?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Raw milk preservation encourages for dairy producers and milk processing plants. This study aimed to investigate the preservative and microbial inhibitory potentials of lactoperoxidase systems (LPs), at natural storage temperature. The milk was collected from a Friesian dairy cows farm right after hand milking from individual cows, bulked to a 50-l milk can and sampled 6 l of milk. Raw milk was divided as activated and un-activated (control). LP enzyme was activated within 2 hours after milking by adding sodium thiocyanate 14 ml (1 mg/ml) and 10 ml hydrogen peroxide (1 mg/ml) per liter. Acidity development and microbial counts were assessed. Results revealed that LPs activation had significantly (P < 0.05) extended the shelf life 8 hours. Activated milk had titratable acidity (0.19) and pH (6.52) value within the acceptable level of Ethiopian Standard up to 12 hours, while lower pH (6.24) and higher titratable acidity (0.21) were recorded in control milk. Activated milk was significantly (p < 0.05) lower in the total bacteria, yeast and mould, coliform and Staphylococcus counts. After 6 hours, total bacterial count in the control group increased to 7.36 log cfu/ml, while in activated milk it was 6.24 log cfu/ml and significantly lower (p < 0.05) from the initial count. LPs decreased the coliform count by 0.45 log cfu/ml as compared to 0.49 log cfu/ml increased in control group. LPs reduced in the Staphylococcus count by 23.13% after 12 hours of storage. LPs can be used to prolong the shelf life and safeguard the microbiological quality of raw cow’s milk where cooling facilitates are unavailable.
PUBLIC INTEREST STATEMENT
The Ethiopian dairy sector is highly dominated by smallholder dairy producers. Milk production and quality are frequently mishandled in relation to handling practices, collection logistics, high ambient tropical temperature, and the production of small volume. Milk processors are challenged with the supply of inferior quality. However, demand for high quality of milk and dairy products has grown steadily and continues to grow. Linking the rural smallholder dairy farmers with the milk processors by providing high-quality inputs is extremely important. Lactoperoxidase system is a viable preservation alternative for milk where cooling facilities are inaccessible. Therefore, this study was aimed to determine the preservation potential and microbiological inhibition of lactoperoxidase activation of raw cow’s milk in Hawassa Great African Rift Valley.
1. Introduction
Preservation of raw milk encourages for dairy farmers and milk processing industries to concentrate around centralized locations to minimize wastage. It also decreases transportation cost by facilitating the collection of raw milk from more remote areas at delayed frequencies (Srisaikham et al., Citation2017). Refrigeration is the preferred method for preventing the deterioration of raw milk until processed or consumed. However, the refrigeration system may not be available to all smallholder dairy producers in rural areas in developing countries. The refrigeration systems are also expensive with high purchasing and running costs and technical problems with a lack and unreliable electricity power supply (Food and Agriculture Organization [FAO], Citation2013).
In Ethiopia, the traditional smallholder dairy producers dominate the milk production system by contributing 97–98% to the annual milk production (Ayza & Olikamo, Citation2020; Demissu et al., Citation2014; FAO, Citation2017). The milk quality is affected by factors related to husbandry practices, collection logistics, high ambient tropical temperatures and the production of small batches. All these have affected the quality of milk delivered to the milk processing plants. The low quality and unsteady milk supply results in a supply deficit, with dairy processors operating below capacity (Farrell, Citation2021). The poor quality of the supplied milk caused by poor handling, absence of cooling facilities, unstable power supply and low supply of milk within the formal system (Brasesco et al., Citation2019; Gebreselassie, Citation2020). Collaborative efforts to support smallholder farmers are needed to improve milk supply and quality (Farrell, Citation2021).
Under such condition, alternative preservation methods are required to maintain the quality of raw milk until transported from the milk producers to the site of processing plants. The best alternative could be using the approved (FAO/WHO, Citation2005), safe and natural milk preservation technique by activating milk LPs. Lactoperoxidase system has a wide application in the dairy industry, particularly in raw milk preservation in situations where prompt refrigeration is difficult, and especially in developing countries (Silva et al., Citation2020).
Naturally, normal bovine milk contains approximately 70 indigenous enzymes, including lactoperoxidase (Walstra et al., Citation2006). Lactoperoxidase is a natural constituent of milk synthesized and released from alveolar epithelial cells of the mammary gland (Zeynep et al., Citation2016). LPs activation comprises three essential components: the enzyme lactoperoxidase, thiocyanate, and hydrogen peroxide (Golmohamadi et al., Citation2016; Silva et al., Citation2020). Lactoperoxidase enzyme, however, requires the addition of different concentrates of hydrogen peroxide and thiocynate to be activated. Therefore, the exogenous addition of substrates is essential to prolong the antimicrobial activity of the system (Puspitarini et al., Citation2013). Köksal et al. (Citation2016) indicated that in the presence of hydrogen peroxide, the system catalyzes the conversion of thiocyanate to hypothiocyanate, which is antibacterial. Lactoperoxidase also oxidizes thiocyanate to hypothiocyanous acid (HOSCN). At the pH of fresh milk HOSCN is detached and exists mainly in the form of hypothiocyanate ions (OSCN−). This agent reacts specifically with free sulfhydryl groups, inactivating several vital metabolic enzymes and blocking their metabolism and ability to multiply (Bafort et al., Citation2014).
