Abstract
Due to the increasing need for food security across the world, it has become urgent to develop a combination of different agricultural inputs that can enhance higher crop productivity under plant growth limiting environment. Thus, the effects of a cellulose decomposing bacterium (Geobacillus stearothermophilus SSK-2018), humic acid, and wheat straw were evaluated on the growth of Cucurbita pepo L. (squash) under an arid land condition during a two-year (2020 and 2021) field trials. Laboratory analysis revealed that G. stearothermophilus SSK-2018 was a gram-positive bacterium capable of promoting plant growth by producing indole-3-acetic acid as well as enzymatic activities including cellulase, amylase, protease, gelatinase, and phosphatase. Consequently, the inoculation of SSK-2018 significantly increased (P ≤ 0.05) squash’s fresh stem, leaf, and root weights by 14.70%, 18.63% and 11.13%, respectively, compared to the uninoculated control. Humic acid significantly (P ≤ 0.05) increased fresh stem, leaf, and root weights by 78.78%, 62.5% and 63.74%, respectively, compared to the control. Wheat straw also significantly increased (P ≤ 0.05) fresh stem, leaf, and root weights by 52.68%, 30.32% and 42.30%, respectively, compared to the control. Furthermore, the combinations of the treatments, including humic acid and straw, SSK-2018 and straw, and humic acid, SSK-2018 and straw, significantly increased the growth of squash. Soil properties including bulk density, moisture content, and basic cations were also significantly (P ≤ 0.05) improved by humic acid, SSK-2018 and wheat straw. This study suggests that like NPK, bio-organic treatments including SSK-2018, humic acid and straw, can equally improve plant’s fresh stem, leaf, and root weights as well as soil’s bulk density, moisture content, and basic cations (K+, Ca2+, Na+, and Mg2+). Therefore, the present study recommends the continuous use of bio-organic amendments to achieve a sustainable improvement in plant growth and soil properties under arid land conditions. However, the continuous use of soil bio-organic amendments should be regularly monitored for potential increase in greenhouse gas emissions that may be at par with that of N fertilizers.
1. Introduction
By 2050, projections show that the world will be a habitat for 9.8 billion people (United Nations Department of Economics and Social Affairs, UNDESA, Citation2017). To meet the increasing global food demand, developing a consortium of agricultural inputs that can enhance higher crop productivity, especially in plant growth-limiting environments like arid lands, is crucial (Bello et al., Citation2021). Attainment of optimal crop yield in arid lands is limited due to the predominantly sandy nature of the soils, harsh climatic conditions (hot, dry, and windy), salinity, and drought. Importantly, the soils are characteristically poor owing to their low cation exchange capacity, organic matter content, nutrients, water-holding capacity, and high evapotranspiration (Al-Omran et al., Citation2005). Particularly, in most regions in Saudi Arabia, there is a high scarcity of water for agricultural purposes due to very low rainfall amount which rarely exceeds 100 mm annually (Alkolibi, Citation2002). It has recently been reported that sustainable crop production in Saudi Arabia is majorly hampered by the harsh climate and water scarcity (Ma et al., Citation2022). Therefore, for an enhanced crop production in Saudi Arabia, it is imperative to develop effective farming practices that can promote organic matter accumulation with improved water conservation and soil structure over time. Effective farming practices involving the use of mineral fertilizers, organic amendments, and microbes can sustainably improve soil health and food production and reduce the negative impacts of agriculture on the environment (Cozzolino et al., Citation2021; George et al., Citation2016).
The utilization of organic materials like straw, humic acid, and manure can improve soil physicochemical properties, organic carbon, and microbial activities, and prevent eutrophication and groundwater pollution with inorganic fertilizers (Antonious et al., Citation2022). On addition to arid land soils and other soil types, humic substances (for example, humins, humic, and fulvic acids) act as soil conditioners that can control several processes including microbial activity, plant nutrition and growth, plant root initiation and emergence, soil aggregate stability, and nitrogen and carbon cycling in the agroecosystem (Dobbss et al., Citation2010; Piccolo et al., Citation2018; Sutradhar & Fatehi, Citation2023). The main sources of commercial humic acid are lignite, leonardite, peat and sometimes, sediments from rivers (Nieweś et al., Citation2022) and extraction from composted plant residues (Monda et al., Citation2018; Sutradhar & Fatehi, Citation2023). Furthermore, the inoculation of beneficial microbes alongside the application of soil organic amendments has a high potential in improving the plant-soil microbiome (Arif et al., Citation2020). Nutrient uptake and yield are promoted due to the integrated use of humic substances and beneficial soil microbes (Thomason et al., Citation2020).
Soil cellulolytic bacteria are groups of beneficial microbes that are capable of decomposing organic (cellulose-rich) materials such as wheat straw into nutrients that can be taken up by plants (Bello et al., Citation2022b and b). Enzymatic hydrolysis of cellulose by microorganisms is aided by the secretion of cellulase (Bhardwaj et al., Citation2021). Enzymes such as cellulase, amylase, phosphatase, dehydrogenase, invertase and urease are secreted by soil beneficial microbes to aid the mineralization of organic matter leading to the transformation of nutrients in organic form to inorganic form (Angelovicova et al., Citation2014; Veres et al., Citation2015). The presence of these microorganisms is a key indicator of improved soil health as they enhance higher nutrient uptake while integrating unused nutrients into the soil reservoir of nutrients—soil organic matter (Antonious et al., Citation2022). Cellulose decomposing microbes are also capable of plant growth-promotion and are good supplements to inorganic fertilizer application as they promote soil nutrient-use efficiency and nutrient cycling (Neumann & Römheld, Citation2012). Although these microorganisms are capable of decomposing plant residues, attention must be paid to carbon dioxide (CO2) emissions that may be given off during the decomposition process. Greenhouse gas emissions such as CO2, and methane (CH4) can occur during straw decomposition under field conditions due to the transformation of cellulose into soil organic matter (Allen et al., Citation2020; Sun et al., Citation2022; Weil & Brady, Citation2017).
Cucurbita pepo L. also commonly known as “squash” is a vegetable crop that is widely grown across the globe (Saudi Arabia inclusive) due to its versatility and adaptations to varying climatic conditions in relation with the different varieties (Abd El-Mageed et al., Citation2016; Bello et al., Citation2022b; Gichana et al., Citation2019). However, squash has a high need for adequate nutrients which is mostly met by the application of unsustainable, non-renewable, and non-enviro-friendly chemical fertilizers. The cultivation of squash is even more challenging under arid land conditions due to the poor nature of the soils and environmental conditions. Therefore, to seek for sustainable substitutes or supplements in the cultivation of squash in Saudi Arabia, this study investigated the effects of organic amendments (humic acid and wheat straw) and a soil beneficial bacterium (Geobacillus stearothermophilus SSK-2018) on squash growth and soil properties. G. stearothermophilus SSK-2018 have been previously described as a cellulose decomposing bacterium (CDB) and further probed in this study for its cellulose decomposing traits in the laboratory (Bello et al., Citation2022a). Under the arid conditions of the current study, humic acid can act as a biostimulant that can have immediate effect on plant growth and soil conditioning for improved crop yield. Likewise, unlike other types of beneficial soil bacteria, CDB only needs little nitrogen to biodegrade organic materials. In addition, the use of wheat straw in the current study can help preserve soil moisture and allow for the proliferation of beneficial soil microbes. Therefore, the overall aim of this study is to contribute knowledge and practices towards the enhancement of agroecosystem functioning and increased food security in an arid environment by employing microbial inoculants in conjunction with soil organic amendments.
2. Materials and methods
2.1. Field location, layout, and experimental design
In 2019/20 and 2020/21 planting seasons, two different field trials were conducted to evaluate the impacts of a cellulose decomposing bacterium (Geobacillus stearothermophilus SSK-2018), humic acid, and wheat straw on the growth of squash and soil properties of an arid land. The field trials were conducted at the Soil Science Field, King Abdulaziz University’s Research Farm, Hada Al-Sham, Saudi Arabia (Supplementary Figure S1). The field study was conducted using a split-split plot design replicated three times. The treatments’ arrangement in the field was completely randomized and had three rates of humic acid (0, 50, and 100 L ha−1) as the main plots, and two rates of bacterium inoculation (with and without) as the sub plots. At the sub-sub plots, there were three rates of wheat straw (0, 20 and 30 t ha−1 straw) and one rate of NPK 20:20:20 fertilizer at 600 kg ha−1. NPK 20:20:20 was added as a treatment in the sub-sub plots to allow for the evaluation of the performance of the treatments (bio-organic amendments) compared to the conventional practice of mineral fertilizer application.
