Abstract
There is currently a great deal of information available about the toxic effects of oxyfluorfen on soil biochemical activity and microbial biodiversity. However, there is no information about how this herbicide affects plant growth promoting bacteria (PGPB) or their properties, such as biofilm formation, nitrogen fixation, siderophore and auxin production, and phosphate solubilisation. As such in this study an agricultural soil was polluted with the oxyfluorfen herbicide every 30 days at a dose of 4 L ha−1 for a total period of 90 days. During this experimental period, the dehydrogenase activity was determined, a count and isolation of cultivable bacteria was performed, and the PGPB and their properties were characterized. The results indicated that oxyfluorfen inhibits the dehydrogenase activity, with this inhibition increasing with herbicide concentration in the soil (69.9% compared to non-polluted soil). It also causes changes in the population diversity of cultivable bacteria in soils. Regard to the evolution of isolated PGPB, it was found that oxyfluorfen induces the growth of nitrogen-fixing, biofilm-forming, and siderophore-producing bacteria, while negatively affecting the growth of auxin-producing and phosphate-solubilising bacteria. These results suggest that oxyfluorfen modulates the properties of PGPB in a concentration-dependent manner.
1. Introduction
Pesticides currently play an important role in agriculture as they enhance crop development and productivity by minimizing the economic losses caused by weeds, insects and pathogens (Tejada et al., Citation2016, Citation2017). However, excessive pesticide use is affecting the environment negatively since it is deteriorating soil quality (biochemical activity and microbial biodiversity) and increasing the contamination of surface and groundwater as a result of diffuse pollution (e.g., run-off and leaching) (Khalid et al., Citation2020; Ogura et al., Citation2021; Yu et al., Citation2019).
Oxyfluorfen is a herbicide used worldwide (Sheng et al., Citation2020) that inhibits protoporphyrinogen oxidase (PPO), thereby increasing the levels of protoporphyrin IX in the plant cells (Wang et al., Citation2016). High amounts of this compound cause peroxidation of the fatty acids in the cell membrane, thereby weakening it and resulting in a loss of pigmentation in the leaves, necrosis, and death of the plant (Liu et al., Citation2016). This herbicide has a degradation rate of 35–138 days (depending on the environmental conditions), thus meaning that it persists in soils (Lewis et al., Citation2016). Indeed, several authors have described the pollution of surface and ground water with this herbicide as a consequence of run-off, and even its accumulation in crops (Sondhia, Citation2010; Tudi et al., Citation2021; Zhang et al., Citation2018). In addition, the Environmental Protection Agency (EPA) has classified oxyfluorfen as carcinogenic for humans (EPA, Citation2002). In Spain, oxyfluorfen is currently applied in quantities of up to 150 g·ha−1·year−1 and its approval has been renewed until 2024, although it is a candidate for substitution by another less toxic herbicide (MAPA, Citation2022).
In addition to crop and human damage, oxyfluorfen also affects soil microorganisms, altering their activities and their biodiversity (Gómez et al., Citation2014; Rodríguez-Morgado et al., Citation2014; Tejada et al., Citation2022). These microorganisms are essential for good crop development given their multiple interactions with plants, especially those that promote plant growth, which are known as PGPB (Plant Growth Promoting Bacteria) (Chamkhi et al., Citation2022). Such bacteria exhibit numerous properties involved in the enhancement of plant growth, such as their ability to facilitate the absorption of nutrients, improve plant development, and protect plants against different biotic and abiotic stressors, while also acting as natural fertilizers (Goswami et al., Citation2016; Navarro-Torre et al., Citation2020; Olanrewaju et al., Citation2017).
These plant growth-promoting (PGP) properties include phosphate solubilisation, a property. Through this property, bacteria can solubilise phosphates by producing organic acids, hydroxyl ions, protons, and carbon dioxide, thus making phosphorous more readily available for plants (Alori et al., Citation2017; Goswami et al., Citation2015). Nitrogen fixation is another key PGP property involved in made more available the nitrogen uptake by plants. Nitrogen-fixing bacteria can fix nitrogen from the atmosphere (not assimilated by plants) and turn it into ammonia, which is assimilated by plants, thanks to the enzyme nitrogenase (Backer et al., Citation2018; Goswami et al., Citation2015). To make iron available for plants, some PGPB produce siderophores, small molecular weight peptides that are able to chelate Fe3+ ions with high affinity and form a siderophore-Fe+3 complex that is easily assimilated by plants via specific receptors (Alori & Babalola, Citation2018). In addition, this property also acts as a biocontrol by preventing phytopathogen growth (Shen et al., Citation2013).
The PGPB can also synthesize and produce phytohormones such as auxins, gibberellins, cytokinins, ethylene, and abscisic acid, with the former, in particular indole-3-acetic acid (IAA), being the most abundant phytohormone produced by bacteria (Goswami et al., Citation2015; Navarro-Torre et al., Citation2020; Olanrewaju et al., Citation2017). IAA is involved in root elongation, thus meaning that IAA producing bacteria can improve the root architecture in the way that plants have more accessibility to water and nutrients (Alemneh et al., Citation2020; Turan et al., Citation2016). Finally, biofilm formation is another important trait present in some PGPB. During this process, the bacteria form a biofilm around that may help to protect them against biotic and abiotic stress and favour nutrient uptake (Rossi, Citation2020). In light of the above, it can be concluded that a decrease or disappearance of this bacterial function from soils due to oxyfluorfen contamination is likely to result in a marked loss of soil quality, thus preventing it from being used for agricultural purposes (Filimon et al., Citation2021).
We hypothesized that oxyfluorfen herbicide negatively affects the biochemical activity of the soil as well as PGPB bacteria. However, we do not know how it can affect the various PGPB properties. For this reason, the main goal of this study was to observe the evolution of soil biochemical activity and the changes in the PGPB population in an agricultural soil affected by oxyfluorfen. To that end, the microbial activity of soil treated with different doses of oxyfluorfen was determined and then the cultivable bacteria were isolated, estimating the different species and analysing their PGP properties at the different doses.
To determine the microbial activity of the soil, the dehydrogenase enzymatic activity was used. This enzyme is related to oxidative phosphorylation, making it a good indicator of microbial activity (Tejada et al., Citation2023).
