Abstract
Phosphorus (P) plays a tremendous and determinant role in plant growth. However, its availability is hampered by the presence of metallic ions in soils. Therefore, the present work has been initiated to analyze the P-solubilization, organic acid, and phosphatase production potency of Bacillus velezensis AR1. Quantification of P-solubilization ranged from 150.6 to 491.3 and from 2.8 to 54.4 mg L−1 in the tricalcium phosphate (Ca-P) and aluminum phosphate (Al-P) medium, respectively. High-performance liquid chromatography analysis of organic acid detection showed variations, whereby 446.88 and 173.73 mg L−1 of succinic and 2-keto gluconic acids in the medium supplemented by Ca-P, and 12.59 and 11.83 mg L−1 of 2-keto gluconic and fumaric acids in the medium supplemented by Al-P were produced, respectively. There were also remarkable extracellular acid and alkaline phosphatase activities. Furthermore, inoculated and uninoculated 5- and 12-day-old culture broths of Ca-P and Al-P were applied to investigate their effect on pea plant growth. The result indicated that the highest plant height and tissue dry weight from the 5-day-old Al-P-modified culture (Al-5), and the highest root length from the 12-day-old Al-P-modified culture (AL-12) were recorded due to the solubilized phosphorus in the culture broth, even compared to the standard fertilizer. Similarly, the highest total nitrogen and total phosphorus were recorded due to Ca-P12 and Ck, respectively. These results concluded that the bacteria isolate B. velezensis AR1 could be developed into a bioinoculant for phosphate solubilization.
1. Introduction
Among the three major primary plant nutrients, phosphorus (P) is one of the most immobile nutrients in soils (Holford, Citation1997). A report indicated that P deficiency alone limits crop productivity in 30–40% of the world’s arable land (Vance et al., Citation2003). Surprisingly, 75–90% of the total P fertilizers applied to the soil is estimated to precipitate as metal cation complexes (Stevenson & Cole, Citation1986). This causes a large sum of phosphate accumulation in the soil. From this point of view, the accumulated phosphates in agricultural soils had been roughly claimed to be enough to sustain maximum crop yields for about 100 years (Goldstein et al., Citation1993). However, the precipitation hinders the solubility of phosphate due to the presence of iron and aluminum in acidic soils and calcium in neutral and alkaline soils at large (Ponmurugan & Gopi, Citation2006). Therefore, the most critical question is the availability mechanism of P which seriously needs intensive work in order to have an alternate solution to overcome this problem.
Interestingly, the rhizosphere hosts a wide variety of microorganisms due to the stimulation by various root exudates, and they can promote greater nutrient absorption and transfer to plants through diverse mechanisms (Kavamura & Esposito, Citation2010). One of these mechanisms is by increasing the bioavailability of naturally existing soil nutrients (Van Der Heijden et al., Citation2008). More specifically, the phosphate-solubilizing abilities of soil microorganisms have proved to be an economically sound alternative to the more expensive phosphatic fertilizers. Several research reports have documented the fact that most of these microorganisms perform remarkable P and zinc solubilization activities, thereby improving plant growth and yields (De Freitas et al., Citation1997; Oliveira et al., Citation2009; Sharma et al., Citation2014). As previously asserted by many reports, various species of bacteria, fungi, and actinomyces, which can solubilize P, can also produce several organic acids as one of the major mechanisms of solubilization (Goldstein, Citation1994; Kavanagh, Citation2011; Lal, Citation2002). Furthermore, P-solubilizing microorganisms have also demonstrated remarkable acid and alkaline phosphatase activities that can play an enormous role in solubilizing organic P sources (Hwangbo et al., Citation2016; Kapri & Tewari, Citation2010; Oliveira et al., Citation2009; Ponmurugan & Gopi, Citation2006).
Bacillus velezensis is a primary plant-growth-promoting rhizobacterium that has been reported to have enormous potential to support crop production in agricultural applications (Fan et al., Citation2018; Meng et al., Citation2016). A recent report indicated that B. velezensis Ag75 could solubilize P and increased maize plant growth and yield (Mosela et al., Citation2022). Similarly, due to its greater potential in rock phosphate solubilization, B. velezensis has been reported to increase 50% phosphate and nitrate in soil and 80% in wheat leaves (Afzal et al., Citation2023). In our current study, B. velezensis AR1 isolated from a saline area of soil in Muan, Republic of Korea (Regassa et al., Citation2018), has been investigated for its inorganic phosphate solubilization, organic acid production, phosphatase activities, and plant growth promotion.