2.2. Land preparation, planting, irrigation, and other cultural practices
The experimental site was prepared by first ploughing the land, then followed by harrowing, levelling, and marking out of the field into 72 plots according to the experimental layout (Bello et al., Citation2022b; Supplementary Figure S2). Planting was done by direct seeding using seeds of hybrid variety of Lebanese squash and the plant spacing was 1 m between rows and 0.5 m between plants, and each plot was 6 m2 with 12 plant stands. The study area being an arid environment with a range of 0 to 23.6 mm rainfall per month during the growing season, a drip irrigation system was deployed in providing water to the plants (Bello et al., Citation2022a). The field trials were monitored throughout the growing periods to ensure that sufficient water was available for plant’s uptake and weeds as well as pests were controlled appropriately.
2.3. Treatments application
The bio-organic amendments (humic acid, G. stearothermophilus, and wheat straw) were applied differently as required. Humic acid (made from leonardite) was applied by diluting the concentrated Humacareliquid, according to the given rates (50 and 100 L ha−1), in water before spraying on the respective plots. The composition of humic acid in Humacare was 12% (w/w) as supplied by the manufacturer. The humic acid application at each rate was made in four equal doses at 15 days interval each after planting. The humic acid used in the study was regarded as a biostimulant due to its overall effect on squash performance from our previous studies (Bello et al., Citation2022b and b). Wheat straw was incorporated into the soil at a depth of 0–30 cm about one month before planting was carried out. Wheat straw incorporation was immediately followed by bacterium inoculation to enhance faster decomposition of straw and establishment of the bacterium in the soil before planting (Supplementary Figure S3). The inoculum of G. stearothermophilus was prepared in the laboratory as described and contained 2.5 × 106 colony forming units at the time of inoculation. The field inoculation of G. stearothermophilus was made at a close range to the soil by spraying. Plots containing wheat straw were regularly watered and turned over to allow for easy decomposition and aeration to support microbial activities. NPK 20:20:20 fertilizer was applied in a similar way to humic acid. The chemical constituents of humic acid and wheat straw used in this study have been previously characterized in the studies of Bello et al. (Citation2022b) and b. In 2021, the same plots and field layout as 2020 was maintained to validate the effect of treatments over time.
2.4. Soil analysis
2.4.1. Initial soil analysis and properties
Samples were collected at 0 to 30 cm soil depth for physicochemical properties analyses according to the methods described by Pansu and Gautheyrou (Citation2006). The initial soil analyses included soil texture, electrical conductivity (EC), pH, bulk density (BD), particle density (PD), porosity, moisture content, soil organic matter (OM) as well as nitrogen, phosphorus (P2O5), potassium (K+), calcium (Ca2+), sodium (Na+) and magnesium (Mg2+). Some of the soil initial properties have been previously described in the study of Bello et al. (Citation2022b). The most probable number (MPN) of bacteria in the experimental soil was determined using the spread plate method. The initial soil physicochemical properties are as shown in (Table ).
Table 1. Physicochemical and biological properties of the experimental soil
2.4.2. Final soil analysis
Representative soil samples per treatment were taken and analyzed for pH, EC, soil moisture content, porosity, and basic cations (Ca, Mg, K and Na) according to the methods described by Pansu and Gautheyrou (Citation2006).
2.5. Laboratory characterization of cellulose decomposing bacterium (Geobacillus stearothermophilus SSK-2018)
2.5.1. Morphological characteristics
To study the morphological characters of G. stearothermophilus, a dilute cell suspension was prepared by suspending loopful of 3–5 days old culture in sterilized distilled water and streaked on nutrient agar (NA) plates. These plates were incubated at 24°C for 3–5 days and colony characters of bacteria viz., colour and shape were recorded.
2.5.2. Physiological and biochemical tests
The physiological and biochemical tests were performed with G. stearothermophilus. Physiological tests (motility, gram stain, and spore formation) were determined according to Bergey’s Manual of Determinative Bacteriology (Hensyl, Citation1994). Biochemical characterization like catalase test (He et al., Citation1983), cellulase, amylase, protease activities (Rodarte et al., Citation2011; Sands, Citation1990), gelatin liquefaction (Stapp (Citation1961), optimum pH test (Sands, Citation1990), casein hydrolysis (Cruickshank et al., Citation1975), esculin hydrolysis and urease test (Schaad et al., Citation2001), starch hydrolysis (Barrit, Citation1936) and phosphate solubilization, and indole acetic acid (IAA) production (Abo-Elyousr et al., Citation2019) were performed.
2.6. Statistical analysis
Collected data on fresh weights of leaf, stem, and root as well as some soil physicochemical properties were statistically analyzed using SAS 9.3 Software (SAS, 2011). Treatment means that were statistically different from each other were separated using LSD at the 95% significant level. The two-way interaction graphs of treatments were plotted with the aid of GraphPad Prism 9.3.1 software. The three-way interaction graphs were plotted using the R program (ggplot) 4.1.2 (R Core Team, Citation2021).
3. Results and discussion
3.1. Effect of treatments on squash growth
The development and growth of squash plants under field conditions requires adequate management and use of effective agricultural inputs (Supplementary Figure S4). The application of humic acid significantly (P ≤ 0.05) increased fresh stem, leaf, and root weights (Table ). 100 L ha−1 humic acid significantly increased fresh stem weight compared to the control and 50 L ha−1 of humic acid by 78.78% and 38.74%, respectively, in 2019/20 season and by 73.68% and 28.89%, respectively, in 2020/21 season. Similarly, humic acid at the rate of 100 L ha−1 significantly increased fresh root weight compared to the control and 50 L ha−1 of humic acid by 63.74% and 22.87%, respectively, in 2019/20 season and by 61.04% and 27.22%, respectively, in 2020/21 season. The application of humic acid has been reported to cause significant improvement in plant growth (Monda et al., Citation2018). Humic acid can induce changes in plant roots by enhancing the emergence of lateral roots and increasing root length and hair density (Nardi et al., Citation2017; Olivares et al., Citation2017). The inoculation of SSK-2018 in both seasons significantly (P ≤ 0.05) improved fresh stem, leaf, and root weights. In 2019/20 and 2020/21 seasons, fresh leaf weights were increased due to the inoculation of SSK-2018 by 18.63% and 21.29%, respectively compared to the control. Likewise, the inoculation of SSK-2018 significantly improved fresh root weights by 11.13% and 10.52%, respectively, compared to the control (uninoculated treatment) in 2019/20 and 2020/21 planting seasons. The inoculation of beneficial microbes can enhance plant growth by increasing nutrient availability for root uptake while causing positive significant changes in the root structure (Adesemoye & Kloepper, Citation2009; Hansen et al., Citation2020).
Table 2. Effect of treatments on the stem, leaf, and root fresh weights of squash during the 2019/20 and 2020/21 planting seasons
The application of wheat straw and NPK in the sub-sub plots significantly (P ≤ 0.05) improved fresh stem, leaf, and root weights compared to 0 t ha−1 straw (control treatment; Table ). Wheat is an exhaustive crop with high uptake of nutrients from the soil during growth (Akhtar et al., Citation2014). A significant portion of these nutrients may be locked up in the plant residues after grain harvest and made available for other plant’s use on decomposition in the soil environment. NPK application significantly increased fresh stem weight by 80.69%, 52.68% and 25.44% compared to 0 (control), 20, and 30 t ha−1 wheat straw, respectively, in 2019/20 season. However, the percentage of increment in fresh stem weight was lower in 2020/21 season compared to 2019/20 season. In 2019/20 and 2020/21 seasons, the application of NPK increased fresh leaf weight by 45.96% and 21.03%, respectively, compared to the control. Likewise, in 2019/20 and 2020/21 seasons, 30 t ha−1 straw significantly enhanced fresh leaf weight compared to the control by 15.73% and 15.24%, respectively. Plant residues like wheat straw are biodegradable agricultural by-products that can be used to sustainably improve fertility of soils for increased crop production (Nigussie et al., Citation2021).