2. Materials and methods
2.1. Design of the experimental assay
An Arenic Calcaric Regosol (WRB, Citation2014) agricultural soil from southwestern Spain was employed in this study. This soil contains 616 ± 62 g kg−1 sand, 123 ± 29 g kg−1 silt, and 260 ± 35 g kg−1 clay. The soil pH was 7.9 ± 0.2, and it contained 17.2 ± 1.8 g kg−1 organic matter, and 0.93 ± 0.07 g kg−1 N. The methodology used to determine these physico-chemical parameters is described in Rodríguez-Morgado et al. (Citation2014).
A 500-g sample of dried and sieved soil (<2 mm) was preincubated in 1-L glass bottles for 7 days at 30–40% of the maximum water-holding capacity, as per the methodology described by Tejada (Citation2009). Subsequently, the soil was mixed with 4 L·ha−1 oxyfluorfen (commercial formulation Fenfen; Lainco, S.A., Spain) in the half of the bottles. Distilled water was then added to each glass bottle until 60% of the maximum water-holding capacity.
In detail, the treatments carried out were:
S, control soil non-polluted with oxyfluorfen
S + Ox, soil polluted with oxyfluorfen
In the S+Ox treatment, the addition of oxyfluorfen was repeated after 30 and 60 days from the first addition. Glass bottles containing polluted and non-polluted soil (n = 3) were deposited randomly and placed in an incubation chamber at 25 ± 1 °C. During the experiment (90 days), distilled water was added to maintain the soil at 60% of its maximum water-holding capacity.
Samples were taken from each bottle at 30, 60, and 90 days from the start of the experiment and deposited in labelled bottles for the subsequent analysis (determination of oxyfluorfen concentration, dehydrogenase activity, and bacterial isolation). Soil samples were stored at −20º C until the analysis.
2.2. Soil analysis
2.2.1. Soil oxyfluorfen
The soil oxyfluorfen concentration was determined by chromatography following the procedure described in Gómez et al. (Citation2014), with previous extraction of the herbicide as per the protocol described in Anastassiades et al. (Citation2003).
2.2.2. Soil dehydrogenase activity
Soil dehydrogenase activity was determined by the reduction of 2-p-iodo-3-nitrophenyl-5-phenyl-tetrazolium chloride to iodonitrophenyl formazan according to the method of Trevors et al. (Citation1982) as modified by García et al. (Citation1993).
2.2.3. Isolation of cultivable bacteria
Cultivable bacteria were isolated from the different samples collected at different times following the protocol described in Navarro-Torre et al. (Citation2016). Briefly, soil was resuspended in sterile 0.9% saline solution for 10 min by shaking. Then, 10−1, 10−2, and 10−3 dilutions were plated on TSA (Tryptic Soy Agar; Scharlau, Spain) plates in duplicate and incubated at 28 °C for 48 h. To determine the total bacterial population in each soil sample, colony forming units (CFU) were counted for the 10−3 dilution. After that, different colonies from all dilutions were separated onto different TSA plates based on their colour, size, and morphology. Pure cultures were stored at −80°C in 15% glycerol.
2.2.4. Analysis of bacterial population by Box-PCR
Bacterial population from the different levels of contamination was analysed by Box-PCR. Thus, DNA extraction was performed using the EZNA® Bacterial DNA kit (Omega Bio-Tek, United States) following the instructions supplied by the manufacturer. Box-PCR was performed using the Maxime™ PCR PreMix kit (i-Taq™) (Intron Biotechnology, Korea) and BOX A1R primer (Flores-Duarte et al., Citation2022). PCR conditions were the following: initial denaturation at 94 °C for 2 min, 30 cycles of denaturation at 94 °C for 20 s, annealing at 52 °C for 20 s, extension at 72 °C for 1 min, and final extension at 72 °C for 5 min. Electrophoresis was performed in a 1.5% (w/v) agarose gel and a voltage of 70 V for 2 h. After the electrophoresis, the gel was photographed and analysed using the PyElph software package (v. 1.4) (Pavel & Vasile, Citation2012). Finally, the similarities were presented as dendrograms using an unweighted pair-group method with arithmetic mean (UPGMA), as suggested by Pavel and Vasile (Citation2012).
2.2.5. Determination of plant growth promoting properties in vitro
Phosphate solubilisation, siderophore and indole-3-acetic acid (IAA) production, nitrogen fixation, and biofilm formation were the PGPB properties assayed in this work.
Phosphate solubilisation was determined by growing the isolates in NBRIP (National Botanical Research Institute’s phosphate growth medium) plates at 28 °C for 5 days (Nautiyal, Citation1999). A transparent halo around the bacterial growth indicated that the bacteria solubilise phosphate. In positive samples, the halo was measured to semi-quantify the solubilisation.
For siderophore production, bacteria were incubated in CAS (chrome azurol S) plates at 28°C for 5 days in the dark (Schwyn & Neilands, Citation1987). In this case, an orange halo confirmed that the bacteria produced siderophores, and this halo was measured to semi-quantify the production.
The IAA production by the isolates was assayed using a colorimetric method. Thus, bacteria were incubated in TSB (Tryptic Soy Broth; Scharlau, Spain) supplemented with L-tryptophan (100 mg·L−1) at 28 °C for 3 days. After incubation, the culture was centrifuged and the supernatant mixed with Salkowski reagent (Gordon & Weber, Citation1951) for 20 minutes and IAA-producing bacteria showed a pink coloration. To quantify the IAA production by each strain, the absorbance was measured using a spectrophotometer (GeneQuant® 1300; Biochrom Ltd., United Kingdom) at 535 nm and the IAA quantification was performed using a pattern curve. IAA pattern curve was performed using 1 mL of different known IAA concentrations mixed with 4 mL Salkowski reagent. The absorbance was recorded at 535 nm. Different known IAA concentrations were prepared from an IAA stock solution (200 mg·L−1).
Nitrogen fixation was tested on NFA (nitrogen-free agar) plates at 28 °C for 5 days (Ji et al., Citation2014). Bacterial growth after incubation indicated that the bacteria could fix nitrogen from the atmosphere.
Finally, biofilm formation was studied in the isolates in a similar manner to Del Castillo et al. (Citation2012). Briefly, biofilm formation was determined in a six-well plate using TSB as culture medium, incubating at 28 °C for 4 days. After that time, the position of the biofilm in the well was determined (surface or bottom), then wells were washed with distilled water and stained with 0.01% violet crystal for 20 minutes to detect the bacterial biomass adhered to the wall of the wells (ring formation).
