?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study investigated the effect of gaseous ozone (O3) exposure time on the postharvest quality and shelf-life of mangoes. “Keitt” mango fruit harvested at physiological maturity was exposed to 0.25 mg/L of O3 for 12, 24,36, or 48 hours, and the control fruit were untreated. Fruit were thereafter stored at 10 ℃ for three weeks and ripened at ambient temperature for one week. Postharvest parameters such as mass loss, decay incidence, firmness, total soluble solids (TSS), titratable acidity (TA), and carotenoid content were assessed at weekly intervals. The findings showed that the physiological weight loss of untreated fruit (30.92%) was significantly higher (p <0.05) compared to O3 (12 h), O3 (24 h), O3 (36 h), and O3 (48 h) treated fruit, which was 28.49%, 25.90%, 20.54%, and 20.50%, respectively. Fruit treated with O3 (36 h) significantly maintained firmness, delayed TSS accumulation, and decreased loss of TA. The untreated fruit had a higher decay incidence compared to other treatments at the end of storage. Moreover, the total carotenoid content was notably higher in O3 (48 h) treated fruit during storage. Overall, the results demonstrated that the shelf-life of mango fruit was longer under the 36 h and 48 h treatments. These research findings indicate that O3 could be used effectively to maintain the postharvest quality and extend the shelf-life of mango fruit. Therefore, O3 (36 h) is recommended as a cost-effective postharvest treatment for “Keitt” mangoes.
1. Introduction
Mango is a vital fruit due to its high nutritional properties and economic value (Hmmam et al., Citation2021). The fruit contains essential dietary compounds such as proteins, vitamins, flavonoids, phenolics, and carotenoids (Maldonado-Celis et al., Citation2019). It is highly perishable with a relatively short shelf-life due to high respiration rate and ethylene production leading to biochemical changes (Hmmam et al., Citation2021; Kaur et al., Citation2020). The increased respiration rate during mango ripening leads to enzyme activities, increased aroma, decreased firmness, and color changes (Hmmam et al., Citation2021). Various postharvest technologies are used to maintain fresh produce quality, including ozone (O3), pulsed electric field, heat treatment, ultraviolet irradiation, and chemical treatment.
Ozone is produced by electric corona discharge or ultraviolet radiation (Pandiselvam et al., Citation2019). It has high oxidizing power and antimicrobial properties that are effective against an extensive spectrum of microorganisms (Brodowska et al., Citation2018; Contigiani et al., Citation2018). The benefit of O3 is that it doesn’t leave chemical residues that pose threats to the environment and human health. Ozone is an unstable gas that decomposes rapidly to form highly reactive oxidative radicals such as superoxide anion and hydroxyl radical (Perry & Yousef, Citation2011). It is speculated that the free radicals attribute to the augmented oxidizing power of O3. The radicals are highly unstable with a short lifespan. They react with other molecules to gain the missing electron (Brodowska et al., Citation2018). Therefore, the United States of America Food and Drug Administration (FDA, 2001) declared O3 as Generally Recognized as Safe (GRAS).
Ozone extends the shelf-life of fresh produce by delaying respiration rate, ripening, and senescence. For instance, Chen et al. (Citation2020) reported that O3 (15.008 mg m− 3) reduced the respiration rate in “the west mi 25” cantaloupes during storage at 4 ℃ for 42 days. Ozone oxidizes ethylene in fresh produce during storage (Aslam et al., Citation2020). The respiration rate influences biochemical changes that initiate cell wall degradation processes, resulting in loss of firmness and ripening. Previous research has shown that O3 maintains firmness in various horticultural crops (Chen et al., Citation2020). For instance, Panou et al. (Citation2021) reported that gaseous O3 (1.0 mg/L, 40 min) maintained firmness in “Carbarosa” strawberries during storage at 1 ℃ for 16 days. Fruit softening is characterized by the modification of cell wall components, including hemicellulose, pectin, and polysaccharides leading to loss of fruit firmness (Toti et al., Citation2018). Enzyme activities play an essential role in cell wall degradation. For instance, Cardenas-Perez et al. (Citation2018) reported an increase in enzyme activities of Polygalacturonase (PG) and pectin methylesterase (PME) during mango ripening. Furthermore, the cell wall pectin methyl esters are hydrolyzed by the PME enzyme (Toti et al., Citation2018). While, the PG enzyme degrades pectic polysaccharides into water-soluble galacturonides resulting in fruit softening (Cardenas-Perez et al., Citation2018).
The carotenoid content in mango fruit is a good source of pro-vitamin A (3894 IU/100 g) (Lebaka et al., Citation2021). During fruit ripening, mango changes color from green to yellow-orange due to carotenoid biosynthesis (Liang et al., Citation2020). Ali et al. (Citation2014) reported that gaseous O3 (5 mg/L, 96 h) enhanced the β-carotene content in “Sekaki” papaya during storage at 25 ℃ for 14 days. Ozone has been shown to be effective in the carotenoid content in fresh produce (de Almeida Monaco et al., Citation2016; Minas et al., Citation2010).
Ozone is an eco-friendly postharvest technology and could provide a potential solution in maintaining fruit quality and extending the shelf-life of mango fruit. Therefore, preserving fruit quality is one of the critical solutions in sustaining food and nutritional security. This study evaluated the influence of O3 on the physicochemical quality attributes and carotenoid content of mango fruit (cv. Keitt) during cold storage.
