Abstract
The global health sector is changing with more consensus towards the consumption of natural products to improve the health of mankind. As a result, the nutrition market diversified with the inclusion of new herbal products with each passing day. Myristica fragrans is a traditional spice used to enrich food with a specific aroma in the cuisines of every region. It has two distinctive parts, i.e. nutmeg (seed) & mace (outer covering) that are used for the same purpose but possess different health benefits. It contains significant amounts of active components that are beneficial in reducing oxidative stress, lipid peroxidation, and allergenic responses. In the current review, efforts were made to elucidate the theoretical background of nutmeg and its health benefits. The antioxidant and antimicrobial potential of nutmeg has been discussed in length and their commercial applications have been highlighted. The health benefits associated with diabetes mellitus, cardiovascular diseases, inflammatory disorders, cancer insurgence, neurodegeneration, etc. are in the limelight of the article. The evidence portraying the negative aspects of nutmeg consumption and their critical analysis is discussed in length. Any plant’s positive and negative aspects depend on its rich phytochemistry and pharmaco-kinetics.
1. Introduction
The dawn of the 21st century has witnessed the peak of research and development that spread to every nick and corner of life. In the same era, drastic changes in dietary patterns led to the worsening of health indicators. The changes were coupled with a higher incidence of metabolic disorders that reverted the focus of the medical experts toward natural products and diet-health linkages. Accordingly, nutritionists deliberated to get a clear insight into the issues and significance of innovative and targeted approaches that can cloak the nutritional and medicinal needs of the people (Wink, Citation2022). The old proverb “diet be the medicine and medicine be the diet” resurfaced with more vigor and resultant health experts in collaboration with nutritionists coined terms like functional and nutraceutical foods. Researchers across the globe conducted targeted research to elucidate the potential of the inclusion of some traditional remedies. Even, some governments conducted intensive research and declared certain areas of their countries as herbal valleys. Nowadays, herbal products and their bioactive compounds are an important component of the global nutrition market resulting in the retrieval of the lost legacy of traditional medicines. According to WHO, 80% of total world’s population is dependent on herbal plants (Jamshidi-Kia et al., Citation2018). Medicines from plants are safe, effective, and have fewer side effects (Yadav & Agarwala, Citation2011). Many herbal plants like Ginger (Zingiber officinale), Mulathi (Glycyrrhiza glabra), Cumin seeds (Nigella sativa), Pepper (Piper nigrum), and Ashwaganda (Withania somnifera) are used in every corner of the world for treating innumerable health diseases and show highest efficacy. Myristica fragrans are one of the valuable therapeutic plants for treating health disorders and offer numerous benefits (Nguyen et al., Citation2021; Selling, Citation2021; Singh et al., Citation2010).
Myristica fragrans is commonly known as “nutmeg” and belongs to the family Myristiceae in the order Magnoliales which comprises around 150 genera and many species. Myristica species are natives of Eastern Indonesia, Sri Lanka, and India (Loizzo et al., Citation2016; Thangaselvabai et al., Citation2011) and they are now cultivated in a lot of countries of the world (Pal et al., Citation2011). It is a common spice in many food items such as sauces, meats, soups, and confectionery stuff (Gupta et al., Citation2013; Leontowicz et al., Citation2006). M. fragrans is the chief source of three commercial goods such as nutmeg, mace (fleshy aril), and essential oil, which are broadly used for flavoring purposes in many dishes and offer valuable health effects (Weerakoon et al., Citation0000).
In the current review, efforts were made to elucidate the theoretical background of nutmeg and its health benefits. The valuable effects of nutmeg against different diseases and some risks associated with its consumption are discussed in separate headings. The nutritional composition, phytochemistry, and pharmaco-kinetics of nutmeg have been discussed in length. The detailed discussion has been done under the following headings.
1.1. Methodology for systemic selection of articles
Literature was collected from the databases of Google Scholar, Google search engine, Pubmed, Science direct, Scoopus, and MDPI. The articles were searched through the keywords of nutmeg, Myristica fragrans, Nutritional composition, Phytochemistry, Pharmacokinetics, Anti-cancer, Anti-fungal, antibacterial, Neurological and psychoactive properties, Therapeutic potential, antioxidant, anti-inflammatory, and cardioprotective effect of nutmeg.
1.1.1. Myristica fragrans: Taxonomical characterization
Nutmeg is a spice herb discovered by the Portuguese and was originated in Indonesia. Dutch broadcasted the significance of M. fragrans. Nutmeg derives from the Latin word nux muscats means “musky nut” (Gupta & Rajpurohit, Citation2011). It is known by many other names in different countries of the world such as Jaiphal in Hindi, Urdu, and Marathi, Jaepatri in Bengali, Jafal in Kashmiri, and Pala in Indonesian, while in English, it is named Nutmeg (Ali et al., Citation2018). Nutmeg grows well on rich soils and in regions with an annual rainfall of 2000–3000 mm/year (Orwa et al., Citation2009). M. fragrans requires a warm climate and survives best at 18–34ºC. Moreover, altitude above sea level (0–700 m) is optimum for the proper growth of the plant (Saputro et al., Citation2016). A brief taxonomy of Myristica fragrans is given in Table (Kumari et al., Citation2021)
Table 1. Taxonomic description
Myristica fragrans is a fragrant evergreen tree that grows to a height of 5–13 m, and occasionally 20 m. Watery red sap or pink sap can be seen in the bark (Sultana et al., Citation2018). The pointed alternating dark green leaves (5–15 cm × 2–7 cm) with a shining top surface are placed along the branches on 1 cm long stems. Flowers are waxy, bell-shaped, pale yellow, and single-sexed. Male flowers are 5–7 mm in length and are found in groups of 1 to 10 while female flowers are 1 cm long and exist in a bunch of 1 to 3 flowers per group. Furthermore, nutmeg fruit is ~6–9 cm long and is smooth, drooping, fleshy, and yellow (Asgarpanah & Kazemivash, Citation2012). It is interesting to mention that the nutmeg flowering initiates at the age of 9 years lasts up to 75 years and usually bears 2–3 crops/year. M. fragrans produces two spices nutmeg and mace, respectively (Kusuma et al., Citation2020; Shafiei et al., Citation2012). Nutmeg is a seed present in the fruit and mace is red covering (aril) on the seed. M. fragrans seeds are broadly ovoid (about 2–3 cm in length), whitish, and fleshy, and there are also red-brown veins transversely on their surface. When it is fresh, aril (mace) is scarlet bright, but when it is dry it becomes brittle and yellowish-brown in color (Kiritikar & Basu, Citation1999; Pal et al., Citation2011). It has a pleasant fragrance and is used as an ingredient to give a slightly warm taste to the cuisines (Babua et al., Citation2021). M. fragrans seeds require 3–6 weeks to dry and then they are all set to use for medicinal purposes (Jaiswal et al., Citation2009).
1.2. Nutritional composition and characterization
Nutmeg contains appreciable quantities of carbohydrates, dietary fiber, energy, and proteins. Moreover, it contains fat-soluble vitamins such as A, E and C. It is predominantly rich in copper, iron, magnesium, phosphorus sodium, potassium, manganese, calcium, and zinc (Agbogidi & Azagbaekwe, Citation2013). In this regard, Nkwocha Chinelo et al. (Citation2018) evaluated the African nutmeg (Monodora myristica) for its proximate composition i.e. carbohydrate (35.92%), crude fiber (19.00 ± 1.00%), moisture (14.50 ± 0.52%), crude protein (12.09 ± 0.52%), crude lipid (16.00 ± 1.00%), and total ash (2.50 ± 0.50%). Previously, Sharathchandra et al. (Citation2023) reported lower values for fiber 10.91 ± 0.3 mg/g, higher lipids (23.28%), and moisture contents (51.03 ± 0.25 mg/g), and energy level (1591.8 KJ/100 g). Besides, mineral content (mg/100 g) was also recorded, i.e. Zn (1.52 ± 0.11), Al (4.98 ± 0.68), Cu (0.19 ± 0.02), Na (17.66 ± 0.32), Fe (21.71 ± 0.52), Mn (1.05 ± 0.35), P (112.03 ± 4.63), K (869.64 ± 4.03), Ca (416.01 ± 1.42), and Mg (86.96 ± 4.01). The biochemistry of the nutmeg fixed oil is of interest due to the presence of myristic acid, stearic, palmitic, linoleic, and lauric acids, and these are nutmeg fixed oil’s main components (Duarte et al., Citation2011). Description of the phytochemical analysis indicated saponins, carbohydrates, tannins, proteins, amino acids, terpenoids, and flavonoids presence.
The phytochemicals present in the nutmeg are mainly centered in its essential oil. The production of essential oil of M. fragrans was recently studied for seed, mace, kernel, and leaf at 5.21%, 6.24%, 3.16%, and 8.10%, respectively. Main fractions in the plant were found to be sesquiterpenes (1.21%–16.76%), phenyl-propenes (1.96%–28.61%), and monoterpenes (53.77%–94.82%), in all the parts of the plant. Myristicin (2.7%), safrole (1.4%), limonene (3.7–8.32%), terpine-4-ol (5.80–11.83%), β-pinene (7.30–9.72%), α-pinene (9.40–18.04%), and a large portion of Sabinene (19.07–41.70%) were notable phytochemicals present in the essential oil of (Ashokkumar et al., Citation2020; Hoda et al., Citation2020; Pal et al., Citation2011; Zachariah et al., Citation2008). Monika-Thakur (Citation2014) demonstrated that M. fragrans active components have antimicrobial, antifungal, antioxidant, and antidepressant properties. Francis et al. (Citation2014) removed different M. fragrans chemical constituents like sabinene, myristicin, eugenol, elemicin, and safrole, along with natural repellents and insecticides to control Lasiodermaserricorne (Du et al., Citation2014).
1.3. Phytochemistry
Phytochemicals are bioactive compounds present in plants like fruits, vegetables, leaves, roots, flowers, and medicinal plants that work with fibers and nutrients to go as a defense system against infection or more precisely protect against disease (Krishnaiah et al., Citation2007; Saxena & Patil, Citation2012). Joseph and George (Citation2014) examined the M. fragrans phytochemical properties of phytosterols, saponins, flavonoids, tannins, proteins, and alkaloids as well as antibacterial and antimicrobial activities (Pal et al., Citation2011).
Plants are well known to have diverse secondary metabolites and leading phytochemical constituents like glycosides, phenolics, saponins, flavonoids, tannins, steroids, and alkaloids (Saxena & Patil, Citation2012). The utilization of phytochemical components with firm antimicrobial characteristics is of importance in the therapeutic medicine system. Nutmeg is a small bundle with several major advantages. It is utilized for the healing and prevention of known conditions (Sharathchandra eta al., Citation2023). M. fragrans phytochemicals such as machilin A enacted p38 mitogen-initiated protein kinase by osteoblast separation just as some lignans like licarin B, licarin A, mesodihydroguaiaretic acid, machilin F, myristargenol, and safrole improve anabolic action in metabolism of bone (Lee et al., Citation2009).
Subjective phytochemical analysis has shown that steroids were distinguished in higher quantity, terpenoids, and saponin were in moderate amounts, whereas tannin, alkaloid, and phenol were detected in little quantity. Besides anthroquinone, anthocyanins and glycoside were not identified. Similarly, the results of the study showed that terpenoid was detected in the quantity of (19.00 ± 3.18%), saponins (12.04 ± 3.33%), and steroid (32.75 ± 5.42%), whereas phenol (0.46 ± 0.10%), an alkaloid (2.75 ± 0.82%) was detected in low amount (Nkwocha Chinelo et al., Citation2018). Phytochemical profiling done by various researchers reported compounds including licarin, neolignan, verrucosin, nectandrin, elemicin, myristicin, myristicin, isoelemicin, surinamensin, methoxylicarin A, malabaricone, myrislignan, dihydro-benzofuran, β-pinene, α-pinene, sabinene, phenylpropanoids, methyl eugenol, eugenol, elemicin, methyl isoeugenol, triterpenoids, phenolic aldehyde, safrole, virolane, erythrosurinamensin, and diaryl phenyl propanoid (Chiu et al., Citation2016; Du et al., Citation2014; Francis et al., Citation2014; Morikawa et al., Citation2016).