After the determination of these PGP properties, the quantification of the number of bacteria showing each property was analysed in order to observe changes in the PGP properties caused by oxyfluorfen.
2.2.6. Statistical analyses
Statistical analyses were performed using Statistica software version 6.0 (StatSoft Inc.). The normality of the results was determined by applying the Kolmogorov—Smirnov test. Dehydrogenase activity, number of colonies, and number of different isolated strains were compared using a one-way ANOVA to determine de effect of oxyfluorfen and the interaction. On the other hand, the evolution of the different PGP properties, the IAA and siderophores production, and the phosphate solubilisation were analysed using GLM. In both cases, Fisher test (LSD) was carried out as post hoc test.
3. Results
3.1. Effect of different oxyfluorfen concentrations on the microbial population in affected soil
The oxyfluorfen concentration increased in each taken sample due to repeated application of the herbicide (Figure ).
Figure 1. Evolution of soil oxyfluorfen concentration (mean ± standard error, n = 3) during the experimental period.
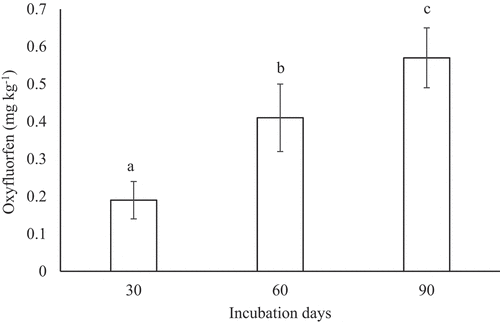
Figure shows the evolution of dehydrogenase activity in non-polluted and herbicide-contaminated soil during the experimental period. Successive application of oxyfluorfen to the soil caused a significant (p < 0.05) decrease in dehydrogenase activity throughout the incubation period. Compared with the non-polluted soil, this enzymatic activity decreased by 57.7% in the polluted soil at 30 days, 65.4% at 60 days and 69.9% at the end of the experimental period.
Figure 2. Evolution of dehydrogenase activity (μg INTF g−1 h−1) (mean ± standard error, n = 3) in control and oxyfluorfen-polluted soil during the experiment. Columns with the same letter(s) do not present significant differences (p > 0.05). INTF: 2-p-iodo-3-nitrophenyl formazan.
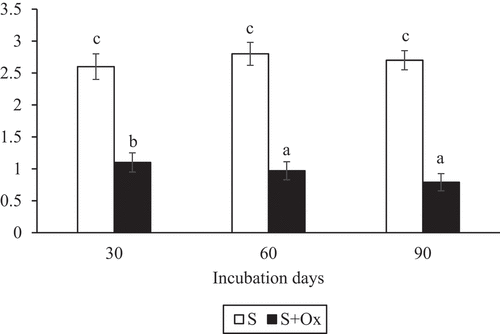
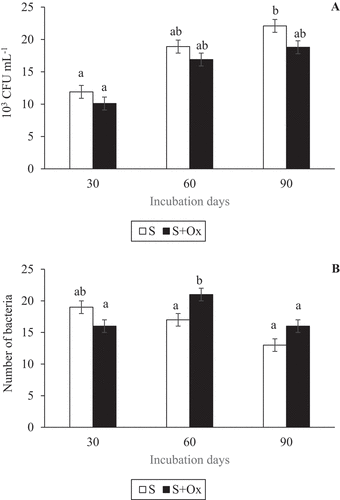
After the negative effect of oxyfluorfen on the dehydrogenase activity had been confirmed, cultivable bacteria were isolated from each sample in order to observe the effect of oxyfluorfen on them. The number of CFU·mL−1 in both soils increased with the passage of time, although soils polluted with oxyfluorfen presented a lower value than control soils for each sample taken, although the difference was not significant (Figure ). This result demonstrates that oxyfluorfen negatively affects the size of the cultivable microbial population.
Figure 3. Cultivable bacterial population (mean ± standard error, n = 3) in control and oxyfluorfen-polluted soil during the experiment. (a) Number of colony forming units (CFU) per mL; (b) Number of bacteria. Columns with the same letter(s) do not present significant differences (p > 0.05).
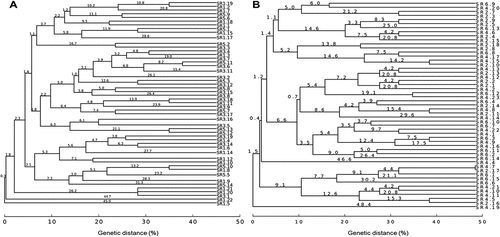
A total of 55 bacteria from control soil and 58 bacteria from oxyfluorfen-polluted soil, which were apparently different, were isolated. However, the Box-PCR results indicated that there were 49 different bacterial profiles in the control soil and 53 different bacterial profiles in the polluted soil (Figure ). The number of different bacteria isolated from the soil increased at 60 and 90 days after the oxyfluorfen addition (Figure ), thus indicating a higher diversity of bacteria in the presence of the herbicide.
Figure 4. Box-PCR dendrograms. (a) Strains isolated from control soils after 30 days; (b) Strains isolated from polluted soils with oxyfluorfen after 30 days; (C) strains isolated from control soils after 60 days; (d) Strains isolated from polluted soils with oxyfluorfen after 60 days; (e) Strains isolated from control soils after 90 days; (f) Strains isolated from polluted soils with oxyfluorfen after 90 days.
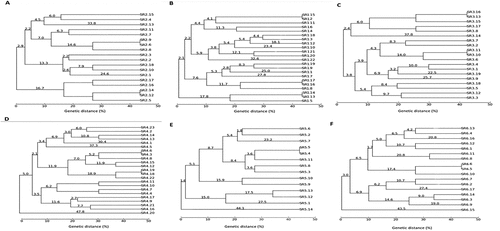
As can be seen Figure , the population of cultivable bacteria changed over time for both the control and oxyfluorfen-polluted soils, with the exception of two strains (SR2.17 and SR4.7) isolated from soil polluted with oxyfluorfen at days 30 and 60, which showed the same Box-PCR profile (Figure ).
Figure 5. Cultivable bacterial populations during the experiment. (a) Dendrogram for all the strains isolated from control soils after 30 (strains SR1), 60 (strains SR3), and 90 (strains SR5) days; (b) Dendrogram for all the strains isolated from soils polluted with oxyfluorfen after 30 (strains SR2), 60 (strains SR4), and 90 (strains SR6) days.