2. Material and methods
2.1. Evaluation for insoluble nutrient solubilization
2.1.1. Identification of Bacillus velezensis AR1
The molecular identification of the isolate AR1, used in this work, was conducted using 16S rRNA gene sequence and submitted to NCBI, which revealed that it has close similarity with Bacillus velezensis CR502 (accession number AY603658) in NCBI database. The bacteria cell was maintained in 50%:50% tryptic soy broth (TSB) medium and glycerol and stored in a deep freezer at −78 ± 2°C for future use.
2.1.2. Qualitative analysis of P solubilization
Qualitative analysis of P-solubilization was assayed on Pikovskaya (PVK) agar medium supplemented with inorganic tricalcium phosphate and aluminum phosphate (Pikovskaya, Citation1948). Briefly, the medium contained L−1: glucose, 10 g; Ca3(PO4)2 (Ca-P), 5 g, or AlPO4 (Al-P), 2 g; (NH4)2SO4, 0.5 g; NaCl, 0.2 g; MgSO4·7H2O, 0.1 g; KCl, 0.2 g; yeast extract, 0.5 g; MnSO4·H2O, 0.002 g; FeSO4·7H2O, 0.002 g; and agar, 2%. The agar medium was spot inoculated with 10 µL fresh (24 h old) B. velezensis culture at the center and incubated at 30°C for 7 days. A clear halo zone surrounding the cell was considered a positive indicator for P-solubilization, and the P-solubilization index was also calculated as the ration of total diameter (colony + halo zone) to the colony diameter (Premono et al., Citation1996).
2.1.3. Quantitative analysis of phosphate solubilization
Quantitative P-solubilization assay was performed by using PVK medium with slight modifications. Briefly, the medium contained L−1: glucose, 10 g; Ca3(PO4)2 (Ca-P), 5 g; (NH4)2SO4, 0.5 g; NaCl, 0.2 g; MgSO4·7H2O, 0.1 g; KCl, 0.2 g; yeast extract, 0.5 g; MnSO4·H2O, 0.002 g; FeSO4·7H2O, 0.002 g; and crab shell powder, 0.5 g. The Ca3(PO4)2 in the liquid medium was replaced with 2 g L−1 AlPO4 (Al-P). The initial pH in all the media was adjusted to 7 ± 0.02 prior to sterilization. A single bacteria colony grown overnight in a TSB agar culture plate at 28 ± 2°C was transferred in triplicate to flasks containing 100 mL liquid medium and incubated at 30°C. Samples were removed once every day until day 12, followed by centrifugation at 12,300 × g and 4ºC for 10 min. Then, 50 µL of the supernatant and 950 µL of double distilled water were transferred to test tubes, and 1.0 mL of the working reagent, which include 0.4 g of ascorbic acid, 100 mL of solution 725 (made up of 1.0 g of bismuth sub-carbonate dissolved in 68 ml of sulfuric acid and added to 300 ml of distilled water, combined with 20 g of ammonium molybdate dissolved in 68 ml of sulfuric acid and added to 300 ml of distilled water) and 100 mL of distilled water, was added. After 20 min of reaction, the phosphate solubilization was determined using a spectrophotometer at 725 nm (Reis et al., Citation2008). To quantify the solubilization ability, a calibration curve was created with increasing phosphoric acid concentrations, using a stock solution (20 mg mL−1).
2.2. Quantitative analysis of phosphatase activity
For phosphatase enzyme activity analysis, 150 µL of cell-free culture filtrate was used and assayed according to a modified protocol (De Freitas et al., Citation1997). Aliquots of each sample were added to 0.48 mL modified universal buffer, pH 6.5 or pH 11, for assay of acid or alkaline phosphatase, respectively, and 0.12 mL of 0.05 M p-nitrophenyl phosphate (pNPP; Sigma Chemical Company, USA) solution, and then incubated for 1 h at 37°C. The yellow color was measured at 410 nm (Tabatabai & Bremner, Citation1969), and the resulting phosphatase activities were calculated from the standard calibration curve.
2.3. Quantitative analysis of culture pH
Simultaneous analysis of culture pH was also conducted daily using a pH meter (pH 200L) to study acidification trends of the organic acid secreted by the isolate. The measurement was repeatedly read until a constant value was achieved for precision purposes.
