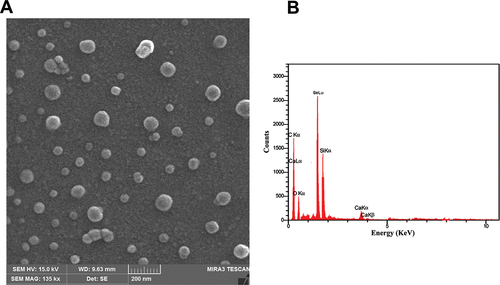
FE-SEM verified the SeNPs’ produced morphology, demonstrating the formation of uniformly sized spherical particles (about 50 ± 1.23 nm in diameter) (Figure ). Similarly, the EDS profile demonstrated that a high Se signal was absorbed, as shown in Figure , that reveals the purity and the complete chemical composition of SeNPs.
3.4. DLS and ZP analysis
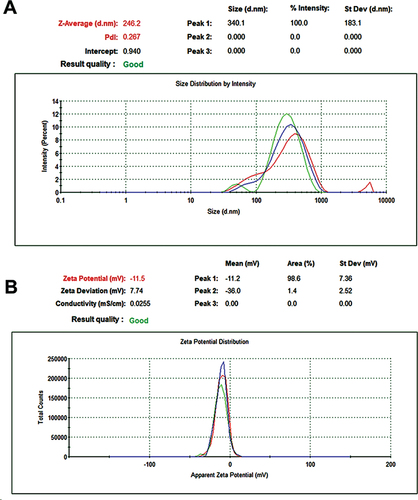
Data suggests that SeNPs produced from V. arctostaphylos fruit extract have a hydrodynamic diameter of 246.2 ± 4.51 nm (mean± SD) (Figure ). The hydrodynamic diameter of a particle in a solution is a measurement that accounts for the particle’s size and shape in addition to the thickness of the solvent layer. SeNPs produced from V. arctostaphylos fruit extract were found to have a zeta potential of − 11.5 ± 1.24 mV (mean± SD) (Figure ). The Polydispersity Index (PDI) for V. arctostaphylos fruit extract-made SeNPs was calculated to be about 0.267. The particle size distribution index (PDI) quantifies the degree to which a sample’s particles are distributed unevenly. If the PDI is little, the particle sizes are rather uniform, whereas if it’s large, there’s a wide range of sizes.
3.5. Antibacterial activity
3.5.1. MIC and MBC determination
Resazurin was used as an oxidation-reduction indicator to measure cell growth. Different green synthesized SeNPs and V. arctostaphylos (L.) extract were tested to determine their MIC and MBC values as shown in (Tables ). The minimal inhibitory concentration (MIC) was calculated as the concentration of the test substance required to prevent the colour shift from blue to pink, indicative of bacterial presence. Our results showed that the effectiveness of the green-produced SeNPs against S. aureus, E. coli, and C. diphtheria ranged from zero when the MIC value was large (0.5 mg/ml) to a significant effect when the MIC value was low (0.015625 mg/ml). When the well’s content was serially diluted from the point where no resazurin colour change was seen (MIC value), the bacterial count dropped dramatically, as described in (Tables ). From 0.5 mg/ml to 0.03125 mg/ml, green-synthesized SeNPs showed a wide MIC. The minimum inhibitory concentration (MIC) was found to be 0.03125 mg/ml. This was the cutoff employed in this study to determine the antibacterial activity of green-synthesized SeNPs and the percentage of viable bacteria (live/dead cells).
Table 2. Estimation of the MIC and MBC by resazurin microdilution method of green synthesized SeNPs and ciprofloxacin antibiotic against Staphylococcus aureus, Escherichia coli, and Corynebacterium diphtheriae
Table 3. Estimation of the MIC and MBC by resazurin microdilution method of extract and ciprofloxacin antibiotic against Staphylococcus aureus, Escherichia coli, and Corynebacterium diphtheriae
3.5.2. Antibacterial inhibition zone
The results of the inhibitory effect of green synthesized SeNPs and V. arctostaphylos (L.) fruit extract are shown in Tables and Figure . According to the findings, the sensitivity of various bacterial species to the green-produced SeNPs varies from that of the V. arctostaphylos (L.) extract. All bacterial isolates were found to be inhibited, though to varying degrees, by the green synthesized SeNPs. The green synthetic SeNPs were the least active, producing a zone of inhibition that was 12 ± .087 mm in diameter at a concentration of 0.03125 mg/ml (Table and Figure ). The most significant antibacterial activity was found against S. aureus, E. coli, and C. diphtheria, respectively. All concentrations showed antibacterial activity, but the high concentration revealed a high inhibition zone. The current study results showed a highly significant (p < 0.05) effect of green synthesized nanoparticles in a concentration-dependent manner on S. aureus, C. diphtheria, and E. coli respectively, where the more concentration SeNPs gave the more significant effect. The results that the 0.5 mg/ml of SeNPs gave an inhibition zone of 36 ± 1.63, 29.66 ± 1.25, and 33 ± 1.63 on S. aureus, E. coli, and C. diphtheria, respectively. The excellent effect was on E. coli, where the inhibition zones of 0.5, 0.25, and 0.125 are better or similar to the effect of ciprofloxacin. In the same context, the effect on S. aureus, the SeNPs gave a better or similar effect as ciprofloxacin, especially in the high concentrations of SeNPs. Regarding the effect of the fruit extract of V. arctostaphylos (L.) against S. aureus, E. coli, and C. diphtheria showed no significant zone inhibition compared to the high effect of ciprofloxacin (Table and Figure ).
Figure 6. The zone inhibition effect of different concentrations of the green synthesized nanoparticles and the fruit extract on the Staphylococcus aureus, Escherichia coli, and Corynebacterium diphtheriae.
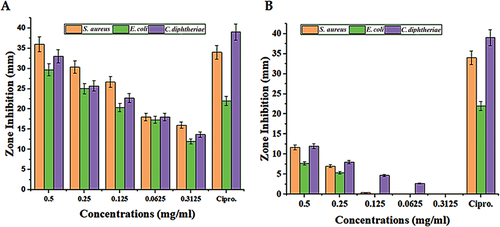
Table 4. The zone inhibition effect of different concentrations of the green synthesized nanoparticles and the Vaccinium arctostaphylos (L.) fruit extract on the Staphylococcus aureus, Escherichia coli, and Corynebacterium diphtheriae
Table 5. The zone inhibition effect of different concentrations of the Vaccinium arctostaphylos (L.) fruit extract on the Staphylococcus aureus, Escherichia coli, and Corynebacterium diphtheriae
Table 6. Determination of the viable, injured, and dead cell percentage after treatment with different concentrations of green synthesized SeNPs using propidium iodide stain and flow cytometry
3.5.3. Live/dead cell viability assay
Flow cytometry (FCM) performed on propidium iodide (PI) was used to underestimate the viability (live/dead cell) of bacterial strains. Three different concentrations of green synthetic SeNPs were tested in accordance with the MIC. At 0.5 MIC concentration, 16%, 19%, and 20% of E. coli, S. aureus, and C. diphtheria cells were dead, respectively. E. coli, S. aureus, and C. diphtheriae had relative death rates of 19%, 33%, and 44% at 1 MIC, and 20%, 20%, and 19% at 2 MIC (Table and Figures ). While the AV extract showed no or non-significant effect on the bacterial strain, where the percentage of the dead cell was no more than 2.2% in different concentrations (Table and Figures ).