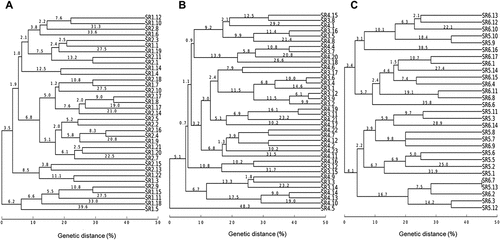
Similar results were observed when the cultivable bacterial populations from control and polluted soil were compared after 30, 60, and 90 days from the first treatment with oxyfluorfen (Figure ). Thus, both treatments showed different bacterial populations for all samples taken. However, one species of bacteria was isolated from each soil treatment at day 90 (strain SR5.13 and SR6.7 for control and polluted soil, respectively; Figure ).
Figure 6. Cultivable bacterial populations in control and polluted soils at the same time points. (a) Dendrogram for all the strains isolated from control soils (strains SR1) and soils polluted with oxyfluorfen (strains SR2) after 30 days; (b) Dendrogram for all the strains isolated for control soils (strains SR3) and soils polluted with oxyfluorfen (strains SR4) after 60 days; (c) Dendrogram for all the strains isolated from control soils (strains SR5) and soils polluted with oxyfluorfen (strains SR6) after 90 days.
3.2. Evolution of the PGPB in soils with different oxyfluorfen concentrations
In order to observe the possible effect of oxyfluorfen doses on the PGPB, all strains isolated were characterized to determine their PGP properties. All strains showed at least one PGP property, except strain SR4.5, which showed none of them (Table ). The number of PGP properties per strain was maintained at the different oxyfluorfen doses, with no significant differences (Table ; GLM, LSD test, p > 0.05).
Table 1. PGP properties of the strains isolated from control and those polluted with oxyfluorfen at different times
Table 2. PGP properties exhibited by the same species at different oxyfluorfen concentrations in soils
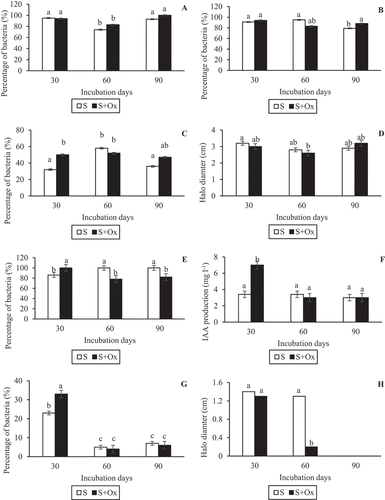
The effect of oxyfluorfen on each PGP property tested also was studied (Figure ). The percentage of bacteria with biofilm formation capacity was very similar at 30 days from the first contamination with oxyfluorfen. However, this percentage increased by 9% and 7% in polluted soil after 60 and 90 days, respectively (Figure ). The number of nitrogen-fixing bacteria was also similar at 30 days from soil contamination. This number decreased by 12% after 60 days, but after 90 days the percentage of nitrogen-fixing bacteria was higher in polluted soil than in the control treatment (Figure ). With regard to the percentage of bacteria that produce siderophores, this number was higher in polluted soil, with the exception of soil samples at 60 days (Figure ). In addition, in soil with the highest concentration of oxyfluorfen, the isolates produced more siderophores (1.13-fold), showing chelating halos larger than those produced by bacteria isolated from control soil, although the differences were not significant (Figure ). In contrast, the presence of high concentrations of oxyfluorfen in soils had a negative effect on the number of IAA-producing bacteria, with the percentage of bacteria decreasing by 22% at 60 days and 18% at 90 days (Figure ). A similar negative effect was observed in the amount of IAA produced by these bacteria, although the differences were not significant (Figure ). However, in soils containing only small doses of oxyfluorfen (at 30 days), the percentage of IAA-producing bacteria, and the amount of IAA produced by them, increased significantly. Finally, the number of phosphate-solubilising bacteria increased in the presence of small doses of oxyfluorfen, but when the herbicide concentration was
Figure 7. Evolution of the PGP properties (mean ± standard error, n = 3) in isolated bacteria for control and polluted soils during the experimental period. (a) Percentage of bacteria with biofilm-formation capacity; (b) Percentage of bacteria with nitrogen-fixation capacity; (c) Percentage of bacteria with siderophore-production capacity; (d) Siderophores production (chelation halo of chelation) by bacteria; (e) Percentage of bacteria with IAA-production capacity; (f) IAA production by bacteria; (g) Percentage of bacteria with phosphate-solubilisation capacity; (h) Phosphate solubilisation (solubilisation halo of solubilisation) by bacteria. Columns with the same letter(s) do not present significant differences (p > 0.05).
4. Discussion
Our results indicate that application of the oxyfluorfen herbicide caused an inhibition in soil dehydrogenase enzymatic activity. These results are in line with those obtained by Franco-Andreu et al. (Citation2016), Ávila-Pozo et al. (Citation2021) and Tejada et al. (Citation2022), who observed a significant toxic effect of oxyfluorfen on this intracellular enzymatic activity.
This inhibition of dehydrogenase activity increased throughout the experiment due to an increase in the concentration of herbicide in the soil, thereby confirming the high persistence of oxyfluorfen in soils (Barba et al., Citation2017; Tejada et al., Citation2022). Indeed, this herbicide does not degrade after 30 days of application to the soil, thus meaning that it accumulates after the next application.
Similar to the dehydrogenase activity, the application of oxyfluorfen to soil decreased the PGPB population. In addition, during the experiment, the number of different strains increased in those soils polluted with oxyfluorfen. This finding suggests that the presence of oxyfluorfen in soils produces changes in the microbial population, thereby increasing the bacterial diversity. The bacterial population studies performed for the strains isolated show that the microbial population changed during the 90 days of the experiment and was different in control and polluted soils. Other authors have also described changes to the microbial population after the application of oxyfluorfen (Mohamed et al., Citation2011; Tejada et al., Citation2022), showing that these changes depend on the oxyfluorfen concentration. The findings of Singh et al. (Citation2020) are also in agreement, and these authors also propose that the modification of the population also depends on the microbial species in soils affected by the herbicide. Microorganism species in soils are very sensitive to any environmental changes (Starke et al., Citation2019; Zhou et al., Citation2020) and evolve to adapt to the new environmental conditions and survive. The change in the microbial population in this study could due to this bacterial adaptation and colonization by oxyfluorfen-resistant species and/or species that degrade the herbicide to use it as a nutrient source (Castillo Diaz et al., Citation2016; Zhao et al., Citation2016).