2.4. Organic acid analysis
To trace the possible reason for the P-solubilization potency of the AR1 isolate, extracellular organic acid secretion of the isolate was analyzed by using a high-performance liquid chromatography (HPLC) method. The HPLC analysis was performed according to a previous method (Davies et al., Citation2016), with slight modification. Briefly, the bacterium was grown in PVK broth for 7 days. The cells were removed from the broth by membrane filter (0.2 µm pore size, Hyundai Micro Co., Ltd, Republic of Korea), following centrifugation at 16,000 × g at 4°C for 10 min and stored at −20°C for later use. Then, 10 mM of organic acid standard was prepared by mixing 2 mL each of 50 mM acetate, fumaric, 2-ketogluconic, α-ketoglutaric, and oxalic and succinate acids stock solutions (all chemicals from Sigma Chemical Company). The organic acids in the filtered solution were detected using a Shimadzu LC-10A HPLC with a UV–Vis detector at 210 nm and an RS pak KC-811 (7.8 mm ID × 300 mm L) column made by Shodex. The operating conditions consist of 8 mM H2SO4, as a mobile phase at a constant (isocratic) elution of 0.65 mL min−1, with injection volume of 20 µL, run for 20 min at 25°C column temperature. Finally, typical chromatograms generated by the detector were identified by comparing retention times (RT) to those of known standards. Concentrations of the detected organic acids were calculated from standard calibration curves.
2.5. Pot experiments
Pot experiment was conducted at soil microbiology laboratory of Chonnam National University to analyze the effect of the Bacillus velezensis AR1-solubilized P-nutrient on the growth and nutrient uptake of pea plants (Pisum sativum L.). Briefly, pea seeds were surface disinfected by 70% ethanol followed by sodium hypochlorite. Disinfected seeds were washed with sterile water four to five times and germinated on Petri plates consisting of a wet sterile filter paper. Six days later, seedlings with equal size and conditions were selected and transplanted in plastic pots filled with pure sand sterilized in an autoclave at 121°C for 15 min. The pots were randomly arranged with three replicates on a bench and closely monitored for 5 days of seedling establishment. Five days later, the PVK culture broth, collected during P-solubilization assay, was applied as follows: blank (nutrient medium incubated without strain AR1); bacteria culture broth 5 days after incubation (DAI 3, 4, and 5 days pooled); and bacteria culture broth 12 DAI were used as treatment. One hundred milliliter PVK medium modified with 1.533 g KH2PO4 was used as a standard control. Note that the PVK culture used for this experiment was inoculated with B. velezensis AR1 and modified with 50 mMol NaCl prior to application. The treatments were applied three times at weekly intervals. A month after transplantation, physiological data, namely, shoot and root length, and dry weight were collected. The role of B. velezensis AR1 in enhancing N and P content was analyzed from the plant tissues. This experiment was repeated, and the result presented are average of the two. The concentrations of total N and P were determined from ground shoot tissue. Shoot samples were dried at 70°C for 72 h and ground for nutrient analysis. For analysis of the nutrients, 1 g plant sample was put in a Kjeldahl flask and digested for 6 h with 10 mL 50% perchloric acid and 1 mL concentrated sulfuric acid. Then, the samples were put on a heated plate at 80°C, 100°C, 150°C, 180°C, and 250°C each for 30 min until the color of the digest became white. Then, the samples were cooled at room temperature and filtered with Whatman filter paper no. 2 twice and the volume was made to 100 mL. The total nitrogen was determined following Kjeldahl method (Kirk, Citation1950), and total P was calorimetrically quantified using a vanadate method (Kitson & Mellon, Citation1944).
2.6. Statistical analysis
All data were processed statistically using SAS Software 9.4 (SAS Institute, 2012). Mean comparisons were performed using the protected least significant difference (p < 0.05) t-test.
3. Results
3.1. Qualitative analysis of nutrient solubilization
Prior to quantification analysis, P-solubilization activity of B. velezensis AR1 was first conducted at the agar plate level with plates containing PVK medium supplemented with Al-P and Ca-P. Based on the clear zone formation on their respective agar plates, the bacterium strain was identified as Al-P and Ca-P solubilizing with solubilization index (SI) values of 3.0 and 2.43, respectively.
3.2. Quantification of P-solubilization
P-solubilization activity trends of B. velezensis AR1 had been conducted by growing the agent in the medium modified with hardly soluble inorganic P-sources. This was targeted to trace the maximum P-solubilization period and its increasing and/or decreasing patterns. As tabulated in Table , there was statistically significant (p ≤ 0.05) P-solubilization variation over the period of incubation. In the case of both Ca-P and Al-P, the highest solubilizing activities were recorded at day 3 after incubation, which was increased by more than 3-fold and 2.75-fold compared to 1 DAI and 2 DAI, respectively. In both growth media, P-solubilization was observed to decline up to 9 DAI, but abruptly increased at 10 DAI. However, inconsistent solubilization patterns have been observed in both media.