M. fragrans contains calculable bioactive components that are useful in improving allergenic reactions, repressing lipid peroxidation, and decreasing oxidative stress (Gupta et al., Citation2013; Morikawa et al., Citation2016). Elimicin, safrole, myristicin, myristic acid, and Trimyristin from nutmeg are accounted for their medicinal properties (Wiart, Citation2007). Because of nutmeg collection and widespread along with strongest biological actions, it is now used as herbal medicine and a kitchen spice in many countries, especially in China and India (Asgarpanah & Kazemivash, Citation2012).
1.4. Pharmacokinetics of Myristica fragrans
During the recent decade, (ADMET) absorption, distribution, metabolism, excretion, and toxicity have been brought into early drug development processes rather than sequential development because pharmacokinetics and ADMET are responsible for clinical trial failure. For absorption and permeability screening, many in vitro plans have been made, out of which common is the Caco-2 cell monolayer model.
The penetrability and transport in the intestine of 10 neolignans that were isolated from M. fragrans were contemplated, among 10 neolignans; the type of neolignans 8-O-4′- displayed elevated permeability, whereas the neolignans of benzofuran-type disclosed poor permeability. Amongst, almost eight neolignans were transported through passive diffusion. These results showed that neolignans of 8-O-4′-type are much-assimilated compounds (Yang et al., Citation2010). Gao et al. (Citation2017) determined eugenol, iso-psoralen, bakuchiol, and psoralen, centralizations to explore the M. fragrant pharmacokinetics process. As per the exploratory results, the M. fragrans compatibility can remarkably affect the pharmacokinetic cycle of principle components and in vivo extend their distribution and speed up their metabolism and elimination.
Statistical analyses indicate that Myrislignan (neolignan extracted from M. fragrans) and podophyllotoxin pharmacokinetic activities in rat models have major differences between the two groups (Zhu et al., Citation2016). Sohn et al. (Citation2007) stated that Anwuligan, 2, 3 - dibenzylbutane lignan of M, fragrans has been revealed to hold a wider pharmacological effects range.
Liquid chromatography-mass spectrometry technique was applied to tissue distribution and pharmacokinetics study of anwuligan after intragastric and intravenous administration. The outright bioavailability is about 16.2%, which addresses the current first-pass effect. After the administration in an intragastric way, enterohepatic dissemination was found. Anwuligan might be distributed rapidly in numerous tissues as well as kept in high concentration in the liver (Song et al., Citation2019).
The blood–brain barrier permeability of 12 lignans and three phenolic malabaricones of nutmeg were investigated. Malabaricones had poor permeabilities, while Benzonfuran-type, arylnaphthalene-type, and dibenzylbutane-type lignans had low to moderate permeabilities, and heterocyclic and neolignan had moderate-high permeabilities, among the 15 compounds studied. To nectandrin B, verrucosin, acetic acid derivation, and 5-methoxy-dehydrodiisoeugenol an efflux was counted in and the primary carrier for 9, 8 (P-glycoprotein). Dependency of concentration and time tests showed the principle transport mechanism for myrislignan 7 and 8 and neolignans dehydrodiisoeugenol, was passive diffusion (Wu et al., Citation2016). A technique was created to examine (+)—licarin A and diastereomers isolicarin A in the blood of rats after administration from the intravenous route. Lower quantification and detection limits were 0.10 µg/mL and 0.05 for isolicarin A and 0.25 and 0.05 Microgram/mL for (+)—licarin A. This analysis method was efficiently applied to the pharmacokinetics study of (+)—licarin A and diastereomers isolicarin A in rodent blood.
1.5. Oil chemistry
Oils from the promising medicinal plant Myristica fragrans hold a lot of active components that are liable for several health benefits (Maya et al., Citation2004). Adewole et al. (Citation2013) represented the results of fatty acids such as eicosanoic acid, oleic acid, stearic acid, and palmitic acid; results indicated that the plants possess fundamental fatty acids that are useful for both infants and adults. The seed oil of African nutmeg physicochemical properties results in the mean value of these properties such as acid value (66.50 mg KOH g/1), peroxide value (4.13 ± 0.40), free fatty acids (33.26 mg/g-1), specific gravity (1.464 g cm/3 refractive index (1.477), iodine value (101.61 mg of Iodine g/1), saponification value (414.53 mg KOH g/1) and yellow color demonstrated that nutmeg oil (African) is somehow a kind of dry oil and may not be healthy for cooking purposes (Burubai et al., Citation2009).
1.6. Pharmacological properties
Myristica fragrans have a wide range of bioactive components that play a central role in the traditional medicine system and have been recommended for treating numerous chronic diseases, due to their significant therapeutic effects. Nutmeg’s major constituents as kernel, mace, seed, and leaf are likewise used in aroma and pharmaceutical industries (Ashokkumar et al., Citation2020). Some of the health benefits of nutmeg are listed below.
1.7. Antioxidant properties
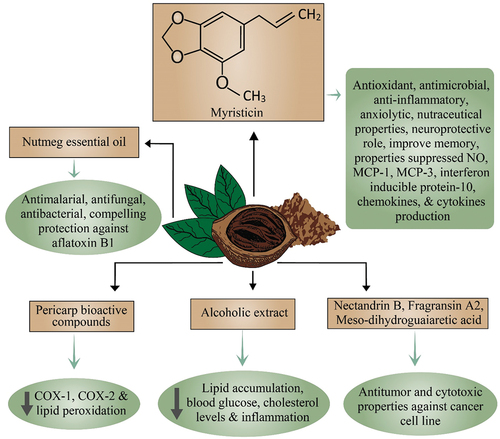
Antioxidants play a crucial role in the human body by hindering the oxidation process and thus contributing to the improvement of health (Shahidi & Zhong, Citation2015). It was accounted that in M. fragrans pericarp bioactive compounds limited cyclooxygenase enzymes COX-2 and COX-1 production bioassay method and lipid peroxidation (Zhang et al., Citation2015). A study reported that M. fragrans contains cytotoxicity, anti-angiogenic, and antioxidant activities (Piaru et al., Citation2012). Cao et al. (Citation2015) announced that M. fragrans hinders the production of nitric oxide. Additionally, nutmeg showed strong antioxidant and hepatoprotective properties against isoproterenol-induced hepatotoxicity and oxidative stress (Kareem et al., Citation2013).
M. fragrans extracts of acetone and ethanol have antioxidant activities with the method of (FRAP) and (DPPH) assay due to myristicin, sabinene, and eugenol presence (Assa et al., Citation2014; Gupta et al., Citation2013). In another research, mace extracts of nutmeg obtained EC50 values of 13.41 g/ml and 12.44 g/ml, respectively, for DPPH and ABTS antioxidant tests, exhibiting radical antioxidant and scavenging properties (Suthisamphat et al., Citation2020). Vangoori Yakaiah and Kavimani (Citation2019) clearly showed 88% inhibition of DPPH free radicals at the standard dose of 5 mg/mL. The standard ascorbic acids also showed a similar inhibition (~90%) that depicts the significance of the MF extract as a potential antioxidant.
The current era of nutrition usually revolves around determining the molecular targets of bioactive compounds. In a study, Zhao et al. (Citation2020) examine the nutmeg alcohol extract effects on lipid synthesis and inflammation inhibition. They reported that nutmeg alcohol extract treatment downregulated the sterol-regulating element-connecting protein 1c and gene expression of fatty acid synthase thus decreasing the accumulation of lipids in cells. It was found that nutmeg alcohol extract remarkably decreased liver function indexes, blood glucose, lipid accumulation, cholesterol levels, and inflammation levels in mice. The root of nutmeg has a strong potential as an antioxidant agent as compared to other plant parts of nutmeg. Strong antioxidant activity was confirmed by DPPH activity with a 12.67 ppm IC50 value (Ginting et al., Citation2020).
1.8. Anti-cancer properties
Anticancer action is due to the effect of some biological or chemical substances to inhibit or reverse carcinogenic advancement. Many plants have been reported in recent years that showed anticancer activity; results support their use as chemotherapeutic agents and Myristica fragrans is one of the most used anti-cancer agents with beneficial satisfying effects (Ginting et al., Citation2021; Saydam & Nalkiran, Citation2021).
M. fragrans is quite effective for various cancer types of treatment such as colon cancer breast cancer and skin papilloma (Piras et al., Citation2012). Gupta et al. (Citation2013) demonstrated that nutmeg holds antitumor and anticancer activities against different types of cancers. M. fragrans anti-inflammatory properties were determined in the fibroblast cell culture which was then treated with viral dsRNR mimetic.
Thuong et al. (Citation2014) indicated that lignans like nectandrin B, fragransin A2, meso-dihydroguaiaretic acid, and mace lignan, showcased their antitumor activity and cytotoxic against the cancer cell lines. The root of nutmeg has solid potential as the anticancer agent in the nutmeg plant parts as a dependent MFHR, MIT assay which showed moderate activity of anticancer against MCF-7 cell lines (Ginting et al., Citation2020).
Phytochemicals, for example, quercetin, and myristicin, found in the ethanolic extract of nutmeg hinder cytokines production and nitric oxide just as they contain inflammatory properties (Dewi et al., Citation2015; Lee & Park, Citation2011). LPS-induced nitric oxide suppression in RAW264.7 cells and its toxicity were found to be protective against stomach cancer when M. fragrans mace extract was used (Suthisamphat et al., Citation2020).
1.9. Antifungal and antibacterial properties
At present, infectious diseases are the leading cause of death around the world. Almost half of the deaths reported in temperate countries are due to bacterial contamination. But now, antibiotics and many drugs are helpful in the treatment of several infectious diseases (Ayoola et al., Citation2008).
Myristica fragrans have anti-secretory and anti-diarrheal properties against various microbes and pathogens (Grover et al., Citation2002). Two antimicrobial resorcinol malabaricone C and malabaricone B were separated from the mace, which is the outer covering of the seed of M. fragrans. Orabi et al. (Citation1991) showed that mace and nutmeg disclosed strong antifungal and antibacterial properties. It is valuable to show the inhibitory activity against the bacterial strain of Streptococcus mutants and to treat period ontic (Jangid et al., Citation2014). Shafiei et al. (Citation2012) researched the M. fragrans antibacterial capacity against various bacterial strains just as it is extremely effective against different microorganisms. Furthermore, Dorman and Deans (Citation2000) also examined the M. fragrans extracts to establish that it has antifungal and antibacterial properties. It repressed the bacterial spores’ development and was utilized as an antiseptic, disinfectant, and food preservative. Akinboro et al. (Citation2012) studied M. fragrans anti-mutagenic and mutagenic effects on Mus musculus and Salmonella typhimurium. Likewise, Radwan et al. (Citation2014) demonstrated that it is effective as an antifungal agent against plant microorganisms like Phomopsis viticola and Phomopsis obscurans. Nutmeg alkaloids have been shown to have antibacterial activity against Gram-positive and Gram-negative bacteria (Azeez et al., Citation2019).
Myristica fragrans leaf extracts a good source of tannin are associated with in vitro inhibition of Ceratobasidium ramicola (a phytopathogenic fungus) growth. The 1% concentration of Tannin extract of M. fragrans produced radial inhibition by 27.0% at 24 h of incubation (Firmansyah et al., Citation2019). In a recent study, the essential oil of nutmeg showed antimalarial properties against Plasmodium falciparum D6 (Ibrahim et al., Citation2020). Conversely, in situ, the efficacy of nano encapsulated and un-encapsulated (essential oil of Myristica fragrans) showed compelling protection against contamination of aflatoxin B1 and fungal pervasion. Un-encapsulated MFEO displayed fungitoxicity against food-borne molds and restrained aflatoxin B1 discharge by Aspergillus flavus however nano-embodied MFEO indicated better inhibitory impact and fungitoxicity on the aflatoxin biosynthesis (Das et al., Citation2020).