3.2. Two-way interaction effects of treatments on fresh weights of squash
3.2.1. Humic acid and wheat straw or NPK interaction
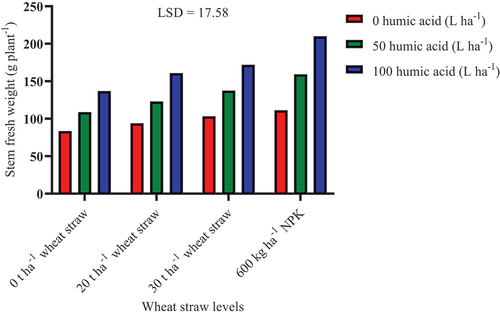
Humic acid application at 100 L ha−1 in combination with NPK and soil incorporation of straw at 30 and 20 t ha−1 significantly (P ≤ 0.05) improved fresh stem weight by 52.92%, 25%, and 17.22%, respectively, compared to the combination of 0 L ha−1 humic acid and 0 t ha−1 straw (control treatment; Figure ). Likewise, humic acid (50 L ha−1) in combination with NPK and wheat straw incorporation at 30 t ha−1 significantly improved fresh stem weight by 46.51% and 26.49%, respectively. Humic acid can promote the degradation of straw thereby enhancing the accumulation of soil organic matter for increased plant growth (Ghanney et al., Citation2023). However, without the application of humic acid, it was only NPK that significantly improved fresh stem weight than the control by 33.12%. This indicates that humic acid can work synergistically with both inorganic and organic sources of nutrients to improve plant growth. In addition to being a biostimulant that can directly support plant growth, humic acid application may also aid the decomposition of wheat straw by the soil native microbial population thereby resulting in a cumulative increase in growth.
3.2.2. Geobacillus stearothermophilus SSK-2018 and wheat straw or NPK interaction
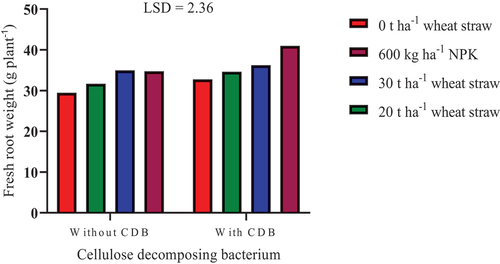
The inoculation of SSK-2018 combined with NPK application increased fresh root weight significantly (P ≤ 0.05) by 17.93% compared to NPK application without SSK-2018 inoculation (Figure ). Likewise, SSK-2018 inoculation in combination with 20 t ha−1 straw increased fresh root weight significantly by 9.28% compared to 20 t ha−1 straw without SSK-2018 inoculation. Furthermore, with SSK-2018 inoculation, the application of NPK improved fresh root weight compared to 30 and 20 t ha−1 of straw and sole SSK-2018 inoculation by 13.11%, 18.38% and 25.02%, respectively. These findings show the importance of synergy between SSK-2018 and straw or NPK in improving fresh root weight of squash over their sole application. The interaction of SSK-2018 with straw may improve the decomposition and mineralization of organic materials and biogeochemical cycling of nutrients that can lead to increased plant growth (Chaparro et al., Citation2012; Lou et al., Citation2011). NPK interaction with SSK-2018 in the rhizosphere can decrease the soil C:N ratio, to favour the activities of the bacterium in plant growth-promotion, in addition to direct uptake of N by plant’s root.
3.3. Effect of three-way interaction of treatments on fresh leaf weight of squash
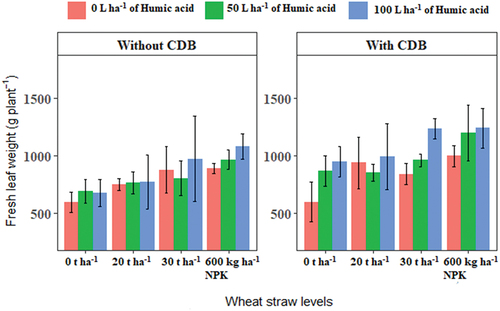
Results obtained show that interaction of humic acid, SSK-2018 and wheat straw increased the fresh leaf weight of squash significantly at P ≤ 0.05 (Figure ). The inoculation of SSK-2018 in the presence of the combination of 600 kg ha−1 NPK with 100 L ha−1 of humic acid application was the most effective treatments interaction with significant impact on leaf weight. This effectiveness was followed by that of the combination of SSK-2018 with 100 L ha−1 of humic acid and 30 t ha−1 of wheat straw. The growth of wheat has also been shown to be significantly improved by the integrated inoculation of Pseudomonas plecoglossicida 2,4-D with humic acid application (Feoktistova et al., Citation2022). Similarly, the growth of maize was highly improved when humic acid was jointly applied with Rhizobium cellulosilyticum or Burkholderia gladioli which are both plant growth-promoters (Melo et al., Citation2018). Humic acid may contain up to 4% nitrogen, 57% organic carbon and 1% phosphorus which is sufficient to support optimal plant growth in the presence of other organic inputs (Akhtar et al., Citation2014). Moreover, in the presence of CDB, most of the treatment combinations outperformed those without CDB inoculation. A recent study has established that the use of bio-organic amendments composing of biochar mixed with organic materials such as sewage sludge, vermicompost, manure and organic fertilizer had significant effects on squash growth, and soil properties (Antonious et al., Citation2022). Another study has also indicated that the joint application of humic acid, a soil bacterium (Pseudomonas fluorescens) and fertilizers, sustainably improved leaf biomass and other growth and yield parameters of cabbage (Verma et al., Citation2014).
3.4. Characterization of cellulose decomposing bacterium
Increased crop yield and soil fertility from traditional agricultural practices involving the application of inorganic and organic fertilizers can be sustainably attained via the introduction of beneficial soil microbes (Cozzolino et al., Citation2021). Table presents a summary of the characterization of the CDB used in this study. G. stearothermophilus SSK-2018 is a gram-positive bacterium (Supplementary Figure S5) with positive reactions for cellulase activity, starch hydrolysis, amylase activity, protease activity, casein hydrolysis and phosphate solubilization (Supplementary Figure S6 a-f). The components of cellulases responsible for cellulose hydrolysis include endoglucanases—breakdown the strong β-glycosidic bonds to give smaller chains of cellulose with free ends, cellobiohydrolases (exoglucanases)—breakdown cellulose into cellobiose, and β-glucosidase—breakdown cellobiose into glucose monomers (Esteghlalian et al., Citation2002). The test for gelatin liquefaction was also positive and the bacterium (G. stearothermophilus) also showed the potential for IAA production. Thus, G. stearothermophilus SSK-2018 was regarded as a beneficial soil microbe with the capability for plant growth-promotion.