Table 1. Phosphorus solubilization and acid and alkaline phosphatase activity patterns of Bacillus velezensis AR1 in a medium supplemented with tricalcium phosphate (Ca-P) and aluminium phosphate (al-P). Values in a column followed by the same letter are statistically not different (p ≥ 0.05) from each other. Data presented are averages of two experiments with replications of each. DIA indicates days after incubation. Phosphatase activity is reported as unit per milliliter of para-nitrophenyl phosphate (p-NPP). LSD is least significant difference at 5%
3.3. Acid and alkaline phosphatase activities
Simultaneous study of phosphatase activity was determined by measuring the amount of liberated para-nitrophenol (р-NP) from para-nitrophenyl phosphate (р-NPP). In both the inorganic P-source supplemented media, there were statistically significant (p ≤ 0.05) differences in phosphatase activities of strain AR1. Interestingly, the data tabulated in Table revealed that the bacteria could produce both the acid and alkaline phosphatase in pronounced amounts. The highest and the lowest acid phosphatase activities, in the case of Ca-P-modified medium, were detected at 5 DAI and 1 DAI, respectively. Activity values of 107.74 U mL−1 followed by 100.26 U mL−1 were recorded for the alkaline phosphatase at 3 and 4 DAI, respectively. The strain AR1 showed the highest acid phosphatase activity at 6 DAI followed by the preceding incubation period in the medium modified with Al-P, whereas the alkaline phosphatase activities of the strain AR1 at 2, 3, 4, and 12 DAI were of similar value.
3.4. Quantification of media pH
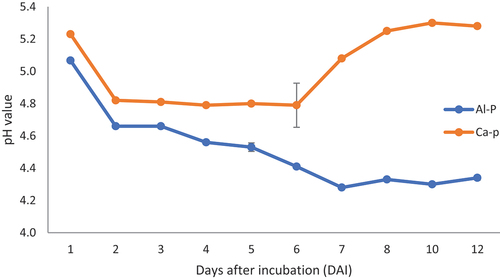
During the courses of experimentation, prior to centrifuging the samples for P-solubilization and enzymatic assays, pH of the culture broth had been tested in triplicate and recorded. There was a significant (p < 0.05) decline in the pH of both the test media. In both growth media, the pH dropped faster immediately after the first day of incubation (Figure ). The Ca-P containing medium pH dropped from 7.0 ± 0.02 to 5.23 after 24 h and continued to drop to 4.79 at 4 DAI. From 4 DAI to 6 DAI, the pH remained constant and then showed inconsistent increment (Figure ). The increment in pH after 6 days could probably be attributed to the increased reaction of the solubilized calcium in the medium. Similar trend has been observed in Al-P-containing medium wherein the pH was decreased by about 3 units at 6 DAI, after which the statistical value remained constant (Figure ).
3.5. Organic acid secretion by strain AR1
With the desire to discover the most probable reason behind solubilization of the hardly soluble inorganic phosphate by B. velezensis AR1, analysis of organic acids was made using HPLC. A total of six common authentic organic acids, viz. acetic, fumaric, 2-ketogluconic, α-ketoglutaric, and oxalic and succinic acids with RT of 15.16, 9.38, 10.18, 16.52, 8.30, and 12.62, respectively, were employed for the purpose of comparison (Fig. S1A). As tabulated in Table , the secreted organic acids in the PVK media supplemented with aluminium and tricalcium phosphate showed variations in terms of composition and concentration.
Table 2. Organic acid secreted by Bacillus velezensis AR1 and their concentrations (conc) presented in milligram per liter. Al-P and Ca-P represent medium supplemented with aluminum and calcium phosphates, respectively. Concentrations were calculated from their respective calibration curves indicated on Supplementary Fig. 1D. ND means not detected
For instance, in the aluminum phosphate supplemented medium, the bacterium could secrete four of the tested organic acids, of which α-ketoglutaric acid production was the highest followed by fumaric acid (Fig. S1B and Table ). On the other hand, five of the tested organic acids were detected in the medium supplemented by Ca-P, with similar RT to the authentic standards (Fig. S1C). Maximum production of succinic acid followed by α-ketogluconic and 2-ketogluconic acids, respectively, was observed (Table ). In contrast, the highly concentrated organic acid (succinic) detected in the medium consisting of Ca-P showed the least concentration in the medium supplemented by Al-P, and fumaric acids in Ca-P supplemented medium too. In addition to the major peaks, certain unidentified peaks were also detected in both medium (Fig. S1 A,B).
3.6. In vivo pot experiment
The in vivo experiment was conducted to investigate whether the strain AR1-solubilized P in the bacterium culture broth could comparatively support pea plant growth. Physiological data from a month-old plant tabulated in Table indicated that there were statistically significant (p < 0.05) differences among the treatments for total root length, plant fresh weight, tissue total nitrogen (TN) and phosphorus (TP). The highest total root length was observed at Al-P12 followed by the Al-P5 treatment. Comparable plant dry weight was tabulated for Al-P5 and Ca-P5. The highest TN and TP were recorded for Ca-P12 and the control (Ck), respectively. On the other hand, there were statistically no significant (p > 0.05) differences among the treatments in plant height. However, there were about 2.35 and 2.9 cm higher height recorded for Al-P5 and Al-P12 respectively, compared to Al-Pi. Similarly, there was an increment of about 1.7 and 2 cm plant height for Ca-P5 and Ca-P12, respectively, compared to Ca-Pi. Others remain statistically equivalent.