Milk quality is highly determined by microbial load (Amakelew et al., Citation2015; Fatine et al., Citation2012). The knowledge bases and the practical needs should for farmers, processors and consumers to increase the use of the LPs (Ponce, Citation2010). Staphylococcus is frequently associated with milk born disease due to the ability of some strains (e.g., toxins produced by S. aureus) in producing heat stable toxins which can result health risk (Worku et al., Citation2012). The LPs are both bactericidal and bacteriostatic for S. aures, Pseudomonas aeruginosa and Escherichia coli in milk (Shariat et al., Citation2015). The LPs preservation technique is an economically viable option to significantly reduce milk losses and increase milk availability (Kirdar & Atamer, Citation2021; Silva et al., Citation2020). However, little work has been done in the Ethiopian Rift Valley climate regarding milk preservation using LPs compared to developing tropical counties like Kenya. This study was, thus, designed to assess improvement in shelf life and microbial inhibitory potential of LPs activation in raw cow’s milk at room temperature under farmers’ storage condition in the Ethiopia Rift Valley Climate.
2. Materials and methods
2.1. Description of the experiment site
The experimental study was carried out at Hawassa University College of Agriculture. Hawassa is situated in the Great African Rift Valley at about 275 km south of Addis Ababa capital of Ethiopia. Hawassa is located at 6° 83’ to 7° 17’N and 38°24’ to 38° 72’E. It has an altitude of 1694 m.a.s.l. Its annual rainfall ranges from 700 to 1200 mm. The minimum and maximum temperatures are 13.5°C and 27.6°C, respectively (NMA-Hawassa Branch Directorates, 2012).
2.2. Milk sources and lactoperoxidase activation procedures
Freshly milked cow’s milk was collected from Hawassa University's Friesian dairy cow farm right after hand milking from individual cows, bulked in 50-l milk can, and sampled 6 l of milk (three L of milk per treatment). The raw milk sample was grouped into LPs activated and un-activated, representing the control group. Lactoperoxidase enzyme activation was made within 2 hours after milking by adding 14 ml of freshly prepared solution of 1 mg/ml sodium thiocyanate per liter of milk as thiocyanate (SCN) ion source. Following 1 min of detailed mixing, 10 ml of freshly prepared solution of 1 mg/ml hydrogen peroxide was added and thoroughly mixed for 1 min according to the methods outlined by (International Dairy Federation [IDF], Citation1988).
2.3. Experimental arrangement of treatments
The current study had two treatments: LPs activated and un-activated as control and five levels of evaluation times (0, 3, 6, 9, and 12 h). The shelf of milk was evaluated until it got spoiled. Titratable acidity, pH, and microbial growth in both activated and un-activated milk groups at the same temperature (25 ± 2°C) were recorded.
2.4. Laboratory analysis
2.4.1. Physical test
An alcohol test (68%) is used to check milk’s freshness, fitness, and shelf life for further processing (Kurwijila et al., Citation1992). Evaluation of LPs fermentation characteristics of milk was monitored by measuring the titratable acidity and pH (Helen & Seifu, Citation2007). pH was measured with a pH meter (Orion research Inc., Cambridge, MA). Lactic acid production was expressed in percent lactic acid. It was determined by titration of 9 ml of milk sample by addition of three drops of phenolphthalein indicator until the color changed to light pink with NaOH (0.1N). Percent lactic acid was expressed as outlined by Kakar et al. (Citation2013) using the following formula.
2.4.2. Microbial analysis
In determining microbiological counts, appropriate agars were used (Table ). Serial dilution was made by adding 1 ml of milk samples into sterile test tubes containing nine-milliliter peptone water up to a serial dilution of 10−7 and systematically mixed. Appropriate decimal dilutions were chosen carefully to would give the predictable number of colonies on a plate (between 30 and 300). Samples with triplicate were placed on a petri-dish and then molten agar medium (15–20 ml) was poured onto the petri-dish and mixed carefully (Yousef & Carlstrom, Citation2003).
Table 1. Species of microorganisms, growth media and incubation durations
2.5. Statistical analysis
The variability between treatments was analyzed with SAS software version 9.4 (SAS Institute, USA). First, the colony-forming units per milliliter (CFU/ml) were transforming in to natural logarithms. General Linear Model producers of SAS were used to determine statistical differences (P < 0.05) between treatments from repeated measures. The Differences in means were separated through the Duncan test at a 5% level. The laboratory experiment was done in triplicates.
3. Results
3.1. Shelf life, fermentation and microbial population counts
3.1.1. Preservation potential of system for raw cow’s milk
In the alcohol test, the formation of flocculates indicated that milk is not fresh; rather, it has turned sour and is not suitable for pasteurization and further processing. The Ethiopian standards (ES 3471) state that alcohol test should be negative (Ethiopian Standard [ES], Citation2009) for fresh milk. The result from the current study revealed that activation of LPs extended significantly (P < 0.05) the shelf life of treated milk. The control milk group was acceptable for only up to 8 ± 0.577 SE hours with a negative alcohol test. However, activated milk remained acceptable up to 16.33 ± 2.028 SE hours of storage. Therefore, activating the LPs of cow’s milk would give the milk extra 8 hours.
3.1.2. Acidity development in stored raw milk
The pH value of the control milk sample dropped more quickly than the treated milk samples (Figure ). pH level between the control and LPs activated milk samples showed a significant difference because LPs control of acidity development in milk by inhibiting the activity of lactic acid bacteria (LAB). Under the current study, LPs activation enabled milk to be within the acceptable standard pH level of 6.52 for 12 hours.