Table 3. Morphological, physiological, and enzymatic characterization of Geobacillus stearothermophilus SSK-2018
3.5. Soil analysis
3.5.1. Soil pH, bulk density, electrical conductivity, gravimetric moisture content and porosity
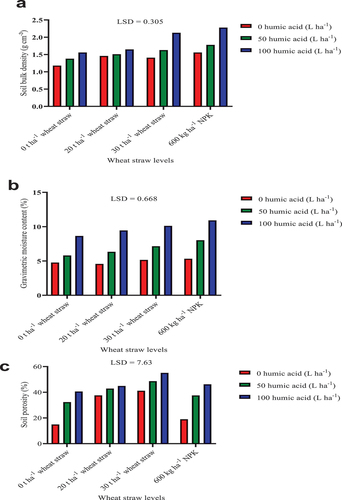
The soil analysis results presented in this study are those of the 2020/21 planting season. The application of humic acid did not significantly (P ≤ 0.05) affect soil pH, and EC, and CDB inoculation also did not significantly (P ≤ 0.05) had effect on the soil pH (Table ). However, soil pH was observed to be significantly higher in the 600 kg ha−1 NPK and 0 t ha−1 wheat straw (control) plots compared to the 30 and 20 t ha−1 wheat straw applied plots. This is contrary to the study of Jia et al. (Citation2022) where NPK was found to decrease soil pH in arid regions of China. The lower soil pH in wheat straw applied plots may be due to the release of ammonia (NH3) during straw decomposition which reacts with soil water vapour to form ammonium hydroxide (NH4OH) that increases soil acidity (Ghanney et al., Citation2023; Ogunwande et al., Citation2008). Although there were no significant differences in the EC obtained from the wheat straw treatments, it is expected that the decomposition of straw may result in the release of mineral salts that can increase soil EC (Ghanney et al., Citation2023). Likewise, CDB inoculation had significant effect on the soil EC and BD. It has been suggested that microbial inoculants are promising tools in the improvement of agroecosystem functioning for increased food security (Bello et al., Citation2018; Calvo et al., Citation2014; Hansen et al., Citation2020). Lower soil EC and BD are positive indicators of soil quality that can enhance plant growth under arid land conditions. The soil BD was also significantly higher in the respective control plots compared to the individual treatments including humic acid and wheat straw. Plots with 100 L ha−1 humic acid treatment had an average BD of 1.40 g cm−3 which was statistically significantly (P ≤ 0.05) lower than the average BD of 1.90 g cm−3 obtained in the control plots. Soils with lower BD enhance quick emergence of plant seedlings and increased proliferation of the root system through the rhizosphere. Expectedly, wheat straw incorporation significantly reduced soil BD compared to NPK application and 0 t ha−1 wheat straw. Soil incorporation of straw can improve soil fertility with an increased humus content and aggregate stability (Yang et al., Citation2020; Zhang et al., Citation2014).
Table 4. Effect of treatments on soil pH, electrical conductivity, bulk density, gravimetric moisture content, and porosity in 2020/21 planting season
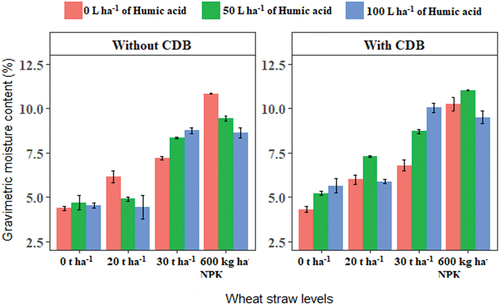
Soil gravimetric moisture content and porosity were improved (P ≤ 0.05) significantly by humic acid, SSK-2018 and wheat straw (Table ). 100 L ha−1 humic acid significantly improved soil moisture content by 97.38% and 43.13% compared to 0 L ha−1 humic acid (control) and 50 L ha−1 humic acid, respectively. The application of humic acid may reduce soil water evaporation thereby enhancing higher soil moisture content in the rhizosphere (Goel & Dhingra, Citation2021). Similarly, soil incorporation of 30 t ha−1 wheat straw improved soil moisture content by 26.01% and 18.97% compared to 0 t ha−1 of straw (control) and NPK, respectively. Soil porosity was improved significantly (P ≤ 0.05) by the application of 50 and 100 L ha−1 humic acid. Likewise, SSK-2018 inoculation significantly (P ≤ 0.05) improved soil porosity by 29.18% than the uninoculated treatment. The incorporation of 30 t ha−1 wheat straw significantly improved soil porosity by 64.96% and 41.09%, respectively, compared to the control and NPK application. Similarly, soil incorporation of 20 t ha−1 straw significantly (P ≤ 0.05) increased soil porosity by 42.75% and 22.09%, respectively, compared to 0 t ha−1 straw (control) and NPK application.
3.5.2. Interaction effect of humic acid and Geobacillus stearothermophilus SSK-2018 on soil moisture content
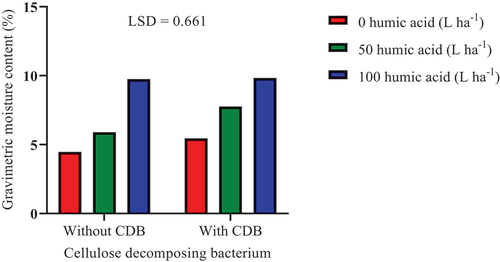
The inoculation of SSK-2018 in conjunction with humic acid (100 L ha−1) significantly (P ≤ 0.05) improved soil moisture content by 120.13% and 80.55%, respectively, compared to the control (0 L ha−1 humic acid without SSK-2018 inoculation) and the sole application of 100 L ha−1 humic acid without SSK-2018 inoculation (Figure ). Furthermore, 50 L ha−1 humic acid application in combination with SSK-2018 inoculation significantly improved soil moisture content than the control (0 L ha−1 humic acid without SSK-2018 inoculation) and the sole application of 50 L ha−1 humic acid without SSK-2018 inoculation. Humic substances can act as a media for microbial inoculant proliferation in the soil (Canellas & Olivares, Citation2014). Humic acid contains bioactive substances (for example, auxin) that can further promote the effectiveness of beneficial microbes in plant growth-promotion (Cozzolino et al., Citation2021).
3.5.3. Effect of three-way interaction of treatments on soil moisture content
The concept of jointly using organic and inorganic fertilizers or amendments with the inclusion of plant growth promoting microbes in crop cultivation and management of soil resources is currently a global practice (Bello & Yusuf, Citation2021; Vanlauwe et al., Citation2010). In the current study, the three-way interaction of the treatments significantly (P ≤ 0.05) impacted the gravimetric soil moisture content (Figure ). Optimal soil moisture is essential for easy dissolution of soil nutrients, proliferation of roots in the rhizosphere and accelerated plant growth (Lee et al., Citation2017). The applications of 50 L ha−1 humic acid and 600 kg ha−1 NPK combined with SSK-2018 inoculation significantly improved soil moisture content compared to all other treatment combinations with SSK-2018 inoculation. This was followed by the applications of 100 L ha−1 humic acid and 30 t ha−1 of wheat straw combined with SSK-2018 inoculation. In the presence of SSK-2018 inoculation and 20 t ha−1 straw, 50 L ha−1 humic acid improved soil moisture content significantly (P ≤ 0.05) compared to the control and 100 L ha−1 humic acid. Overall, treatments interaction involving humic acid in combination with SSK-2018 and straw outperformed those treatments without humic acid application in the combination. The obtained results indicate that there is significant synergy among the treatments (humic acid, CDB and wheat straw) in the improvement of soil moisture which is a key constraint to crop growth under arid land conditions.
3.5.4. Effect of three-way interaction of treatments on soil pH
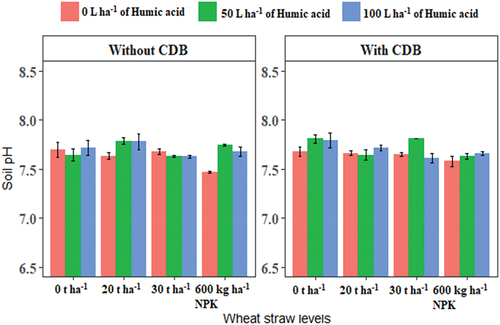
The three-way interaction effects of imposed treatment on soil pH were highly variable (Figure ). With SSK-2018 inoculation and 100 L ha−1 humic acid application, NPK application led to a significantly (P ≤ 0.05) higher soil pH. NPK in conjunction with manure application have been demonstrated to increase soil pH where NPK application alone decreased it (Jia et al., Citation2022). Likewise, in the current study, in the absence of SSK-2018 inoculation and humic acid application, NPK application resulted in a significantly reduced soil pH than all other treatments combination. This is expected as N containing fertilizers are known with soil acidification through reduction in the soil pH. In the presence of SSK-2018 inoculation and 50 L ha−1 humic acid, 30 t ha−1 wheat straw incorporation increased the soil pH compared to the control and 100 L ha−1 humic acid. This observation was, however, reversed in the absence of SSK-2018 inoculation where the control (0 L ha−1 humic acid) significantly increased the soil pH compared to 50 and 100 L ha−1 humic acid. Microbial degradation of straw may result in ammonification which produces ammonium ion (NH4+) that are alkaline in nature and can cause the soil pH to increase (Ghanney et al., Citation2023).