Furthermore, M. fragrans extract was found to have an antibacterial effect against Klebsiella pneumoniae, Proteus vulgaris, and Staphylococcus aureus, when used regularly. Salmonella typhimurium, Bacillus subtilis and Pseudomonas aeruginosa were all resistant to M. fragrans extract (Ibrahim & Oyinloye, Citation2011). Myristica fragrans (nutmeg) seeds extract and (AgNPs) silver nanoparticles are accounted for to be viable against (MDR) multidrug-resistant Salmonella enterica in the antibiofilm activity. In one study, nutmeg aqueous seed concentrate displayed 87% antibiofilm activity, on the other hand, the biosynthesized silver nanoparticles showed antibiofilm activity of 99.1% (Balakrishnan et al., Citation2020). In the comparative study, Cherian et al. (Citation2019) featured that nutmeg leaf ester (MFLE) zinc oxide nanoparticles (MFLE-ZnONPs) are utilized as environmentally friendly and cost-effective antibiotic agents against the MDR clinical isolates. Antibacterial effectiveness was assessed by checking the development of 15 bacterial strains which were treated by various dilution ranges of hydrolats and essential. Excipient essential oil inhibited P. multocida, S. mutants, and E. faecalis growth, though the oil was effective against the strain (Matulyte et al., Citation2020). Adewole et al. (Citation2013) conducted a study to notice the antibacterial activity of nutmeg against the standard of streptomycin. The antimicrobial screening result indicated the oil was extremely sensitive against tried isolates. Following the incubation duration of 24 hours, the inhibition zone against Staphylococcus aureus (11 mm), Bacillus subtilis (8 mm), and Escherichia coli (11 mm). The outcome compares well with the standard of streptomycin; the inhibition zone result against E. coli of the sample (11 mm) was higher than that recorded for the standard of streptomycin (7 mm) (Adewole et al., Citation2013). Nurjanah et al. (Citation2017) studied nutmeg oil’s antibacterial impact against pathogenic microbes (gram-positive bacteria: Staphylococcus epidermis, Staphylococcus aureus, and gram-negative bacteria: Salmonella Typhi, Shigella Dysenteriae) (Setty et al., Citation2020). Resistance design was researched by the method of in vitro disc diffusion utilizing the essential oil concentration of 100%, 80%, 60%, 40%, and 20%. The result suggested that two essential oils of nutmeg countered all bacterial species. The highest zone of inhibition for nutmeg oil was achieved on 60% convergence (12.96 mm for S. aureus, 16.79 mm for S. epidermis, 13.46 mm for S. Dysenteriae and 16.50 mm for S. Typhi), while on the Sulawesi nutmeg oil the result on concentration of 100% (18.84 mm for S. aureus, 16.54 mm for S. epidermis, 17.84 mm for S. Dysenteriae, and 12.54 mm for S. typhi. Similarly, Sipahelut et al. (Citation2019) determined the antifungal and antibacterial action of flesh oil of nutmeg fruit inhibiting the action growth of food-destroying fungi, food-destroying bacteria, and pathogenic bacteria. The tested bacteria incorporate Escherichia coli and Staphylococcus aureus, while the tested fungi are Fusarium moniliforme and Aspergillus flavus. The outcomes indicated that the nutmeg fruit flesh oil might suppress the isolates’ growth at entire concentration. A higher concentration of the oil of nutmeg fruit flesh increases the inhibition zone of bacterial growth. Flesh-derived oil utilization showed compact antibacterial action against every tested bacterium. In any case, it showed feeble antifungal activity against every tested fungus (Sipahelut et al., Citation2019). But the malabaricones C and B and resorcinols, separated from the mace of nutmeg by exhibiting strong antifungal and antibacterial activities (Khare, Citation2008). Potential antifungal properties were studied by Hoda et al. (Citation2020) against Aspergillus fumigatus demonstrated that the M. fragrans extract of hexane inhibited the production of melanin by 76.09%, decreased the ergosterol content by 83.63% and A. fumigatus cell hydrophobicity by 72.2% at MEC of about 0.078 mg/mL. It was tracked that the mace ethanolic concentrate displayed antimicrobial action against six strains with MIC upsides of 125–250 μg/ml and H. pylori (Suthisamphat et al., Citation2020). Another study was conducted by Firempong et al. (Citation2016) to decide Calabash nutmeg seeds, bark, root, and leaves extract against antifungal activity (Monodora myristica). The M. myristica ethanolic extracts contained a compound mixture of triterpenoids, flavonoids, saponins, alkaloids, general glycosides, steroids, and tannins. Roots and seeds of nutmeg were best in repressing the C. albicans growth. Most plant extracts antifungal activities were also the same as to Clotrimazole drug (standard).
Wang et al. (Citation2019) contemplated a Myristica fragrans essential oil (MFEO), contrasting the β-CD/MFEO antimicrobial activity and essential oil against Bacillus subtilis, the yeast Saccharomyces cerevisiae, Klebsiella pneumoniae, Escherichia coli, Staphylococcus epidermidis, and Staphylococcus aureus. Results indicated that the antimicrobial impact was improved after the inclusion of complex formation. Ansory et al. (Citation2020) reported nutmeg major antimicrobial compounds 1–4-terpineol, safrole, 2-β-pinene, α-pinene, sabinene, and myristicin. Results indicated that MBC and MIC on the Shigella sp. essential oil of nutmeg showed 12.11 mm inhibitory impacts at 1.25%, division 4 has 9.67 mm at 1.25%, and portion 2 at 2.5% displayed 5.88 mm inhibitory impacts. Escherichia coli MBC and MIC nutmeg oils exhibit inhibitory impacts (10.89 mm) at 2.5%; part one showed 10.22 mm at 10%, and part 4 at 2.5% has 10.11 mm inhibitory impacts.
1.10. Neurological properties
The neurological disorder is mainly characterized by loss of memory, behavioral disorder, loss of interest in daily activities, and cognitive disability. This disorder is increasing daily in developed countries and is most common in older people (Orhan et al., Citation2004).
Myristica fragrans neuropharmacological activities in (CNS) are actually because of their bioactive components (Sultana et al., Citation2018). Methanolic extract of M. fragrans seed on neuroprotective effect on the scopolamine-prompted impacts have been reported. Methanolic extract treatment shielded the subject cortex scopolamine effects by turning around the poisonousness markers effects. Surprisingly, the Methanolic extract neuroprotective impact was practically identical to that applied by antioxidant NAC reference (Al-Quraishy et al., Citation2020). The anxiogenic property of Hexane and Trimyristin extract of nutmeg was evaluated in the rat models through the whole board paradigm and elevated plus maze. The seeds concentrated by hexane additionally exhibited stimulant action in rats by swim and tail tests by suspension (Asgarpanah & Kazemivash, Citation2012). The ethyl acetic acid fraction indicated high metal chelating and neuroprotectivity ability. Results affirmed the 5, 7-diacetyl chrysin, and trimyristin in the comparing fraction (Omidpanah et al., Citation2022). Dhingra and Sharma (Citation2006) showed that n-hexane extracts of Myristica fragrans seeds exhibited a significant anti-depressant effect by using tail suspension test (TST) and forced swim test (FST) in rat models. Nutmeg extract administration displayed a significant decline in the immobility of mice. Furthermore, a 10 mg/kg dose of nutmeg showed similar effects to standard drugs.
The Myristica fragrans Houtt seed’s activity anti-cholinesterase was assessed. Compounds, for example, (hydroxy-3’− methoxyphenyl hydroxypropyl benzene) indicated the effective action with an IC50 estimation of 35.1 microM, trailed by [11 (malabaricone C), methylenedioxyphenyl—dimethyl (dihydroxyphenyl)- butane] with IC50 estimations of 44.0 pM and 42.1, respectively. This is the primary report on M. fragrans seeds anticholinesterase properties. M. fragrans may be effective in the treatment of Alzheimer’s disease, according to the findings (Cuong et al., Citation2014). Extract of Myristica fragrans has shown anxiogenic and antidepressant effects. Myristicin in the nutmeg is termed a standard aromatic constituent of essential oil. In this specific context, McCall (Citation2011) revealed myristicin anxiolytic properties and its possible interactivity with the GABAA receptor site. Afterward, Sonavane et al. (Citation2002) reported that myristicin plays a role at the GABAA receptor site apart from the benzodiazepine site. Phytochemical compounds of M. fragrans, elemicin, and myristicin, offer pain-relieving effects, a mild narcotic, and antianxiety by actuation of neurotransmitters dopamine and serotonin (Dhingra & Sharma, Citation2006; Leiter et al., Citation2011; Romana et al., Citation2020).
Nagaraju et al. (Citation2013) contemplated M. fragransin anxiolytic impact open field model utilizing various parameters. They discovered that high dose has more anxiolytic action than little dosages. Three compounds, malabaricone C, 5’- methoxylicarin A, and licarin A, were discovered to be generally dynamic in the inhibition of FAAH in the rodent anxiety model. These compounds have high potency and FAAH selectivity just as anxiolytic activity (El-Alfy et al., Citation2019). Patricia et al. (Citation2019) suggested that the DCM (as the nutmeg powder was fractionated using Dichloromethane (DCM)) has high memory-improving activity, which might be because of the enhancement of cholinergic transmission and antioxidant activity. The lessening of ketamine impact by DCM may potentially result from oxidative stress attenuation and NMDA receptor interceded neurotransmission (Patricia et al., Citation2019).
Ghorbanian et al. (Citation2019) evaluated the composition of nutmeg using GC-MS and they claimed that there are three main components, i.e. myristicin (11.17%), myristic acid (39.93%), and elemicin (22.16%). They further suggested that nutmeg extract pre-treatment successfully decreased cell death, diminished seizure behavior, and enhanced glial initiation that is trailed by the administration of PTZ. Taking everything into account, nutmeg extract may be viewed as a helpful supplementary agent in the treatment of epilepsy through its neural loss attenuation and glial activation
1.11. Antidiabetic properties
Diabetes mellitus is an enduring metabolic condition characterized by elevated glucose levels and the insufficient secretion or action of insulin. Generally, many drugs are available in the market for the management of diabetes and its complications (Abebe et al., Citation2014). Apart from their valuable therapeutic effects, these drugs have surplus adverse effects such as hypoglycemia, secondary failure, and cell death (Suji & Sivakami, Citation2003). In a study, Tahrani et al. (Citation2010) demonstrated that special consideration has been paid to alternative remedies for the treatment of diabetes using herbal plants and their phytochemical components.
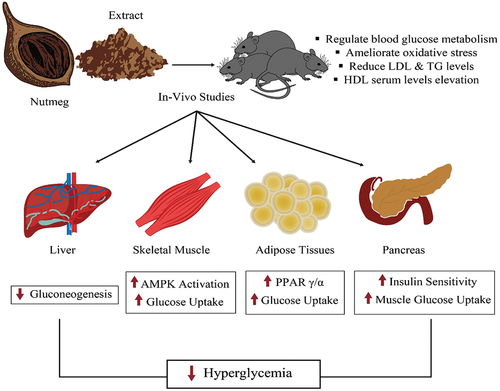
Nguyen et al. (Citation2010) inspected M. fragrans; they found lignans of (THF) tetrahydrofuran which are successful in diminishing levels of LDL, adipose tissue mass, and body weight. It has properties of anti-obese nature because of the essence of adenosine monophosphate (AMP) initiated protein kinase enactment system.
