Abstract
A potting experiment was conducted was carried out during the two consecutive seasons of 2020/2021 and 2021/2022 to investigate the impact of various bio-stimulant treatments, specifically salicylic acid (SA), ascorbic acid (AA), and Moringa oleifera leaf extract (MLE), as well as their combinations on plant growth, flowering and chemical composition of Dahlia pinnata plants cultivated in sandy soils. The study utilized an experimental design followed by a complete block design with three replications having eight treatments as follows: control, 300 mg/l SA, 300 mg/l AA, 10 mg/l MLE, SA+AA, SA+MLE, AA+MLE, and SA+AA+MLE. The results indicated that the application of various bio-stimulant treatments, either individually or in combination, resulted in enhanced vegetative growth characteristics, flowering attributes, and tuberous root production, as well as improved chemical constituents when compared to untreated plants. In addition, the most effective treatment combinations were found to be MLE + SA + AA, followed by SA + MLE, based on overall outcomes.
1. Introduction
Dahlia pinnata belongs to the family Asteraceae with tuberous roots and attractive large flowers. It is one of the most commonly grown and popular flowering plants worldwide. It is also a common cut flower used in spring and summer bouquets and for medicinal purposes. Dahlia blooms in the summer season, which makes it compete strongly with the other ornamental summer flowering plants. Previous research has demonstrated the importance of natural fertilizers in improving the growth and flowering of ornamental plants and bulbs. El-Naggar (Citation2010) proved that all biofertilizer treatments significantly increased total chlorophylls, root dry weight, dry and fresh bulb weights, bulb circumference and flowering parameters, vegetative growth, and K, P, and N contents of Narcissus tazetta plants. Moreover, in order to achieve enhanced growth, flowering attributes, bulb yield, and chemical composition of the Narcissus tazetta plant, it is advisable to incorporate compost into the cultivation soil along with the supplementation of either seaweed extracts or elements chelators (Rizk & Elngar, Citation2020).
There is a trend towards clean organic farming to replace the wasteful use of chemical fertilizers. The primary objective is to uphold a pristine environment devoid of contaminants, as evidenced by the implementation of chemical fertilizers and their impact on human well-being. Therefore, the utilization of organic alternatives in the field of agriculture is widely regarded as a viable approach to protect the environment and mitigate pollution (Ramadan et al., Citation2002). There is currently an observable inclination towards expanding the agricultural sector in Egypt, primarily through the process of land reclamation. Most of these lands frequently encounter challenges such as water scarcity, drought, salinity, and inadequate fertility (El-Khalifaa et al., Citation2022). The influence of organic matter on soil’s biological, chemical, and physical characteristics has been widely acknowledged for an extended period of time. There exists a prevailing tendency to enhance the resilience of plants against diverse stressors through the augmentation of antioxidant enzymes (Paciolla et al., Citation2019). However, AA is a small molecule dissolved in water that acts as an antioxidant and serves as a primary substrate in the cyclic pathways for detoxifying as well as neutralizing oxygen radicals and single superoxide (Alves et al., Citation2021). When AA is added, drought stress resistance is induced, and the harmful effect of oxidative stress is reduced (Khazaei & Estaji, Citation2020). Moreover, the application of AA was found to have an impact on both the qualitative and quantitative features of basil plants when subjected to drought stress (Hamidi et al., Citation2022).
SA has been widely recognized for its impact on various physiological and biochemical processes, and its crucial role in the regulation of plant productivity and growth has been extensively studied (Hayat et al., Citation2010). It may be recommended to add SA to improve Vigna mungo plant productivity (Muthulakshmi & Lingakumar, Citation2016). The application of SA through foliar spraying at a concentration of 3 mM resulted in earliness, increased number of flower clusters, and improved vegetative growth in strawberry cultivars (Mohamed et al., Citation2017).
Moringa leaves have antioxidant properties and contain plant growth stimulants like cytokinin as well as zeatin, which induces elongation and cell division. It also contains many enzymes and works to protect the plant cells from the harmful reactive oxygen species impact (Siddhuraju & Becker, Citation2003; Taiz & Zeiger, Citation2010; Yang et al., Citation2006; Yasmeen et al., Citation2013). Hassan et al. (Citation2020) studied the effects of MLE on the mitigation of salt stress in Rosa damascene and indicated that spraying with MLE improved the growth parameters, proline content, and chlorophyll content under salt stress. Given the aforementioned findings and recognizing the significance of the dahlia plant, the objective of this study is to investigate the impact of foliar application of SA and AA, along with MLE, on the morphological characteristics, flowering patterns, and biochemical reactions of dahlia plants cultivated in sandy soil.