2. Materials and methods
2.1. Fruit material
Mango fruit (cv. Keitt) were harvested at physiological maturity from Goedgelegen Farm (25° 778ʹS, 30° 447ʹE) of Westfalia (Pty) LTD (Tzaneen, South Africa). Fruit were couriered overnight under cold storage to the laboratory at the University of KwaZulu-Natal (Pietermaritzburg Campus, 29° 624ʹS, 30° 403ʹE). On arrival at the laboratory, fruit were graded for uniformity according to color and size (850–1000 g), and thereafter stored in carton boxes at 10 ℃ and 90 % relative humidity (RH) for three weeks mimicking shipment to European markets. The fruit were ripened at ambient temperature (25 ℃) and 60 % RH for one week.
2.2. Ozone treatment
The fruit were treated with 0.25 mg/L gaseous O3 equipped with an ozone analyzer (Corona discharge ozone generator, Ozone Purification Technology, Johannesburg, South Africa) during cold storage. Mango fruits were intermittently treated with O3 for 12 (day 0), 24 (day 0 & 7), 36 (day 0, 7 & 14), or 48 h (day 0, 7, 14 & 21). After each O3 treatment, the fruit was removed from a O3 cold room and stored without O3 at 10 ℃, and control fruit were untreated. A screening study was conducted to evaluate the effect of various O3 exposure times on mango fruit quality. The fruit were subjected to gaseous O3 at various levels (i.e., Level 1: 12 h; Level 2: 24 h; Level 3: 26 h, and Level 4: 48 h) during cold storage. The exposure times to O3 used in the current study were selected based on the biochemical parameters of the screening experiment which was conducted in 2018.
2.3. Fruit firmness and physiological weight loss (PWL)
Fruit firmness, expressed in Newton (N), was measured on the opposite side of the fruit’s equatorial region using a handheld firmness tester with tip dimension of 0.25 cm2, (HP-FFF, Bareiss, Germany). Fruit were weighed on arrival at the laboratory and after every seven days. The same fruit were weighed throughout the storage period. The PWL percentage was calculated using the equation by Akhtar et al. (Citation2010):
Where A indicates the fruit weight before storage and B means the fruit weight after storage.
2.4. Fruit color
Color changes in mango pulp were determined as described by González-Aguilar et al. (Citation2001) using a chromameter (Chroma Meter, Konica Minolta Sensing, INC., Japan). They were expressed as Luminosity (L), a* (red to green), b* (yellow to blue), °Hue angle (H), and Chromaticity (c) values. In triplicates, readings were done on the surface of half-cut mango pulp. Prior to scanning fruit, the chromameter was calibrated by scanning a white reference brick, y = 0.3215, Y = 87.0, X = 0.3.
2.5. Total soluble solids (TSS) and titratable acidity (TA)
Fruit were peeled, cut into cubes, and homogenized using a blender. The puree was triple=filtered using a muslin cloth, and the juice was used for determination of TSS and TA levels. The TA was tested using automated titration (Rondolino G 20’s Compact Titrator, Mettler Toledo, Schwerzenbach, Switzerland). Prior to titration, the sensor was calibrated using buffer standards with pH 7 and 4, with the slope and zero point (offset): Slope: − 59,16 mV/pH @ 25°C and Zero point: ±0 mV or pH 7.00. Juice (8 mL) was mixed with distilled water (40 mL) and titrated with 0.1 M of sodium hydroxide (NaOH) to the endpoint (pH 8.1) and expressed as % malic acid using equation (2)
TSS was analyzed using a digital refractometer (B+S RFM340+ refractometer, Bellingham and Stanley Ltd, Tunbridge Wells, Kent, United Kingdom) and reported as a percentage.
2.6. Decay index (DI)
For each treatment, nine fruits were evaluated for the DI on the peel. Fruits were classified into four groups according to their severity (Score 0–4). Score 0 (none) means no visible decay, score 1 means that there is few (1%) scattered decay, score 2 means that 2–20 % of fruit surface is covered by decay, score 3 means that there is 21–50 % decay incidence while score 4 means that there’s > 50% fruit decay. The DI index was calculated using equation 3 as defined by Khaliq et al. (Citation2016)
Where DS, NFEC, NTF, and HDS are disease scale, number of fruit in each class, number of total fruit, and highest disease, respectively.
2.7. Total carotenoid content
Total carotenoid content was measured as described by More and Rao (Citation2019) with modifications. In triplicates, 1 g of the sample was added to 14 mL of hexane: acetone (3:2 v/v) and incubated for 1.5 h in the dark. Thereafter, samples were centrifuged (Avanti J-265 XP, Beckaman Coulter, Indianapolis, USA) at 10 000 rpm for 10 min at 4 ℃. The extract was transferred to a volumetric flask and adjusted to 25 mL using hexane: acetone. The absorbance was measured at 450 nm using the spectrophotometer (Shimadzu Scientific Instruments INC., Columbia USA) against hexane: acetone as a blank. The total carotenoid content was calculated using equation 4, and results were expressed as µg g− 1 DM.
Where OD450 is the sample absorbance at 450 nm.
2.8. Determination of fruit shelf-life
The shelf-life of mango fruit was determined as described by Chamnan et al. (Citation2019). Mango fruit were evaluated for firmness and decay incidence for seven days after cold storage. The fruit were deemed unmarketable when the decay incidence was more than 0.20.
2.9. Statistical analysis
Data was subjected to analysis of variance (ANOVA) using GenStat statistical software (GenStat®, 18 edition, VSN International, UK). Means were separated using using Fischer’s least significant difference (LSD) at 5 %. Pearson correlation coefficient was used to determine relationships between physicochemical parameters.