3.5.5. Interaction effects of humic acid and wheat straw on soil physicochemical properties
The interaction of humic acid and wheat straw had significant (P ≤ 0.05) effect on soil BD, gravimetric moisture content, and porosity (Figure ). The interactions of 50 L ha−1 humic acid with either NPK, 30 and 20 t ha−1 of straw reduced the soil pH significantly compared to the interactions of 100 L ha−1 humic acid with either NPK or straw (Figure ). However, the control treatment (0 L ha−1 humic acid and 0 t ha−1 straw) had significantly lowered soil BD compared to treatments that received the different rates of humic acid and wheat straw. Similarly, 100 L ha−1 humic acid interaction with either NPK or 20 and 30 t ha−1 of straw improved gravimetric moisture content significantly by 128.66%, 111.92% and 97.91%, respectively, compared to the control treatment (Figure ). The interaction of 100 L ha−1 humic acid with either NPK or 20 and 30 t ha−1 of wheat straw significantly improved soil porosity by 208.75%, 200.47%, and 268.27%, respectively, compared to the combination 0 L ha−1 humic acid and 0 t ha−1 wheat straw (control) (Figure ). Furthermore, 100 L ha−1 humic acid application combined with either NPK or wheat straw application at 20 and 30 t ha−1 significantly improved soil porosity by 143.14%, 136.61%, and 190.01%, respectively, compared to NPK application alone without humic acid. Gravimetric moisture content and porosity are key indicators of water availability for plant uptake. The ability of the treatments to improve soil moisture and porosity is an important process in the survival of plants under arid conditions. When water is unavailable for plant uptake, leave and shoot growth are severely impacted (Hsiao & Jackson, Citation1999).
3.6. Soil basic cations (calcium, magnesium, potassium, and sodium)
3.6.1. Effect of treatments on soil basic cations
The effects of treatments on soil exchangeable cations (Ca2+, Mg2+, K+ and Na+) are as shown in Table . Most of the results obtained from the individual treatments followed the same trend where humic acid, CDB and straw all had significant (P ≤ 0.05) effect on the soil exchangeable soil cations (Ca, Mg and K) except Na. The application of humic acid at 100 L ha−1 significantly increased Ca content in the soil compared to 0 (control) and 50 L ha−1 humic acid by 95.31% and 52.78%, respectively. The application of humic substances has been suggested to aid the transport of nutrients from soils to the plant root for uptake (Goel & Dhingra, Citation2021). Furthermore, the inoculation of CDB increased soil exchangeable Ca by 13.90% compared to the uninoculated control treatment. 30 t ha−1 wheat straw incorporation increased soil exchangeable Ca significantly (P ≤ 0.05) by 28.58%, 16.21%, and 7.99% compared to 0 t ha−1 of straw (control), NPK, and 20 t ha−1 straw, respectively. In addition, 20 t ha−1 straw incorporation increased soil exchangeable Ca significantly by 19.07% compared to 0 t ha−1 of straw (control).
Table 5. Effect of treatments on soil basic cations in 2020/21 planting season
For soil exchangeable Mg, the application of 100 L ha−1 humic acid increased it by 55.34% and 23.05%, respectively, compared to the control and 50 L ha−1 humic acid (Table ). Wheat straw incorporation at 30 t ha−1 increased soil exchangeable Mg significantly (P ≤ 0.05) by 20.12%, 11.69% and 5.51% compared to 0 t ha−1 straw (control), NPK, and 20 t ha−1 straw, respectively. In addition, 20 t ha−1 straw incorporation increased soil exchangeable Mg significantly (P ≤ 0.05) by 13.83% compared to 0 t ha−1 straw (control). Easily decomposable constituents of straw such as proteins and sugars stimulate soil bacterial activities which can enhance the availability of soil nutrients (Paterson et al., Citation2011).
3.6.2. Two-way interaction effect of treatments on soil basic cations
3.6.2.1. Cellulose decomposing bacterium and humic acid
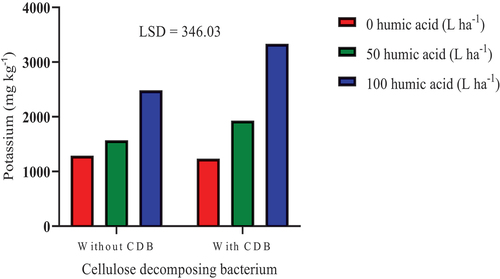
The combination of CDB inoculation with 100 L ha−1 humic acid increased soil exchangeable K significantly (P ≤ 0.05) by 170.01% and 158.77%, respectively, compared to sole inoculation of CDB and the control (Figure ). Furthermore, CDB inoculation in conjunction with 100 L ha−1 humic acid increased K significantly by 34.30% compared to sole application of 100 L ha−1 humic acid. Moreover, CDB inoculation combined with 100 L ha−1 humic acid application increased soil exchangeable K significantly (P ≤ 0.05) by 72.82% compared to CDB inoculation combined with 50 L ha−1 humic acid. The application of humic acid combined with the inoculation of some beneficial microbes (Bacillus amyloliquefaciens and Pseudomonas spp.) have been demonstrated to improve nutrient uptake in a potted soil experiment (Cozzolino et al., Citation2021).
3.6.2.2. Humic acid and wheat straw or NPK
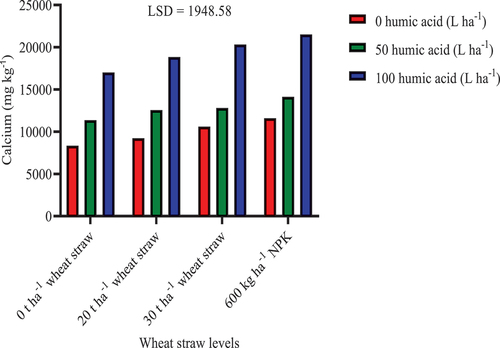
The combination of humic acid (100 L ha−1) with NPK, 20 t ha−1, and 30 t ha−1 wheat straw significantly increased soil exchangeable Ca by 157.17%, 125.85%, and 143.46%, respectively, compared to the control (Figure ). Furthermore, the combination of humic acid (100 L ha−1) with NPK, 20 t ha−1, and 30 t ha−1 wheat straw significantly increased Ca by 102.8%, 77.68%, and 91.53%, respectively, compared to the sole application of 30 t ha−1 wheat straw. Similarly, 100 L ha−1 humic acid with NPK, 20 t ha−1, and 30 t ha−1 wheat straw increased Ca significantly by 85.50%, 62.52%, and 75.19%, respectively, compared to the sole application of NPK. The study of Akhtar et al. (Citation2014) has also demonstrated that combined humic acid and wheat straw application is capable of soil nutrients availability.
3.6.2.3. Geobacillus stearothermophilus SSK-2018 and wheat straw or NPK
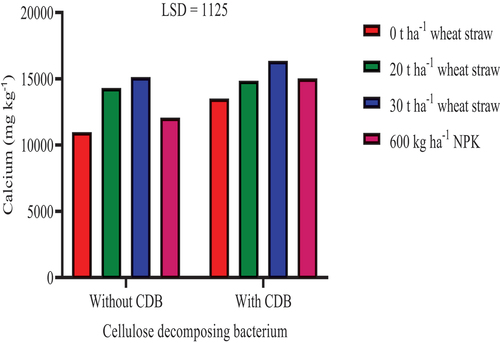
The inoculation of SSK-2018 in conjunction with either the application of NPK or wheat straw increased soil exchangeable Ca significantly at P ≤ 0.05 (Figure ). The inoculation of SSK-2018 in conjunction with either the application of NPK, or 20, and 30 t ha−1 straw increased soil exchangeable Ca significantly by 25.39%, 24.57%, and 35.58%, respectively, compared to SSK-2018 inoculation without wheat straw application. The integrated application of straw with bacteria can improve soil quality and provide soil with resistance to diseases by suppressing the survival of pathogenic microorganisms in the rhizosphere which may hinder plant growth (Liu et al., Citation2021). In an arid environment in China, the application of straw alone did not improve soil properties (increased soil aggregate stability and soil organic matter accumulation). This was, however, reversed when Bacillus amyloliquefaciens was co-applied with straw (Liu et al., Citation2021).