Figure 1. Lps effects in determining pH and titratable acidity of cow’s milk maintained at 25±2°C. TAA- Titratable acidity of LPs treated milk; TAC – titratable acidity of control milk samples. pHA- pH value of lactoperoxidase activated milk sample. pHC- pH value of control sample. Samples with acidity >0.20% recorded as rejected.
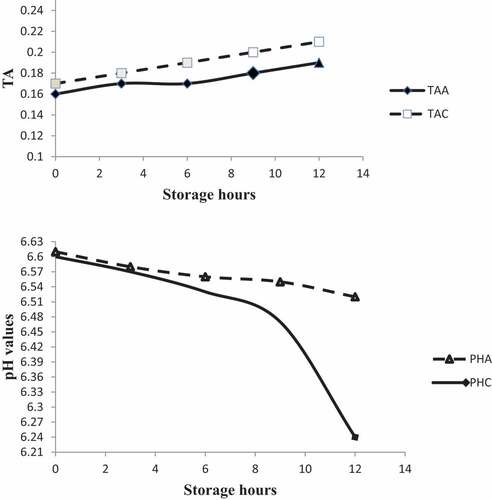
According to the Ethiopian standards fresh cow milk should have a pH value of 6.5 to 6.7 (ES, Citation2009). Milk delivered at processing plants is often checked on arrival for acidity since milk acidity is an essential indicator of milk quality. Milk having acidity above specific limits is downgraded or rejected since high acidity reduced its keeping quality and heat stability. As presented in Figure , lactoperoxidase activated milk has a titratable acidity level that is within the normal limits of raw milk (0.19) up to 12 hours as compared to control which had 0.21% titratable acidity.
3.1.3. Microbial inhibition potential of lacto peroxidase system for cow’s milk
3.1.3.1. Total bacterial count
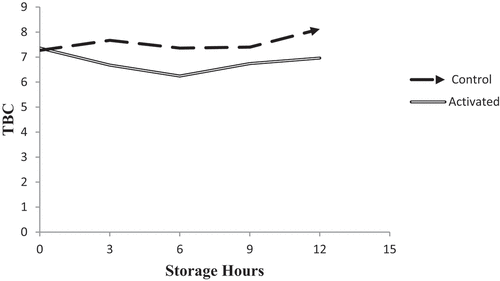
The result revealed that the total bacterial count in LPs activated milk samples was significantly lowered (P < 0.05%) than that of the control milk samples at 3, 6 and 12 hours (Figure ). However, the total bacterial count was comparable in LPs treated and control samples after 9 hours of storage. Total bacterial count in the control group increased to 8.12 log CFU/ml after 12 hours of storage, while in LPs activated milk sample, the total bacterial load was suppressed to log 6.96 cfu/ml, which is even lower than the initial count. LPs significantly (p < 0.05) lowered the count after 6 hours of storage (Figure ).
3.1.3.2. Coliform count
The coliform count was lower (P < 0.05) in LPs activated samples than from the control milk after 6, 9 and 12 hours of storage. Activated milk had an initial count of 5.72 log cfu/ml, which raised to only 5.89 log cfu/ml after 3 hours and reduced to 5.27 log 10 cfu/ml at 6 hours, 5.81 log 10 cfu/ml at 9 hours and 6.18 log cfu/ml at 12 hours. In comparison, the control milk was recorded with an initial count of 5.73 log 10 cfu/ml, were growing to 6.12 log 10 cfu/ml at three hours, 6.22 log 10 cfu/ml at six hours, 7.08 log 10 cfu/ml at 9 hours and 7.59 log 10 cfu/ml/ml at 12 h (Table ) LPs maintained the initial coliform count up to 12 hours of storage. However, in the control group, coliform counts increased significantly (P < 0.05) throughout storage times.
Table 2. Lps activation effects on coliform counts raw cow’s milk stored at 25 ± 2°C for 12 h
3.1.3.3. Lactic Acid Bacteria (LAB)
The present study found that LP enzyme activation did not cause a significance (P > 0.05) impact on the LAB load although there was a reduction after 3, 6 and 9 hours of storage by 2.63%, 1.47% and 6.49%, respectively, between treated and control milk samples (Table ). Significant differences were also not observed for LAB counts among counts of different storage hours for both activated and control milk. This might be due to the bacteriostatic effects of LPs on the LAB. LPs suppress the growth of LAB and affect the rate of acid production (Parveen et al., Citation2016). The effect of LPs on LAB obtained in this study agrees with the finding by Ay and Bostan (Citation2016), who reported that LPs had a bacteriostatic effect on the LAB without a significant impact on their load. A review by Seifu et al. (Citation2005) also revealed that antimicrobial compounds or agents formed by the lactoperoxidase system show antibacterial activity and reduced starter activities during the manufacturing of fermented dairy products.