2. Materials and methods
Two pot experiments were performed to study the effects of different biostimulants, namely AS, AA, and moringa leaf extract, and their combinations on D. pinnata biochemical activities, flowering, and growth. The investigation was carried out over two consecutive seasons, specifically in 2020/2021 and 2021/2022, within sandy soil located at a privately owned farm (Damanhour-Beheira Governorate-Egypt (31° 04 °N, 30° 47’ °E). The experimental soil’s chemical and physical characteristics are depicted in (Table ). The tuberous roots of D. pinnata, precisely the summer flowering type, were obtained from a commercial nursery located in El Kanater El Khayria, Cairo, Egypt. Rooted cuttings measuring 10–15 cm in length and weighing 15–20 g were submerged in a solution containing fungicide. These cuttings were then planted on February 15th for both seasons, using PVC pots with a diameter of 30 cm. The pots were filled with sandy soil. Following the planting process, the tuberous roots were adequately irrigated in the pots. One month later, all plants were pinched to stimulate better branching. In this study, a randomized complete block design (RCBD) was employed, consisting of four replicates and eight treatments as follows: control, SA at a rate of 300 mg/L., AA at a rate of 300 mg/L, moringa leaf extract at a rate of 10 g/l MLE, SA+AA, SA+MLE, AA+MLE, and SA+AA+MLE. Different treatments were applied as a foliar spray one week after plant pinching and repeated three times at 15-day intervals. Fertilization of all experimental units of dahlia plants was done with 4 g calcium superphosphate, 4 g calcium nitrate, and 2 g/pot potassium sulfate (applied in a split manner) at 30 and 45 days following planting.
Table 1. Chemical and physical properties of the sandy soil under study
2.1. Preparing of MLE
M. oleifera fresh leaves were harvested from healthy trees grown in Al-Marashda Station, Agriculture Research Center, Qena Governorate. Fresh leaves (10 g) were mixed with (1 L) of distilled water by a house mixer. The solution was subsequently purified by wringing the solution (utilizing a mutton cloth). The extract was re-filtered using No. 2 Whatman filter paper (Fuglie, Citation2000). The extract was utilized within 5 h after extracting and cutting. Determination of the mineral compositions of Moringa leaves is shown in Table .
Table 2. Mineral compositions of moringa leaves
2.2. Measurements
Measurements were taken for the vegetative traits as branch number/plant, plant height (cm), leaf number/plant, shoot dry weight (g), flower diameter (cm), flowering traits as flowering date (days), flower dry and fresh weight (g), number of flowers/plant, length (cm), diameter (mm), and fresh and dry weight (g) of flowering stalk, and for tuberose roots as number of tuberose roots/plant and tuberous roots fresh weight/plant (g).
2.3. Chemical analysis
The measurement of the chlorophyll content in dahlia leaves was conducted in milligrams per 100 grams of fresh weight of the sixth leaf below the terminal bud, at the stage when color is visible, following the methodology outlined by Moran and Porath (Citation1980). The quantification of total carbohydrate content in dry leaves was conducted following Dubious (Citation1956). Nitrogen, phosphorus, and potassium contents (%) in the leaves were determined according to Westerman (Citation1990). The statistical software program SAS (SAS Institute, Citation1999) was employed to conduct a statistical analysis of the collected data in order to assess the variations between the different treatments. The distinctions among treatments were examined through a one-way analysis of variance (ANOVA), with the least significant differences (L.S.D.) test implemented at a significance level of 5% (p < 0.05).
3. Results and discussion
3.1. Growth characteristics
According to Tables , each of the four vegetative traits of growth, plant height, branch leaf/plant, leaf number/branch, and shoot dry weight/plant were significantly increased in both seasons as a result of biostimulants application in comparison with untreated plants. However, higher growth parameters were registered with the three mixed bio-stimulant treatments (SA+AA+MLE), followed by SA+MLE, compared to the other treatments (in both seasons). The plant height values that were observed to be the highest were 77.87 cm and 79.03 cm in the first season and 79.03 cm and 72.20 cm in the second season, respectively. These values were obtained using the combination of SA+AA+MLE in the first season and SA+MLE in the second season. The recorded values of the number of branches per plant in the first and second seasons were highest for the treatments SA+AA+MLE and SA+MLE, with measurements of 5.53 and 5.40 cm and 5.20 and 5.10 cm, respectively. The observed increase in the number of leaves/branch (22.07 and 21.70), as well as the subsequent increase (20.73 and 20.43), can be attributed to the application of SA+AA+MLE and SA+MLE treatments during the first and second seasons, respectively. Similarly, the higher weight of dry shoot (51.83 and 51.73), followed by (47.77 and 48.50), can be attributed to the same treatments in the respective seasons.