Table 3. Bacteria-solubilized P effect on plant growth and tissue nutrient content. Values in the column followed by the same letter are statistically not different (p > 0.05). The values presented are means of three replications. Note that Ck is the control that received KH2 PO4, Al-Pi is aluminum phosphate without AR1; Al-P5 is the AR1 culture broth of days 3, 4, and 5 (pooled); Al-P12 culture broth at day 12. The same for Ca. PH is plant height, RL is root length, DW is dry weight, TN is total nitrogen, and TP is total phosphorus
4. Discussion
Nutrient uptake improvement of hardly soluble minerals such as tricalcium phosphate and aluminum phosphate enhances plant growth. The tricalcium phosphate and aluminum phosphate are produced by metal cation complex in neutral-to-calcareous and acidic soils, respectively, when 75–90% inorganic phosphate fertilizers are precipitated in a soil and become non-available to the growing plants (Flatian et al., Citation2021; Sharma et al., Citation2013). The only mechanism to extract the bioavailable phosphate from such complexed sources is through microbial solubilization activities (Shen et al., Citation2011). Furthermore, P concentration in soil solution is very low as soluble phosphate (Sharma et al., Citation2013). Several works have also been published regarding high inorganic and organic P-solubilization capacity of bacteria, mainly Bacillus species. For instance, soluble P production of up to 211 mg L−1 from AlPO4, and Ca3(PO4)2 (Oliveira et al., Citation2009) up to 483 ± 5 mg L−1 from poultry bones, fish bones, and ash (Saeid et al., Citation2018), and 3.35 g L−1 from CaHPO4 (dos Santos et al., Citation2015) have been reported. In the current work, B. velezensis AR1 could solubilize about 491 and 54 mg L−1 of Ca-P and Al-P, respectively. This infers that the strain AR1 has high P-solubilizing potential that could be exploited for solubilization of fixed phosphates present in a soil. Microbes perform P-solubilizing activities via the release of mineral dissolving compounds, namely organic acids, siderophores, protons, hydroxyl ions, CO2, and through liberation of extracellular enzymes as well as biological P-mineralization (McGill & Cole, Citation1981). The isolate AR1 demonstrated remarkable acid and alkaline phosphatase activities. In the Ca-P-modified medium, comparatively higher alkaline phosphatase activity was evidenced, while in the Al-P-modified medium, higher acid phosphatase activity was evidenced. The production of high phosphatase enzyme infers that the isolate has a promising potential to be used for the solubilization of organic P sources in the soil or compost. Findings indicated that 19 in B. velezensis CBMB205 (Hwangbo et al., Citation2016), 11 in B. velezensis Ag75 (Mosela et al., Citation2022), and recently 4 in B. velezensis YC89 (Xie et al., Citation2023) phosphatase genes that are involved in P-solubilization activities have been identified. Several Bacillus species and Trichoderma have also been reported to produce both the acid and alkaline phosphatase (De Freitas et al., Citation1997; Kapri & Tewari, Citation2010). Although it cannot be the main cause of P-solubilization, a previous work reported the induction of extracellular phosphatase activity only in the presence of tricalcium phosphate (Kapri & Tewari, Citation2010). In the current works, analysis of the Pearson correlation results showed positive relation between the alkaline phosphatase activity and Ca-P and Al-P solubilization (P = 0.025, R = 0.732 and P = 0.111, R = 0.535, respectively).
The analysis of the growth medium modified with P-sources revealed that the pH started declining immediately after bacterium inoculation within 24 h. The essential part of this acidification could be attributed to the consumption of glucose from the growth media and to the production of the organic acids, which is the most reported mechanism for solubilization of inorganic phosphate (Mardad et al., Citation2013). However, the decline in pH did not show any correlation with P-solubilization.