Figure 7. Determination of the live/dead percentage of Escherichia coli after treating with different concentrations of green synthesized SeNPs and Vaccinium arctostaphylos (L.) extract using propidium iodide stain and flow cytometry.
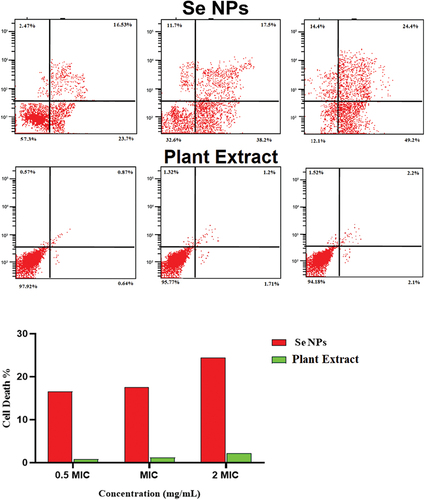
Figure 8. Determination of the live/dead percentage of Staphylococcus aureus after treating with different concentrations of green synthesized SeNPs and Vaccinium arctostaphylos (L.) extract using propidium iodide stain and flow cytometry.
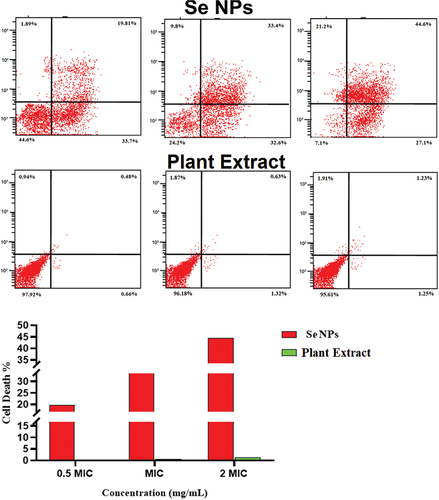
Figure 9. Determination of the live/dead percentage of C. diphtheriae after treating with different concentrations of green synthesized SeNPs and Vaccinium arctostaphylos (L.) extract using propidium iodide stain and flow cytometry.
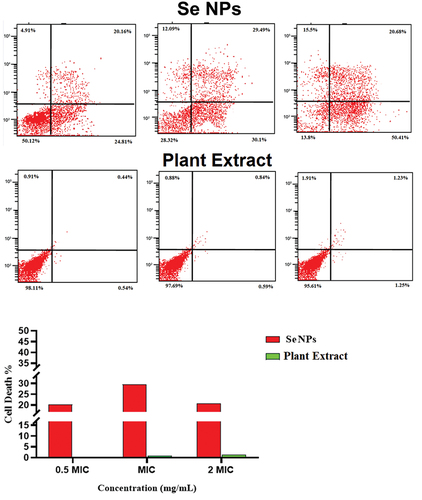
Table 7. Determination of the viable, injured, and dead cell percentage after treatment with different concentrations of Vaccinium arctostaphylos (L.) fruit extract using propidium iodide stain and flow cytometry
4. Discussion
Biological techniques, often known as “green strategies,” for producing various kinds of nanoparticles using a variety of biological resources, such as plants, algae, bacteria, and other biological components (Alhujaily et al., Citation2022). The biogenic synthesis of NPs through the use of plant extracts has earned considerable praise due to the non-toxic nature of the process and the low cost of natural capping agents (Alvi et al., Citation2021; Prasad & Selvaraj, Citation2014). Because to its high antioxidant, anti-inflammatory, and antihyperlipidemic characteristics, V. arctostaphylos (L.) fruit extract is utilized in a wide range of traditional medicine treatments (Barut et al., Citation2019; Mohtashami et al., Citation2019). Green synthesis of SeNPs using V. arctostaphylos L. plant extract involves the reduction of Se+4 to elemental selenium (34Se) using the phytochemicals present in the plant extract (Gharbavi et al., Citation2022; Singh et al., Citation2021). The fabricated SeNPs are characterized by biocompatibility and stability, as well as potential activity in biomedicine, agriculture, and environmental remediation (Abu-Elghait et al., Citation2021; Hashem et al., Citation2022; Salem et al., Citation2021, Citation2022). Our work used a UV-vis spectrophotometer to direct the absorbance band at 274 nm, resulting in an orange-red colour change, to identify the usage of V. arctostaphylos L. fruit extract as a capping agent in the synthesis of SeNPs. Previous studies have revealed that biologically generated SeNPs exhibit a distinct absorption band at 274 nm (Gharbavi et al., Citation2022; Mellinas et al., Citation2019). The extent of the transformation from clear to orange-red is determined by the amount of extract used and the period of incubation. As a result, raising the extract ratio results in a higher absorption point, indicating that symmetrical nanoparticles were created (Alvi et al., Citation2021; Anu et al., Citation2020). The biomolecules in the plant extract that are responsible for decreasing and stabilizing the SeNPs have been identified by FTIR analysis, which has been utilized to study the chemical composition of the surface of green synthesized SeNPs (Gharbavi et al., Citation2022; Sowndarya et al., Citation2017). The presence of—OH and—NH functional groups in the FTIR spectrum indicates that these groups are involved in the reduction of selenium ions to SeNPs (Gharbavi et al., Citation2022). The—C=O peak corresponds to the carbonyl group present in flavonoids and other polyphenolic compounds present in the plant extract that act as reducing agents for SeNPs synthesis. The—C–H peak corresponds to aliphatic hydrocarbons present in the plant extract that contribute to the stabilization of SeNPs (Ishak et al., Citation2019). In addition to providing information on the functional groups involved in SeNPs synthesis, FTIR analysis can also be used to study the interaction between SeNPs and biomolecules such as proteins and DNA. The FTIR spectrum of SeNPs-protein or SeNPs-DNA complexes shows characteristic shifts in the peaks corresponding to the functional groups involved in the interaction, providing insights into the nature and strength of the interaction (Shahabadi et al., Citation2021). Several studies have reported the use of FTIR analysis to characterize green synthesized SeNPs. For example, Mulla and his colleagues investigated the green synthesis of SeNPs using leaf extract of Azadirachta indica and characterized the nanoparticles using various techniques including FTIR spectroscopy (Mulla et al., Citation2020). The FTIR spectrum showed peaks corresponding to various functional groups such as hydroxyl (−OH), carbonyl (-C=O), and amine (−NH2) groups, indicating that these biomolecules were involved in reducing and stabilizing the SeNPs (Safdar et al., Citation2023). Another study reported the green synthesis of SeNPs using leaf extract of Pelargonium zonale and characterized the nanoparticles using FTIR analysis (Fardsadegh et al., Citation2019). In a study by (Kora & Rastogi, Citation2016) green synthesis of SeNPs was carried out using Pseudomonas aeruginosa ATCC 27,853 and characterized using various techniques including FTIR spectroscopy. FESEM analysis clearly distinguished the synthetic SeNPs made from fruit extract. In accordance with previous research, FESEM pictures reveal that the vast majority of NPs are spherical and uniformly distributed (Gharbavi et al., Citation2022; Sowndarya et al., Citation2017) with an average size of 50 nm (Singh et al., Citation2021). The study also reported that the size and shape of SeNPs could be controlled by varying the concentration of Azadirachta indica leaf extract. Another study conducted by Kumar et al. (Citation2019) (Kumar