Vangoori Yakaiah and Kavimani (Citation2019) evaluated the effects of anti-obesity MF extracts in cafeteria diet-induced obese rats. The 10 weeks of the feeding of MF extract MFE 200 mg/kg and 400 mg/kg reduced the weight of the rats by 9.29% and 12.87%, respectively. The higher doses were more effective and can be comparable to the standard drug or list at 50 mg. The results might be due to the inhibition of some digestive enzymes especially pancreatic lipase thus resulting in reduced absorption of lipids ultimately reducing the energy intake.
M. fragrans seed extract showed strong antidiabetic properties by controlling hyperglycemia in artificially induced diabetic rats (Somani & Singhai, Citation2008). Likewise, Lestari et al. (Citation2012) indicated that the anti-diabetic effect agonist PPAR γ/α present in the nutmeg extract. Arulmozhi et al. (Citation2007) demonstrated that the hydroalcoholic extract of nutmeg has been reported to be effective against chlorpromazine-induced increased glucose levels and triglyceride advancements in animal models. Oral administration of extracts reduced metabolic abnormalities as well as glucose levels. Similarly, Myristica fragrans extract significantly reduced the raised cholesterol and triglyceride levels of rats. So, it was confirmed that nutmeg extract improved the hyperglycemic condition and irregular fatty acid metabolism in rat models.
The nutmeg extract administration to the diabetic rats caused a significant drop in the low-density lipoprotein, malondialdehyde levels, and total cholesterol and triglycerides levels. Whereas a remarkable elevation was noticed in the HDL serum levels and antioxidant capacity (Pashapoor et al., Citation2020).
Myristica fragrans seed extract’s hypoglycemic potential in the in vivo study was determined with glimepiride. This combination of therapy with glimepiride remarkably reduced the level of glucose as compared to the standard (glimepiride). Dynamic simulation on a molecular basis revealed that complexes of macelignan γ and α were more stable than those complexes of pioglitazone (Nasreen et al., Citation2020).
The antidiabetic effect of nutmeg was investigated by Lestari et al. (Citation2019), which indicated that the level of blood glucose levels decreased by 20% after two days of therapy, 30% after four days of therapy, and 40% after six days of treatment with nutmeg extracts. Luciferase assay indicated that without safrole, as per dose-dependent action, nutmeg improved the PPAR α and γ activities, which denoted the expected mechanism in bringing down the glucose levels. M. fragrans extracts of petroleum ether diminished blood glucose levels in a normal diet, glucose diet, and alloxan-prompted diabetic rodents (Sultana et al., Citation2018). The hypo-glycemic impact might be because of the insulin release potentiation from beta-cells. Orally administered extract additionally smothered the glucose level expansion by the loading of glucose. This impact may be because of diminishing intestinal glucose assimilation rate pancreatic discharge potentiation or glucose take-up increase. The concentrate expanded the body weight in diabetic rodents, which may be because of better glycemic control and increased levels of insulin secretion (Somani & Singhai, Citation2008).
The Myristica fragrans and its products can be used in conjunction with potential antidiabetic drugs, e.g. glimepiride. In this context, Nasreen et al. (Citation2020) reported the effect of combined therapy including nutmeg extract and glimepiride that drastically decreased blood glucose levels. Figure presents a thematic diagram illustrating the anti-diabetic properties of nutmeg.
1.12. Anti-inflammatory activity
Inflammation is the body’s natural, defensive response to damaged tissue resulting from toxic chemicals, physical trauma, or microbes. Acute and chronic inflammations are the two most common types of inflammation. For millennia, medicinal plants have been a source of a wide range of active substances, which have been widely employed as crude material to treat a variety of inflammatory disorders (Kumar et al., Citation2013).
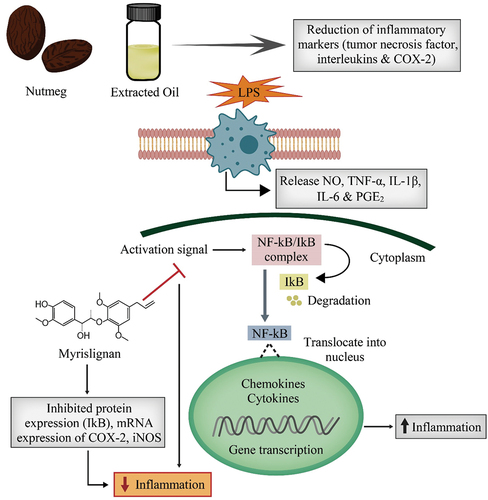
Many researchers reported the anti-inflammatory action of nutmeg as well as its extracted oil. A study described that nutmeg reduced the cytokines such as tumor necrosis factor and interleukin. The extract also reduced the cyclooxygenase-2 pathway and contributed to the significant reduction of inflammatory markers (Mueller et al., Citation2010).
The anti-inflammatory activity of flavonoid-rich fractions from M. fragrans’s ethanolic extract was confirmed, demonstrating the plant’s importance as an anti-inflammatory medicine (Akinwunmi & Oyedapo, Citation2014). Myrislignan, a phytochemical component of M. fragrans inhibited the protein expressions and mRNA expression of cyclooxygenase-2 and inducible iNOS in LPS-stimulated macrophage cells. The results disclosed that myrislignan exerts strong anti-inflammatory properties in LPS-stimulated macrophage cells by impeding the NF-kB signaling pathway activation (Jin et al., Citation2012). Figure presents a thematic diagram illustrating the anti-inflammatory properties of nutmeg.
BATM (Citation2016) detected that ethanolic extract from wood, nutmeg, and mace showed an anti-inflammatory effect, but the extract from wood revealed more powerful anti-inflammatory action than others. Lee and Park (Citation2011) evaluated that myristicin holds the strong anti-inflammatory property.
According to the researchers, Myristicin suppressed the production of NO, monocyte chemotactic protein (MCP)-1, MCP-3, interferon-inducible protein-10, IL-6, IL-10, calcium, macrophage inflammatory protein, granulocyte-macrophage colony-stimulating factor, and leukemia inhibitory factor. Results disclosed that myristicin has powerful anti-inflammatory effects by reducing the chemokines, growth factors, and cytokines.
1.13. Miscellaneous health benefits
For quite a while, utilization of M. fragrans in conventional medicines as an abortifacient, emmenagogue, narcotic, stimulant, and carminative. Nutmeg is additionally endorsed for various ailments treatment, like diarrhea, decreased appetite, muscle spasms, and rheumatism (Khare, Citation2008). Therapeutically, nutmeg contains strong antimicrobial properties. It is viable in killing off various cavity-causing microorganisms in the mouth. Nutmeg such as cloves contains a compound, eugenol that benefits the heart. Myristicin present in the nutmeg has appeared to repress an enzyme in the cerebrum that adds to Alzheimer’s illness and is utilized to improve memory. It is utilized in small doses to lessen flatulence (intestinal gas or excessive stomach), help assimilation, and improve craving (Jaiswal et al., Citation2009). Nutmeg can assist with combatting asthma. It is additionally used to loosen up muscles. Nutmeg oil drops can likewise be blended in with nectar to treat indigestion, chronic diarrhea, gastroenteritis, and nausea. In homeopathy, the use of nutmeg to treat depression and anxiety is evident. Essential oils of nutmeg are excellent antimicrobial sources. The restorative usage of essential oils is turning out to be popular in light of their fewer side effects and low obstruction towards microorganisms (Saxena & Patil, Citation2012; Suthisamphat et al., Citation2020).
M. fragrans’s anti-oxidant and anti-inflammatory capabilities have been shown to boost learning and memory in mice (Parle et al., Citation2004). Hayfaa et al. (Citation2013) stated that the alkaloid concentrate of the seed kernel of ground nutmeg significantly declined the writhing reactions in rat models.
A previous study reported the hepatocurative effect of water extract of nutmeg. Hepatotoxicity was induced by isoproterenol and the nutmeg extract improved the liver enzymes together with hepatocurative effects (Kareem et al., Citation2013). Similar results have been reported by Zhao et al. (Citation2020) that nutmeg’s alcohol extract upgraded nonalcoholic fatty liver disease (NAFLD). An in vivo study by the author showed that nutmeg extract considerably reduced inflammation, body weight, cholesterol levels, blood glucose, and mainly lipid accumulation resulting in an effective hepatocurative agent. Investigations reported that nutmeg can fight against hepatotoxicity because of a particular phytochemical compound identified as myrislignan, which protects against liver damage condition. Chloroform, methanol, petroleum ether, and n-hexane fractions of Myristica fragrans (200 and 400 mg/kg body weight) reported anti-inflammatory and analgesic properties. Results showed that all extracts inhibited the carrageenan-induced inflammation but the methanol extract displayed significant results (90.9%). The petroleum ether showed good analgesic action (60.7%) among other extracts but was not as effective as diclofenac sodium (Standard) (76.3%). So, it was concluded that several nutmeg extracts hold anti-inflammatory and analgesic potential (Bhuiyan et al., Citation2018). Kelble (Citation2005) exposed the nutmeg anti-aggregatory and hypolipidemic effects in an animal model. They discovered that it lessens the LDL and VLDL levels and diminishes the levels of cholesterol in the liver and heart. Likewise, Sharma et al. (Citation1995) prompted M. fragrans extract in hypercholesterolemic animals to contemplate the hypolipidemic impact which likewise inhibits cholesterol accumulation, fats in the heart and liver, etc.
The administration of M. fragrans extract for constant 21 days reduced the arthritic indices induced by complete fruend’s adjuvant (CFA). While 300 mg/kg extract significantly reduced the TNF-α levels. Extract administration reduced cartilage destruction and showed powerful anti-arthritic action (Najafzadeh et al., Citation2014). Chung et al. (Citation2012) reported that after the extraction of some bioactive components such as safrole and myristicin from the ethanolic extract of Myristica fragrans, it is considered to be a strong nutraceutical candidate for the treatment of skin diseases such as atopic dermatitis.
An antiulcer property of Myristica fragrans seed extract was reported by Sattar et al. (Citation2019) in rat models. Administration of extract for a constant 15 days reduced the gastrointestinal lesions by 41.68%. The pH of gastrointestinal contents of extract-treated rats was increased (4.25 ± 0.25) when compared to the control group (2.25 ± 0.25). Moreover, the extract also upgraded the Ulcer index (41.74%) and the acidity of the extract-treated group was diminished. Results suggested that Myristica fragrans exhibited noteworthy gastroprotective action against ethanol-induced ulcers.
Hydroalcoholic extract of M. fragrans reduced the lipoprotein levels in artificially induced triglyceride and glucose elevations in rat models. Doses of 450 mg/kg for 7 days improved the metabolic abnormalities as well as extract reduced the triglyceride level by 47%, and cholesterol by 66.7%. According to the findings, nutmeg extract improved impaired lipid metabolism in mouse models (Arulmozhi et al., Citation2007). Mary et al. (Citation2012) reported the cytotoxic property of the essential oil Myristica fragrans against epidermal skin cancer cells and breast cancer cells. According to Morita et al. (Citation2003), myristicin is amongst the most active phytochemical components reported to have a strong hepato-curative effect. Figure presents a thematic diagram illustrating the miscellaneous health benefits of nutmeg.
1.14. Psychoactive potential
Nutmeg is a common spice used in the kitchen and for many other medicinal purposes, it also acts as an analgesic, antioxidant, antimicrobial, and aphrodisiac agent yet little is thought about the harmful impacts of nutmeg (Beckerman & Persaud, Citation2019). Phytochemical screening aftereffects uncovered phytates, oxalates, steroids, tannins, saponins, flavonoids, glycosides, and cyanogenic glycosides, presence, and the antinutrient components which included cyanogenic glycosides (0.32 ± 0.08), saponins (1.58 ± 0.60), phytates (4.08 ± 0.10), tannins (0.64 ± 0.08), oxalates (1.05 ± 0.02) propose that Nutmeg (African) is usually considered to be safe to use.