3.6.3. Effect of three-way interaction of treatments on soil exchangeable magnesium (Mg)
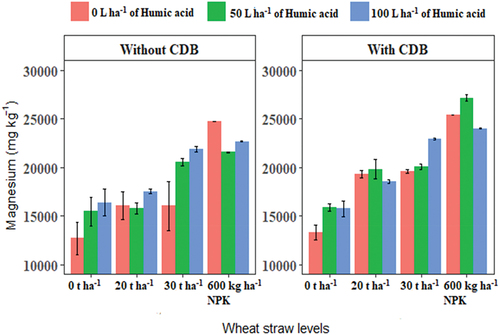
Humic acid, SSK-2018 and wheat straw combination significantly (P ≤ 0.05) improved Mg availability in the soil (Figure ). 50 L ha−1 humic acid and NPK combined with SSK-2018 inoculation increased soil Mg significantly (P ≤ 0.05) compared to all other treatment combinations. The inoculation of plant growth-promoting bacteria alongside the application of humic acid has been shown to boost nutrient uptake by plant roots (Melo et al., Citation2018; Pishchik et al., Citation2016). 30 t ha−1 straw incorporation in combination with 100 L ha−1 humic acid and SSK-2018 inoculation increased soil exchangeable Mg significantly compared to 0 t ha−1 (control) and 20 t ha−1 of wheat straw. The combination of humic acid and CDB with wheat straw soil incorporation may result in faster mineralization of wheat straw through the acidification of the rhizosphere as both humic acid and CDB can cause the plant to secrete IAA (Chaiharn & Lumyong, Citation2011). Humic acid in addition to organic acid released during CDB degradation of wheat straw may promote the availability of residual soil nutrients. The combined application of humic-rich organic amendments with the inoculation of plant beneficial microbes improves soil microbial diversity, crop nutrient uptake, growth, and yield (Cozzolino et al., Citation2021; Maji et al., Citation2017). Due to the positive impact of humic acid on plant roots, humic acid may influence higher microbial colonization of plants to aid an extensive root system capable of tapping available nutrients in the rhizosphere compared to inoculated plants without humic acid application. Overall, humic acid efficiency in this study may be attributed to the presence of active substances (phytohormones) which can be utilized directly by plants while simultaneously promoting soil conditioning through enhancing the proliferation of beneficial microbes for increased mineralization of wheat straw and improved squash performance.
4. Conclusion
In the current study, the effects of humic acid, G. stearothermophilus SSK-2018 and wheat straw were evaluated on squash growth and soil properties. G. stearothermophilus SSK-2018 was a cellulose decomposing bacterium (CDB) that enhances plant growth through the production of indole-3-acetic acid, along with various enzymatic activities including cellulase, amylase, protease, gelatinase, and phosphatase. This study demonstrated that G. stearothermophilus SSK-2018, humic acid, and wheat straw significantly improved the fresh weights of stems, leaves, and roots compared to the control. Furthermore, the combinations of treatments involving humic acid and straw, SSK-2018 and straw, as well as humic acid, SSK-2018, and straw, significantly boost squash growth. The study also demonstrated that soil properties, including bulk density, moisture content, and basic cations, were significantly improved by humic acid, SSK-2018, and wheat straw and their different combinations. However, the improvements obtained from the treatments were at par with that of NPK fertilizer application. Therefore, this study suggests that bio-organic amendments should be used consistently for a longer period (more than two seasons) to sustainably improve soil properties that would lead to significant crop performance compared to the use of inorganic fertilizer. However, the continuous use of soil bio-organic amendments should be regularly monitored for potential increase in greenhouse gas emissions that may be like that of nitrogen-containing fertilizers. This would mean that the use of bio-organic amendments should be closely monitored for improved straw management practices that can reduce the emissions of CO2 and CH4 without any negative effect on crop growth and yield.
Supplemental Material
Download MS Word (5.6 MB)Acknowledgments
This research work was funded by Institutional Fund Projects under grant no. (IFPIP: 685-155-1443). The authors gratefully acknowledge technical and financial support provided by the Ministry of Education and King AbdulAziz University, DSR, Jeddah, Saudi Arabia. Notes on contributors Suleiman Kehinde Bello had his PhD in Field Crops and Soil Science from the Department of Arid Land Agriculture, Faculty of Environmental Sciences, King Abdulaziz University, Jeddah, Saudi Arabia. Samir Gamil Al-Solaiman is a Professor of Soil Chemistry and Fertility at the Department of Arid Land Agriculture, Faculty of Environmental Sciences, King Abdulaziz University, Jeddah, Saudi Arabia. Kamal Ahmed Mohamed Abo-Elyousr is a Professor of Plant Pathology at the Department of Arid Land Agriculture, Faculty of Environmental Sciences, King Abdulaziz University, Jeddah, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/23311932.2023.2246182.
Additional information
Funding
References
- Abd El-Mageed, T. A., Semida, W. M., & Abd El-Wahed, M. H. (2016). Effect of mulching on plant water status, soil salinity and yield of squash under summer-fall deficit irrigation in salt affected soil. Agricultural Water Management, 173, 1–20. https://doi.org/10.1016/j.agwat.2016.04.025
- Abo-Elyousr, K. A. M., Khalil Bagy, H. M. M., Hashem, M., Alamri, S. A. M., & Mostafa, Y. S. (2019). Biological control of the tomato wilt caused by Clavibacter michiganensis subsp. michiganensis using formulated plant growth-promoting bacteria. Egyptian Journal of Biological Pest Control, 29(1), 54. https://doi.org/10.1186/s41938-019-0152-6
- Adesemoye, A. O., & Kloepper, J. W. (2009). Plant–microbes interactions in enhanced fertilizer-use efficiency. Applied Microbiology and Biotechnology, 85(1), 1–12. https://doi.org/10.1007/s00253-009-2196-0
- Akhtar, K., Shah, S. N. M., Ali, A., Zaheer, S., Wahid, F., Khan, A., Shah, M., Bibi, S., & Majid, A. (2014). Effects of humic acid and crop residues on soil and wheat nitrogen contents. American Journal of Plant Sciences, 5(9), 1277–1284. https://doi.org/10.4236/ajps.2014.59141
- Alkolibi, F. M. (2002). Possible effects of global warming on agriculture and water resources in Saudi Arabia: Impacts and responses. Climate Change, 54(1/2), 225–245. https://doi.org/10.1023/A:1015777403153
- Allen, J., Pascual, K. S., Romasanta, R. R., Trinh, M. V., Thach, T. V., Hung, N. V., Sander, B. O., & Chivenge, P. (2020). Rice straw management effects on greenhouse gas emissions and mitigation options. In M. Gummert, N. Hung, P. Chivenge, & B. Douthwaite (Eds.), Sustainable rice straw management (pp. 145–159). Springer International Publishing. https://doi.org/10.1007/978-3-030-32373-8_9