Production of organic acid is one of the principal biomechanisms by which microorganisms solubilize hardly soluble inorganic P minerals as reported by several researchers (Atlas & Bartha, Citation1997; Rashid et al., Citation2004; Rodrı́guez & Fraga, Citation1999; Whitelaw et al., Citation1999). Acidification of culture supernatant is another biochemical base for P-solubilization mechanisms (Perez et al., Citation2007). Furthermore, the existence of P-solubilizing genes is the main biochemical base of the P-solubilizing bacteria (Hwangbo et al., Citation2016; Mosela et al., Citation2022; Xie et al., Citation2023). Some of the organic acids are produced by extracellular oxidation of glucose to gluconic acid and 2-ketogluconic acid via the direct oxidation pathway, which provides the biochemical basis for highly efficacious calcium phosphate solubilization (Goldstein & Krishnaraj, Citation2007). Different species of organisms produce varying amount and types of organic acids that either directly dissolve the mineral P as a result of anion exchange of the phosphate ion by the acid anion or chelate the P-associated Fe, Al, and Ca ions (Omar, Citation1997; Vazquez et al., Citation2000). Production of acetic, fumaric, and succinic acids by Bacillus amyloliquefaciens and Bacillus licheniformis has previously been reported (Vazquez et al., Citation2000). Similarly, production of oxalic, 2-ketogluconic, and succinic acids by P-solubilizing Bacillus spp., LAB1, LAB2, LAB4, LAB5, LAB6, and LAB7 were also reported (Banik & Dey, Citation1983). Similarly, in the current work, B. velezensis AR1 produced a high amount of succinic acid in the medium supplemented with Ca-P, which was not detected in the Al-P-supplemented medium. Other secondary organic acid compounds such as acetic, fumaric, 2-ketogluconic, α-ketoglutaric, and oxalic acids were also detected in both media. However, as to my knowledge, there has been no reported data on the organic acid secretion capacity of B. velezensis.
Improvements in the tissue P percent and plant biomass due to biofertilization with P-solubilizing rhizobacteria and insoluble rock phosphate had been reported (De Freitas et al., Citation1997). The results of the current work are also in agreement with this finding insofar that application of AR1 containing broth showed improvement in root length, fresh weight, and tissue N and P contents. It has also been revealed in a report that Ca-P solubilizing Trichoderma significantly improved the root and shoot length and plant weight of chickpea (Cicer arietinum L.) (Kapri & Tewari, Citation2010).
Therefore, with its remarkable solubilization of hardly soluble nutrients, production of enzymes and varying organic acids, B. velezensis AR1 is useful in overall soils with unavailable form of P and can be recommended as a potent plant nourishing and biofertilizer agent in the field of sustainable agriculture.
Conflict of Interest
The author declares that there is no conflict of interest.
Supplemental Material
Download MS Word (237.8 KB)Acknowledgement
Chonnam National University and, especially, soil microbiology laboratory personnel are acknowledged for providing reagents and free bench supply for the study. Professor John Fisher deserves gratitude for his proofreading.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/23311932.2023.2276561
Additional information
Funding
References
- Afzal, A., Bahader, S., Ul Hassan, T., Naz, I., & Din, A. U. (2023). Rock phosphate solubilization by plant growth-promoting Bacillus velezensis and its impact on wheat growth and yield. Geomicrobiology Journal, 40(2), 131–10. https://doi.org/10.1080/01490451.2022.2128113
- Atlas, R. M., & Bartha, R. (1997). Microbial ecology. Benjamin/Cummings Science Publishing.
- Banik, S., & Dey, B. K. (1983). Phosphate-solubilizing potentiality of the microorganisms capable of utilizing aluminium phosphate as a sole phosphate source. Zentralblatt für Mikrobiologie, 138(1), 17–23. https://doi.org/10.1016/S0232-4393(83)80060-2
- Davies, F. K., D’Adamo, S., & Posewitz, M. C. (2016). HPLC analysis of secreted organic acids. Bio-protocol, 6(8), e1786. https://doi.org/10.21769/BioProtoc.1786
- De Freitas, J. R., Banerjee, M. R., & Germida, J. J. (1997). Phosphate-solubilizing rhizobacteria enhance the growth and yield but not phosphorus uptake of canola (Brassica napus L.). Biology and Fertility of Soils, 24(4), 358–364. https://doi.org/10.1007/s003740050258
- dos Santos, C. R. L., Cristina M. C. J. I., Antônio S. M., Luiz S. E., & Carlos C. A. (2015). Isolation and selection of P-solubilizing and IAA-synthesizing microorganisms from the rhizosphere of Guanandi (Calophyllum brasiliensis). African Journal of Agricultural Research, 10, 4455–4460. https://doi.org/10.5897/AJAR2015.9635
- Fan, B., Wang, C., Song, X., Ding, X., Wu, L., Wu, H., Gao, X., & Borriss, R. (2018). Bacillus velezensis FZB42 in 2018: The gram-positive model strain for plant growth promotion and biocontrol. Frontiers in Microbiology, 9. https://doi.org/10.3389/fmicb.2018.02491
- Flatian, A. N., Anas, I., & Sutandi, A. (2021). The ability of some microbes to solubilize the hardly soluble phosphorous and potassium from various sources in vitro. In Paper presented to the IOP Conference Series: Earth and Environmental Science, 1st International Conference on Sustainable Tropical Land Management, 2000, September 16–18, Bogor, Indonesia (Vol. 648).