Volatile oils present in nutmeg contain a derivative of alkyl benzene named myristicin. The myristicin acid is that it goes about as a weak inhibitor of monoamine oxidase and some segments of myristicin have serotonin agonist-like structures. Myristicin might be processed to show compounds like amphetamine with psychedelic impacts comparable to tolysergic acid (Forrester, Citation2005). As Myristicin is considered a hallucinogenic agent, it very well may be utilized as a modest psychedelic intoxicant, while successive utilization can prompt lethal occurrences that result in organ failure and affect cardiovascular muscles. M. fragrans essential oil is portrayed by phenylpropanoids, terpenoids, and terpenes presence, for example, safrole and methyl eugenol which are viewed as carcinogenic and genotoxic (Lanari et al., Citation2018).
The nutmeg effect on the central nervous system may be excitatory or depressant. Pathways of serotonin and dopaminergic may assume a part. Elemicin and myristicin have been considered to be nutmeg chemical compounds liable for their CNS impacts (Beckerman & Persaud, Citation2019). During rodent trials, Myristicin was found to produce focal inhibition of monoamine oxidase (Hategan & Bourgeois, Citation2013). These fixings have both psychotropic and anticholinergic. Common effects of CNS incorporate stupor, seizures, incoherent speech, hallucinations (visual, tactile, and auditory), hyperactivity, headache, giddiness, euphoria, drowsiness, dizziness, confusion, and anxiety (Pytte & Rygnestad, Citation1998).
Research led by Demetriades et al. (Citation2005) indicates the poisoning of nutmeg to be rare yet most likely underreported and ought to cause strong psychotic side effects such as CNS neuromodulatory signs that may emulate to some degree anticholinergic hyperstimulation. One substance that nutmeg possesses is safrole. Past studies indicate that safrole is present in nutmeg, which has negative effects on human wellbeing. Safrole has been accounted to be a potential hepatocarcinogen (Yang et al., Citation2018). A study reported that 4 g/kg of nutmeg causes abnormal behavior signs, which include dizziness, hyperactivity, and unstable gait in animal models. Moreover, alkaloid administration improved the strange behavior (Hayfaa et al., Citation2013).
1.15. Safety and toxicity
There are not many cases of nutmeg overdose and abuse. Nutmeg effects can be observed after the ingestion around the time of 0.5 to 8 h and go away in about 1–2 days (Akinboro et al., Citation2011). Nutmeg in low doses shows no noticeable response of a neurological or physiological nature, but nutmeg (both freshly ground and raw) in large doses, as well oil of Nutmeg oil, impart some Psychoactive effects, which derive from mechanisms of Anticholinergic- attributes to Elemicin and Myristicin (Ehrenpreis et al., Citation2014; McKenna et al., Citation2004). The nutmeg toxic dose is about 2–3 teaspoons, while less than one spoon consumption can initiate similar effects to the toxic episode of anticholinergic. Likewise, larger consumption, a minimum of 2 tablespoons, results in psychotic effects which include delusions and hallucinations. To define this 1 teaspoon is equivalent to 5 g and 1 tablespoon is equal to 15 g. Persistent use of nutmeg causes severe mental illness which is identified by distorted thinking and emotions (Hategan & Bourgeois, Citation2013). Myristicin a psychoactive substance and monoamine oxidase inhibitor can initiate generalized body pain, eventual Dehydration, Nausea, Palpitations, and Convulsions when it is ingested in large quantities. Poisonings of nutmeg happen due to unintentional ingestion and also its recreational use intentionally (Ehrenpreis et al., Citation2014).
Nutmeg substance intoxication varies individually and occurs with different side effects, such as amnesia, eye irritation, dry mouth, dizziness, nausea, headaches, confusion, anxiety, or excited delirium (Ehrenpreis et al., Citation2014). It takes several hours for intoxication before the experience of maximum effect is attained. The toxic effects of nutmeg can be sustained for many days (Demetriades et al., Citation2005; Ehrenpreis et al., Citation2014)
It is rarely reported that nutmeg overdose can be a cause of death, especially when it is combined with different drugs. Fatal poisoning incidents individually from myristicin or nutmeg are uncommon (Ehrenpreis et al., Citation2014). As per Stein et al. (Citation2001), the first nutmeg poisoning case was perceived in 1576 when an English pregnant lady reported prompt inebriety by 10–12 nutmeg ingestion (about 70–84 g) (Rahman et al., Citation2015).
2. Conclusion
The extract from different fractions of Myristica fragrans is used for several pharmaceutical properties. The majority of studies are focused on the phytochemical constituents of nutmeg responsible for a range of therapeutic impacts such as antibacterial, anticancer, antioxidant, antifungal, antidiabetic, antidepressant activity, hepatoprotective, anti-obesity, anti-inflammatory, cardioprotective, and many other therapeutic effects. According to the literature, myristicin is one of the most active compounds accountable for many health benefits. More struggles are required to revise the customary uses of nutmeg, its remedial activity as well and the mechanism of action. Much experimental work on this plant has been done in rodent models but very limited clinical data are available, so more investigations are needed to evaluate its efficacy on the human body. However, nutmeg is a storehouse of therapeutically active constituents that are accountable for many positive effects. Therefore, this plant stimulates researchers to search more about its unique pharmaceutical properties. This study highlights the potential of M. fragrans for usage in therapeutic medications and provides a theoretical foundation for future research on nutmeg and its pharmacological features.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abebe, S. M., Berhane, Y., & Worku, A. (2014). Barriers to diabetes medication adherence in North West Ethiopia. Springerplus, 3(1), 1–21. https://doi.org/10.1186/2193-1801-3-195
- Adewole, E., Ajiboye, B. O., Idris, O. O., Ojo, O. A., Onikan, A., Ogunmodede, O. T., & Adewumi, D. F. (2013). Phytochemical, antimicrobial and Gc-Ms of African nutmeg (Monodora Myristica). Phytochemical, Antimicrobial and Gc-Ms of African Nutmeg (Monodora Myristica), 2(5), 1–8.
- Agbogidi, O. M., & Azagbaekwe, O. P. (2013). Health and nutritional benefits of nutmeg (mystica fragrans houtt.). Scientia Agriculturae, 1(2), 40–44.
- Akinboro, A., Mohamed, K. B., Asmawi, M. Z., Othman, A. S., Ying, T. H., & Maidin, S. M. (2012). Mutagenic and antimutagenic assessment of methanol leaf extract of Myristica fragrans (Houtt.) using in vitro and in vivo genetic assays. Drug and Chemical Toxicology, 35(4), 412–422. https://doi.org/10.3109/01480545.2011.638300
- Akinboro, A., Mohamed, K. B., Asmawi, M. Z., Sulaiman, S. F., & Sofiman, O. A. (2011). Antioxidants in aqueous extract of Myristica fragrans (Houtt.) suppress mitosis and cyclophosphamide-induced chromosomal aberrations in Allium cepa L. cells. Journal of Zhejiang University Science B, 12(11), 915–922. https://doi.org/10.1631/jzus.B1000315
- Akinwunmi, K. F., & Oyedapo, O. O. (2014). In vitro anti-inflammatory evaluation of African nutmeg (Monodora myristica) seeds. Methodology, 8(3), 167–174. https://doi.org/10.9734/EJMP/2015/17853
- Ali, M. A., Hamiduddin, M. Z., & Ikram, M. (2018). Phyto-pharmacological potential of Jaiphal (Myristica fragrans Houtt): A spice of medicinal importance and its utilization in Unani Medicine. International Journal Green Pharm, 12(1), 26–36.
- Al-Quraishy, S., Dkhil, M. A., Abdel-Gaber, R., Zrieq, R., Hafez, T. A., Mubaraki, M. A., & Abdel Moneim, A. E. (2020). Myristica fragrans seed extract reverses scopolamine-induced cortical injury via stimulation of HO-1 expression in male rats. Environmental Science and Pollution Research, 27(11), 12395–12404. https://doi.org/10.1007/s11356-020-07686-8
- Ansory, H. M., Fitriani, I. N., & Nilawatii, A. (2020). May. Chemical separation and antibacterial activity of nutmeg seed essential oil against Shigella sp. and Escherichia coli ATCC 25922. IOP Conference Series: Materials Science & Engineering, 846(1), 012005. IOP Publishing: https://doi.org/10.1088/1757-899X/846/1/012005
- Arulmozhi, D. K., Kurian, R., Veeranjaneyulu, A., & Bodhankar, S. L. (2007). Antidiabetic and antihyperlipidemic effects of Myristica fragrans. In animal models. Pharmaceutical Biology, 45(1), 64–68. https://doi.org/10.1080/13880200601028339
- Asgarpanah, J., & Kazemivash, N. (2012). Phytochemistry and pharmacologic properties of Myristica fragrans Hoyutt.: A review. African Journal of Biotechnology, 11(65), 12787–12793. https://doi.org/10.5897/AJB12.1043
- Ashokkumar, K., Vellaikumar, S., Muthusamy, M., Dhanya, M. K., & Aiswarya, S. (2020). Compositional variation in the leaf, mace, kernel, and seed essential oil of nutmeg (Myristica fragrans Houtt.) from the Western Ghats, India. Natural Product Research, 36(1), 1–4. https://doi.org/10.1080/14786419.2020.1771713
- Assa, J. R., Widjanarko, S. B., Kusnadi, J., & Berhimpon, S. (2014). Antioxidant potential of flesh, seed and mace of nutmeg (Myristica fragrans Houtt). International Journal of ChemTech Research, 6(4), 2460–2468.
- Ayoola, G. A., Folawewo, A. D., Adesegun, S. A., Abioro, O. O., Adepoju-Bello, A. A., & Coker, H. A. B. (2008). Phytochemical and antioxidant screening of some plants of apocynaceae from South West Nigeria. African Journal of Plant Science, 2(10), 124–128.
- Azeez, B. A. A., Sebah, F. S., & Inaam, M. N. (2019). Alrubayae, study of antibacterial and antifungal efficacy of alkaloid isolated from nutmeg (Myristica Fragrans). Journal of Pure & Applied Microbiology, 13(4), 2105–2110. https://doi.org/10.22207/JPAM.13.4.22
- Babua, B., Pa, A. K., Ia, D. V., & Aa, A. P. (2021). A Comprehensive review on bioactive and therapeutic potential of Indian nutmeg Myristica fragrans (Houtt). Advances in Bioscience and Biotechnology Research, 1.
- Balakrishnan, S., Ibrahim, K. S., Duraisamy, S., Sivaji, I., Kandasamy, S., Kumarasamy, A., & Kumar, N. S. (2020). Antiquorum sensing and antibiofilm potential of biosynthesized silver nanoparticles of Myristica fragrans seed extract against MDR Salmonella enterica serovar typhi isolates from asymptomatic typhoid carriers and typhoid patients. Environmental Science and Pollution Research, 27(3), 2844–2856. https://doi.org/10.1007/s11356-019-07169-5
- BATM, S. C. (2016). Bioactivities of ethanolic extracts of three parts (wood, nutmeg and mace) from Myristica fragrans Houtt. J Med Assoc Thai, 99(4), S124–S130.
- Beckerman, B., & Persaud, H. (2019). Nutmeg overdose: Spice not so nice. Complementary Therapies in Medicine, 46, 44–46. https://doi.org/10.1016/j.ctim.2019.07.011
- Bhuiyan, M. S. A., Lee, D. H., Kim, H. J., Cho, S. H., Yang, B. S., Kim, S. D., & Lee, S. H. (2018). Estimates of genetic parameters for fatty acid compositions in the longissimus dorsi muscle of Hanwoo cattle. 12(4), 675–683. https://doi.org/10.1017/S1751731117001872
- Burubai, W., Amula, E., Daworiye, P., Suowari, T., & Nimame, P. (2009). Proximate composition and some technological properties of African nutmeg (Monodora myristica) seeds. Electronic Journal of Environmental, Agricultural and Food Chemistry, 8(5), 396–402.