Table 3. Lps activation effects on the LAB counts (log 10cfu/ml) of raw cow’s milk stored at 25 ± 2°C
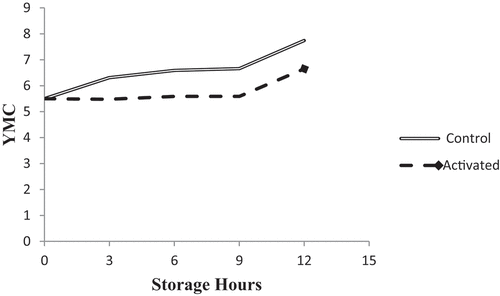
3.1.3.4. Yeast and Mold Counts (YMC)
Differences in YMC between the treatments under the current study revealed anti-yeast-mold activity of the LPs. A decrease in yeast and mould count by 0.83, 1.00, 1.07 and 1.09 cfu/ml were observed in LPs activated samples at 3, 6, 9 and 12 hours of storage, respectively, as compared to the control group, as shown in Figure . Initially, at 0 hour of storage yeast and mould counts were found to be log 5.50 cfu/ml in both samples. However, as storage time increased, significant differences were recorded between activated and control, and significantly (P < 0.05) lower counts were counted for LPs activated samples.
3.1.3.5. Staphylococcus counts
Activation of LP enzyme had affected Staphylococcus count with a slight reduction by 1.55% in the beginning, increasing its antibacterial potential after hours of storage and excreting its maximum effect at 12 hours by reducing 23.13%. In the control milk sample, among the average counts of Staphylococcus at different storage hours significantly higher count was recorded at 6 hours of storage. However, within LPs activated milk at different storage hours, there were no significant differences (P < 0.05) in the Staphylococcus count. This could be attributed to the inhibitory and deleterious effects of LPs activation on the Staphylococcus count for 12 hours of storage (Table ).
Table 4. The LPs activation effects on Staphylococcus count of raw milk stored at 25 ± 2°C
4. Discussion
The present study revealed that activating of the LPs of Cow milk would give the milk extra 8 hours. Previous works also reported that the LPs maintained the milk qualities and significantly extended its shelf life without refrigeration until milk can be processed. A study by Pokharel and Laldas (Citation2012) on station indicated that LPs significantly increased the shelf life of raw cow’s milk with the extended average shelf life of 6 hours at room temperature (25 ± 2°C), which is lower by 2 hours as compared to the current study result. A study by Assen et al. (2023) revealed the LPs prolonged the shelf-life of milk and provided sufficient time for the milk to be stored, transported, and sold in its fresh state. On-farm study in the central highlands of Ethiopia by Firew et al. (Citation2013) reported that under room temperature (22.5°C) storage conditions, the milk treatment by the lactoperoxidase system extended the shelf life of milk by 7.5 hours than control milk. Helen and Seifu (Citation2007) also reported that LPs with container smoking increased the shelf life of treated milk for additional 7 hours. Fanta et al. (Citation2019) reported that the LPs can extend the shelf life of cow’s milk ranging from 6 to 12 hours without deterioration. A study by Gershom and Ssemakula (Citation2017) also reported that the shelf life of raw up to 7 to 8 hours at 30°C by lactoperoxidase system. The differences observed among the different reports could be related to the initial quality of milk and the storage temperature differences (Lara-Aguilar & Alcaine, Citation2019; Silva et al., Citation2020).
Lactoperoxidase activation enabled milk to be within the acceptable standard pH level of 6.52 up to 12 hours. The finding is agrees with previous studies (Campos-Vallejo et al., Citation2017; Firew et al., Citation2013; Puspitarini et al., Citation2013). Puspitarini et al. (Citation2013) revealed that the pH level of activated milk was in the range of quality standard of fresh milk 6.49, while the pH of milk from the non-activated group fell below the standard level of fresh milk. The use of the LPs activation preserved a stable pH in treated milk with 6.74, 6.73 and 6.68, at 0, 4 and 8 hours of storage, respectively as compared to control, which had pH value of 6.71, 6.4 and 6.13 at 0, 4 and 8 hours of storage, respectively (Campos-Vallejo et al., Citation2017). Control milk shows quicker quality deterioration in terms of the shelf life than the LPs activated milk (Firew et al., Citation2013).
With respect to milk acidity level, LPs keep the acidity of the milk in normal condition up to 12 hours. A study indicated that LPs retained the acidity of the milk normal condition up to 0.18% for 12 hours, while control milk exceeded this value after some hours at ambient temperature (Campos-Vallejo et al., Citation2017). Galeano Lopez (Citation2012) also demonstrated that LPs conserved milk had acidity in the normal range, between 0.15% and 0.17% of lactic acid. In control milk, the acidity gradually increased to 0.25% from 0.18% which falls outside the normal range. A study by Helen and Seifu (Citation2007) reported that the LPs delay acid development up to 7-hour storage at 22°C to 23°C. Ponce (Citation2007) also found that the LPs were inhibiting the fermentation of milk carbohydrate lactose to lactic acid, proteolysis and lipolysis preserving and stabilizing the milk components for 12 hours. LPs affect the rate of acid production for the reason that it suppresses the growth of LAB (Parveen et al., Citation2016).
The current study results indicated that LPs inhibited the rapid multiplication of microflora initially present in milk. This might be due to the essentially bacteriostatic (stop/inhibited bacterial growth) nature of the LPs (Silva et al., Citation2020). The oxidation of substrate SCN in the presence of hydrogen peroxide, to an unstable product hypothiocyanate (OSCN0, which has antibacterial properties, also damages the inner membrane of bacterial cells, causing leakage and interruption of uptake of nutrients, leading to lysis and death of the organisms. The oxidation of—SH groups in the bacterial cytoplasmic membrane causes a loss of the ability to transport glucose and in leakage of potassium ions, amino acids and peptides (Al-Baarri et al., Citation2019; Sisecioglu et al., Citation2010).