- Goldstein, A. H. (1994). Involvement of the quinoprotein glucose dehydrogenase in the solubilization of exogenous phosphates by gram-negative bacteria. Phosphate in microorganisms: Cellular and molecular biology (pp. 197–203). ASM Press.
- Goldstein, A. H., & Krishnaraj, P. U. (2007). Phosphate solubilizing microorganisms vs. phosphate mobilizing microorganisms: What separates a phenotype from a trait? In Paper presented to the First International Meeting on Microbial Phosphate Solubilization, Dordrecht, Springer. https://doi.org/10.1007/978-1-4020-5765-6_31
- Goldstein, A. H., Rogers, R. D., & Mead, G. (1993). Mining by microbe. Bio/technology, 11(11), 1250. https://doi.org/10.1038/nbt1193-1250
- Holford, I. C. R. (1997). Soil phosphorus: Its measurement, and its uptake by plants. Soil Research, 35(2), 227–40. https://doi.org/10.1071/S96047
- Hwangbo, K., Um, Y., Ki, Y. K., Madhaiyan, M., Sa, T. M., & Lee, Y. (2016). Complete genome sequence of Bacillus velezensis CBMB205, a phosphate-solubilizing bacterium isolated from the rhizoplane of rice in the Republic of Korea. Genome Announcements, 4(4). https://doi.org/10.1128/genomeA.00654-16
- Kapri, A., & Tewari, L. (2010). Phosphate solubilization potential and phosphatase activity of rhizospheric Trichoderma spp. Brazilian Journal of Microbiology, 41(3), 787–795. https://doi.org/10.1590/S1517-83822010005000001
- Kavamura, V. N., & Esposito, E. (2010). Biotechnological strategies applied to the decontamination of soils polluted with heavy metals. Biotechnology Advances, 28(1), 61–69. https://doi.org/10.1016/j.biotechadv.2009.09.002
- Kavanagh K. (2011)Biology and Applications (2nded., Vol. 125). John Wiley & Sons.
- Kirk, P. L. (1950). Kjeldahl method for total nitrogen. Analytical Chemistry, 22(2), 354–358. https://doi.org/10.1021/ac60038a038
- Kitson, R. E., & Mellon, M. G. (1944). Colorimetric determination of phosphorus as molybdivanadophosphoric acid. Industrial & Engineering Chemistry Analytical Edition, 16(6), 379–383. https://doi.org/10.1021/i560130a017
- Lal, L. (2002). Phosphatic biofertilizers: Agrotech Pub. Academy.
- Mardad, I., Serrano Delgado, A., & Soukri, A. (2013). Solubilization of inorganic phosphate and production of organic acids by bacteria isolated from a Moroccan mineral phosphate deposit. African Journal of Microbiology Research, 7, 626–635. https://doi.org/10.5897/AJMR12.1431
- McGill, W. B., & Cole, C. V. (1981). Comparative aspects of cycling of organic C, N, S and P through soil organic matter. Geoderma, 26(4), 267–286. https://doi.org/10.1016/0016-7061(81)90024-0
- Meng, Q., Jiang, H., & Hao, J. J. (2016). Effects of Bacillus velezensis strain BAC03 in promoting plant growth. Biological Control, 98, 18–26. https://doi.org/10.1016/j.biocontrol.2016.03.010
- Mosela, M., Andrade, G., Massucato, L. R., de Araújo Almeida, S. R., Nogueira, A. F., de Lima Filho, R. B., Zeffa, D. M., Mian, S., Higashi, A. Y., Shimizu, G. D., Teixeira, G. M., Branco, K. S., Faria, M. V., Giacomin, R. M., Scapim, C. A., & Gonçalves, L. S. A. (2022). Bacillus velezensis strain Ag75 as a new multifunctional agent for biocontrol, phosphate solubilization and growth promotion in maize and soybean crops. Scientific Reports, 12(1). https://doi.org/10.1038/s41598-022-19515-8
- Oliveira, C. A., Alves, V. M. C., Marriel, I. E., Gomes, E. A., Scotti, M. R., Carneiro, N. P., Guimaraes, C. T., Schaffert, R. E., & NMH, S. (2009). Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biology and Biochemistry, 41(9), 1782–1787. https://doi.org/10.1016/j.soilbio.2008.01.012
- Omar, S. A. (1997). The role of rock-phosphate-solubilizing fungi and vesicular–arbusular-mycorrhiza (VAM) in growth of wheat plants fertilized with rock phosphate. World Journal of Microbiology and Biotechnology, 14(2), 211–218. https://doi.org/10.1023/A:1008830129262
- Perez, E., Sulbaran, M., Ball, M. M., & Andres Yarzabal, L. (2007). Isolation and characterization of mineral phosphate-solubilizing bacteria naturally colonizing a limonitic crust in the south-eastern Venezuelan region. Soil Biology and Biochemistry, 39(11), 2905–2914. https://doi.org/10.1016/j.soilbio.2007.06.017
- Pikovskaya, R. I. (1948). Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Microbiologiya, 17(7), 362–370. https://doi.org/10.4236/as.2019.103028
- Ponmurugan, P., & Gopi, C. (2006). In vitro production of growth regulators and phosphatase activity by phosphate solubilizing bacteria. African Journal of Biotechnology, 5(4), 348–350.