3. Results
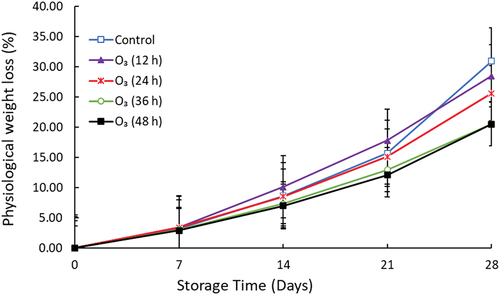
3.1. Fruit PWL
Fruit PWL (%) increased sharply during storage in treated and untreated mango fruit (Figure ). The results demonstrated that O3 significantly (p < 0.05) reduced the PWL percentage. On day 14 , fruit treated with O3 24 h (8.45%), had a high mass loss compared to O3 48 h (6.98%). At the end of storage, untreated fruits had the highest PWL (30.92%) compared to O3 (12 h), O3 (24 h), and O3 (36 h) which was 28.49%, 25.90%, and 20.54%, respectively. The treatment means of control and O3 (12 h) were not significantly different at the end of storage.
3.2. Fruit firmness
Fruit firmness decreased significantly (p < 0.01) in all treatments during storage (Table ). The fruit treated with O3 (24 and 36 h) maintained higher firmness from day 14 till the end of storage. Compared to other treatments, the control fruit and O3 (12 h) had low fruit firmness at the end of storage. However, no significant difference was observed between treatment means of O3 (24 h), O3 (36 h), and O3 (48 h).
Table 1. Effect of gaseous ozone (0.25 mg/L) on mango fruit texture (N) stored at 10 ℃ for three weeks and one-week shelf-life at ambient temperature
3.3. Fruit color
Ozone treatments significantly affected the color of mango pulp during storage. There was no significant difference between treatment means regarding the °Hue angle and b* value of mango fruit. The lightness significantly (p < 0.05) decreased in all treatments during storage (Table ). On day 14, fruit treated with O3 (12 h) had a high L* value compared to other treatments. There was no significant difference between the treatment means of O3 (24 h), O3 (36 h), and O3 (48 h) on day 14. At the end of storage, fruit treated with O3 (48 h) had a high L* value compared to O3 (12 h), O3 (36 h) and the control. The chroma values varied among treatments during storage (Table ). Chroma significantly (p < 0.05) decreased in all treatments from day 14 till the end of storage. The chroma of O3-treated fruit and control was significantly different at the end of cold storage. However, increasing the O3 exposure time decreased the chroma value of mango fruit. The fruit treated with O3 (24 h) and control had the highest chroma compared to other treatments at the end of storage. The a* value of mango fruit significantly (p < 0.05) increased in all treatments during the storage period (Table ). There was no significant difference between the treatment means of all treatments at days zero and seven. The untreated fruit had a high a* value compared to other treatments at the end of cold storage. At the end of storage, untreated fruit had a high a* value compared to O3 (12 h), O3 (24 h), O3 (48 h), and O3 (36 h).
Table 2. Changes in the L* value of mango fruit treated with O3 and stored at 10 ℃ for three week and one-week shelf-life at ambient temperature
Table 3. Changes in the chroma of mango fruit treated with gaseous ozone (0.25 mg/L) and stored at 10 ℃ for three weeks and one-week shelf-life at ambient temperature
Table 4. Changes in the a* value of mango fruit treated with gaseous ozone (0.25 mg/L) and stored at 10 ℃ for three weeks and one-week shelf-life at ambient temperature
3.4. Fruit pulp TSS and TA
The increase in TSS and a decrease in TA, play an important role in mango fruit ripening. In this study, TSS gradually increased during storage in both control and O3-treated fruit (Table ). The TSS of ozone-treated fruit was significantly (p < 0.05) different from that of the control. At day 14, there was no significant difference between the treatment means of O3-treated fruit. At the end of storage, the highest TSS was observed in untreated fruit and O3 (12 h), which was 17.38 and 17.45, respectively. The TA of untreated and treated mango fruit significantly decreased during storage. Fruit treated with O3 (36 h) had significantly (p < 0.05) higher TA compared to other treatments on day 14.
Table 5. Effect of gaseous ozone (0.25 mg/L) on mango fruit TSS (%) and TA during storage at 10 ℃ for three weeks and one-week shelf-life at ambient temperature
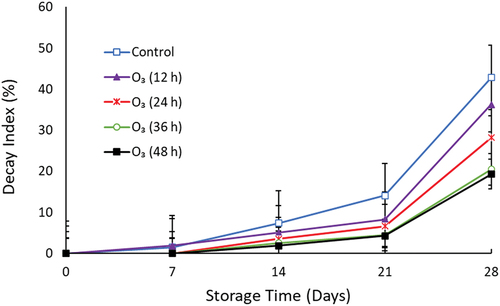
3.5. Decay index
The decay index of mango fruit varied between treatments during the storage period (Figure ). Ozone treatment significantly (p < 0.05) decreased the decay of mango fruit. The fruit showed signs of decay from day seven in control treatment and O3 (12 h). Untreated fruit had a higher decay incidence compared to other treatments from day 14 until the end of storage. At the end of storage, untreated fruit had high decay (42.83%) compared to O3 (12 h), O3 (24 h), O3 (36 h), and O3 (48 h), which were 36.26%, 28.22%, 20.52%, and 19.29%, respectively.