Regarding total bacterial count, the result from the current study is similar to the findings reported by Hamid and Musa (Citation2013), where significant differences in total bacterial count due to the LPs activation were reported to be associated with killing and inhibition effects. In another study, LPs supported bacterial growth to be controlled for 12 hours stored at 25°C and 24 hours stored at 16°C (Munsch-Alatossava et al., Citation2016). Similarly, Helen and Seifu (Citation2007) indicated that in LPs, activated milk was decreased in the total bacterial count after 7 hours of storage compared to the initial count, with the suggestion that activation can keep the quality of raw cow’s milk for at least 7 hours at ambient temperature. A study by Ismail et al. (Citation2016) also revealed significant variation between the control and LPs activated milk in total bacterial counts. Lactoperoxidase enzyme activating exhibited decreased microbial population, and has been reported to be an effective milk preservative method mainly in the area where there is a lack of cooling facilitates (Silva et al., Citation2020; Srisaikham et al., Citation2017).
LPs inhibited the rapid multiplication of coliform counts at ambient storage conditions. The current study results agree with Ponce (Citation2010), who found that activating the LPs had affected the total coliform counts; at the beginning LPs had the bacteriostatic effect with slightly reduction of the bacterial load. As the storage time increased, LPs showed its bactericidal effect. Similarly, LPs treated milk showed bactericidal effects on coliform counts in cow and camel milk (Bekele et al., Citation2017). Under the current study, the activation of lactoperoxidase enzyme caused a decrease in the count by 7.87% after 6 hours of storage at ambient temperature compared to the initial coliform counts as shown in Table . The study by Helen and Seifu (Citation2007) also found that a decrease in coliform count (0.57 log units) was observed in LPs after 7 hours of storage as compared to the initial count. Although LPs reduced or/and inhibited the rapid multiplication, initial microbial quality plays a role. Studies by Pokharel and Laldas (Citation2012) and Ponce (Citation2010) indicated that integrating LPs with hygienic milk production and clean packages is reliable and economically suitable alternative for preserving raw milk. Thus, good hygienic milking practices and handling conditions should not be ignored.
In the current study, as shown in Figure , yeast-mould counts increased as the storage time increased for different rate. This could be related to the milk’s low pH or higher acidity, which creates a favorable environment for their growth (Ledenbach & Marshall, Citation2009). In line with the current finding, Campos-Vallejo et al. (Citation2017) revealed that mould was higher in milk without addition of the LPs activators than milk treated with LPs at 0, 4 and 8 hours of storage. Similarly, Ay and Bostan (Citation2016) reported the lactoperoxidase system activation led to a significant reduction in the number of yeast. LPs showed an apparent antifungal activity as it reduces the initial counts of yeast and mould (Popper & Knorr, Citation1997; Saad et al., Citation2013; Welk et al., Citation2009). Other report also revealed that LPs elicit antimicrobial activities against various milk spoilage microorganisms, including bacteria, yeast and moulds (Jooyandeh et al., Citation2011). Production of intermediate products from the lactoperoxidase system with antibacterial properties has been indicated to be bactericidal effects for some spoilage yeast and pathogenic microorganisms (Corbo et al., Citation2009; Gould, Citation2001; United States Food and Drug Administration [USFDA], Citation2011).
LPs activation possesses inhibitory effect on Staphylococcus counts, deleterious effect on Staphylococcus counts until 12 hours of storage. The current finding is in line with the result of Ponce (Citation2010) reported an 8.45% reduction was observed at 12 hours post activation in Staphylococcus count. The LPs activation has antimicrobial properties against a wide range of pathogenic microorganisms in milk (Jooyandeh et al., Citation2011). Reports indicate that LPs preservation of raw milk resulted in a significant variation of Staphylococcus count between activated and control groups at 8 h of storage at room temperature (Campos-Vallejo et al., Citation2017).
The lactoperoxidase systems, an antimicrobial system naturally present in milk that is activated by H2O2, has been used to inhibit the microbial outgrowth in raw milk in the areas where refrigeration is not viable (Lara-Aguilar & Alcaine, Citation2019). Generally, the results obtained from the current experimental work revealed that LPs activation was effective in arresting bacteria viability. However, different microbial groups show a different degree to the LPs activation, it might due the differences in cell wall structure and different barrier properties (Sarr et al., Citation2018; Seifu et al., Citation2005).
5. Conclusions
LPs activation has positively effects on shelf life and has been proven to be a better way to maintain the initial raw milk quality at room temperature condition maintain. Preservation of the raw cow’s milk by LPs maintained the pH, titratable acidity and microbiological characteristics at ambient temperature under the Ethiopian Rift Valley. Under the present study, LPs activation in cow’s milk gave the milk extra 8 hours. Moreover, lactoperoxidase treatment has resulted in decreased microbial counts. From this, it can be concluded that LPs activation provides an opportunity to preserve milk for smallholder rural dairy farmers who do not have access to milk cooling facilities. Coupled with good milking practices and handling conditions, activating LPs can be considered an effective milk preservative method. Moreover, LPs have a great role in linking rural dairy farmers with formal milk markets without the need of cooling milk in hot climates.