- Premono, M. E., Moawad, A. M., & Vlek, P. L. G. (1996). Effect of phosphate-solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Journal of Environmental Protection, 9(3), 2152–2219.
- Rashid, M., Khalil, S., Ayub, N., Alam, S., & Latif, F. (2004). Organic acids production and phosphate solubilization by phosphate solubilizing microorganisms (PSM) under in vitro conditions. Pakistan Journal of Biological Sciences: PJBS, 7(2), 187–196. https://doi.org/10.3923/pjbs.2004.187.196
- Regassa, A. B., Taegyu, C., & Seong Lee, Y. (2018). Supplementing biocontrol efficacy of Bacillus velezensis against Glomerella cingulata. Physiological and Molecular Plant Pathology, 102, 173–179. https://doi.org/10.1016/j.pmpp.2018.03.002
- Reis, M. R., Silva, A. A., Guimarães, A. A., Costa, M. D., Massenssini, A. M., & Ferreira, E. A. (2008). Ação de herbicidas sobre microrganismos solubilizadores de fosfato inorgânico em solo rizosférico de cana-de-açúcar. Planta Daninha, 26(2), 333–341. https://doi.org/10.1590/S0100-83582008000200009
- Rodrı́guez, H., & Fraga, R. (1999). Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnology Advances, 17(4–5), 319–339. https://doi.org/10.1016/S0734-9750(99)00014-2
- Saeid, A., Prochownik, E., & Dobrowolska-Iwanek, J. (2018). Phosphorus solubilization by Bacillus species. Molecules, 23(11), 2897. https://doi.org/10.3390/molecules23112897
- Sharma, S., Kaur, M., & Prashad, D. (2014). Isolation of fluorescent Pseudomonas strain from temperate zone of Himachal Pradesh and their evaluation as Plant Growth Promoting Rhizobacteria (PGPR). The Bioscan, 9(1), 323–328.
- Sharma, S. B., Sayyed, R. Z., Trivedi, M. H., & Gobi, T. A. (2013). Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus, 2(1), 1–14. https://doi.org/10.1186/2193-1801-2-587
- Shen, J., Yuan, L., Zhang, J., Li, H., Bai, Z., Chen, X., Zhang, W., & Zhang, F. (2011). Phosphorus dynamics: From soil to plant. Plant Physiology, 156(3), 997–1005. https://doi.org/10.1104/pp.111.175232
- Stevenson, F. J., & Cole, M. A. (1986). Cycles of the soil John Wiley and Sons (2nd ed.). John Wiley & Sons, Inc.
- Tabatabai, M. A., & Bremner, J. M. (1969). Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biology and Biochemistry, 1(4), 301–307. https://doi.org/10.1016/0038-0717(69)90012-1
- Vance, C. P., Uhde‐Stone, C., & Allan, D. L. (2003). Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytologist, 157(3), 423–447. https://doi.org/10.1046/j.1469-8137.2003.00695.x
- Van Der Heijden, M. G., Bardgett, R. D., & Van Straalen, N. M. (2008). The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology Letters, 11(3), 296–310. https://doi.org/10.1111/j.1461-0248.2007.01139.x
- Vazquez, P., Holguin, G., Puente, M. E., Lopez-Cortes, A., & Bashan, Y. (2000). Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biology and Fertility of Soils, 30(5–6), 460–468. https://doi.org/10.1007/s003740050024
- Whitelaw, M. A., Harden, T. J., & Helyar, K. R. (1999). Phosphate solubilisation in solution culture by the soil fungus Penicillium radicum. Soil Biology and Biochemistry, 31(5), 655–665. https://doi.org/10.1016/S0038-0717(98)00130-8
- Xie, L., Liu, L., Luo, Y., Rao, X., Di, Y., Liu, H., Qian, Z., Shen, Q., He, L., & Li, F. (2023). Complete genome sequence of biocontrol strain Bacillus velezensis YC89 and its biocontrol potential against sugarcane red rot. Frontiers in Microbiology, 14, 1180474. https://doi.org/10.3389/fmicb.2023.1180474