The increase in oxyfluorfen concentration in the soil modified the different properties of the PGPB analysed. Thus, bacteria that form biofilms, fix nitrogen, and produce siderophores increased in number in the presence of high concentrations of oxyfluorfen. This increased biofilm production in affected soils could be a response to oxyfluorfen toxicity because bacteria are covered by extracellular polymeric substances that they secrete, thus making them more resistant to stress (Rossi, Citation2020). The proliferation of this PGPB type is very beneficial for agricultural soils because bacteria form a biofilm around roots and facilitate nutrient uptake by plants. Moreover, the biofilm formed around the roots protects plants against biotic and abiotic stresses (Bhagat et al., Citation2021; Rossi, Citation2020) and they could be very helpful to the crops development in agricultural soils affected by this herbicide. With regard to the nitrogen-fixing bacteria, Das and Debnath (Citation2006) also reported that the presence of oxyfluorfen in soils seems to promote the proliferation of this bacterial function. In addition, these authors observed that the addition of oxyfluorfen increases the nitrogen-fixing capacity by bacteria and the nitrogen availability in soils. Similar results were founded in soils affected by imazethapyr and flumioxazin and in this study, the increase in this activity was related to the increase in the relative abundance of the genus Bradyrhizobium (Araujo et al., Citation2023). Several authors proposed that the degradation pathway for oxyfluorfen by bacteria implicates a reduction of nitro group to an amino group and some of the metabolites produced after degradation were rich in nitrogen (4-nitrobenzene-1,3-diol, 4-amino-3-ethoxyphenol, N-(2-ethoxy-4-hydroxyphenyl) acetamide, 3-ethoxy-a-nitrophenol, among others) (Castillo Diaz et al., Citation2016; Zhao et al., Citation2016). The presence of these metabolites could be the reason why the bacteria related with the nitrogen metabolism increase the number in the presence of oxyfluorfen. In any case, this is very important in agricultural soils because nitrogen is a poorly available nutrient and an increase in this PGPB type therefore elevates the nitrogen content in soils, thereby improving their fertility (Zeng et al., Citation2022). Siderophore-producing bacteria were also isolated in greater numbers from soils treated with a high concentration of oxyfluorfen than from the control soil. In addition, under these conditions, the quantity of siderophores produced by bacteria was also higher, although the difference was not significant. This result implies that when the oxyfluorfen concentration in soils increases, siderophore-producing bacteria, and therefore siderophore production, also increase. This fact could be due because metabolites produced by oxyfluorfen degrading bacteria can act as siderophores or siderophores precursors. However, there are no studies that support this hypothesis.
In contrast, the presence of oxyfluorfen in soils negatively affected some PGPB properties, such as IAA production and phosphate solubilisation. Although IAA-producing bacteria increased in number at the lowest oxyfluorfen level, as the herbicide concentration increased, this bacterial function was also affected. One of the effects of glyphosate (another herbicide) in plants is the interruption of the synthesis of three aromatic amino acids and one of them is tryptophan, the precursor of IAA (Maeda & Dudareva, Citation2012). This presumption is according to the results showed in this study although in bacteria instead plants. Studies of oxyfluorfen effects are very scarce in this topic, but maybe this herbicide affects also the synthesis of tryptophan or interrupts any IAA pathway in bacteria in a similar way that glyphosate. A reduction in IAA producers has a negative effect on agricultural soils as this PGP property can be very useful for promoting crop growth (Alemneh et al., Citation2020; Turan et al., Citation2016) and for limiting weed growth (Kang et al., Citation2020; Park et al., Citation2015). With regard to phosphate solubilisers, the behaviour of these bacteria showed a similar pattern to that for IAA producers. In addition, phosphate solubilisation decreased abruptly with an increase in herbicide concentration. This effect of the oxyfluorfen could be due to for the inhibition of the phosphatase activities as described Sheeba et al. (Citation2011) in their study, where two cyanobacteria decreased the acid and alkaline phosphatase activities in increased concentrations of oxyfluorfen. However, the opposite effect was observed by Das and Debnath (Citation2006) and Das et al. (Citation2003). Both these studies found that the number of phosphate-solubilising bacteria and solubilised phosphate increased after the application of 0.12 kg·ha−1 of oxyfluorfen. These different results may be due to the different doses of herbicide applied to the soils, thus suggesting that the population of phosphate-solubilising bacteria, and their activity, could be modified depending on the oxyfluorfen concentration in soils. In addition, a bulk soil was used for the experiment in this study, whereas Das and Debnath (Citation2006) and Das et al. (Citation2003) used the rhizosphere of rice (Oryza sativa), and plant rhizospheres are known to have a higher PGPB activity than bulk soils (Chamkhi et al., Citation2022).
Finally, the effect of oxyfluorfen was observed on two bacterial strains belonging to the same species. These strains were isolated at different concentrations of oxyfluorfen. The results show that the presence of oxyfluorfen, and its concentration, can modify the PGP activity in the same species of bacterium, as also occurs in the presence of other stressors such as high concentrations of heavy metals (Mesa et al., Citation2015; Navarro-Torre et al., Citation2016).
5. Conclusions
It can be concluded that the continuous application of oxyfluorfen to soil increases the inhibition of soil dehydrogenase activity and the PGPB population. These results show the high toxicity of oxyfluorfen in terms of both soil biochemical activity and PGPB.
With regard to the evolution of isolated PGPB, it has been shown that oxyfluorfen induces the growth of nitrogen-fixing, biofilm-forming, and siderophore-producing bacteria, while negatively affecting the growth of auxin-producing and phosphate-solubilising bacteria.