Table 3. Influence of biological substances on branch number/plant and plant height (cm) of D. pinnata under sandy soils during the two seasons of 2021 and 2022
Table 4. Influence of biological substances on number of leaves/branch and shoot dry weight/plant (g) of D. pinnata under sandy soils during the two seasons of 2021 and 2022
The present study indicated that applying the different plant growth stimulators improved dahlia plants’ growth, flowering, and tuberous roots compared to their controls. When comparing the effects of the three growth stimulants separately, we note that the most significant effect was a result of spraying dahlia plants with MLE, followed by SA in both seasons. Also, in the case of the dual treatments of two substances, the addition of MLE increases the effectiveness of spraying in improving growth parameters, flowering and tuberous roots compared to other double treatments. However, the most pronounced effects were due to the mix of the three substances: SA, AA, and MLE. Many previous studies proved the positive effects of MLE on different plants (Ahmad et al., Citation2022; Ahmed et al., Citation2021; Alkuwayti et al., Citation2020; Yap et al., Citation2021).
3.2. Flowering traits
The bio-stimulant treatments significantly affected the number of flowers/plant, flower diameter, flowering date, stalk length, stalk diameter, stalk fresh weight, flower fresh weight, and flower dry weight (Tables ). However, higher values of flower number/plant (31.00 and 30.00), diameter of flower (12.63 and 13.40 cm), flowering date (127.67 and 130.67 days), stalk diameter (6.23 and 6.07 mm), stalk fresh weight (40.00 and 42.00 g), flower fresh weight (25.10 and 25.30 g) and flower dry weight (4.80 and 4.80 g) were obtained with SA+AA+MLE treatment (in the first and second seasons), respectively. In contrast, higher values of stalk length (44.00 and 45.00 cm), followed by (43.00 and 44.00 cm), were due to SA+AA+MLE and AA+MLE (in the first and second seasons), respectively.
Table 5. Influence of biological substances on flower number/plant and flower diameter (cm) of D. pinnata under sandy soils during the two seasons of 2021 and 2022
Table 6. Influence of biological substances on flowering date (day) and stalk length (cm) of D. pinnata under sandy soils during the two seasons of 2021 and 2022
Table 7. Influence of biological substances on stalk diameter (mm) and stalk fresh weight (g) of D. pinnata under sandy soils during the two seasons of 2021 and 2022
Table 8. Influence of biological substances on the dry and fresh weight (g) of D. pinnata flower under sandy soils during the two seasons of 2021 and 2022
Zahra et al. (Citation2022) indicated that the growth traits and milk thistle plant (Silybum marianum) secondary metabolites were improved with the application of AA, thiourea, and MLE by upregulating secondary metabolites accumulation. Conversely, Hassan et al. (Citation2020) illustrated that spraying Rosa damascena with MLE improved the growth attributes and chlorophyll content. They suggested that MLE had an efficient antioxidant system that scavenged reactive oxygen species and endured considerable protection against salt-induced oxidative damage under newly reclaimed soils. They investigated Moringa oleifera extract as a biostimulant on Ocimum basilicum plants, and they found that spraying with 10% extract induced vegetative growth traits such as shoots’ dry and fresh weight, shoot length, and branch number/plant (Hassanein et al., Citation2019). Moreover, the positive effect of SA on the growth characteristics of dahlia plants aligns with those previously reported by many researchers on different crops (Amin et al., Citation2007; Khodary, Citation2004; Mohamed et al., Citation2017; Stevens et al., Citation2006; Szepesi, Citation2005; Wang et al., Citation2022; Yildirim et al., Citation2008).
3.3. Tuberous roots
The number of tuberous roots and the fresh weight of tuberous roots in dahlia plants were found to be significantly influenced by various biostimulant treatments in comparison to the control treatment during both the first and second seasons, as indicated in Table . The tuberous roots exhibited the highest values of 7.73 and 7.73, respectively, in the first and second seasons when subjected to the combined application of SA+AA+MLE and SA+MLE. Additionally, values of 6.70 and 6.80 were observed in the same order for the respective treatments. In addition, it was observed that the fresh weight of tuberous roots per plant exhibited higher values of 369.00 and 366.33 g in the first season and 344.67 and 345 g in the second season, respectively when treated with a combination of SA+AA+MLE and SA+MLE.