- Cao, G. Y., Xu, W., Yang, X. W., Gonzalez, F. J., & Li, F. (2015). New neolignans from the seeds of Myristica fragrans that inhibit nitric oxide production. Food Chemistry, 173, 231–237. https://doi.org/10.1016/j.foodchem.2014.09.170
- Cherian, T., Ali, K., Fatima, S., Saquib, Q., Ansari, S. M., Alwathnani, H. A., Al-Khedhairy, A. A., Al-Shaeri, M., & Musarrat, J. (2019). Myristica fragrans bio-active ester functionalized ZnO nanoparticles exhibit antibacterial and antibiofilm activities in clinical isolates. Journal of Microbiological Methods, 166, 105716. https://doi.org/10.1016/j.mimet.2019.105716
- Chiu, S., Wang, T., Belski, M., & Abourashed, E. A. (2016). HPLC-guided isolation, purification and characterization of phenylpropanoid and phenolic constituents of nutmeg kernel (Myristica fragrans). Natural Product Communications, 11(4), 1934578X1601100416. https://doi.org/10.1177/1934578X1601100416
- Chung, H. C., Kim, M. S., Mun, S. Y., Sa, B. K., Chung, J. Y., Kim, D. U., & Hwang, J. K. (2012). Effect of oral administration of nutmeg extract on american house dust mite (dermatophagoides farinae) extract-induced atopic dermatitis-like skin lesions in NC/Nga mice. Food Science and Biotechnology, 21(2), 559–564. https://doi.org/10.1007/s10068-012-0071-8
- Cuong, T. D., Hung, T. M., Han, H. Y., Sik Roh, H., Seok, J. H., Lee, J. K., Jeong, J. Y., Choi, J. S., Kim, J. A., & Min, B. S. (2014). Potent acetylcholinesterase inhibitory compounds from Myristica fragrans. Natural Product Communications, 9(4), 1934578X1400900418. https://doi.org/10.1177/1934578X1400900418
- Das, S., Singh, V. K., Dwivedy, A. K., Chaudhari, A. K., Upadhyay, N., Singh, A., & Dubey, N. K. (2020). Fabrication, characterization and practical efficacy of Myristica fragrans essential oil nanoemulsion delivery system against postharvest biodeterioration. Ecotoxicology and Environmental Safety, 189, 110000. https://doi.org/10.1016/j.ecoenv.2019.110000
- Demetriades, A. K., Wallman, P. D., McGuiness, A., & Gavalas, M. C. (2005). Low cost, high risk: Accidental nutmeg intoxication. Emergency Medicine Journal, 22(3), 223–225. https://doi.org/10.1136/emj.2002.004168
- Dewi, K., Widyarto, B., Erawijantari, P. P., & Widowati, W. (2015). In vitro study of Myristica fragrans seed (nutmeg) ethanolic extract and quercetin compound as anti-inflammatory agent. International Journal of Research in Medical Sciences, 3(9), 2303–2310. https://doi.org/10.18203/2320-6012.ijrms20150621
- Dhingra, D., & Sharma, A. (2006). Antidepressant-like activity of n-hexane extract of nutmeg (Myristica fragrans) seeds in mice. Journal of Medicinal Food, 9(1), 84–89. https://doi.org/10.1089/jmf.2006.9.84
- Dorman, H. D., & Deans, S. G. (2000). Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. Journal of Applied Microbiology, 88(2), 308–316. https://doi.org/10.1046/j.1365-2672.2000.00969.x
- Duarte, R. C., Fanaro, G. B., Koike, A. C., & Villavicencio, A. L. C. (2011). Irradiation effect on antifungal potential Myristica fragrans (nutmeg) essential oil, a preliminary study.
- Du, S. S., Yang, K., Wang, C. F., You, C. X., Geng, Z. F., Guo, S. S., Deng, Z. W., & Liu, Z. L. (2014). Chemical constituents and activities of the essential oil from Myristica fragrans against cigarette beetle lasioderma serricorne. Chemistry & Biodiversity, 11(9), 1449–1456. https://doi.org/10.1002/cbdv.201400137
- Ehrenpreis, J. E., DesLauriers, C., Lank, P., Armstrong, P. K., & Leikin, J. B. (2014). Nutmeg poisonings: A retrospective review of 10 years experience from the Illinois poison center, 2001–2011. Journal of Medical Toxicology, 10(2), 148–151. https://doi.org/10.1007/s13181-013-0379-7
- El-Alfy, A. T., Abourashed, E. A., Patel, C., Mazhari, N., An, H., & Jeon, A. (2019). Phenolic compounds from nutmeg (Myristica fragrans Houtt.) inhibit the endocannabinoid-modulating enzyme fatty acid amide hydrolase. Journal of Pharmacy and Pharmacology, 71(12), 1879–1889. https://doi.org/10.1111/jphp.13174
- Firempong, C. K., Andoh, L. A., Akanwariwiak, W. G., Addo-Fordjour, P., & Adjakofi, P. (2016). Phytochemical screening and antifungal activities of crude ethanol extracts of red-flowered silk cotton tree (bombax buonopozense) and calabash nutmeg (Monodora myristica) on Candida albicans. Journal of Microbiology and Antimicrobials, 8(4), 22–27. https://doi.org/10.5897/JMA2015.0350
- Firmansyah, M. A., Jayanegara, A., & Wijayanto, N. (2019). In vitro biological control of Ceratobasidium ramicola by using tannin extracts from Acacia villosa, Myristica fragrans, Acacia mangium, and Calliandra calothyrsus leaves. Brazilian Journal of Biology, 80, 235–239. https://doi.org/10.1590/1519-6984.184912
- Forrester, M. B. (2005). Nutmeg intoxication in Texas, 1998–2004. Human & Experimental Toxicology, 24(11), 563–566. https://doi.org/10.1191/0960327105ht567oa
- Francis, K. S., Suresh, E., & Nair, M. S. (2014). Chemical constituents from Myristica fragrans fruit. Natural Product Research, 28(20), 1664–1668. https://doi.org/10.1080/14786419.2014.934236
- Gao, J. R., Xu, S. Z., Han, Y. Q., Wei, L. B., Jiang, H., Song, J. M., & Xue, X. (2017). Determination and pharmacokinetics of main components for psoralea corylifolia-Myristica fragrants drug pair by using UPLC-MS/MS. Zhongguo Zhong Yao Za Zhi= Zhongguo Zhongyao Zazhi= China Journal of Chinese Materia Medica, 42(9), 1782–1786. https://doi.org/10.19540/j.cnki.cjcmm.2017.0071
- Ghorbanian, D., Ghasemi-Kasman, M., Hashemian, M., Gorji, E., Gol, M., Feizi, F., Kazemi, S., Ashrafpour, M., & Moghadamnia, A. A. (2019). Myristica fragrans Houtt extract attenuates neuronal loss and glial activation in pentylenetetrazol-induced kindling model. Iranian Journal of Pharmaceutical Research: IJPR, 18(2), 812. https://doi.org/10.22037/ijpr.2019.1100670
- Ginting, B., Mustanir M, M., Nurdin N, N., Maulidna M, M., Murniana M, M., & Safrina S, S. (2021). Evaluation of antioxidant and anticancer activity of Myristica fragrans Houtt. Bark. Pharmacognosy Journal, 13(3), 780–786. https://doi.org/10.5530/pj.2021.13.99
- Ginting, B., Saidi, N., Simanjuntak, M., & Simanjuntak, P. (2020). Lignan compound isolated from n-Hexane extract myristica fragrans Houtt root as antioxidant and antitumor activities against MCF-7 cell lines data. Data in Brief, 31, 105997. https://doi.org/10.1016/j.dib.2020.105997
- Grover, J. K., Khandkar, S., Vats, V., Dhunnoo, Y., & Das, D. (2002). Pharmacological studies on Myristica fragrans–antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods and Findings in Experimental and Clinical Pharmacology, 24(10), 675–680. https://doi.org/10.1358/mf.2002.24.10.802317
- Gupta, A. D., Bansal, V. K., Babu, V., & Maithil, N. (2013). Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). Journal of Genetic Engineering & Biotechnology, 11(1), 25–31. https://doi.org/10.1016/j.jgeb.2012.12.001
- Gupta, A. D., & Rajpurohit, D. (2011). Antioxidant and antimicrobial activity of nutmeg (Myristica fragrans). In Nuts and seeds in health and disease prevention (pp. 831–839). Elsevier.
- Hategan, A., & Bourgeois, J. A. (2013). Out of the cupboard and into the clinic: Nutmeg-induced mood disorder. Current Psychiatry, 12(12), E1–E2.
- Hayfaa, A. A. S., Sahar, A. M. A. S., & Awatif, M. A. S. (2013). Evaluation of analgesic activity and toxicity of alkaloids in Myristica fragrans seeds in mice. Journal of Pain Research, 6, 611. https://doi.org/10.2147/JPR.S45591
- Hoda, S., Vermani, M., Joshi, R. K., Shankar, J., & Vijayaraghavan, P. (2020). Anti-melanogenic activity of Myristica fragrans extract against Aspergillus fumigatus using phenotypic based screening. BMC Complementary Medicine and Therapies, 20(1), 1–13. https://doi.org/10.1186/s12906-020-2859-z
- Ibrahim, M. A., Cantrell, C. L., Jeliazkova, E. A., Astatkie, T., & Zheljazkov, V. D. (2020). Utilization of nutmeg (Myristica fragrans Houtt.) seed hydrodistillation time to produce essential oil fractions with varied compositions and pharmacological effects. Molecules, 25(3), 565. https://doi.org/10.3390/molecules25030565
- Ibrahim, T. A., & Oyinloye, B. O. J. (2011). Antibacterial activity of herbal extracts against multi drug resistant strains of bacteria from clinical origin by 1 ta ibrahim, 2 bo opawale and 3 jma oyinloye. Life Sciences Leaflets, 15, 490–498.
- Jaiswal, P., Kumar, P., Singh, V. K., & Singh, D. K. (2009). Biological effects of Myristica fragrans. Annual Review of Biomedical Sciences, 11, 21–29. https://doi.org/10.5016/1806-8774.2009v11p21
- Jamshidi-Kia, F., Lorigooini, Z., & Amini-Khoei, H. (2018). Medicinal plants: Past history and future perspective. Journal of Herbmed Pharmacology, 7(1), 1–7. https://doi.org/10.15171/jhp.2018.01
- Jangid, K. R. I. T. I. K. A., Jayakumar, N. D., & Varghese, S. S. (2014). Achievable therapeutic effects of Myristica fragrans (nutmeg) on periodontitis a short review. International Journal of Pharmacy and Pharmaceutical Sciences, 6(5), 591–594.
- Jin, H., Zhu, Z. G., Yu, P. J., Wang, G. F., Zhang, J. Y., Li, J. R., Ai, R. T., Li, Z. H., Tian, Y. X., Zhang, W. X. J. J., & Wu, S. G. (2012). Myrislignan attenuates lipopolysaccharide‐induced inflammation reaction in murine macrophage cells through inhibition of NF‐κB signalling pathway activation. Phytotherapy Research, 26(9), 1320–1326. https://doi.org/10.1002/ptr.3707
- Joseph, J., & George, M. (2014). Antimicrobial susceptibility of selected medicinal fruit-Myristica fragrans. Scholars Research Library, 6(6), 396–402.
- Kareem, M. A., Gadhamsetty, S. K., Shaik, A. H., Prasad, E. M., & Kodidhela, L. D. (2013). Protective effect of nutmeg aqueous extract against experimentally-induced hepatotoxicity and oxidative stress in rats. Journal of Ayurveda and Integrative Medicine, 4(4), 216. https://doi.org/10.4103/0975-9476.123704
- Kelble, A. (2005). Spices and type 2 diabetes. Nutrition & Food Science.