These results suggest that PGPB respond different to oxyfluorfen pollution, with properties that may be affected negatively and/or positively.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alemneh, A. A., Zhou, Y., Ryder, M. H., Denton, M. D., Denton, M., & Zhou, Y. (2020). Mechanisms in plant growth-promoting rhizobacteria that enhance legume–rhizobial symbioses. Journal of Applied Microbiology, 129(5), 1133–17. https://doi.org/10.1111/jam.14754
- Alori, E. T., & Babalola, O. O. (2018). Microbial inoculants for improving crop quality and human health in Africa. Frontiers in Microbiology, 9, 2213. https://doi.org/10.3389/fmicb.2018.02213
- Alori, E. T., Glick, B. R., & Babalola, O. O. (2017). Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Frontiers in Microbiology, 8, 971. https://doi.org/10.3389/fmicb.2017.00971
- Anastassiades, M., Lehotay, S. J., Stajnbaher, D., & Shenck, F. J. (2003). Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “Dispersive solid-phase extraction” for the determination of pesticide residues in produce. Journal of AOAC International, 86(2), 412–431. https://doi.org/10.1093/jaoac/86.2.412
- Araujo, A. S. F., Pertile, M., Costa, R. M., Costa, M. K. L., de Aviz, R. O., Mendes, L. W., de Medeiros, E. V., da Costa, D. P., Melo, V. M. M., & Pereira, A. P. A. (2023). Short-term responses of plant growth-promoting bacterial community to the herbicides imazethapyr and flumioxazin. Chemosphere, 328, 138581. https://doi.org/10.1016/j.chemosphere.2023.138581
- Ávila-Pozo, P., Parrado, J., Caballero, P., Díaz-López, M., Bastida, F., & Tejada, M. (2021). Use of slaughterhouse sludge in the bioremediation of an oxyfluorfen-polluted soil. International Journal of Environmental Health Research, 15(4), 723–731. https://doi.org/10.1007/s41742-021-00351-z
- Backer, R., Rokem, J. S., Ilangumaran, G., Lamont, J., Praslickova, D., Ricci, E., Subramanian, S., & Smith, D. L. (2018). Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Frontiers in Plant Science, 871, 1473. https://doi.org/10.3389/fpls.2018.01473
- Barba, S., Villaseñor, J., Rodrigo, M. A., & Cañizares, P. (2017). Effect of the polarity reversal frequency in the electrokinetic-biological remediation of oxyfluorfen polluted soil. Chemosphere, 177, 120–127. https://doi.org/10.1016/j.chemosphere.2017.03.002
- Bhagat, N., Raghav, M., Dubey, S., & Bedi, N. (2021). Bacterial exopolysaccharides: Insight into their role in plant abiotic stress tolerance. Journal of Microbiology and Biotechnology, 31(8), 1045–1059. https://doi.org/10.4014/jmb.2105.05009
- Castillo Diaz, J. M., Delgado-Moreno, L., Núñez, R., Nogales, R., & Romero, E. (2016). Enhancing pesticide degradation using indigenous microorganisms isolated under high pesticide load in bioremediation systems with vermicomposts. Bioresource Technology, 214, 234–241. https://doi.org/10.1016/j.biortech.2016.04.105
- Chamkhi, I., El Omari, N., Balahbib, A., El Menyiy, N., Benali, T., & Ghoulam, C. (2022). Is —— the rhizosphere a source of applicable multi-beneficial microorganisms for plant enhancement? Saudi Journal of Biological Sciences, 29(2), 1246. https://doi.org/10.1016/j.sjbs.2021.09.032
- Das, A. C., & Debnath, A. (2006). Effect of systemic herbicides on N2-fixing and phosphate solubilizing microorganisms in relation to availability of nitrogen and phosphorus in paddy soils of West Bengal. Chemosphere, 65(6), 1082–1086. https://doi.org/10.1016/j.chemosphere.2006.02.063
- Das, A. C., Debnath, A., & Mukherjee, D. (2003). Effect of the herbicides oxadiazon and oxyfluorfen on phosphates solubilizing microorganisms and their persistence in rice fields. Chemosphere, 53(3), 217–221. https://doi.org/10.1016/S0045-6535(03)00440-5
- Del Castillo, I., Hernández, P., Lafuente, A., Rodríguez-Llorente, I. D., Caviedes, M. A., & Pajuelo, E. (2012). Self-bioremediation of cork-processing wastewaters by (chloro)phenol-degrading bacteria immobilised onto residual cork particles. Water Research, 46(6), 1723–1734. https://doi.org/10.1016/j.watres.2011.12.038
- EPA. (2002) . Office of pesticide programs. U. US EPA - Pesticides - Fact Sheet for Oxyfluorfen.
- Filimon, M. N., Roman, D. L., Bordean, D. M., & Isvoran, A. (2021). Impact of the herbicide oxyfluorfen on the activities of some enzymes found in soil and on the populations of soil microorganisms. Agronomy, 11(9), 1702. https://doi.org/10.3390/agronomy11091702
- Flores-Duarte, N. J., Mateos-Naranjo, E., Redondo-Gómez, S., Pajuelo, E., Rodriguez-Llorente, I. D., & Navarro-Torre, S. (2022). Role of nodulation-enhancing rhizobacteria in the promotion of Medicago sativa development in nutrient-poor soils. Plants, 11, 1164. https://doi.org/10.3390/plants11091164
- Franco-Andreu, L., Gómez, I., Parrado, J., García, C., Hernández, T., & Tejada, M. (2016). Behavior of two pesticides in a soil subjected to severe drought. Effects on soil biology. Applied Soil Ecology, 105, 17–24. https://doi.org/10.1016/j.apsoil.2016.04.001
- García, C., Hernández, T., Costa, F., Ceccanti, B., & Masciandaro, G. (1993). The dehydrogenase activity of soils and ecological marker in processes of perturbed system regeneration. In J. Gallardo-Lancho (Ed.), XI International Symposium Environmental Biogeochemistry (pp. 89–100).