et al., Citation2019) reported the synthesis of SeNPs using an aqueous extract of Trigonella foenum-graecum seeds. FESEM analysis showed that the synthesized SeNPs were spherical in shape with an average size of 20–30 nm. The study also reported that the fenugreek seed extract acted as both a reducing agent and stabilizing agent for SeNPs. In addition to plant extracts, other green methods such as microbial synthesis have also been used for SeNPs synthesis. A study conducted by (Bharathi et al., Citation2020) investigated the synthesis of SeNPs using Bacillus subtilis bacteria. FESEM analysis revealed that the synthesized SeNPs were spherical in shape with an average size of 20–50 nm. The study also reported that the bacterial cells acted as both a reducing agent and stabilizing agent for SeNPs. Dynamic light scattering (DLS) analysis was performed to determine the size distribution of the synthesized SeNPs. The results showed that the average hydrodynamic diameter of SeNPs was 124 nm with a polydispersity index (PDI) of 0.26, indicating a narrow size distribution. The zeta potential (ZP) of SeNPs was found to be −24.5 mV, indicating that the particles were stable and had a high degree of repulsion (Abbas et al., Citation2021; Gharbavi et al., Citation2022). Se nanoparticles are effective against both gram-positive and gram-negative bacteria. In a variety of experimental conditions, Se nanoparticles are effective against a wide variety of bacteria, including S. aureus and E. coli (Boroumand et al., Citation2019; Tran et al., Citation2015; Vahdati & Tohidi Moghadam, Citation2020). Antifungal action has been demonstrated in previous studies for biogenic SeNPs manufactured with Bacillus spp (Filipović et al., Citation2021; Shakibaie et al., Citation2015). The SeNPs formulations demonstrated in this work were also more effective against Gram-positive bacteria. The composition of the bacterial wall differs significantly between the two types, which may explain the observed behaviour (Kędziora et al., Citation2018). Similar findings have been reported by several studies (Guisbiers et al., Citation2016; Rangrazi et al., Citation2020). In addition (Cremonini et al., Citation2016), show that the green produced selenium nanoparticles had high antibacterial activity against clinical isolates of P. aeruginosa, but less effectiveness against C. albicans. Several investigations comparing the antibacterial activity of green- and chemically-synthesized SeNPs have indicated that the former is significantly more effective (Mariadoss et al., Citation2022). They predicted that a synergistic impact between the capping agent and SeNPs enhanced antibacterial efficacy. Green-synthesized SeNPs coated with Chitosan have been shown to exhibit enhanced antibacterial activity at MICs of 137 and 274 g/mL against E. faecium and E. faecalis, respectively; however, they had no effect on Gram-negative K. pneumonia (Rangrazi et al., Citation2020). Several researchers hypothesized that the improved adhesion to bacterial surfaces gives positively charged NPs a higher opportunity to suppress bacterial growth (Alghuthaymi, Citation2022; Filipović et al., Citation2021). Other study reported that large concentrations of negatively charged NPs can result in significant antibacterial activity because of molecular crowding (Arakha et al., Citation2015). The negatively charged SeNPs are more effective in inhibiting bacterial growth as compared to positively charged SeNPs (Alghuthaymi, Citation2022). Several studies have shown that negatively charged SeNPs can inhibit the growth of various bacterial strains (Escobar-Ramírez et al., Citation2021; Vahdati & Tohidi Moghadam, Citation2020). For instance, a study conducted by (Galić et al., Citation2020) investigated the effect of negatively charged SeNPs on the growth of E. coli and S. aureus. The results showed that the negatively charged SeNPs significantly inhibited the growth of both bacterial strains in a dose-dependent manner. The study also suggested that the negatively charged SeNPs could disrupt the cell membrane of bacteria, leading to their death. Another study conducted by (Wang et al., Citation2022) investigated the effect of negatively charged SeNPs on the growth of P. aeruginosa. The results showed that the negatively charged SeNPs significantly inhibited the growth of P. aeruginosa and disrupted its biofilm formation. The study suggested that the negatively charged SeNPs could inhibit bacterial growth by disrupting the cell membrane and inducing oxidative stress. Furthermore, a study conducted by (Khiralla & El-Deeb, Citation2015) investigated the effect of negatively charged SeNPs on the growth of S. typhimurium. The results showed that the negatively charged SeNPs significantly inhibited the growth of S. typhimurium and reduced its motility and biofilm formation. The study suggested that the negatively charged SeNPs could inhibit bacterial growth by disrupting the cell membrane and interfering with bacterial motility. Our study was in agreement with other studies have investigated the effect of SeNPs on the growth of C. diphtheriae. One such study conducted by (Ao et al., Citation2022) found that SeNPs inhibited the growth of C. diphtheriae in a dose-dependent manner. The study also found that SeNPs reduced the biofilm formation ability of C. diphtheriae, which is an important virulence factor for the pathogen. May be the mode of action of the SeNPs on the virulence of C. diphtheriae is similar to the silver nanoparticles that investigated by Arana et al. (Citation2021) who found that Silver NPs reduced the expression of various virulence genes in C. diphtheriae, including those involved in toxin production and iron acquisition and inhibit the ability of C. diphtheriae to adhere to and invade host cells.