Acknowledgments
The authors are grateful to all dairy laboratory staff of Hawassa University College of Agriculture School of Animal and Range Sciences for their support and kindness during the laboratory work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Kedir Awol
Kedir Awol is a lecturer in the Department of Animal science at Raya University, Maichew, Ethiopia. He has conducted several courses for Animal Science and Agro economic Department students. His current areas of research interest are livestock products and by-products processing milk production and constraints, quality characteristics of milk and dairy value chain and marketing and so on.
Mestawet Taye
Mestawet Taye (PhD) is an Associate Professor at Hawassa University, College of Agriculture, School of Animal and Range Sciences, Hawassa, Ethiopia. She has conducted various research activities on animal breeding, dairy technology and dairy production.
Binyam Kassa
Binyam Kassa is working in ET ALIM consulting, Addis Ababa, Ethiopia. He has conducted various research activities on milk preservation, dairy technology and dairy production.
References
- I. D. F. (1988). Code of practice for the preservation of raw milk by the lactoperoxidase system. In Bulletin No (Vol. 234). International Dairy Federation.
- Al-Baarri, A. N., Damayanti, N. T., Legowo, A. M., Tekiner, E. H. J., & Hayakawa, S. (2019). Enhanced antibacterial activity of lactoperoxidase–thiocyanate–hydrogen peroxide system in reduced-lactose milk whey. International Journal of Food Science, 2019, 1–12. https://doi.org/10.1155/2019/8013402
- Amakelew, S., Eshetu, M., Animut, G., & Gebeyew, K. (2015). Microbial quality of cow milk in Dawa Chefa District, Amhara Region, Ethiopia. Advances in Dairy Research, 3(2), 135. https://doi.org/10.4172/2329-888X.1000135
- Ay, M., & Bostan, K. (2016). Effects of activated LPS on microbiological quality of raw milk. Kafkas üniversitesi Veteriner Fakültesi Dergisi. https://doi.org/10.9775/kvfd.2016.15993
- Ayza, A., & Olikamo, Y. (2020). A review on the role of smallholders’ milk production in Ethiopia. Research and Reviews: Journal of Food and Dairy Technology, 8(3), 1–7. https://www.rroij.com/open-access/investigating-the-relationship-between-aspects-of-support-and-performance-amongdairy-employees.pdf
- Bafort, F., Parisi, O., Perraudin, J. P., & Jijakli, M. H. (2014). Mode of action of lactoperoxidase as related to its antimicrobial activity: A review. Enzyme Research, 2014, 1–13. https://doi.org/10.1155/2014/517164
- Bekele, A., Mitiku, E., Yonas, H., & Egon, B. H. (2017). Activation of LPS: Evaluation of the acidification rate, microbial quality, and shelf life of camel and cow milk. East African Journal of Sciences, 11(2), 107–116.
- Brasesco, F., Asgedom, D., & Sommacal, V. (2019). Strategic analysis and intervention plan for cow milk and dairy products in the agro-commodities procurement zone of the pilot integrated agro-industrial park in central-Eastern Oromia, Ethiopia. FAO. www.fao.org/3/ca4200en/CA4200EN.pdf
- Campos-Vallejo, M., Puga-Torres, B., Núñez-N, L., De la Torre-Duque, D., Morales-Arciniega, S., & Vayas, E. (2017). Evaluation of the use of sodium thiocyanate and sodium Percarbonate in the activation of the LPs in the conservation of raw milk without refrigeration in the Ecuadorian Tropics. Food and Nutrition Sciences, 8(5), 526–534. https://doi.org/10.4236/fns.2017.85036
- Corbo, M. R., Bevilacqua, A., Campaniello, D., D’Amato, D., Speranza, B., & Sinigaglia, M. (2009). Prolonging microbial shelf life of foods through the use of natural compounds and non-thermal approaches – a review. International Journal of Food Science and Technology, 44(2), 223–241. https://doi.org/10.1111/j.1365-2621.2008.01883.x
- Demissu, H., Fekadu, B., & Gemeda, D. (2014). Dairy productive potential, challenges and production opportunities of horro and their F1 JerseyCrossbred cows: A case of Guduru livestock production and research center and its surroundings, West Oromia, Ethiopia. Science, Technology and Arts Research Journal, 3(4), 79–84. https://doi.org/10.4314/star.v3i4.11
- Ethiopian Standard (ES). (2009). Unprocessed whole/raw cow milk specification. Ethiopian Standard Agency, (2nd ed.). ( ES: 3460:2009).
- Fanta, D. G., Hani, S., Fufa, D., Takele, B. T., & Dinka, A. (2019). Assessment of the effect of activated lactoperoxidase system on keeping quality of raw cow milk under different climatic zones of Ethiopia. Journal of Dairy & Veterinary Sciences, 10(5), 555796. https://doi.org/10.19080/JDVS.2019.10.555796
- FAO/WHO. (2005). Benefits and potential risks of the LPS on raw milk preservation report of FAO/WHO technical meeting. FAO Headquarters,
- Farrell, J. (2021). Dairy in Ethiopia. Application of the supply chain analysis for Nutrition (SCAN) tool. Global Alliance for Improved Nutrition (GAIN). Briefing Paper #7. https://doi.org/10.36072/bp.7
- Fatine, H., Abdelmoula, E., Doha, B., & Hinde, H. (2012). Bacterial quality of informally marketed raw milk in Kenitra city, Morocco. Pakistan Journal of Nutrition, 11(8), 662–669. https://doi.org/10.3923/pjn.2012.760.767
- Firew, K., Zelalem, Y., Getnet, A., Tadesse, B., Yohannes, G., Rahel, N., & Binyam, K. (2013). Evaluation of LP-system as raw milk preservative at different storage temperature conditions in the central highlands of Ethiopia. Livestock Research for Rural Development, 25, 4. http://www.lrrd.org/lrrd25/4/kass25068.htm
- Food and Agriculture Organization (FAO). (2013). The LPS. Scientific excellence Industry applicability Strategic networking Global influence IDF Factsheet –January, 2013.