- Gómez, I., Rodríguez-Morgado, B., Parrado, J., García, C., Hernández, T., & Tejada, M. (2014). Behavior of oxyfluorfen in soils amended with different sources of organic matter. Effects on soil biology. Journal of Hazardous Materials, 273, 207–214. https://doi.org/10.1016/j.jhazmat.2014.03.051
- Gordon, S. A., & Weber, R. P. (1951). Colorimetric estimation of indoleacetic acid. Plant Physiology, 26(1), 192–195. https://doi.org/10.1104/pp.26.1.192
- Goswami, D., Parmar, S., Vaghela, H., Dhandhukia, P. C., Thakker, J. N., & Moral, M. T. (2015). Describing Paenibacillus mucilaginosus strain N3 as an efficient plant growth promoting rhizobacteria (PGPR). Cogent Food & Agriculture, 1(1), 1000714. https://doi.org/10.1080/23311932.2014.1000714
- Goswami, D., Thakker, J. N., Dhandhukia, P. C., & Tejada Moral, M. (2016). Portraying mechanics of Plant Growth Promoting Rhizobacteria (PGPR): A review. Cogent Food & Agriculture, 2(1), 1127500. https://doi.org/10.1080/23311932.2015.1127500
- Ji, S. H., Gururani, M. A., & Chun, S. C. (2014). Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiological Research, 169(1), 83–98. https://doi.org/10.1016/j.micres.2013.06.003
- Kang, S. M., Bilal, S., Shahzad, R., Kim, Y. N., Park, C. W., Lee, K. E., Lee, J. R., & Lee, I. J. (2020). Effect of ammonia and indole-3-acetic acid producing endophytic Klebsiella pneumoniae YNA12 as a bio-herbicide for weed inhibition: Special reference with evening primroses. Plants, 9(6), 761. https://doi.org/10.3390/plants9060761
- Khalid, S., Shahid, M., Murtaza, B., Bibi, I., Naeem, M. N. A., Niazi, N. K., & Niazi, N. K. (2020). A critical review of different factors governing the fate of pesticides in soil under biochar application. Science of the Total Environment, 711, 134645. https://doi.org/10.1016/j.scitotenv.2019.134645
- Lewis, K. A., Tzilivakis, J., Warner, D. J., & Green, A. (2016). An international database for pesticide risk assessments and management. Human & Ecological Risk Assessment, 22(4), 1050–1064. https://doi.org/10.1080/10807039.2015.1133242
- Liu, Y. C., Qu, R. Y., Chen, Q., Yang, J. F., Cong-Wei, N., Zhen, X., & Yang, G. F. (2016). Triazolopyrimidines as a new herbicidal lead for combating weed resistance associated with acetohydroxyacid synthase mutation. Journal of Agricultural & Food Chemistry, 64(24), 4845–4857. https://doi.org/10.1021/acs.jafc.6b00720
- Maeda, H., & Dudareva, N. (2012). The shikimate pathway and aromatic amino acid biosynthesis in plants. Annual Review of Plant Biology, 63(1), 73–105. https://doi.org/10.1146/annurev-arplant-042811-105439
- MAPA. (2022). Lista comunitaria de sustancias activas aprobadas, excluidas y en evaluación comunitaria, sustancias de bajo riesgo, sustancias candidatas a la sustitución y lista de sustancias básicas. Pub. L. No. Regalmento (CE) No 1107/2009 (2022). Ministerio de Agricultura, Pesca y Alimentación.
- Mesa, J., Mateos-Naranjo, E., Caviedes, M. A., Redondo-Gómez, S., Pajuelo, E., & Rodríguez-Llorente, I. D. (2015). Scouting contaminated estuaries: Heavy metal resistant and plant growth promoting rhizobacteria in the native metal rhizoaccumulator Spartina maritima. Marine Pollution Bulletin, 90(1–2), 150–159. https://doi.org/10.1016/j.marpolbul.2014.11.002
- Mohamed, A. T., El Hussein, A. A., El Siddig, M. A., & Osman, A. G. (2011). Degradation of oxyfluorfen herbicide by soil microorganisms biodegradation of herbicides. Biotechnology, 10(3), 274–279. https://doi.org/10.3923/biotech.2011.274.279
- Nautiyal, C. S. (1999). An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiology Letters, 170(1), 265–270. https://doi.org/10.1111/j.1574-6968.1999.tb13383.x
- Navarro-Torre, S., Bessadok, K., Flores-Duarte, N. J., Rodríguez-Llorente, I. D., Caviedes, M. A., & Pajuelo, E. (2020). Helping legumes under stress situations: Inoculation with beneficial microorganisms. In M. Hasanuzzaman (Ed.), Legume crops. https://doi.org/10.5772/INTECHOPEN.91857
- Navarro-Torre, S., Mateos-Naranjo, E., Caviedes, M. A., Pajuelo, E., & Rodríguez-Llorente, I. D. (2016). Isolation of plant-growth-promoting and metal-resistant cultivable bacteria from Arthrocnemum macrostachyum in the Odiel marshes with potential use in phytoremediation. Marine Pollution Bulletin, 110(1), 133–142. https://doi.org/10.1016/j.marpolbul.2016.06.070
- Ogura, A. P., Lima, J. Z., Marques, J. P., Sousa, L. M., Rodrigues, V. G. S., & Espíndola, E. L. G. (2021). A review of pesticides sorption in biochar from maize, rice, and wheat residues: Current status and challenges for soil application. Journal of Environmental Management, 300, 113753. https://doi.org/10.1016/j.jenvman.2021.113753
- Olanrewaju, O. S., Glick, B. R., & Babalola, O. O. (2017). Mechanisms of action of plant growth promoting bacteria. World Journal of Microbiology & Biotechnology, 33(11), 197. https://doi.org/10.1007/s11274-017-2364-9
- Park, J. M., Radhakrishnan, R., Kang, S. M., & Lee, I. J. (2015). IAA producing Enterobacter sp. I-3 as a potent bio-herbicide candidate for weed control: A special reference with lettuce growth inhibition. Indian Journal of Microbiology, 55(2), 207. https://doi.org/10.1007/s12088-015-0515-y
- Pavel, A. B., & Vasile, C. I. (2012). PyElph - a software tool for gel images analysis and phylogenetics. BMC Bioinformatics, 13(1), 9. https://doi.org/10.1186/1471-2105-13-9
- Rodríguez-Morgado, B., Gómez, I., Parrado, J., & Tejada, M. (2014). Behaviour of oxyfluorfen in soils amended with edaphic biostimulants/biofertilizers obtained from sewage sludge and chicken feathers. Effects on soil biological properties. Environmental Science and Pollution Research, 21(18), 11027–11035. https://doi.org/10.1007/s11356-014-3040-3
- Rossi, F. (2020). Beneficial biofilms for land rehabilitation and fertilization. FEMS Microbiology Letters, 367(21), fnna184. https://doi.org/10.1093/femsle/fnaa184
- Schwyn, B., & Neilands, J. B. (1987). Universal chemical assay for the detection and determination of siderophores. Analytical Biochemistry, 160(1), 47–56. https://doi.org/10.1016/0003-2697(87)90612-9
- Sheeba, P. S. V., Kumar, S. P., Mohan, P. S., & Mohan Prasad, S. (2011). Differential physiological and biochemical responses of two cyanobacteria Nostoc muscorum and Phormidium foveolarum against oxyfluorfen and UV-B radiation. Ecotoxicology & Environmental Safety, 74(7), 1981–1993. https://doi.org/10.1016/j.ecoenv.2011.07.006
- Sheng, E., Lu, Y., Tan, Y., Xiao, Y., Li, Z., & Dai, Z. (2020). Oxidase-mimicking activity of ultrathin MnO2 nanosheets in a colorimetric assay of chlorothalonil in food samples. Food Chemistry, 331, 127090. https://doi.org/10.1016/j.foodchem.2020.127090
- Shen, X., Hu, H., Peng, H., Wang, W., & Zhang, X. (2013). Comparative genomic analysis of four representative plant growth-promoting rhizobacteria in Pseudomonas. BMC Genomics, 14(1), 1–20. https://doi.org/10.1186/1471-2164-14-271
- Singh, M. K., Singh, N. K., & Singh, S. P. (2020). Impact of herbicide use on soil microorganisms. In P. Singh, S. K. Singh, & S. M. Prasad (Eds.), Plant responses to soil pollution (pp. 179–194). Springer.