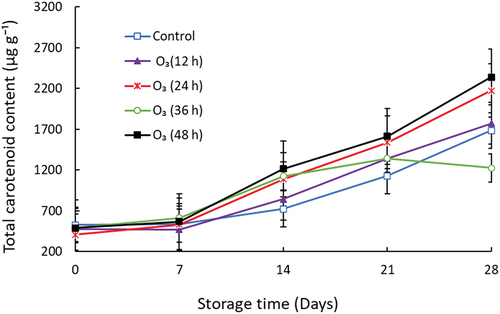
3.6. Total carotenoid
The total carotenoid content of mango fruit notably increased during storage (Figure ). Ozone treatment significantly (p < 0.05) enhanced the carotenoids of mango fruit. The highest carotenoid content was observed in O3 (48 h) from day 14 till the end of storage. On day 14, there was no significant difference between the treatment means of control, O3 (12 h), O3 (24 h), and O3 (36 h). Fruit treated with O3 (48 h) had high carotenoid content compared to O3 (24 h), O3 (12 h), and control at the end of the storage period.
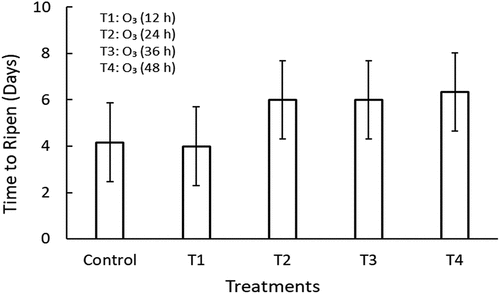
3.7. Fruit shelf-life
Ozone treatment significantly (p < 0.05) extended fruit shelf-life of mangoes. The shelf-life of the treated fruit ranged between four and six days. Untreated fruit ripened faster compared to other treatments (Figure ). However, no significant differences were observed between the ripening time of O3 (36 h) and (48 h).
3.8. Relationship between DI and mango fruit physicochemical parameters
Fruit firmness was negatively correlated to TSS (R2 = −0.72), PWL % (R2 = 0.89), carotenoids (R2 = −0.63) and DI (R2 = −0.67). The TSS was positively correlated to PWL % (R2 = 0.73) and a* value (R2 = 0.67) (Table ). A positive correlation was observed between PWL %, carotenoids (R2 = 0.76), and DI (R2 = 0.66). The TA was negatively correlated to PWL % (R2 = −0.73) and carotenoids (R2 = −0.62). Luminosity was positively correlated to the chroma (R2 = 0.86), while a weak correlation was observed between firmness and luminosity (R2 = 0.51).
Table 6. Pearson correlations coefficient of firmness, TSS, TA, carotenoids, DI, L*, and chroma in mango fruit
4. Discussion
4.1. Fruit PWL
The mass is an essential parameter as fresh horticultural produce is sold based on weight. Thus, it is imperative that postharvest treatments minimize the loss of water and fruit mass during storage. da Silva et al. (Citation2020) reported that aqueous O3 (3 mg/L) decreased the weight loss in “Palmer” mango during storage at 20 ℃ for 20 days. Physiological moisture loss occurs in fresh produce due to increased transpiration and respiration rate. The decreased fruit weight loss in O3-treated fruit could be due to retarded respiration rate (Zhang et al., Citation2011). In mango fruit, shriveling occurs when weight loss increases above 10 % and the marketability of the fruit decreases (Barman et al., Citation2014; Vázquez-Celestino et al., Citation2016). Current results indicate that the marketability of untreated fruit decreased from day 14 of storage. On the other hand, the marketability of fruit treated with O3 for 36 and 48 h decreased after 21 days of cold storage.
4.2. Fruit firmness
Loss of firmness occurs in mango fruit postharvest due to biochemical modifications resulting in softening (Yashoda et al., Citation2006). The current study showed that O3 delayed loss of firmness for 14 days in mango fruit. Similarly, Zhang et al. (Citation2021) reported that O3 (1 mg/L, for 5 h) maintained the firmness of cantaloupe melon during storage at 4 ℃ for 30 days. The retained firmness could be attributed to O3 retarding modification of cell wall degrading enzymes such as polygalacturonase and β-galactosidase. Minas et al. (Citation2014) reported that O3 (0.3 µL/L−1) inhibited the enzyme activities of polygalacturonase and endo-1,4-β-glucanase/1,4-β-glucosidase in “Hayward” kiwifruit during storage. Zhang et al. (Citation2021) reported that O3 (1 mg/L, for 5 h) down-regulated pectinesterase and polygalacturonase gene expression in cantaloupe melon. The enhanced firmness could be attributed to O3 inhibiting cell wall modifying enzymes, thus decreasing fruit softening and extending shelf-life.
4.3. Fruit color
The color changes from green to yellow occur in mango fruit postharvest, indicating ripening. The O3 treatment effectively maintained the interior color of mango fruit during storage. These findings corroborate those of Jia et al. (Citation2020), who reported that O3 (200 mg m− 3) retained the L* value in “Jin Qiu hong” peach. Ozone treatment maintained a high L* value in “Carbarosa” strawberries during storage (Panou et al., Citation2021). The color changes from green to orange-yellow were observed in both treated and untreated fruit. However, untreated fruit showed higher a* values than ozone-treated fruit. Panou et al. (Citation2021) reported a low a* value in “Carbarosa” strawberries treated with O3 (1 ppm). Similarly, Bolel et al. (Citation2019) observed decreased chroma values in “Hicaznar” pomegranates treated with O3 (4 mg/L). The high chroma values in ozone-treated fruit could be attributed to O3 delaying metabolic processes involved in color pigment degradation. Current results indicate that O3 (36 h) delayed color changes in mango fruit. Chroma suggests the intensity of the color that is affected by pigment saturation (Sousa et al., Citation2021). The changes in color from green to yellow could be attributed to chlorophyll degradation and accumulation of pigments. Mango fruit ripening is characterized by decrease in chlorophyll content. The decline in chlorophyll could be attributed to the thylakoids collapsing inside the cloroplasts.