- Al-Omran, A. M., Sheta, A. S., Falatah, A. M., & Al-Harbi, A. R. (2005). Effect of drip irrigation on squash (Cucurbita pepo) yield and water-use efficiency in sandy calcareous soils amended with clay deposits. Agricultural Water Management, 73(1), 43–55. https://doi.org/10.1016/j.agwat.2004.09.019
- Angelovicova, L., Lodenius, M., Tulisalo, E., & Fazekasova, D. (2014). Effect of heavy metals on soil enzyme activity at different field conditions in middle spis mining area (Slovakia). Bulletin of Environmental Contamination and Toxicology, 93(6), 670–675. https://doi.org/10.1007/s00128-014-1397-0
- Antonious, G. F., Dawood, M. H., Turley, E. T., & Trivette, T. G. (2022). Soil amendments enhanced summer squash yield, fruit composition, quality, and soil enzymes activity. Agricultural Science, 13(06), 684–701. https://doi.org/10.4236/as.2022.136045
- Arif, I., Batool, M., & Schenk, P. M. (2020). Plant microbiome engineering: Expected benefits for improved crop growth and resilience. Trends in Biotechnology, 38(12), 1385–1396. https://doi.org/10.1016/j.tibtech.2020.04.015
- Barrit, M. M. (1936). The intensification of the Voges-Proskauer reaction by the addition of α-naphthol. Journal of Pathology and Bacteriology, 42(2), 441. https://doi.org/10.1002/path.1700420212
- Bello, S. K., Alayafi, A. H., AL-Solaimani, S. G., & Abo-Elyousr, K. A. (2021). Mitigating soil salinity stress with gypsum and bio-organic amendments: A review. Agronomy, 11(9), 1735. https://doi.org/10.3390/agronomy11091735
- Bello, S. K., AL-Solaimani, S. G., & Abo-Elyousr, K. A. (2022a). The effect of bio-organic amendments on the fruit weight and quality of summer squash under arid land conditions. Gesunde Pflanzen, 75(4), 1–15. https://doi.org/10.1007/s10343-022-00802-3
- Bello, S. K., Al-Solaimani, S. G., & Abo-Elyousr, K. A. (2022b). Squash yield, water-use efficiency and nitrate accumulation as influenced by the application of humic acid, Geobacillus stearothermophilus SSK-2018 and wheat straw in an arid land condition. Horticulturae, 8(7), 588. https://doi.org/10.3390/horticulturae8070588
- Bello, S. K., & Yusuf, A. A. (2021). Phosphorus influences the performance of mycorrhiza and organic manure in maize production. Journal of Plant Nutrition, 44(5), 679–691. https://doi.org/10.1080/01904167.2020.1849295
- Bello, S. K., Yusuf, A. A., & Cargele, M. (2018). Performance of cowpea as influenced by native strain of rhizobia, lime and phosphorus in Samaru, Nigeria. Symbiosis, 75(3), 167–176. https://doi.org/10.1007/s13199-017-0507-2
- Bhardwaj, N., Kumar, B., Agrawal, K., & Verma, P. (2021). Green biomimetic synthesis of Ag–TiO2 nanocomposite using Origanum majorana leaf extract under sonication and their biological activities. Bioresources and Bioprocessing, 8(1), 1–34. https://doi.org/10.1186/s40643-020-00357-z
- Calvo, P., Nelson, L., & Kloepper, J. W. (2014). Agricultural uses of plant biostimulants. Plant and Soil, 383(1–2), 3–41. https://doi.org/10.1007/s11104-014-2131-8
- Canellas, L. P., & Olivares, F. L. (2014). Physiological responses to humic substances as plant growth promoter. Chemical and Biological Technologies in Agriculture, 1(1), 1–11. https://doi.org/10.1186/2196-5641-1-3
- Chaiharn, M., & Lumyong, S. (2011). Screening and optimization of indole-3-acetic acid production and phosphate solubilization from rhizobacteria aimed at improving plant growth. Current Microbiology, 62(1), 173–181. https://doi.org/10.1007/s00284-010-9674-6
- Chaparro, J. M., Sheflin, A. M., Manter, D. K., & Vivanco, J. M. (2012). Manipulating the soil microbiome to increase soil health and plant fertility. Biology and Fertility of Soils, 48(5), 489–499. https://doi.org/10.1007/s00374-012-0691-4
- Cozzolino, V., Monda, H., Savy, D., DiMeo, V., Vinci, G., & Smalla, K. (2021). Cooperation among phosphate-solubilizing bacteria, humic acids and arbuscular mycorrhizal fungi induces soil microbiome shifts and enhances plant nutrient uptake. Chemical and Biological Technologies in Agriculture, 8(1), 31. https://doi.org/10.1186/s40538-021-00230-x
- Cruickshank, R., Duguid, J. P., Marmion, B. P., & Swain, R. H. A. (1975). Medical Microbiology (12 Ed.). (Vol. 2). Churchill Livingstone Publishing Company:
- Dobbss, L. B., Pasqualoto Canellas, L., Lopes Olivares, F., Oliveira Aguiar, N., Peres, L. E. P., Azevedo, M., Spaccini, R., Piccolo, A., & Façanha, A. R. (2010). Bioactivity of chemically transformed humic matter from vermicompost on plant root growth. Journal of Agricultural and Food Chemistry, 58(6), 3681–3688. https://doi.org/10.1021/jf904385c
- Esteghlalian, A. R., Mansfield, S. D., & Saddler, J. N. (2002). Cellulases: agents for fiber modification or bioconversion? The effect of substrate accessibility on cellulose enzymatic hydrolyzability. In L. Viikari & R. Lantto (Eds.), Progress in biotechnology (Vol. 21, pp. 21–36). Elsevier. https://doi.org/10.1016/S0921-0423(02)80005-3
- Feoktistova, A., Bakaeva, M., Timergalin, M., Chetverikova, D., Kendjieva, A., Rameev, T., Hkudaygulov, G., Nazarov, A., Kudoyarova, G., & Chetverikov, S. (2022). Effects of humic substances on the growth of Pseudomonas plecoglossicida 2, 4-D and wheat plants inoculated with this strain. Microorganisms [Internet], 10(5), 1066. https://doi.org/10.3390/microorganisms10051066
- George, T. S., Hinsinger, P., & Turner, B. L. (2016). Phosphorus in soils and plants–facing phosphorus scarcity. Plant and Soil, 401(1–2), 1–6. https://doi.org/10.1007/s11104-016-2846-9
- Ghanney, P., Yeboah, S., Anning, D. K., Yang, H., Wang, Y., & Qiu, H. (2023). Moisture-induced effects on lignocellulosic and humification fractions in aerobically composted straw and manure. Fermentation, 9(6), 551. https://doi.org/10.3390/fermentation9060551
- Gichana, Z., Liti, D., Wakibia, J., Ogello, E., Drexler, S., Meulenbroek, P., Ondiba, R., Zollitsch, W., & Waidbacher, H. (2019). Efficiency of pumpkin (Cucurbita pepo), sweet wormwood (Artemisia annua) and amaranth (Amaranthus dubius) in removing nutrients from a smallscale recirculating aquaponic system. Aquaculture International, 27(6), 1767–1786. https://doi.org/10.1007/s10499-019-00442-x
- Goel, P., & Dhingra, M. (2021). Humic substances: prospects for use in agriculture and medicine. In A. Makan (Ed.), Humic substances (pp. 1–21). IntechOpen.
- Hansen, V., Bonnichsen, L., Nunes, I., Sexlinger, K., Lopez, S. R., van der Bom, F. J. T., Nybroe, O., Nicolaisen, M. H., & Jensen, L. S. (2020). Seed inoculation with Penicillium bilaiae and Bacillus simplex affects the nutrient status of winter wheat. Biology and Fertility of Soils, 56(1), 97–109. https://doi.org/10.1007/s00374-019-01401-7
- Hensyl, W. R. (1994). Bergey’s Manual of Systematic Bacteriology, 9th ed. Holt J. G. and Williams S. T., and Williams Wilkins.