The presence of Se ions is primarily responsible for the antibacterial properties of green-synthesized SeNPs (Truong et al., Citation2021; Dang-Bao et al., Citation2022). The increased surface area of SeNPs results in a stronger bactericidal impact. The capacity of Se nanoparticles to bind to key cellular structures is largely responsible for their bactericidal effects, in particular against the SH groups of bacteria. The production of reactive oxygen species (ROS) and free radicals by Se nanoparticles contributes to their toxicity, which includes destroying the bacterial cell wall and blocking the respiratory enzymes. Se nanoparticles destroy bacteria by interfering with their ability to replicate their DNA (X. Huang et al., Citation2016; Zhang et al., Citation2021). The resazurin test is a commonly used method for determining the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of antimicrobial agents (Mann & Markham, Citation1998). This test works by measuring the metabolic activity of microorganisms in the presence of different concentrations of antimicrobial agents. In this test, resazurin, a blue dye, is added to the growth medium along with the antimicrobial agent. The dye changes colour from blue to pink as a result of the metabolic activity of the microorganisms (Elshikh et al., Citation2016).
The MIC is defined as the lowest concentration of an antimicrobial agent that inhibits the visible growth of microorganisms, while the MBC is defined as the lowest concentration that kills 99.9% of the initial inoculum. The resazurin test is a rapid method that can provide results within hours, compared to traditional methods that can take days or even weeks to produce results (Scimone et al., Citation2021). Furthermore, the resazurin test is highly sensitive and can detect small changes in bacterial growth and metabolism. This allows for a more accurate determination of MIC and MBC concentrations. Moreover, the resazurin test is a cost-effective method that requires minimal equipment and reagents, making it an attractive option for laboratories with limited resources (Costa et al., Citation2021). The effect of SeNPs on the live/dead percentage of bacterial cells can be analyzed using propidium iodide (PI) staining and flow cytometry (Palomo-Siguero et al., Citation2016). Propidium iodide is a fluorescent dye that binds to the DNA of dead cells, causing them to emit red fluorescence when excited by a laser. Live cells with intact cell membranes exclude PI and do not fluoresce red. Flow cytometry is a technique that uses lasers to detect and analyse fluorescent signals from individual cells as they pass through a narrow channel. Several studies have reported the use of PI staining and flow cytometry to analyse the live/dead percentage of bacterial cells treated with SeNPs (Bankier et al., Citation2019; Tran et al., Citation2015). For instance, a study conducted (Singh et al., Citation2021) investigated the effect of SeNPs on E. coli and S. aureus using PI staining and flow cytometry. The results showed that SeNPs significantly reduced the viability of both bacterial strains, with a higher proportion of dead cells detected in the treated samples compared to the control. Similarly, a study by (Tran et al., Citation2015) investigated the effect of SeNPs on S. aureus and E. coli using PI staining and flow cytometry. The results showed that SeNPs caused significant damage to the bacterial cell membrane, leading to an increase in dead cells detected by PI staining.
5. Conclusion
Selenium nanoparticles (Se NPs) were synthesized using the biogenic method. The Nanoparticles were successfully synthesized using the fruit extract of V. arctostaphylos (L.) and their characterization was confirmed by UV-vis, FTIR, Zeta potentials, FESEM, and EDX that indicated the obtained SeNPs were spherical and uniform in size 50 ± 1.23 nm. The results of in vitro biocompatibility studies demonstrated that SeNPs produced from the fruit extract of V. arctostaphylos (L.) are biocompatible and therefore safe for use in medical applications. Our study reported that SeNPs exhibit strong antibacterial activity against S. aureus, E. coli, and C. diphtheria. The antibacterial activity of SeNPs is attributed to their ability to generate reactive oxygen species (ROS) that cause oxidative stress and damage to bacterial cells. The ROS generated by SeNPs can induce lipid peroxidation, protein oxidation, DNA damage, and membrane damage in bacterial cells, leading to their death. In addition to their antibacterial activity, SeNPs significantly reduced the live cell percentage of S. aureus and E. coli, and C. diphtheria. These findings suggest that SeNPs could be used as an effective antibacterial agent for the treatment of infectious diseases caused by these bacterial strains.
Author contributions
Conceptualization and methodology, M.A.A.K., H.A.H.; formal analysis, H.T.A., and H.K; investigation and data curation S.A., G.M.S.; validation H.B.M., Y.H.D., visualization Y.H.D., original draft preparation, S.A., H.K., and W.J.A.; writing-review and editing, S.A., G.M.S., A.K.A., A.A. and Y.H.D.; supervision, A.A., S.A., and G.M.S.; project administration, A.K.A., and W.S.J. All authors gave approval to the final version of the manuscript.
Acknowledgments
The authors acknowledge Researchers Supporting Project number (RSP2023R375), King Saud University, Riyadh, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author on reasonable request
Additional information
Funding
Notes on contributors
Mahasen A.A. Khudier
Mahasen A.A. Khudier has a Ph.D. in Veterinary Medicine with a specialty in poultry diseases. She is an assistant professor at the College of Veterinary Medicine, University of Al-Qadisiyah, Iraq. She does research in poultry diseases, including bacterial, viral, and fungal diseases, as well as some research in poultry nutrition.
Hassan A. Hammadi
Hassan A. Hammadi has a M.Sc. in veterinary medicine/poultry diseases. He is an assistant professor at the College of Veterinary Medicine, University of Al-Qadisiyah, Iraq. He works in the field of avian virology.
Hiba T. Atyia
Hiba T. Atyia has a M.Sc. in veterinary medicine/poultry diseases. She is a lecturer in the College of Veterinary Medicine, University of Al-Qadisiyah, Iraq. She works in the fields of poultry diseases and nutrition.
Hassan Al-Karagoly
Hassan Al-Karagoly has a Ph.D. in Veterinary pathology. He is an assistant professor at the College of Veterinary Medicine, University of Al-Qadisiyah, Iraq. He has vast experience in Veterinary pathology, Immunopathology, Molecular pathology, toxicology, DNA sequencing analysis, high-resolution melting analysis, nanotechnology, especially green synthesis applications, and electrospinning.
Salim Albukhaty
Salim Albukhaty has got Ph.D. in Nanobiotechnology. Currently, he is a professor in the Department of Chemistry, College of Science, University of Misan, Iraq. He does research in green chemistry, electrospun, gene, and drug-drug delivery. contact email: [email protected]
Ghassan M. Sulaiman
Ghassan Sulaiman has Ph.D. in Biotechnology. He is a professor at the Division of Biotechnology, Department of Applied Sciences, University of Technology, Baghdad, Iraq. He does research in mechanisms of cell death, cancer research, and cell culture techniques such as flow cytometry.