4.4. Fruit pulp TSS and TA
Total soluble solids (TSS) is a vital indicator of maturity in horticultural crops. In the current study, O3 treatment delayed TSS accumulation. Similar results were reported by Paico et al. (Citation2018) in mango fruit treated with O3. However, Tran et al. (Citation2015) reported that O3 (10 µL L− 1) had no significant effect on TSS of “Nam Dok Mai No.4” mango fruit. The delayed TSS accumulation could be attributed to O3 inhibiting starch conversion into soluble sugars. Ozone causes oxidative shock to sugar metabolism, thus delaying and shifting the metabolic pathway (Panou et al., Citation2021). Proteins responsible for sugar metabolism, particularly sucrose synthase, are abundant in fruit during ripening (Muccilli et al., Citation2009). In the current study, O3 treatment delayed the TA loss. Jia et al. (Citation2020) reported that O3 delayed the loss of TA in “Jin Qiu hong” peach during storage. Chen et al. (Citation2020) reported that high O3 (15.008 mg m− 3) concentration delayed the TA decrease on the exocarp and sarcocarp of “West mi 25” cantaloupes during storage at 4 ℃ for 42 days. Intermittent gaseous O3 treatment was ineffective in maintaining the TA of “Hicaznar” pomegranate stored at 6 ℃ for 4 months (Buluc & Koyuncu, Citation2022). Barboni et al. (Citation2010) reported that O3 delayed the decrease of malic, citric, and quinic acids in “Hayward” kiwifruit. The high TA could be attributed to O3 modifying the metabolic processes. Buluc and Koyuncu (Citation2022) reported that O3 delays the respiration rate, thus maintaining the TA. The organic acids are utilized during respiration, as they donate the hydrogen ion. The TSS and organic acids affect fruit flavor and ripening.
4.5. Decay index
Postharvest decay reduces fruit quality and restricts the growth of the mango industry. The current results showed that high O3 exposure time decreased decay in mango fruit during storage. Similarly, Bambalele et al. (Citation2023) observed that O3 (0.25 mg/L, 36 h) decreased the mycelial growth and disease incidence of Lasiodiplodia theobromae in mango fruit stored at 10 ℃ for 21 days. Ozone (200 mg m− 3) decreased the decay percentage in “Jin Qiu hong” peach during storage (Jia et al., Citation2020). The reduced decay in treated fruit could be attributed to the high oxidizing potential of O3. Ozone enhances the flavonoid content and peroxidase activity in mango, thus increasing the disease resistance of the fruit (Bambalele et al., Citation2023). Ozone is reported to be effective against fungal diseases such as anthracnose, stem-end rot, and grey mold (Savi & Scussel, Citation2014; Terao et al., Citation2019).
4.6. Total carotenoid
The concentration of carotenoids is known to increase during mango fruit ripening. This study showed O3 treatment increased the total carotenoid content from day 14 till the end of storage. Minas et al. (Citation2010) reported that O3 (0.3 µL L− 1) treatment increased the carotenoid content in “Hayward” kiwifruit. In contrast, Toti et al. (Citation2018) reported that O3 (0.3 mg/L) was ineffective in maintaining the carotenoid content of cantaloupe melons during storage. The enhanced carotenoid content in O3-treated mango could be attributed to O3 altering the carotenoid biosynthesis pathway during fruit ripening. Carotenoid biosynthesis occurs through the methylerythritol phosphate pathway (Ma et al., Citation2018). The gene expression of phytoene desaturase, phytoene synthase, ζ-carotene desaturase, zeaxanthin epoxidase, and β-carotene hydroxylase are upregulated during mango fruit ripening and correlates with β-carotene (Ma et al., Citation2018). Ozone down-regulated VviZDS1, VviLBCY2, VviZISO1 and VviCISO1 genes in “ML1 microvine” berries (Campayo et al., Citation2021). These genes are involved in the early stages of carotenoid biosynthesis during fruit ripening. In the current study, the enhanced carotenoid content correlated with increasing orange-yellow color, indicating fruit ripening.
4.7. Fruit shelf-life
Gaseous O3 extended the shelf-life by 25 days in “Daw” longan fruit stored at 5 ℃ for 40 days (Chamnan et al., Citation2022). The current study suggests that gaseous O3 effectively extends the shelf-life of mango fruit. The extended shelf-life could be attributed to O3-inhibiting enzyme activities, biochemical changes, and fruit ripening.
4.8. Relationship between DI and mango fruit physicochemical parameters
Current results revealed a negative correlation between TSS and firmness. Mustapha et al. (Citation2020) showed that firmness was negatively correlated to TSS and TA. According to the correlation analysis, total carotenoid content was positively correlated to the a* value and TSS. Similarly, Hmmam et al. (Citation2021) observed a positive correlation between carotenoids and color in mango fruit. The carotenoid content contributes to the mango fruit color and nutritional value (Hmmam et al., Citation2021; Maldonado-Celis et al., Citation2019).