- Khare, C. P. (2008). Indian medicinal plants: An illustrated dictionary. Springer Science & Business Media.
- Kirtikar, K. R., & Basu, B. D. (1999). Indian medicinal plants (Vol. 3, 2nd ed.). International Book Distributors.
- Krishnaiah, D., Sarbatly, R., & Bono, A. (2007). Phytochemical antioxidants for health and medicine a move towards nature. Biotechnology and Molecular Biology Reviews, 2(4), 97–104.
- Kumar, S., Bajwa, B. S., Kuldeep, S., & Kalia, A. N. (2013). Anti-inflammatory activity of herbal plants: A review. International Journal of Advances in Pharmacy, Biology and Chemistry, 2(2), 272–281.
- Kumari, I., Kaurav, H., & Chaudhary, G. (2021). Myristica fragrans (Jaiphal): A significant medicinal herbal plant. International Journal for Research in Applied Sciences and Biotechnology, 8(2), 213–224. https://doi.org/10.31033/ijrasb.8.2.27
- Kusuma, J., Scarcelli, N., Couderc, M., Mariac, C., Zekraoui, L., & Duminil, J. (2020). Microsatellite markers development for Indonesian nutmeg (Myristica fragrans Houtt.) and transferability to other myristicaceae spp. Molecular Biology Reports, 47(6), 4835–4840. https://doi.org/10.1007/s11033-020-05535-y
- Lanari, D., Marcotullio, M. C., & Neri, A. (2018). A design of experiment approach for ionic liquid-Based extraction of toxic components-minimized essential oil from Myristica fragrans Houtt. Fruits †. Molecules, 23(11), 2817. https://doi.org/10.3390/molecules23112817
- Lee, J. Y., & Park, W. (2011). Anti-inflammatory effect of myristicin on RAW 264.7 macrophages stimulated with polyinosinic-polycytidylic acid. Molecules, 16(8), 7132–7142. https://doi.org/10.3390/molecules16087132
- Lee, S. U., Shim, K. S., Ryu, S. Y., Min, Y. K., & Kim, S. H. (2009). Machilin a isolated from Myristica fragrans stimulates osteoblast differentiation. Planta medica, 75(2), 152–157. https://doi.org/10.1055/s-0028-1112197
- Leiter, E., Hitchcock, G., Godwin, S., Johnson, M., Sedgwick, W., Jones, W., McCall, S., & Ceremuga, T. E. (2011). Evaluation of the anxiolytic properties of myristicin, a component of nutmeg, in the male sprague-dawley rat. AANA Journal, 79(2), 109–114.
- Leontowicz, H., Leontowicz, M., Drzewiecki, J., Haruenkit, R., Poovarodom, S., Park, Y. S., Jung, S. T., Kang, S. G., Trakhtenberg, S., & Gorinstein, S. (2006). Bioactive properties of snake fruit (Salacca edulis reinw) and mangosteen (Garcinia mangostana) and their influence on plasma lipid profile and antioxidant activity in rats fed cholesterol. European Food Research and Technology, 223(5), 697–703. https://doi.org/10.1007/s00217-006-0255-7
- Lestari, K., Diantini, A., Barliana, M. I., Achmad, T. H., Subarnas, A., Abdulah, R., Lesmana, R., & Hwang, J. K. (2019). Potential natural dual agonist PPARα/γ-induced antidiabetic and antidyslipidemic properties of safrole-free nutmeg seed (Myristica fragrans Houtt) extract. The Natural Products Journal, 9(3), 248–253. https://doi.org/10.2174/2210315509666190206122849
- Lestari, K., Hwang, J. K., Kariadi, S. H., Wijaya, A., Ahmad, T., Subarnas, A., & Muchtaridi, M. (2012). Screening for PPAR γ agonist from Myristica fragrans Houtt seeds for the treatment of type 2 diabetes by in vitro and in vivo. Medical and Health Science Journal, 12(3), 7–16. https://doi.org/10.15208/mhsj.2012.37
- Loizzo, M. R., Sicari, V., Tenuta, M. C., Leporini, M. R., Falco, T., Pellicanò, T. M., Menichini, F., & Tundis, R. (2016). Phytochemicals content, antioxidant and hypoglycaemic activities of commercial nutmeg mace (Myristica fragrans L.) and pimento (Pimenta dioica (L.) merr.). International Journal of Food Science & Technology, 51(9), 2057–2063. https://doi.org/10.1111/ijfs.13178
- Mary, H., Tina, A. V., Jeeja, K. J., & Abiramy, M. R. (2012). Phytochemical analysis and anticancer activity of essential oil from Myristica fragrans. International Journal of Current Pharmaceutical Review and Research, 2(4), 188–198.
- Matulyte, I., Jekabsone, A., Jankauskaite, L., Zavistanaviciute, P., Sakiene, V., Bartkiene, E., Ruzauskas, M., Kopustinskiene, D. M., Santini, A., & Bernatoniene, J. (2020). The essential oil and hydrolats from Myristica fragrans seeds with magnesium aluminometasilicate as excipient: Antioxidant, antibacterial, and anti-inflammatory activity. Foods, 9(1), 37. https://doi.org/10.3390/foods9010037
- Maya, K. M., Zachariah, T. J., & Krishnamoorthy, B. (2004). Chemical composition of essential oil of nutmeg (Myristica fragrans Houtt.) acces. Journal of Spices & Aromatic Crops, 13(2), 135–139.
- McCall, S. (2011). Evaluation of the anxiolytic properties of myristicin, a component of nutmeg, in the male sprague-dawley rat. AANA journal, 79(2), 109.
- McKenna, A., Nordt, S. P., & Ryan, J. (2004). Acute nutmeg poisoning. European Journal of Emergency Medicine, 11(4), 240–241. https://doi.org/10.1097/01.mej.0000127649.69328.a5
- Monika-Thakur, A. P. S. (2014). Qualitative phytochemical screening, total phenolic content and antioxidant activity in methanolic extracts of M. fragrans Houtt. (Mace) Journal of Food Science Research, 5(2), 135–138. https://doi.org/10.15740/HAS/FSRJ/5.2/135-138
- Morikawa, T., Hachiman, I., Matsuo, K., Nishida, E., Ninomiya, K., Hayakawa, T., Yoshie, O., Muraoka, O., & Nakayama, T. (2016). Neolignans from the arils of Myristica fragrans as potent antagonists of CC chemokine receptor 3. Journal of Natural Products, 79(8), 2005–2013. https://doi.org/10.1021/acs.jnatprod.6b00262
- Morita, T., Jinno, K., Kawagishi, H., Arimoto, Y., Suganuma, H., Inakuma, T., & Sugiyama, K. (2003). Hepatoprotective effect of myristicin from nutmeg (Myristica fragrans) on lipopolysaccharide/d-galactosamine-induced liver injury. Journal of Agricultural and Food Chemistry, 51(6), 1560–1565. https://doi.org/10.1021/jf020946n
- Mueller, M., Hobiger, S., & Jungbauer, A. (2010). Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chemistry, 122(4), 987–996. https://doi.org/10.1016/j.foodchem.2010.03.041
- Nagaraju, B., Sahar, S. H., Ali, B., Kushnoor, N. Z., Zahra, A., Zothanmawia, C., & Surendranatha, A. (2013). Anxiolytic effect of Myristica fragrans. International Journal of Phytotherapy Research, 3, 1–7.
- Najafzadeh, H., Esmailzadeh, S., Ghorbanpour, M., Farajzadeh, A., & Asadirad, A. (2014). Comparing the effect of Myristica fragrans and flunixin on adjuvant-induced arthritis in rat. Zahedan Journal of Research in Medical Sciences, 16(2), 33–36.
- Nasreen, W., Sarker, S., Sufian, M. A., Opo, F. D. M., Shahriar, M., Akhter, R., & Halim, M. A. (2020). A possible alternative therapy for type 2 diabetes using Myristica fragrans Houtt in combination with glimepiride: in vivo evaluation and in silico support. Zeitschrift für Naturforschung C, 75(3–4), 103–112. https://doi.org/10.1515/znc-2019-0134
- Nguyen, P. H., De Tran, V., Pham, D. T., Dao, T. N. P., & Dewey, R. S. (2021). Use of and attitudes towards herbal medicine during the COVID-19 pandemic: A cross-sectional study in Vietnam. European Journal of Integrative Medicine, 44, 101328. https://doi.org/10.1016/j.eujim.2021.101328
- Nguyen, P. H., Le, T. V. T., Kang, H. W., Chae, J., Kim, S. K., Kwon, K. I., Seo, D. B., Lee, S. J., & Oh, W. K. (2010). AMP-activated protein kinase (AMPK) activators from Myristica fragrans (nutmeg) and their anti-obesity effect. Bioorganic & Medicinal Chemistry Letters, 20(14), 4128–4131. https://doi.org/10.1016/j.bmcl.2010.05.067
- Nkwocha Chinelo, C., Nworah Florence, N., Okagu Innocent, U., & Nwagwe Onyinyechi, R. (2018). Proximate and Phytochemical Analysis of Monodora myristica (African Nutmeg) from Nsukka, Enugu State, Nigeria. Journal of Food and Nutrition Research, 6(9), 597–601. https://doi.org/10.12691/jfnr-6-9-9
- Nurjanah, S., Putri, I. L., & Sugiarti, D. P. (2017). Antibacterial activity of nutmeg oil. KnE Life Sciences, 2(6), 563–569. https://doi.org/10.18502/kls.v2i6.1076
- Omidpanah, S., Vahedi-Mazdabadi, Y., Manayi, A., Rastegari, A., Hariri, R., Mortazavi-Ardestani, E., Eftekhari, M., Khanavi, M., Akbarzadeh, T., & Saeedi, M. (2022). Phytochemical investigation and anticholinesterase activity of ethyl acetate fraction of Myristica fragrans Houtt. seeds. Natural Product Research, 36(2), 610–616. https://doi.org/10.1080/14786419.2020.1788555
- Orabi, K. Y., Mossa, J. S., & El-Feraly, F. S. (1991). Isolation and characterization of two antimicrobial agents from mace (Myristica fragrans). Journal of Natural Products, 54(3), 856–859. https://doi.org/10.1021/np50075a017
- Orhan, I., Şener, B., Choudhary, M. I., & Khalid, A. (2004). Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. Journal of Ethnopharmacology, 91(1), 57–60. https://doi.org/10.1016/j.jep.2003.11.016
- Orwa, C., Mutua, A., Kindt, R., Jamnadass, R., & Simons, A. (2009). Agroforestree Database: A tree reference and selection guide. Version 4. Agroforestree Database: A tree reference and selection guide. Version 4.
- Pal, M., Srivastava, M., Soni, D. K., Kumar, A., & Tewari, S. K. (2011). Composition and anti-microbial activity of essential oil of Myristica fragrans from Andaman Nicobar Island. International Journal of Pharmacy and Life Sciences, 2(10), 1115–1117.
- Parle, M., Dhingra, D., & Kulkarni, S. K. (2004). Improvement of mouse memory by Myristica fragrans seeds. Journal of Medicinal Food, 7(2), 157–161. https://doi.org/10.1089/1096620041224193
- Pashapoor, A., Mashhadyrafie, S., & Mortazavi, P. (2020). Ameliorative effect of Myristica fragrans (nutmeg) extract on oxidative status and histology of pancreas in alloxan induced diabetic rats. Folia Morphologica, 79(1), 113–119. https://doi.org/10.5603/FM.a2019.0052
- Patricia, C. E., Ganiyu, B. A., Benneth, B. A., & Olugbenga, I. E. (2019). Evaluation of the memory enhancing activity of dichloromethane fraction of the methanolic extract of pycnanthus angolensis stem bark on experimental models of memory impairment. Drug Research, 69(10), 551–558. https://doi.org/10.1055/a-0875-3631
- Piaru, S. P., Mahmud, R., Majid, A. M. S. A., & Nassar, Z. D. M. (2012). Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and morinda citrifolia. Asian Pacific Journal of Tropical Medicine, 5(4), 294–298. https://doi.org/10.1016/S1995-7645(12)60042-X
- Piras, A., Rosa, A., Marongiu, B., Atzeri, A., Dessì, M. A., Falconieri, D., & Porcedda, S. (2012). Extraction and separation of volatile and fixed oils from seeds of Myristica fragrans by supercritical CO2: Chemical composition and cytotoxic activity on Caco‐2 cancer cells. Journal of Food Science, 77(4), C448–C453. https://doi.org/10.1111/j.1750-3841.2012.02618.x
- Pytte, M., & Rygnestad, T. (1998). Nutmeg–more than a spice. Tidsskrift for den Norske Laegeforening: Tidsskrift for Praktisk Medicin, ny Raekke, 118(28), 4346–4347.