Yaser H. Dewir
Yaser H. Dewir is a professor at King Saud Univ., Saudi Arabia. He received his MSc from South China Agricultural Univ. (SCAU) China and Ph.D. from Chungbuk National Univ.(CBNU), Korea. He carried out Postdoc. studies in CBNU, Korea and University of KwaZulu-Natal (UKZN), South Africa. His research interests including cell and tissue culture, plant biology and biotechnology. Contact e-mail: [email protected]; [email protected]
Hameed B. Mahood
Hameed B. Mahood has Ph.D. in Chemical and Process Engineering, University of Surrey, UK. He works at the Division of Civil, Chemical, and Environmental Engineering, University of Surrey. He does research in Chemical Engineering. Their current project is 'Energy Engineering, Heat Transfer and Multiphase Flow'.
References
- Abbas, H. S., Abou Baker, D. H., & Ahmed, E. A. (2021). Cytotoxicity and antimicrobial efficiency of selenium nanoparticles biosynthesized by Spirulina platensis. Archives of Microbiology, 203(2), 523–21. https://doi.org/10.1007/s00203-020-02042-3
- Abu-Elghait, M., Hasanin, M., Hashem, A. H., & Salem, S. S. (2021). Ecofriendly novel synthesis of tertiary composite based on cellulose and myco-synthesized selenium nanoparticles: Characterization, antibiofilm and biocompatibility. International Journal of Biological Macromolecules, 175, 294–303. https://doi.org/10.1016/j.ijbiomac.2021.02.040
- Albukhaty, S., Al-Bayati, L., Al-Karagoly, H., & Al-Musawi, S. (2022). Preparation and characterization of titanium dioxide nanoparticles and in vitro investigation of their cytotoxicity and antibacterial activity against Staphylococcus aureus and Escherichia coli. Animal Biotechnology, 33(5), 864–870. https://doi.org/10.1080/10495398.2020.1842751
- Albukhaty, S., Naderi-Manesh, H., Taki, T., & Jabir, M. (2018). Poly- l -lysine-coated superparamagnetic nanoparticles: A novel method for the transfection of pro-BDNF into neural stem cells. Cells Nanomed Biotechnol, 46(sup3), S125–S132. https://doi.org/10.1080/21691401.2018.1489272
- Alghuthaymi, M. A. (2022). Antibacterial action of insect chitosan/gum Arabic nanocomposites encapsulating eugenol and selenium nanoparticles. Journal of King Saud University-Science, 34(7), 102219. https://doi.org/10.1016/j.jksus.2022.102219
- Alhujaily, M., Albukhaty, S., Yusuf, M., Mohammed, M. K., Sulaiman, G. M., Al-Karagoly, H., Alyamani, A. A., Albaqami, J., & AlMalki, F. A. (2022). Recent advances in plant-mediated zinc oxide nanoparticles with their significant biomedical properties. Bioengineering, 9(10), 541. https://doi.org/10.3390/bioengineering9100541
- Alvi, G. B., Iqbal, M. S., Ghaith, M. M. S., Haseeb, A., Ahmed, B., & Qadir, M. I. (2021). Biogenic selenium nanoparticles (SeNps) from citrus fruit have anti-bacterial activities. Scientific Reports, 11(1), 4811. https://doi.org/10.1038/s41598-021-84099-8
- Alyamani, A. A., Albukhaty, S., Aloufi, S., AlMalki, F. A., Al-Karagoly, H., & Sulaiman, G. M. (2021). Green fabrication of zinc oxide nanoparticles using phlomis leaf extract: Characterization and in vitro evaluation of cytotoxicity and antibacterial properties. Molecules, 26(20), 6140. https://doi.org/10.3390/molecules26206140
- Anu, K., Devanesan, S., Prasanth, R., AlSalhi, M. S., Ajithkumar, S., & Singaravelu, G. (2020). Biogenesis of selenium nanoparticles and their anti-leukemia activity. Journal of King Saud University-Science, 32(4), 2520–2526. https://doi.org/10.1016/j.jksus.2020.04.018
- Ao, B., Lv, J., Yang, H., He, F., Hu, Y., Hu, B., Jiang, H., Huo, X., Tu, J., & Xia, X. (2022). Moringa oleifera extract mediated the synthesis of Bio-SeNPs with antibacterial activity against Listeria monocytogenes and Corynebacterium diphtheriae. LWT, 165, 113751. https://doi.org/10.1016/j.lwt.2022.113751
- Arakha, M., Pal, S., Samantarrai, D., Panigrahi, T. K., Mallick, B. C., Pramanik, K., Mallick, B., & Jha, S. (2015). Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Scientific Reports, 5(1), 14813. https://doi.org/10.1038/srep14813
- Arana, L., Gallego, L., & Alkorta, I. (2021). Incorporation of Antibiotics into Solid Lipid Nanoparticles: A Promising Approach to Reduce Antibiotic Resistance Emergence. Nanomaterials, 11, 1251. https://doi.org/10.3390/nano11051251
- Bankier, C., Matharu, R., Cheong, Y., Ren, G., Cloutman-Green, E., & Ciric, L. (2019). Synergistic antibacterial effects of metallic nanoparticle combinations. Scientific Reports, 9(1), 16074. https://doi.org/10.1038/s41598-019-52473-2
- Baran, M. F., Keskin, C., Baran, A., Kurt, K., İ̇̇pek, P., Eftekhari, A., Khalilov, R., Fridunbayov, I., & Cho, W. C. (2023). Green synthesis and characterization of selenium nanoparticles (Se NPs) from the skin (testa) of Pistacia vera L.(Siirt pistachio) and investigation of antimicrobial and anticancer potentials. Biomass Conversion and Biorefinery, Biorefinery.1–11. https://doi.org/10.1007/s13399-023-04366-8
- Barut, B., Barut, E. N., Engin, S., Ö, A., & Sezen, F. S. (2019). Investigation of the Antioxidant, α-Glucosidase Inhibitory, Anti-inflammatory, and DNA Protective Properties of Vaccinium arctostaphylos L. Turkish Journal of Pharmaceutical Sciences, 16(2), 175. https://doi.org/10.4274/tjps.galenos.2018.28247
- Bharathi, S., Kumaran, S., Suresh, G., Ramesh, M., Thangamani, V., Pugazhvendan, S., & Sathiyamurthy, K. (2020). Extracellular synthesis of nanoselenium from fresh water bacteria Bacillus sp., and its validation of antibacterial and cytotoxic potential. Biocatalysis and Agricultural Biotechnology, 27, 101655. https://doi.org/10.1016/j.bcab.2020.101655