- He, L. Y., Sequeira, L., & Kelman, A. (1983). Characteristics of strains of Pseudomonas solanacearum from China. Plant Disease, 67(12), 1357–1361. https://doi.org/10.1094/PD-67-1357
- Hsiao, T. C., & Jackson, R. B. (1999). Interactive effects of water stress and elevated CO2 on growth, photosynthesis, and water-use efficiency. In Carbon dioxide and environmental stress (pp. 3–31). Academic Press. https://doi.org/10.1016/B978-012460370-7/50002-4
- Jia, S., Yuan, D., Li, W., He, W., Raza, S., Kuzyakov, Y., Zamanian, K., & Zhao, X. (2022). Soil chemical properties depending on fertilization and management in china: A meta-analysis. Agronomy, 12(10), 2501. https://doi.org/10.3390/agronomy12102501
- Lee, E. P., Han, Y. S., Lee, S. I., Cho, K. T., Park, J. H., & You, Y. H. (2017). Effect of nutrient and moisture on the growth and reproduction of Epilobium hirsutum L., an endangered plant. Journal of Ecology and Environment, 41(1), 35. https://doi.org/10.1186/s41610-017-0054-z
- Liu, H., Qi, Y., Wang, J., Jiang, Y., & Geng, M. (2021). Synergistic effects of crop residue and microbial inoculant on soil properties and soil disease resistance in a Chinese Mollisol. Scientific Reports, 11(1), 24225. https://doi.org/10.1038/s41598-021-03799-3
- Lou, Y. L., Xu, M. G., Wang, W., Sun, X. L., & Zhao, K. (2011). Return rate of straw residue affects soil organic C sequestration by chemical fertilization. Soil and Tillage Research, 113(1), 70–73. https://doi.org/10.1016/j.still.2011.01.007
- Maji, D., Misra, P., Singh, S., & Kalra, A. (2017). Humic acid rich vermicompost promotes plant growth by improving microbial community structure of soil as well as root nodulation and mycorrhizal colonization in the roots of Pisum sativum. Applied Soil Ecology, 110, 97–108. https://doi.org/10.1016/j.apsoil.2016.10.008
- Ma, C., Johansen, K., & McCabe, M. F. (2022). Monitoring irrigation events and crop dynamics using Sentinel-1 and Sentinel-2 time series. Remote Sensing, 14(5), 1205. https://doi.org/10.3390/rs14051205
- Melo, R. O. D., Oliveira, H. P. D., Silveira, K. C., Baldotto, L. E. B., & Baldotto, M. A. (2018). Initial performance of maize in response to humic acids and plant growth-promoting bacteria. Revista Ceres, 65(3), 271–277. https://doi.org/10.1590/0034-737x201865030007
- Monda, H., Cozzolino, V., Vinci, G., Drosos, M., Savy, D., & Piccolo, A. (2018). Molecular composition of the Humeome extracted from different green composts and their biostimulation on early growth of maize. Plant and Soil, 429(1–2), 407–424. https://doi.org/10.1007/s11104-018-3642-5
- Nardi, S., Ertani, A., & Francioso, O. (2017). Soil–root cross‐talking: The role of humic substances. Journal of Plant Nutrition and Soil Science, 180(1), 5–13. https://doi.org/10.1002/jpln.201600348
- Neumann, G., & Römheld, V. (2012). Rhizosphere chemistry in relation to plant nutrition. In Marschner’s mineral nutrition of higher plants (pp. 347–368). Academic Press. https://doi.org/10.1016/B978-0-12-384905-2.00014-5
- Nieweś, D., Huculak-Mączka, M., Braun-Giwerska, M., Marecka, K., Tyc, A., Biegun, M., Hoffmann, K., & Hoffmann, J. (2022). Ultrasound-assisted extraction of humic substances from peat: Assessment of process efficiency and products’ quality. Molecules, 27(11), 3413. https://doi.org/10.3390/molecules27113413
- Nigussie, A., Dume, B., Ahmed, M., Mamuye, M., Ambaw, G., Berhiun, G., Biresaw, A., & Aticho, A. (2021). Effect of microbial inoculation on nutrient turnover and lignocellulose degradation during composting: A meta-analysis. Waste Management, 125, 220–234. https://doi.org/10.1016/j.wasman.2021.02.043
- Ogunwande, G., Osunade, J., Adekalu, K., & Ogunjimi, L. (2008). Nitrogen loss in chicken litter compost as affected by carbon to nitrogen ratio and turning frequency. Bioresource Technology, 99(16), 7495–7503. https://doi.org/10.1016/j.biortech.2008.02.020
- Olivares, F. L., Busato, J. G., de Paula, A. M., da Silva Lima, L., Aguiar, N. O., & Canellas, L. P. (2017). Plant growth promoting bacteria and humic substances: Crop promotion and mechanisms of action. Chemical and Biological Technologies in Agriculture, 4(1), 1–13. https://doi.org/10.1186/s40538-017-0112-x
- Pansu, M., & Gautheyrou, J. (2006). Handbook of soil analysis. mineralogical, organic and inorganic methods. Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-540-31211-6
- Paterson, E., Sim, A., Osborne, S. M., & Murray, P. J. (2011). Long-term exclusion of plant-inputs to soil reduces the functional capacity of microbial communities to mineralise recalcitrant root-derived carbon sources. Soil Biology & Biochemistry, 43(9), 1873–1880. https://doi.org/10.1016/j.soilbio.2011.05.006
- Piccolo, A., Spaccini, R., Drosos, M., Vinci, G., & Cozzolino, V. (2018). The molecular composition of humus carbon: Recalcitrance and reactivity in soils. In C. Garcia, P. Nannipieri, & T. Hernandez (Eds.), The future of soil carbon (pp. 87–124). Academic Press. https://doi.org/10.1016/B978-0-12-811687-6.00004-3
- Pishchik, V. N., Vorobyov, N. I., Walsh, O. S., Surin, V. G., & Khomyakov, Y. V. (2016). Estimation of synergistic effect of humic fertilizer and Bacillus subtilis on lettuce plants by reflectance measurements. Journal of Plant Nutrition, 39(8), 1074–1086. https://doi.org/10.1080/01904167.2015.1061551
- R Core Team. (2021) R: A language and environment for statistical computing. Retrieved January 31, 2023. https://www.R-project.org/
- Rodarte, M. P., Dias, D. R., Vilela, D. M., & Schwan, R. F. (2011). Proteolytic activities of bacteria, yeasts and filamentous fungi isolated from coffee fruit (Coffea arabica L.). Acta Scientiarum Agronomy, 33(3). https://doi.org/10.4025/actasciagron.v33i3.6734
- Sands, D. C. (1990). Physiological criteria: Determinative tests. In Z. Klement, K. Rudolph, & D.C. Sands (Eds.), Methods in Phytobacteriology (pp. 133–143). Akademiai Kiado.
- Schaad, N. W., Jones, J. B., & Chun, W. (2001). Laboratory guide for identification of plant pathogenic Bacteria (3rd ed.). American Phytopathological Society Press.
- Stapp, C. (1961). Bacterial plant pathogen (Vol. 15). Oxford University Press.
- Sun, N., Gao, C., Ding, Y., Bi, Y., Seglah, P. A., & Wang, Y. (2022). Five-dimensional straw utilization model and its impact on carbon emission reduction in China. Sustainability, 14(24), 16722. https://doi.org/10.3390/su142416722
- Sutradhar, S., & Fatehi, P. (2023). Latest development in the fabrication and use of lignin-derived humic acid. Biotechnology for Biofuels and Bioproducts, 16(1), 38. https://doi.org/10.1186/s13068-023-02278-3
- Thomason, W. E., Evanylo, G. K., Zhang, X., Strickland, M. S., Chim, B. K., & Diatta, A. A. (2020). The synergistic effects of humic substances and biofertilizers on plant development and microbial activity: A review. International Journal of Plant & Soil Science, 32(7), 56–75. https://doi.org/10.9734/ijpss/2020/v32i730306
- United Nations Department of Economics and Social Affairs, UNDESA, (2017). World population projected to reach 9.8 billion in 2050, and 11.2 billion in 2100. Retrieved April 26, 2023. https://www.un.org/en/desa/world-population-projected-reach-98-billion-2050-and-112-billion-2100.html
- Vanlauwe, B., Bationo, A., Chianu, J., Giller, K. E., Merckx, R., Mokwunye, U., Ohiokpehai, O., Pypers, P., Tabo, R., Shepherd, K. D., Smaling, E. M. A., Woomer, P. L., & Sanginga, N. (2010). Integrated soil fertility management: Operational definition and consequences for implementation and dissemination. Outlook on Agriculture, 39(1), 17–24. https://doi.org/10.5367/000000010791169998
- Veres, Z., Kotroczó, Z., Fekete, I., Tóth, J. A., Lajtha, K., Townsend, K., & Tóthmérész, B. (2015). Soil extracellular enzyme activities are sensitive indicators of detrital inputs and carbon availability. Applied Soil Ecology, 92, 18–23. https://doi.org/10.1016/j.apsoil.2015.03.006
- Verma, R., Maurya, B. R., & Meena, V. S. (2014). Integrated effect of bio-organics with chemical fertilizer on growth, yield and quality of cabbage (Brassica oleracea var capitata). Indian Journal Agricultural Science, 84(8), 914–919.
- Weil, R. R., & Brady, N. C. (2017). The nature and properties of soils (15th ed.). ISBN: 978-0133254488. Pearson Education.
- Yang, H. S., Xu, M., Li, Y., Xu, C., Zhai, S., & Liu, J. (2020). The impacts of ditch-buried straw layers on the interface soil physicochemical and microbial properties in a rice-wheat rotation system. Soil and Tillage Research, 202, 104646. https://doi.org/10.1016/j.still.2020.104656
- Zhang, P., Wei, T., Jia, Z. K., Han, Q. F., & Ren, X. L. (2014). Soil aggregate and crop yield changes with different rates of straw incorporation in semiarid areas of northwest China. Geoderma, 230–231, 41–49. https://doi.org/10.1016/j.geoderma.2014.04.007