5. Conclusion
This study revealed that gaseous O3 effectively maintained the physicochemical parameters such as firmness, color, TSS, TA, and carotenoids of mango fruit during storage. The decay of mango fruit was delayed until day 14 of storage by O3 treatment. Current results indicate the O3 treatment should be applied at the pre-climacteric stage for 24 or 36 h to yield superior results. Superior results were observed in treatments that received O3 for 36 and 48 h. However, increasing O3 exposure time from 36 to 48 h yielded similar results in most physicochemical parameters. Therefore, O3 treatment for 36 h should be considered to economize costs. Current results provide the basis for developing postharvest O3 protocols for mango fruit. However, the mode of action for O3 in enhancing fruit quality warrants further investigation.
Author contribution
Conceptualization, A.M., L.S.M., and S.Z.T.; laboratory analysis, N.B.; data curation, N.B., original draft preparation, N.B.; review and editing, A.M., L.S.M., and S.Z.T.; project administration, A.M. All authors have read and agreed to the published version of the manuscript.
Availability of data
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors are grateful to Thokozani Nkosi for technical assistance in the laboratory and for the student financial support provided by Moses Kotane Institute.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
Notes on contributors
Nonjabulo L. Bambalele
Nonjabulo L. Bambalele is a Postdoctoral Researcher in Horticultural Science at the School of Agricultural Earth and Environmental Science at the University of KwaZulu-Natal, KwaZulu-Natal, Pietermaritzburg, South Africa. Her research is focused on the agronomic performance of crops and the use of ozone to maintain the quality of fresh produce.
Asanda Mditshwa
Asanda Mditshwa is an Associate Professor in the discipline of Horticultural Science at the School of Agricultural Earth and Environmental Science at the University of KwaZulu-Natal, KwaZulu-Natal, Pietermaritzburg, South Africa. His research is focused on postharvest physiology and technology with a special interest in finding effective non-chemical technologies such as dynamic controlled atmospheres, ozone, UV-C, and edible coatings for preserving quality and extending shelf-life.
Lembe S. Magwaza
Lembe S. Magwaza is an Associate professor in the discipline of Crop Science at the School of Agricultural Earth and Environmental Science at the University of KwaZulu-Natal, KwaZulu-Natal, Pietermaritzburg, South Africa. He is a C-rated researcher by the National Research Foundation of South Africa. His research is focused on non-destructive technologies of evaluating internal quality of fresh produce; as well as developing strategies to maximize the postharvest shelf life of different crops.
Samson Z. Tesfay
Samson Z. Tesfay is an Associate in the discipline of Horticultural Science at the School of Agricultural Earth and Environmental Science at the University of KwaZulu-Natal, KwaZulu-Natal, Pietermaritzburg, South Africa. His research is focused on fruit biochemistry, plant antioxidants, and climate-smart crops such as moringa.
References
- Akhtar, A., Abbasi, N. A., & Hussain, A. (2010). Effect of calcium chloride treatments on quality characteristics of loquat fruit during storage. Pakistan Journal of Botany, 42, 181–14.
- Ali, A., Ong, M. K., & Forney, C. F. (2014). Effect of ozone pre-conditioning on quality and antioxidant capacity of papaya fruit during ambient storage. Food chemistry, 142, 19–26.
- Aslam, R., Alam, M. S., & Saeed, P. A. (2020). Sanitization potential of ozone and its role in postharvest quality management of fruits and vegetables. Food Engineering Reviews, 12(1), 48–67. https://doi.org/10.1007/s12393-019-09204-0
- Bambalele, N. L., Mditshwa, A., Mbili, N. C., Tesfay, S. Z., & Magwaza, L. S. (2023). The antifungal effect of gaseous ozone on Lasiodiplodia theobromae causing stem-end rot in ‘Keitt’ mangoes. Foods, 12(1), 195. https://doi.org/10.3390/foods12010195
- Barboni, T., Cannac, M., & Chiaramonti, N. (2010). Effect of cold storage and ozone treatment on physicochemical parameters, soluble sugars and organic acids in Actinidia deliciosa. Food Chemistry, 121(4), 946–951. https://doi.org/10.1016/j.foodchem.2010.01.024
- Barman, K., Asrey, R., Pal, R. K., Jha, S. K., & Bhatia, K. (2014). Post-harvest nitric oxide treatment reduces chilling injury and enhances the shelf-life of mango (Mangifera indica L.) fruit during low-temperature storage. Journal of Horticultural Science and Biotechnology, 89(3), 253–260. https://doi.org/10.1080/14620316.2014.11513076
- Bolel, H., Koyuncu, M. A., & ErbaŞ, D. (2019). The combined effect of controlled atmosphere with ozone and prochloraz treatment on storage life and quality of pomegranate cv. Hicaznar Akademik Ziraat Dergisi, 8(2), 195–202. https://doi.org/10.29278/azd.555195
- Brodowska, A. J., Nowak, A., & Smigielski, K. (2018). Ozone in the food industry: Principles of ozone treatment, mechanisms of action, and applications: An overview. Critical Reviews in Food Science and Nutrition, 58(13), 2176–2201. https://doi.org/10.1080/10408398.2017.1308313
- Buluc, O., & Koyuncu, M. A. (2022). Ozone: A promising alternative to prochloraz for cold storage of pomegranate. Journal of Food Science and Technology, 59, 2731–2740. https://doi.org/10.1007/s13197-021-05295-y
- Campayo, A., Savoi, S., Romieu, C., López-Jiménez, A. J., de la Hoz, K. S., Salinas, M. R., Torregrosa, L., & Alonso, G. L. (2021). The application of ozonated water rearranges the Vitis vinifera L. leaf and berry transcriptomes eliciting defence and antioxidant responses. Scientific Reports, 11(1), 1–14. https://doi.org/10.1038/s41598-021-87542-y
- Cardenas-Perez, S., Chanona-Perez, J. J., Guemes-Vera, N., Cybulska, J., Szymanska-Chargot, M., Chylinska, M., Koziol, A., Gawkowska, D., Pieczywek, P. M., & Zdunek, A. (2018). Structural, mechanical and enzymatic study of pectin and cellulose during mango ripening. Carbohydrate Polymers, 196, 313–321. https://doi.org/10.1016/j.carbpol.2018.05.044
- Chamnan, S., Varith, J., Jaturonglumlert, S., Klinkajorn, P., & Phimphimol, J. (2019). The effect of packaging materials on the quality of freshness of longan fumigated with medium concentration-ozone gas. Pertanika Journal of Science & Technology, 27, 159–168.