- Bisht, N., Phalswal, P., & Khanna, P. K. (2022). Selenium nanoparticles: A review on synthesis and biomedical applications. Materials Advances, 3(3), 1415–1431. https://doi.org/10.1039/D1MA00639H
- Boroumand, S., Safari, M., Shaabani, E., Shirzad, M., & Faridi-Majidi, R. (2019). Selenium nanoparticles: Synthesis, characterization and study of their cytotoxicity, antioxidant and antibacterial activity. Materials Research Express, 6(8), 0850d0858. https://doi.org/10.1088/2053-1591/ab2558
- Chinnaraj, S., Palani, V., Maluventhen, V., Chandrababu, R., Soundarapandian, K., Kaliannan, D., Rathinasamy, B., Liu, W.-C., Balasubramanian, B., & Arumugam, M. (2023). Silver nanoparticle production mediated by Goniothalamus wightii extract: Characterization and their potential biological applications. Particulate Science and Technology, 41(4), 517–531. https://doi.org/10.1080/02726351.2022.2123752
- Costa, P., Gomes, A. T., Braz, M., Pereira, C., & Almeida, A. (2021). Application of the resazurin cell viability assay to monitor Escherichia coli and Salmonella typhimurium inactivation mediated by phages. Antibiotics, 10(8), 974. https://doi.org/10.3390/antibiotics10080974
- Cremonini, E., Zonaro, E., Donini, M., Lampis, S., Boaretti, M., Dusi, S., Melotti, P., Lleo, M. M., & Vallini, G. (2016). Biogenic selenium nanoparticles: Characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microbial Biotechnology, 9(6), 758–771. https://doi.org/10.1111/1751-7915.12374
- Dang-Bao, T., Ho, T.-T., Do, B. L., Phung Anh, N., Phan, T. D. T., Tran, T. B. Y., Duong, N. L., Hong Phuong, P., & Nguyen, T. (2022). Green orange peel-mediated bioinspired synthesis of nanoselenium and its antibacterial activity against methicillin-resistant staphylococcus aureus. ACS Omega, 7(40), 36037–36046. https://doi.org/10.1021/acsomega.2c05469
- Dulta, K., Virk, A. K., Chauhan, P., Bohara, P., & Chauhan, P. K. (2022). Nanotechnology and applications. Applications of computational intelligence in multi-disciplinary research (pp. 129–141). Elsevier.
- Elakraa, A. A., Salem, S. S., El-Sayyad, G. S., & Attia, M. S. (2022). Cefotaxime incorporated bimetallic silver-selenium nanoparticles: Promising antimicrobial synergism, antibiofilm activity, and bacterial membrane leakage reaction mechanism. RSC Advances, 12(41), 26603–26619. https://doi.org/10.1039/D2RA04717A
- Elshikh, M., Ahmed, S., Funston, S., Dunlop, P., McGaw, M., Marchant, R., & Banat, I. M. (2016). Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnology Letters, 38(6), 1015–1019. https://doi.org/10.1007/s10529-016-2079-2
- Escobar-Ramírez, M. C., Castañeda-Ovando, A., Pérez-Escalante, E., Rodríguez-Serrano, G. M., Ramírez-Moreno, E., Quintero-Lira, A., Contreras-López, E., Añorve-Morga, J., Jaimez-Ordaz, J., & González-Olivares, L. G. (2021). Antimicrobial activity of Se-nanoparticles from bacterial biotransformation. Fermentation, 7(3), 130. https://doi.org/10.3390/fermentation7030130
- Fardsadegh, B., Vaghari, H., Mohammad Jafari, R., Najian, Y., & Jafarizadeh-Malmiri, H. (2019). Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract. Green Processing and Synthesis, 8(1), 191–198. https://doi.org/10.1515/gps-2018-0060
- Filipović, N., Ušjak, D., Milenković, M. T., Zheng, K., Liverani, L., Boccaccini, A. R., & Stevanović, M. M. (2021). Comparative study of the antimicrobial activity of selenium nanoparticles with different surface chemistry and structure. Frontiers in Bioengineering and Biotechnology, 8, 624621. https://doi.org/10.3389/fbioe.2020.624621
- Galić, E., Ilić, K., Hartl, S., Tetyczka, C., Kasemets, K., Kurvet, I., Milić, M., Barbir, R., Pem, B., Erceg, I., Dutour Sikirić, M., Pavičić, I., Roblegg, E., Kahru, A., & Vinković Vrček, I. (2020). Impact of surface functionalization on the toxicity and antimicrobial effects of selenium nanoparticles considering different routes of entry. Food and Chemical Toxicology, 144, 111621. https://doi.org/10.1016/j.fct.2020.111621
- Garza-García, J. J., Hernández-Díaz, J. A., Zamudio-Ojeda, A., León-Morales, J. M., Guerrero-Guzmán, A., Sánchez-Chiprés, D. R., López-Velázquez, J. C., & García-Morales, S. (2021). The role of selenium nanoparticles in agriculture and food technology. Biological Trace Element, 200(5), Research.1–21. https://doi.org/10.1007/s12011-021-02847-3
- Gharbavi, M., Johari, B., Mousazadeh, N., Rahimi, B., Leilan, M. P., Eslami, S. S., & Sharafi, A. (2020). Hybrid of niosomes and bio-synthesized selenium nanoparticles as a novel approach in drug delivery for cancer treatment. Molecular Biology Reports, 47(9), 6517–6529. https://doi.org/10.1007/s11033-020-05704-z
- Gharbavi, M., Mousavi, M., Pour‐Karim, M., Tavakolizadeh, M., & Sharafi, A. (2022). Biogenic and facile synthesis of selenium nanoparticles using Vaccinium arctostaphylos L. fruit extract and anticancer activity against in vitro model of breast cancer. Cell Biology International, 46(10), 1612–1624. https://doi.org/10.1002/cbin.11852
- Guisbiers, G., Wang, Q., Khachatryan, E., Mimun, L., Mendoza-Cruz, R., Larese-Casanova, P., Webster, T., & Nash, K. (2016). Inhibition of E. coli and S. aureus with selenium nanoparticles synthesized by pulsed laser ablation in deionized water. International Journal of Nanomedicine, 11, 3731. https://doi.org/10.2147/IJN.S106289