- Food and Agriculture Organization (FAO). (2017). Gender assessment of dairy value chains: Evidence from Ethiopia. FAO,(Herego, E, Ed.)
- Galeano Lopez, B. (2012). Efecto del sistema lactoperoxidasa en la conservacion de la leche cruda en dos fincas de la comunidad Monte Rosa, El Rama—RAAS, Nicaragua. Universidad Nacional Agraria de Nicaragua.
- Gebreselassie, N. (2020). Review on challenges and opportunities of milk production in Ethiopia. Food Science, 95, 29–33. https://www.iiste.org/Journals/index.php/FSQM/article/view/51895/53632
- Gershom, N., & Ssemakula, E. (2017). Traditional methods of milk processing and preservation by local farmers in Kashongi sub County Kiruhura District. American Journal of Science, Engineering and Technology, 2(2), 62–71. https://doi.org/10.11648/j.ajset.20170202.12
- Golmohamadi, A., Morra, M. J., Popova, I., & Nindo, C. I. (2016). Optimising the use of Sinapis alba seed meal extracts as a source of thiocyanate (SCN–) for the lactoperoxidase system. LWT - Food Science and Technology, 72, 416–422. https://doi.org/10.1016/j.lwt.2016.05.007
- Gould, G. (2001). Symposium on ‘Nutritional effects of new processing technologies’ New processing technologies: An overview. The Proceedings of the Nutrition Society, 60(4), 463–474. https://doi.org/10.1079/PNS2001105
- Hamid, O. I. A., & Musa, Z. A. B. (2013). Effect of different levels of sodium thiocyanate and percarbonate for activation of lactoperoxidase on the keeping quality of raw milk. Journal of Advanced Scientific Research, 4(1), 27–30.
- Helen, N., & Seifu, E. (2007). Effect of the LPS and container smoking on the microbial quality of cows’ milk produced in Kombolcha woreda, eastern Ethiopia. Livestock Research for Rural Development, 19, 10. http://www.lrrd.org/lrrd19/10/nigu19157.htmairy Federation (IDF).
- Ismail, H. M., El Zubeir, I. Y. M., & Fadlelmoula, A. A. A. (2016). Influence of lactation stage and storage temperature on the activity of Lp enzyme system on microbial load of raw cow’s milk, Annals. Food Science & Technology, 17(1), 239–244. https://api.semanticscholar.org/CorpusID:9154274
- ISO. (1998). Microbiology of food and animal feeding stuffs – Horizontal method for enumeration of Mesophilic LAB. https://www.iso.org/obp/ui/#!iso:std:26853:en
- Jooyandeh, H., Aberoumand, A., & Nasehi, B. (2011). Application of LPS in fish and food products. American-Eurasian Journal of Agricultural & Environmental Sciences, 10(1), 89–96.
- Kakar, M. U. H., Kakar, M. A., Shahwani, M. N., Ahmed, N., Arain, M. A., & Khaskhaili, M. (2013). Stabilization of fresh buffalo milk by activating LPS. Journal of Animal and Plant Sciences, 23(1), 90–93. https://www.researchgate.net/publication/292717349
- Kirdar, S. S., & Atamer, M. (2021). Quality criteria of tulum cheese produced from cow’s milk preserved by activation of lactoperoxidase system. Journal of Food Processing and Preservation, 45(4), e15210. https://doi.org/10.1111/jfpp.15210
- Köksal, Z., Kalin, R., Camadan, Y., Usanmaz, H., Almaz, Z., Gülçin, İ., Gokcen, T., Gören, A. C., & Ozdemir, H. (2016). Secondary sulfonamides as effective lactoperoxidase Inhibitors. Molecules, 22(6), 793. https://doi.org/10.3390/molecules22060793
- Kurwijila, R. L., Hansen, K. K., Macha, I. E., Abdallah, K., & Kadigi, H. J. S. (1992). The bacteriological quality of milk from hand and machine milked dairy herds in Morongo, Tanzania. Journal of African Livestock Research, 2(2), 59–67.
- Lara-Aguilar, S., & Alcaine, D. (2019). Lactose oxidase: A novel activator of the lactoperoxidase system in milk for improved shelf life. Journal of Dairy Science, 102(3), 1933–1942. https://doi.org/10.3168/jds.2018-15537
- Ledenbach, L. H., & Marshall, R. T. (2009). Microbiological spoilage of dairy products. In W. Sperber & M. Doyle (Eds.), Compendium of the microbiological spoilage of foods and beverages (pp. 41–67). Springer Science Business. https://doi.org/10.1007/978-1-4419-0826-1_2
- Marth, E. H. (1978). Standard methods for the examinations of dairy products. American Public Health Association.