- Chen, C., Zhang, H., Zhang, X., Dong, C., Xue, W., & Xu, W. (2020). The effect of different doses of ozone treatments on the postharvest quality and biodiversity of cantaloupes. Postharvest Biology and Technology, 163, 111124. https://doi.org/10.1016/j.postharvbio.2020.111124
- Contigiani, E. V., Jaramillo-Sánchez, G., Castro, M. A., Gómez, P. L., & Alzamora, S. M. (2018). Postharvest quality of strawberry fruit (Fragaria x Ananassa Duch cv. Albion) as affected by ozone washing: Fungal spoilage, mechanical properties, and structure. Food and Bioprocess Technology, 11(9), 1639–1650. https://doi.org/10.1007/s11947-018-2127-0
- da Silva, E., Neto, A. F., Silva, J., Donzeli, V., Costa, M., Costa, J., & Figueiredo, R. (2020). Effects of sanitizers on the control of Alternaria sp. Fungus for ‘Palmer’ mango conservation. The Journal of Agricultural Science, 12(9), 216–226. https://doi.org/10.5539/jas.v12n9p216
- de Almeida Monaco, K., Costa, S. M., Minatel, I. O., Correa, C. R., Calero, F. A., Vianello, F., & Lima, G. P. P. (2016). Influence of ozonated water sanitation on postharvest quality of conventionally and organically cultivated mangoes after postharvest storage. Postharvest Biology and Technology, 120, 69–75. https://doi.org/10.1016/j.postharvbio.2016.05.003
- González-Aguilar, G. A., Buta, J. G., & Wang, C. Y. (2001). Methyl jasmonate reduces chilling injury symptoms and enhances colour development of ‘Kent’ mangoes. Journal of the Science of Food and Agriculture, 81(13), 1244–1249. https://doi.org/10.1002/jsfa.933
- Hmmam, I., Zaid, N. M., Mamdouh, B., Abdallatif, A., Abd-Elfattah, M., & Ali, M. (2021). Storage behavior of “Seddik” mango fruit coated with CMC and guar gum-based silver Nanoparticles. Horticulturae, 7(3), 44. https://doi.org/10.3390/horticulturae7030044
- Jia, X., Li, J., Du, M., Zhao, Z., Song, J., Yang, W., Zheng, Y., Chen, L., & Li, X. (2020). Combination of low fluctuation of temperature with TiO2 photocatalytic/ozone for the quality maintenance of postharvest peach. Foods, 9(2), 234. https://doi.org/10.3390/foods9020234
- Kaur, K., Kaur, G., & Brar, J. S. (2020). Pre-harvest application of hexanal formulations for improving post-harvest life and quality of mango (Mangifera indica L.) cv. Dashehari. Journal of Food Science and Technology, 57(11), 4257–4264. https://doi.org/10.1007/s13197-020-04464-9
- Khaliq, G., Mohamed, M. T. M., Ding, P., Ghazali, H., & Ali, A. (2016). Storage behaviour and quality responses of mango (Mangifera indica L.) fruit treated with chitosan and gum arabic coatings during cold storage conditions. International Food Research Journal, 23(Suppl), S141–S148.
- Lebaka, V. R., Wee, Y.-J., Ye, W., & Korivi, M. (2021). Nutritional composition and bioactive compounds in three different parts of mango fruit. International Journal of Environmental Research and Public Health, 18(2), 741. https://doi.org/10.3390/ijerph18020741
- Liang, M., Su, X., Yang, Z., Deng, H., Yang, Z., Liang, R., & Huang, J. (2020). Carotenoid composition and expression of carotenogenic genes in the peel and pulp of commercial mango fruit cultivars. Scientia horticulturae, 263(2020), 109072. https://doi.org/10.1016/j.scienta.2019.109072
- Maldonado-Celis, M. E., Yahia, E. M., Bedoya, R., Landazuri, P., Loango, N., Aguillon, J., Restrepo, B., & Guerrero Ospina, J. C. (2019). Chemical composition of mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Frontiers in Plant Science, 10, 1073. https://doi.org/10.3389/fpls.2019.01073
- Ma, X., Zheng, B., Ma, Y., Xu, W., Wu, H., & Wang, S. (2018). Carotenoid accumulation and expression of carotenoid biosynthesis genes in mango flesh during fruit development and ripening. Scientia horticulturae, 237, 201–206. https://doi.org/10.1016/j.scienta.2018.04.009
- Minas, I. S., Karaoglanidis, G. S., Manganaris, G. A., & Vasilakakis, M. (2010). Effect of ozone application during cold storage of kiwifruit on the development of stem-end rot caused by Botrytis cinerea. Postharvest Biology and Technology, 58(3), 203–210. https://doi.org/10.1016/j.postharvbio.2010.07.002
- Minas, I. S., Vicente, A. R., Dhanapal, A. P., Manganaris, G. A., Goulas, V., Vasilakakis, M., Crisosto, C. H., & Molassiotis, A. (2014). Ozone-induced kiwifruit ripening delay is mediated by ethylene biosynthesis inhibition and cell wall dismantling regulation. Plant Science: An International Journal of Experimental Plant Biology, 229, 76–85. https://doi.org/10.1016/j.plantsci.2014.08.016
- More, S., & Rao, T. R. (2019). Elicitor-mediated sanitization in combination with edible coatings improve postharvest shelf life and antioxidant potential of mango fruit. Environment Experimental Biology, 17, 107–114. https://doi.org/10.22364/eeb.17.11
- Muccilli, V., Licciardello, C., Fontanini, D., Russo, M. P., Cunsolo, V., Saletti, R., Recupero, G. R., & Foti, S. (2009). Proteome analysis of Citrus sinensis L.(Osbeck) flesh at ripening time. Journal of Proteomics, 73(1), 134–152. https://doi.org/10.1016/j.jprot.2009.09.005
- Mustapha, A. T., Zhou, C., Wahia, H., Amanor-Atiemoh, R., Otu, P., Qudus, A., Fakayode, O. A., & Ma, H. (2020). Sonozonation: Enhancing the antimicrobial efficiency of aqueous ozone washing techniques on cherry tomato. Ultrasonics Sonochemistry, 64, 105059. https://doi.org/10.1016/j.ultsonch.2020.105059
- Paico, M. J. P., Jittanit, W., Jarupan, L., & Chonhenchob, V. (2018). Effect of ozone on prolonging the shelf life of mango and Broccoli during cold storage and distribution. Proceedings of the 21st IAPRI World Conference on Packaging, Zhuhai, China.