- Hashem, A. H., Abdelaziz, A. M., Attia, M. S., & Salem, S. S. (2022). Selenium and nano-selenium-mediated biotic stress tolerance in plants. In Selenium and nano-selenium in environmental stress management and crop quality improvement (pp. 209–226). Springer International Publishing. https://doi.org/10.1007/978-3-031-07063-1_11
- Huang, X., Chen, X., Chen, Q., Yu, Q., Sun, D., & Liu, J. (2016). Investigation of functional selenium nanoparticles as potent antimicrobial agents against superbugs. Acta Biomaterialia, 30, 397–407. https://doi.org/10.1016/j.actbio.2015.10.041
- Huang, Y., Su, E., Ren, J., & Qu, X. (2021). The recent biological applications of selenium-based nanomaterials. Nano Today, 38, 101205. https://doi.org/10.1016/j.nantod.2021.101205
- Ishak, N. M., Kamarudin, S., & Timmiati, S. (2019). Green synthesis of metal and metal oxide nanoparticles via plant extracts: An overview. Materials Research Express, 6(11), 112004. https://doi.org/10.1088/2053-1591/ab4458
- Kalaimurugan, D., Lalitha, K., Durairaj, K., Sivasankar, P., Park, S., Nithya, K., Shivakumar, M. S., Liu, W.-C., Balamuralikrishnan, B., & Venkatesan, S. (2022). Biogenic synthesis of ZnO nanoparticles mediated from Borassus flabellifer (Linn): Antioxidant, antimicrobial activity against clinical pathogens, and photocatalytic degradation activity with molecular modeling. Environmental Science and Pollution Research, 29(57), 86308–86319. https://doi.org/10.1007/s11356-021-18074-1
- Kędziora, A., Speruda, M., Krzyżewska, E., Rybka, J., Łukowiak, A., & Bugla-Płoskońska, G. (2018). Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. International Journal of Molecular Sciences, 19(2), 444. https://doi.org/10.3390/ijms19020444
- Khiralla, G. M., & El-Deeb, B. A. (2015). Antimicrobial and antibiofilm effects of selenium nanoparticles on some foodborne pathogens. LWT-Food Science and Technology, 63(2), 1001–1007. https://doi.org/10.1016/j.lwt.2015.03.086
- Khodadadi, S., Mahdinezhad, N., Fazeli-Nasab, B., Heidari, M. J., Fakheri, B., & Miri, A. (2021). Investigating the possibility of green synthesis of silver nanoparticles using Vaccinium arctostaphlyos extract and evaluating its antibacterial properties. BioMed Research International, 2021, 1–13. https://doi.org/10.1155/2021/5572252
- Kora, A. J., & Rastogi, L. (2016). Biomimetic synthesis of selenium nanoparticles by Pseudomonas aeruginosa ATCC 27853: An approach for conversion of selenite. Journal of Environmental Management, 181, 231–236. https://doi.org/10.1016/j.jenvman.2016.06.029
- Kumar, I., Mondal, M., & Sakthivel, N. (2019). Green synthesis of phytogenic nanoparticles. In Green Synthesis, Characterization and Applications of Nanoparticles (pp. 37–73). Elsevier. 978-0-08-102579-6. https://doi.org/10.1016/C2017-0-02526-0
- Lehtinen, J., Nuutila, J., & Lilius, E. M. (2004). Green fluorescent protein-propidium iodide (GFP-PI) based assay for flow cytometric measurement of bacterial viability. Cytom Part A, 60, 165–172. https://doi.org/10.1002/cyto.a.20026
- Mann, C., & Markham, J. (1998). A new method for determining the minimum inhibitory concentration of essential oils. Journal of Applied Microbiology, 84(4), 538–544. https://doi.org/10.1046/j.1365-2672.1998.00379.x
- Manojkumar, U., Kaliannan, D., Srinivasan, V., Balasubramanian, B., Kamyab, H., Mussa, Z. H., Palaniyappan, J., Mesbah, M., Chelliapan, S., & Palaninaicker, S. (2023). Green synthesis of zinc oxide nanoparticles using Brassica oleracea var. botrytis leaf extract: Photocatalytic, antimicrobial and larvicidal activity. Chemosphere, 323, 138263. https://doi.org/10.1016/j.chemosphere.2023.138263
- Mariadoss, A. V. A., Saravanakumar, K., Sathiyaseelan, A., Naveen, K. V., & Wang, M.-H. (2022). Enhancement of anti-bacterial potential of green synthesized selenium nanoparticles by starch encapsulation. Microbial Pathogenesis, 167, 105544. https://doi.org/10.1016/j.micpath.2022.105544
- Meenambigai, K., Kokila, R., Chandhirasekar, K., Thendralmanikandan, A., Kaliannan, D., Ibrahim, K. S., Kumar, S., Liu, W., Balasubramanian, B., & Nareshkumar, A. (2022). Green synthesis of selenium nanoparticles mediated by Nilgirianthus ciliates leaf extracts for antimicrobial activity on foodborne pathogenic microbes and pesticidal activity against Aedes aegypti with molecular docking. Biological Trace Element Research, 200(6), 2948–2962. https://doi.org/10.1007/s12011-021-02868-y
- Mellinas, C., Jiménez, A., & Garrigós Md, C. (2019). Microwave-assisted green synthesis and antioxidant activity of selenium nanoparticles using Theobroma cacao L. bean shell extract. Molecules, 24(22), 4048. https://doi.org/10.3390/molecules24224048
- Mohammed, H. A., Khan, R. A., Singh, V., Yusuf, M., Akhtar, N., Sulaiman, G. M., Albukhaty, S., Abdellatif, A. A., Khan, M., Mohammed, S. A., & Al-Subaiyel, A. M. (2023). Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development. Nanotechnology Reviews, 12(1), 20220517. https://doi.org/10.1515/ntrev-2022-0517
- Mohtashami, R., Huseini, H. F., Nabati, F., Hajiaghaee, R., & Kianbakht, S. (2019). Effects of standardized hydro-alcoholic extract of Vaccinium arctostaphylos leaf on hypertension and biochemical parameters in hypertensive hyperlipidemic type 2 diabetic patients: A randomized, double-blind and placebo-controlled clinical trial. Avicenna Journal of Phytomedicine, 9(1), 44.