- Radwan, M. M., Tabanca, N., Wedge, D. E., Tarawneh, A. H., & Cutler, S. J. (2014). Antifungal compounds from turmeric and nutmeg with activity against plant pathogens. Fitoterapia, 99, 341–346. https://doi.org/10.1016/j.fitote.2014.08.021
- Rahman, N. A. A., Fazilah, A., & Effarizah, M. E. (2015). Toxicity of nutmeg (myristicin): A review. International Journal on Advanced Science, Engineering and Information Technology, 5(3), 61–64. https://doi.org/10.18517/ijaseit.5.3.518
- Romana, R. K., Sharma, A., Gupta, V., Kaur, R., Kumar, S., & Bansal, P. (2020). Was Hawan designed to fight anxiety-scientific evidences? Journal of Religion and Health, 59(1), 505–521. https://doi.org/10.1007/s10943-016-0345-1
- Saputro, M. A., Andarwulan, N., & Faridah, D. N. (2016). Physical characterization and essential oil properties of West Sumatra mace and nutmeg seed (Myristica fragrans Houtt) at different ages at harvest. Journal of Pharmacognosy & Phytochemistry, 5(6), 371–376.
- Sattar, A., Abdo, A., Mushtaq, M. N., Anjum, I., & Anjum, A. (2019). Evaluation of gastro-protective activity of Myristica fragrans on ethanol-induced ulcer in albino rats. Anais da Academia Brasileira de Ciências, 91(2), 91. https://doi.org/10.1590/0001-3765201920181044
- Saxena, R., & Patil, P. (2012). Phytochemical studies on Myristica fragrance essential oil. Biological Forum – an International Journal, 2, 62–64.
- Saydam, F., & Nalkiran, H. S. (2021). Anticancer effects of a novel herbal combination as a potential therapeutic candidate against lung cancer. European Journal of Integrative Medicine, 48, 101401. https://doi.org/10.1016/j.eujim.2021.101401
- SELLING, W. D. (2021). FUTURE of DIRET SELLING. IMMUNITY.
- Setty, J. V., Srinivasan, I., Sathiesh, R. T., Kale, M., Shetty, V. V., & Venkatesh, S. (2020). In vitro evaluation of antimicrobial effect of Myristica fragrans on common endodontic pathogens. Journal of Indian Society of Pedodontics and Preventive Dentistry, 38(2), 145. https://doi.org/10.4103/JISPPD.JISPPD_214_20
- Shafiei, Z., Shuhairi, N. N., Md Fazly Shah Yap, N., Harry Sibungkil, C. A., & Latip, J. (2012). Antibacterial activity of Myristica fragrans against oral pathogens. Evidence-Based Complementary and Alternative Medicine, 2012, 1–7. https://doi.org/10.1155/2012/825362
- Shahidi, F., & Zhong, Y. (2015). Measurement of antioxidant activity. Journal of Functional Foods, 18, 757–781. https://doi.org/10.1016/j.jff.2015.01.047
- Sharathchandra, K., Srujana, M. G., & Sridhar, K. R. (2023). Nutritional and Functional Attributes of Myristica malabarica Fruits. In Ethnic Knowledge and Perspectives of Medicinal Plants (pp. 287–301). Apple Academic Press.
- Sharma, A. R. T. I., Mathur, R. I. T. U., & Dixit, V. P. (1995). Prevention of hypercholesterolemia and atherosclerosis in rabbits after supplementation of Myristica fragrans seed extract. Indian Journal of Physiology and Pharmacology, 39(4), 407–410.
- Singh, G., Sharma, P. K., Dudhe, R., & Singh, S. (2010). Biological activities of withania somnifera. Annals of Biological Research, 1(3), 56–63.
- Sipahelut, S. G., Patty, J. A., Patty, Z., Kastanja, A. Y., & Lekahena, V. N. J. (2019). The antibacterial and antifungal activity of essential oil derived from the flesh of nutmeg fruit. EurAsian Journal of BioSciences, 13(1), 93–98.
- Sohn, J. H., Han, K. L., Choo, J. H., & Hwang, J. K. (2007). Macelignan protects HepG2 cells against tert‐butylhydroperoxide‐induced oxidative damage. Biofactors, 29(1), 1–10. https://doi.org/10.1002/biof.5520290101
- Somani, R. S., & Singhai, A. K. (2008). Hypoglycaemic and antidiabetic activities of seeds of Myristica fragrans in normoglycaemic and alloxan-induced diabetic rats. Asian Journal of Experimental Sciences, 22(1), 95–102.
- Sonavane, G. S., Sarveiya, V. P., Kasture, V. S., & Kasture, S. B. (2002). Anxiogenic activity of Myristica fragrans seeds. Pharmacology Biochemistry and Behavior, 71(1–2), 239–244. https://doi.org/10.1016/S0091-3057(01)00660-8
- Song, Y., Zhang, Y., Duan, X. Y., Cui, D. W., Qiu, X., Bian, Y., Wang, K. F., & Feng, X. S. (2019). Pharmacokinetics and tissue distribution of Anwuligan in rats after intravenous and intragastric administration by Liquid Chromatography-mass Spectrometry. Molecules, 25(1), 39. https://doi.org/10.3390/molecules25010039
- Stein, U., Greyer, H., & Hentschel, H. (2001). Nutmeg (myristicin) poisoning—report on a fatal case and a series of cases recorded by a poison information centre. Forensic Science International, 118(1), 87–90. https://doi.org/10.1016/S0379-0738(00)00369-8
- Suji, G., & Sivakami, S. (2003). Approaches to the treatment of diabetes mellitus: An overview. Cellular and Molecular Biology (Noisy-le-Grand, France), 49(4), 635–639.
- Sultana, A., Najeeya, A. G., & Anjum, A. (2018). Traditional unani uses with multiple pharmacological activities of aril of Myristica fragrans (mace). CELLMED, 8(2), 6–1.
- Suthisamphat, N., Dechayont, B., Phuaklee, P., Prajuabjinda, O., Vilaichone, R. K., Itharat, A., Mokmued, K., & Prommee, N. (2020). Anti-Helicobacter pylori, Anti-Inflammatory, Cytotoxic, and Antioxidant Activities of Mace Extracts from Myristica fragrans. Evidence-Based Complementary and Alternative Medicine, 2020, 1–6. https://doi.org/10.1155/2020/7576818
- Tahrani, A. A., Piya, M. K., Kennedy, A., & Barnett, A. H. (2010). Glycaemic control in type 2 diabetes: Targets and new therapies. Pharmacology & Therapeutics, 125(2), 328–361. https://doi.org/10.1016/j.pharmthera.2009.11.001
- Thangaselvabai, T., Sudha, K. R., Selvakumar, T., & Balakumbahan, R. (2011). Nutmeg (Myristica fragrans Houtt)-the twin spice-a review. Agricultural Reviews, 32(4), 283–293.
- Thuong, P. T., Hung, T. M., Khoi, N. M., Nhung, H. T. M., Chinh, N. T., Quy, N. T., Jang, T. S., & Na, M. (2014). Cytotoxic and anti-tumor activities of lignans from the seeds of Vietnamese nutmeg Myristica fragrans. Archives of Pharmacal Research, 37(3), 399–403. https://doi.org/10.1007/s12272-013-0185-4
- Vangoori Yakaiah, A. D., & Kavimani, S. (2019). Effect of Myristica fragrans extract on total body composition in cafeteria diet induced obese rats. Bioinformation, 15(9), 657. https://doi.org/10.6026/97320630015657
- Wang, J., Zhang, H. H., Liu, F., Zhang, Y. P., & Zhao, Y. M. (2019). Preparation, characterization and antimicrobial activity of inclusion complexes of Myristica fragrans Hott. essential oil in β-cyclodextrins. Die Pharmazie-An International Journal of Pharmaceutical Sciences, 74(10), 590–594.
- Weerakoon, W. M. T. D. N., Perera, P. R. T., Rajapaksha, M. H., & Liyanage, A., A REVIEW ON BIOACTIVE COMPOUNDS AND PHARMACOLOGICAL ACTIVITIES OF Myristica fragrans AS A MEDICINAL PLANT.
- Wiart, C. (2007). Ethnopharmacology of medicinal plants: Asia and the Pacific. Springer Science & Business Media.
- Wink, M. (2022). Current understanding of modes of action of multicomponent bioactive phytochemicals: Potential for nutraceuticals and antimicrobials. Annual Review of Food Science and Technology, 13(1), 337–359. https://doi.org/10.1146/annurev-food-052720-100326
- Wu, N., Xu, W., Cao, G. Y., Yang, Y. F., Yang, X. B., & Yang, X. W. (2016). The blood-brain barrier permeability of lignans and malabaricones from the seeds of Myristica fragrans in the MDCK-pHamdr cell monolayer model. Molecules, 21(2), 134. https://doi.org/10.3390/molecules21020134
- Yadav, R. N. S., & Agarwala, M. (2011). Phytochemical analysis of some medicinal plants. Journal of Phytology, 3(12), 10–14.
- Yang, X. W., Huang, X., Ma, L., Wu, Q., & Xu, W. (2010). The intestinal permeability of neolignans from the seeds of Myristica fragrans in the caco-2 cell monolayer model. Planta medica, 76(14), 1587–1591. https://doi.org/10.1055/s-0030-1249810
- Yang, A. H., Zhang, L., Zhi, D. X., Liu, W. L., Gao, X., & He, X. (2018). Identification and analysis of the reactive metabolites related to the hepatotoxicity of safrole. Xenobiotica, 48(11), 1164–1172. https://doi.org/10.1080/00498254.2017.1399227
- Zachariah, T. J., Leela, N. K., Maya, K. M., Rema, J., Mathew, P. A., Vipin, T. M., & Krishnamoorthy, B. (2008). Chemical composition of leaf oils of myristica beddomeii (King). Myristica fragrans (Houtt.) and Myristica Malabarica (Lamk.), 17(1), 10–15.
- Zhang, C. R., Jayashree, E., Kumar, P. S., & Nair, M. G. (2015). Antioxidant and anti-inflammatory compounds in nutmeg (Myristica Fragrans) pericarp as determined by in vitro assays. Natural Product Communications, 10(8), 1934578X1501000822. https://doi.org/10.1177/1934578X1501000822
- Zhao, W., Song, F., Hu, D., Chen, H., Zhai, Q., Lu, W., Zhao, J., Zhang, H., Chen, W., Gu, Z., & Wang, G. (2020). The protective effect of Myristica fragrans Houtt. extracts against obesity and inflammation by regulating free fatty acids metabolism in nonalcoholic fatty liver disease. Nutrients, 12(9), 2507. https://doi.org/10.3390/nu12092507
- Zhu, Z., Yang, S., Zhao, W., Li, R., & Zhao, C. (2016). A comparative pharmacokinetic study of myrislignan by UHPLC–MS after oral administration of a monomer and Myristica fragrans extract to rats. Journal of Chromatographic Science, 54(5), 689–696. https://doi.org/10.1093/chromsci/bmv227