- Munsch-Alatossava, P., Quintyn, R., De Man, I., Alatossava, T., & Gauchi, J. P. (2016). Efficiency of N2 gas flushing compared to the LPS at controlling bacterial growth in bovine raw milk stored at mild temperatures. Frontiers in Microbiology, 7, 839. https://doi.org/10.3389/fmicb.2016.00839
- Parveen, R., Kausar, R., Sameen, A., Khan, M. I., & Sana, N. (2016). Effect of activating Lacto Peroxidase system (LPs) on quality and storage Stability of Soft Cheese. Journal of Biotechnology & Biomaterials, 6(2), 224. https://doi.org/10.4172/2155-952X.1000224
- Pokharel, P., & Laldas, S. K. (2012). Study on the extension of shelf-life by activation of inherent LPS in raw cow milk. Journal of Food Science and Technology Nepal, 7, 57–60. https://doi.org/10.3126/jfstn.v7i0.10606
- Ponce, C. P. (2010). LPS under tropical conditions: Use, advantages and limitations in conservation of raw milk and potential applications. Review article Revista de Salud Animal, 32(3), 146–154.
- Ponce, P. (2007). Activación del sistema lactoperoxidasa para la conservación de leche cruda en el trópico americano. Aspectos prácticos y consideraciones toxicológicas. 10-13, San José de las Lajas, La Habana-Cuba.
- Popper, L., & Knorr, D. (1997). Inactivation of yeast and filamentous fungi by the lactoperoxidase-hydrogen peroxide-thiocyanate-system. Die Nahrung, 41(1), 29–33. https://doi.org/10.1002/food.19970410108
- Puspitarini, O. R., Al-Baarri, A. N., Legowo, A. M., Bintoro, P., & Hintono, A. (2013). The activation method of LPs to inhibit microbial activity in fresh milk. Animal Production, 15(2), 119–126.
- Richardson, G. H. (1985). Standard method for the examination of dairy products (15th ed.). American Public Health Association.
- Saad, M. S. A., Zubeir, I., & Fadel Elseed, A. M. A. (2013). Effect of LPs and storage temperature on the keeping quality of sheep milk. Livestock Research for Rural Development, 25, 6. http://www.lrrd.org/lrrd25/6/saad25102.htm
- Sarr, D., Tóth, E., Gingerich, A., & Rada, B. (2018). Antimicrobial actions of dual oxidases and lactoperoxidase. Journal of Microbiology, 56(6), 373–386. https://doi.org/10.1007/s12275-018-7545-1
- Seifu, E., Buys, E. M., & Donkin, E. F. (2005). Significance of LPS in the dairy industry &its potential applications: A review. Trends in Food Science and Technology, 16(4), 137–154. https://doi.org/10.1016/j.tifs.2004.11.002
- Shariat, S. Z., Jafari, N., Tavakoli, N., & Najafi, B. R. (2015). Protection of lactoperoxidase activity with sugars during lyophilisation and evaluation of its antibacterial properties. Research in Pharmaceutical Science, 10, 152–160.
- Silva, E., Oliveira, J., Silva, Y., Urbano, S., Sales, D., Moraes, E., Rangel, A., & Anaya, K. (2020). Lactoperoxidase system in the dairy industry: Challenges and opportunities. Czech Journal of Food Sciences, 38(6), 337–346. https://doi.org/10.17221/103/2020-CJFS
- Sisecioglu, M., Ekrem, K., Murat, C., Hasan, O., Ilhami, G., & Ali, A. (2010). The prohibitive effect of LPS (LPS) on some pathogen fungi and bacteria. African Journal of Pharmacy and Pharmacology, 4(9), 671–677.
- Srisaikham, S., Naoki, I., & Suksombat, W. (2017). The inhibitory effect of sodium thiocyanate, sodium percarbonate ratios on microorganism growth in raw milk samples as an effective treatment to extend milk quality during storage. Songklanakarin Journal of Science and Technology, 39(1), 77–89. DOI: 10.14456/sjst-psu.2017.9.
- U.S. Food and Drug Administration (USFDA). (2011). Spoilage bacteria. U.S Food and Drug Administration FDA.
- Walstra, P., Wouters, J. T. M., & Geurts, T. J. (2006). In dairy Science and Technology. Taylor & Francis Group, LLC. https://doi.org/10.1201/9781420028010
- Welk, A., Meller, C., Schubert, R., Schwahn, C., Kramer, A., & Below, H. (2009). Effect of lactoperoxidase on the antimicrobial effectiveness of the thiocyanate, hydrogen peroxide combination in a quantitative suspension test. Microbiology (Reading, England), 9(1), 134–139. https://doi.org/10.1186/1471-2180-9-134
- Worku, T., Negera, E., Nurfeta, A., & Welearegay, H. (2012). Microbiological quality and safety of raw milk collected from Borana pastoral community, Oromia Regional state. African Journal of Food Science and Technology, 3, 213–222.
- Yousef, A. E., & Carlstrom, C. (2003). Food Microbiology: A laboratorymanual. A John Wiley and Son, Inc.
- Zeynep, K., Ilhami, G., & Hasan, O. (2016). An important milk enzyme: Lactoperoxidase chapter from the book milk proteins – from structure to biological properties and health aspects. World’s largest Science, Technology & Medicine Open Access book publisher pp. 142–156.