- Mulla, N. A., Otari, S. V., Bohara, R. A., Yadav, H. M., & Pawar, S. H. (2020). Rapid and size-controlled biosynthesis of cytocompatible selenium nanoparticles by Azadirachta indica leaves extract for antibacterial activity. Materials Letters, 264, 127353. https://doi.org/10.1016/j.matlet.2020.127353
- Nayak, V., Singh, K. R., Singh, A. K., & Singh, R. P. (2021). Potentialities of selenium nanoparticles in biomedical science. New Journal of Chemistry, 45(6), 2849–2878. https://doi.org/10.1039/D0NJ05884J
- Palomo-Siguero, M., AMa, G., Pérez-Conde, C., & Madrid, Y. (2016). Effect of selenite and selenium nanoparticles on lactic bacteria: A multi-analytical study. Microchemical Journal, 126, 488–495. https://doi.org/10.1016/j.microc.2016.01.010
- Prasad, K. S., & Selvaraj, K. (2014). Biogenic synthesis of selenium nanoparticles and their effect on as (III)-induced toxicity on human lymphocytes. Biological Trace Element Research, 157(3), 275–283. https://doi.org/10.1007/s12011-014-9891-0
- Rangrazi, A., Bagheri, H., Ghazvini, K., Boruziniat, A., & Darroudi, M. (2020). Synthesis and antibacterial activity of colloidal selenium nanoparticles in chitosan solution: A new antibacterial agent. Materials Research Express, 6(12), 1250h1253. https://doi.org/10.1088/2053-1591/ab6a56
- Rudramurthy, G. R., Swamy, M. K., Sinniah, U. R., & Ghasemzadeh, A. (2016). Nanoparticles: Alternatives against drug-resistant pathogenic microbes. Molecules, 21(7), 836. https://doi.org/10.3390/molecules21070836
- Safdar, M., Aslam, S., Akram, M., Khaliq, A., Ahsan, S., Liaqat, A., Mirza, M., Waqas, M., & Ak, W. (2023). Bombax ceiba flower extract mediated synthesis of Se nanoparticles for antibacterial activity and urea detection. World Journal of Microbiology & Biotechnology, 39(3), 80. https://doi.org/10.1007/s11274-022-03513-z
- Salem, S. S. (2022). Bio-fabrication of selenium nanoparticles using Baker’s yeast extract and its antimicrobial efficacy on food borne pathogens. Applied Biochemistry and Biotechnology, 194(5), 1898–1910. https://doi.org/10.1007/s12010-022-03809-8
- Salem, S. S., Badawy, M. S. E., Al-Askar, A. A., Arishi, A. A., Elkady, F. M., & Hashem, A. H. (2022). Green biosynthesis of selenium nanoparticles using orange peel waste: Characterization, antibacterial and antibiofilm activities against multidrug-resistant bacteria. Life, 12(6), 893. https://doi.org/10.3390/life12060893
- Salem, S. S., & Fouda, A. (2021). Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biological Trace Element Research, 199(1), 344–370. https://doi.org/10.1007/s12011-020-02138-3
- Salem, S. S., Fouda, M. M., Fouda, A., Awad, M. A., Al-Olayan, E. M., Allam, A. A., & Shaheen, T. I. (2021). Antibacterial, cytotoxicity and larvicidal activity of green synthesized selenium nanoparticles using Penicillium corylophilum. Journal of Cluster Science, 32(2), 351–361. https://doi.org/10.1007/s10876-020-01794-8
- Scimone, A., Redfern, J., Patiphatpanya, P., Thongtem, T., Ratova, M., Kelly, P., & Verran, J. (2021). Development of a rapid method for assessing the efficacy of antibacterial photocatalytic coatings. Talanta, 225, 122009. https://doi.org/10.1016/j.talanta.2020.122009
- Shahabadi, N., Zendehcheshm, S., & Khademi, F. (2021). Selenium nanoparticles: Synthesis, in-vitro cytotoxicity, antioxidant activity and interaction studies with ct-DNA and HSA, HHb and Cyt c serum proteins. Biotechnology Reports, 30, e00615. https://doi.org/10.1016/j.btre.2021.e00615
- Shakibaie, M., Mohazab, N. S., & Mousavi, S. A. A. (2015). Antifungal activity of selenium nanoparticles synthesized by Bacillus species Msh-1 against Aspergillus fumigatus and Candida albicans. Jundishapur Journal of Microbiology, 8(9). https://doi.org/10.5812/jjm.26381
- Singh, K. R., Nayak, V., Singh, J., Singh, A. K., & Singh, R. P. (2021). Potentialities of bioinspired metal and metal oxide nanoparticles in biomedical sciences. RSC Advances, 11(40), 24722–24746. https://doi.org/10.1039/D1RA04273D
- Soltani, R., Hakimi, M., Asgary, S., Ghanadian, S. M., Keshvari, M., & Sarrafzadegan, N. (2014). Evaluation of the effects of Vaccinium arctostaphylos L. Fruit extract on serum lipids and hs-CRP levels and oxidative stress in adult patients with hyperlipidemia: A randomized, double-blind, placebo-controlled clinical trial. Evidence-Based Complementary and Alternative Medicine, 2014, 1–6. https://doi.org/10.1155/2014/217451
- Sowjanya, P., Srinivasa, B. P., & Lakshmi, N. M. (2015). Phytochemical analysis and antibacterial efficacy of Amaranthus tricolor (L) methanolic leaf extract against clinical isolates of urinary tract pathogens. African Journal of Microbiology Research, 9(20), 1381–1385. https://doi.org/10.5897/AJMR2015.7294
- Sowndarya, P., Ramkumar, G., & Shivakumar, M. (2017). Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artificial Cells, Nanomedicine, and Biotechnology, 45(8), 1490–1495. https://doi.org/10.1080/21691401.2016.1252383
- Tran, P. A., O’Brien-Simpson, N., Reynolds, E. C., Pantarat, N., Biswas, D. P., & O’Connor, A. J. (2015). Low cytotoxic trace element selenium nanoparticles and their differential antimicrobial properties against S. aureus and E. coli. Nanotechnology, 27(4), 045101. https://doi.org/10.1088/0957-4484/27/4/045101
- Truong, L. B., Medina-Cruz, D., Mostafavi, E., & Rabiee, N. (2021). Selenium nanomaterials to combat antimicrobial resistance. Molecules, 26(12), 3611. https://doi.org/10.3390/molecules26123611
- Vahdati, M., & Tohidi Moghadam, T. (2020). Synthesis and characterization of selenium nanoparticles-lysozyme nanohybrid system with synergistic antibacterial properties. Scientific Reports, 10(1), 510. https://doi.org/10.1038/s41598-019-57333-7
- Viskelis, P., Rubinskiene, M., Jasutiene, I., Sarkinas, A., Daubaras, R., & Cesoniene, L. (2009). Anthocyanins, antioxidative, and antimicrobial properties of American cranberry (Vaccinium macrocarpon Ait.) and their press cakes. Journal of food science, 74, 157–161. https://doi.org/10.1111/j.1750-3841.2009.01066.x
- Wang, C., Liu, X., Chen, F., Yue, L., Cao, X., Li, J., Cheng, B., Wang, Z., & Xing, B. (2022). Selenium content and nutritional quality of Brassica chinensis L enhanced by selenium engineered nanomaterials: The role of surface charge. Environmental Pollution, 308, 119582. https://doi.org/10.1016/j.envpol.2022.119582
- Zhang, H., Li, Z., Dai, C., Wang, P., Fan, S., Yu, B., & Qu, Y. (2021). Antibacterial properties and mechanism of selenium nanoparticles synthesized by Providencia sp. DCX. Environmental Research, 194, 110630. https://doi.org/10.1016/j.envres.2020.110630