- Pandiselvam, R., Subhashini, S., Banuu Priya, E., Kothakota, A., Ramesh, S., & Shahir, S. (2019). Ozone based food preservation: A promising green technology for enhanced food safety. Ozone: Science & Engineering, 41(1), 17–34. https://doi.org/10.1080/01919512.2018.1490636
- Panou, A. A., Akrida-Demertzi, K., Demertzis, P., & Riganakos, K. A. (2021). Effect of gaseous ozone and heat treatment on quality and shelf life of fresh strawberries during cold storage. International Journal of Fruit Science, 21(1), 218–231. https://doi.org/10.1080/15538362.2020.1866735
- Perry, J. J., & Yousef, A. E. (2011). Decontamination of raw foods using ozone-based sanitization techniques. Annual Review of Food Science and Technology, 2(1), 281–298. https://doi.org/10.1146/annurev-food-022510-133637
- Savi, G. D., & Scussel, V. M. (2014). Effects of ozone gas exposure on Toxigenic Fungi species from Fusarium, aspergillus and Penicillium Genera. Ozone: Science & Engineering, 36(2), 144–152. https://doi.org/10.1080/01919512.2013.846824
- Sousa, F. F., Pinsetta Junior, J. S., Oliveira, K., Rodrigues, E. C. N., Andrade, J. P., & Mattiuz, B. H. (2021). Conservation of ‘Palmer’ mango with an edible coating of hydroxypropyl methylcellulose and beeswax. Food Chemistry, 346, 128925. https://doi.org/10.1016/j.foodchem.2020.128925
- Terao, D., de Lima Nechet, K., Frighetto, R. T. S., & Sasaki, F. F. C. (2019). Ozonated water combined with heat treatment to control the stem-end rot of papaya. Scientia horticulturae, 257, 108722. https://doi.org/10.1016/j.scienta.2019.108722
- Toti, M., Carboni, C., & Botondi, R. (2018). Postharvest gaseous ozone treatment enhances quality parameters and delays softening in cantaloupe melon during storage at 6 °C. Journal of the Science of Food and Agriculture, 98(2), 487–494. https://doi.org/10.1002/jsfa.8485
- Tran, T. T. L., Aiamla-Or, S., Srilaong, V., Jitareerat, P., Wongs-Aree, C., & Uthairatanakij, A. (2015). Ozone fumigation to delay ripening of mango ‘Nam Dok Mai no 4’. Proceedings of the IInd Southeast Asia Symp. on Quality Management in Postharvest Systems, Vientiane (Laos).
- Vázquez-Celestino, D., Ramos-Sotelo, H., Rivera-Pastrana, D. M., Vázquez-Barrios, M. E., & Mercado-Silva, E. M. (2016). Effects of waxing, microperforated polyethylene bag, 1-methylcyclopropene and nitric oxide on firmness and shrivel and weight loss of ‘Manila’ mango fruit during ripening. Postharvest Biology and Technology, 111, 398–405. https://doi.org/10.1016/j.postharvbio.2015.09.030
- Yashoda, H. M., Prabha, T. N., & Tharanathan, R. N. (2006). Mango ripening: Changes in cell wall constituents in relation to textural softening. Journal of the Science of Food and Agriculture, 86(5), 713–721. https://doi.org/10.1002/jsfa.2404
- Zhang, X., Tang, N., Zhang, H., Chen, C., Li, L., Dong, C., & Cheng, Y. (2021). Comparative transcriptomic analysis of cantaloupe melon under cold storage with ozone treatment. Food Research International (Ottawa, Ont), 140, 109993. https://doi.org/10.1016/j.foodres.2020.109993
- Zhang, X., Zhang, Z., Wang, L., Zhang, Z., Li, J., & Zhao, C. (2011). Impact of ozone on quality of strawberry during cold storage. Frontiers of Agriculture in China, 5(3), 356–360. https://doi.org/10.1007/s11703-011-1053-y