- Sondhia, S. (2010). Persistence and bioaccumulation of oxyfluorfen residues in onion. Environmental Monitoring and Assessment, 162(1–4), 163–168. https://doi.org/10.1007/s10661-009-0784-1
- Starke, R., Jehmlich, N., & Bastida, F. (2019). Using proteins to study how microbes contribute to soil ecosystem services: The current state and future perspectives of soil metaproteomics. Journal of Proteomics, 198, 50–58. https://doi.org/10.1016/j.jprot.2018.11.011
- Tejada, M. (2009). Application of different organic wastes in a soil polluted by cadmium: Effects on soil biological properties. Geoderma, 153(1–2), 254–268. https://doi.org/10.1016/j.geoderma.2009.08.009
- Tejada, M., Gómez, I., Franco-Andreu, L., & Benitez, C. (2016). Role of different earthworms in a soil polluted with oxyfluorfen herbicide. Short-time response on soil biochemical properties. Ecological Engineering, 86, 39–44. https://doi.org/10.1016/j.ecoleng.2015.09.058
- Tejada, M., Gómez, I., Paneque, P., Del Toro, M., García-Quintanilla, A., & Parrado, J. (2023). Use of biostimulants obtained from sewage sludge for the restoration of soils polluted by diuron: Effect on soil biochemical properties. Agronomy, 13(1), 24. https://doi.org/10.3390/agronomy13010024
- Tejada, M., Macias-Benitez, S., Caballero, P., Gómez, I., Paneque, P., & Parrado, J. (2022). Bioremediation of an oxyfluorfen-polluted soil using biostimulants obtained by fermentation processes: Effect on biological properties. Applied Soil Ecology, 170, 929–1393. https://doi.org/10.1016/j.apsoil.2021.104270
- Tejada, M., Morillo, E., Gómez, I., Madrid, F., & Undabeytia, T. (2017). Effect of controlled release formulations of diuron and alachlor herbicides on the biochemical activity of agricultural soils. Journal of Hazardous Materials, 322, 334–347. https://doi.org/10.1016/j.jhazmat.2016.10.002
- Trevors, J. T., Mayfield, C. I., & Inniss, W. E. (1982). Measurement of electron transport system (ETS) activity in soil. Microbial Ecology, 8(2), 163–168. https://doi.org/10.1007/BF02010449
- Tudi, M., Ruan, H. D., Wang, L., Lyu, J., Sadler, R., Connell, D., Chu, C., & Phung, D. T. (2021). Agriculture development, pesticide application and its impact on the environment. International Journal of Environment Research Public Health, 18(3), 1112–1124. https://doi.org/10.3390/ijerph18031112
- Turan, M., Kıtır, N., Alkaya, Ü., Günes, A., Tüfenkçi, S., Yıldırım, E., & Nikerel, E. (2016). Making soil more accessible to plants: The case of plant growth promoting rhizobacteria. In E. C. Rigobelo (Ed.), Plant growth (pp. 61–69). https://doi.org/10.5772/64826
- Wang, B., Wen, X., & Xi, Z. (2016). Molecular simulations bring new insights into protoporphyrinogen IX oxidase/protoporphyrinogen IX interaction modes. Molecular Informatics, 35(10), 476–482. https://doi.org/10.1002/MINF.201600008
- WRB. (2014). World reference base for soil resources 2014. In International soil classification system for naming soils and creating legends for soil maps (pp. 192). Food and Agriculture Organization of the United Nations.
- Yu, H., Zou, W., Chen, J., Chen, H., Yu, Z., Huang, J., Tang, H., Wei, X., & Gao, B. (2019). Biochar amendment improves crop production in problem soils: A review. Journal of Environmental Management, 232, 8–21. https://doi.org/10.1016/j.jenvman.2018.10.117
- Zeng, Q., Ding, X., Wang, J., Han, X., Iqbal, H. M. N., & Bilal, M. (2022). Insight into soil nitrogen and phosphorus availability and agricultural sustainability by plant growth-promoting rhizobacteria. Environmental Science and Pollution Research, 29(30), 45089–45106. https://doi.org/10.1007/s11356-022-20399-4
- Zhang, Q., Zhang, L., Wang, H., Jiang, Q., & Zhu, X. (2018). Simultaneous efficient removal of oxyfluorfen with electricity generation in a microbial fuel cell and its microbial community analysis. Bioresource Technology, 250, 658–665. https://doi.org/10.1016/j.biortech.2017.11.091
- Zhao, H., Xu, J., Dong, F., Liu, X., Wu, Y., Wu, X., & Zheng, Y. (2016). Characterization of a novel oxyfluorfen-degrading bacterial strain Chryseobacterium aquifrigidense and its biochemical degradation pathway. Applied Microbiology and Biotechnology, 100(15), 6837–6845. https://doi.org/10.1007/s00253-016-7504-x
- Zhou, Y., Bastida, F., Zhou, B., Sun, Y., Gu, T., Li, S., & Li, Y. (2020). Soil fertility and crop production are fostered by micro-nano bubble irrigation with associated changes in soil bacterial community. Soil Biology & Biochemistry, 141, 107663. https://doi.org/10.1016/j.soilbio.2019.107663