Table 9. Influence of biological substances on the number of tuberous roots/plant and fresh weight of tuberous roots/plant (g) of D. pinnata under sandy soils during the two seasons of 2021 and 2022
SA plays a pivotal role in regulating numerous vital processes and growth in plants (Blokhina et al., Citation2003; Raskin, Citation1992). The findings suggest that the stimulatory impact of SA can be attributed to its ability to regulate various physiological and biochemical processes in plants. These processes include ion uptake, cell division, cell elongation, sink/source regulation, cell differentiation, protein synthesis, enzymatic activities, photosynthetic activity, and enhancement of antioxidant capacity in plants. SA plays a crucial role in the plant signal transduction pathway, contributing to both local and systemic resistance against various stresses (Meena et al., Citation2001). The stimulatory effect of SA on dahlia plant growth and productivity could be The observed rise in carbohydrate content could potentially be attributed to the activation of the photosynthetic machinery, which may be a result of the stimulatory effect exerted by the biostimulants used on the photosynthetic process. The application of SA may potentially hinder the activity of polysaccharide-hydrolyzing enzymes and/or expedite the conversion of soluble sugars into polysaccharides (Bakry et al., Citation2012). Moreover, the application of exogenous SA has been found to play a role in various plant reactions, such as the regulation and transportation of ions, the closure of stomata (Gunes et al., Citation2005), the permeability of membranes, and photosynthesis (Barkosky & Einhellig, Citation1993). Rehman et al. (Citation2022) reported that the growth of lemongrass was enhanced when treated with different concentrations of SA, particularly at a concentration of 0.5 mM, in comparison to the control (Rehman et al., Citation2022).
Conversely, AA contributes to the plant’s vital functions such as cell division, development and enlargement, and other developmental processes. Furthermore, in accordance with the acidic theory, it has been proposed that it induces the degradation of cell walls, subsequently leading to an increase in cell expansion and the development of cell walls (Pazuky et al., Citation2013). This observation highlights the significance of applying a 300 mg/L AA concentration to dahlia plants, particularly when combined with AA and MLE.
3.4. Chemical constituents
Biological substance application enhances dahlia plants’ total carbohydrates, chlorophylls, and nutrient status by achieving significant increases in these contents compared to their controls. The higher values of total carbohydrate contents (48.63 and 49.13 mg/g D.W.) and total chlorophylls (3.70 and 3.60 mg/g F.W.) were obtained with SA+AA+MLE treatment in the first and second seasons, respectively (Table & Figures ). Higher increases in the contents of N, P, and K were (32.4 and 34.6 %), (25.8 and 28.1%) and (50.0 and 48.4 %) were due to the application of SA+AA+MLE as a foliar spray in the first and second seasons, respectively (Table & Figures ).
Figure 1. Influence of biological substances on total carbohydrate content (mg/g D. W.) of D. pinnata under sandy soils during two seasons of 2022 and 2023.T1 = control, T2 = salicylic acid (SA) at rate of 300 mg/L., T3 = AA at rate of 300 mg/L, T4 = moringa leaf extract at rate of 10 g/l MLE, T5 = SA+AA, T6 = SA+MLE, T7 = AA+MLE and T8 = SA+AA+MLE.
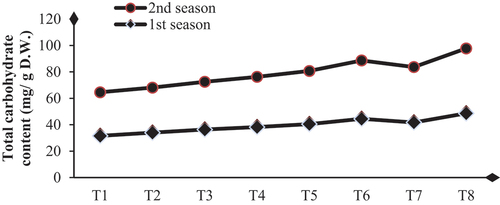
Figure 2. Influence of biological substances on total chlorophyll content (mg/g D. W.) of D. pinnata under sandy soils during two seasons of 2022 and 2023.T1=control, T2=salicylic acid (SA) at rate of 300 mg/L., T3= AA at a rate of 300 mg/L, T4=moringa leaf extract at rate of 10 g/l MLE, T5=SA+AA, T6=SA+MLE, T7=AA+MLE and T8= SA+AA+MLE.
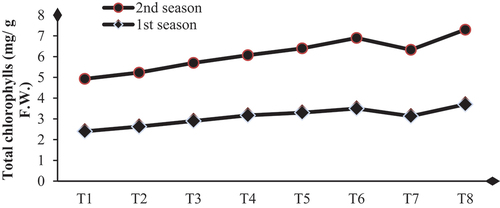
Figure 3. Influence of biological substances on nitrogen content (%) (mg/g D. W.) of D. pinnata under sandy soils during two seasons of 2022 and 2023. T1=control, T2=salicylic acid (SA) at rate of 300 mg/L., T3= AA at rate of 300 mg/L, T4=moringa leaf extract at rate of 10 g/l MLE, T5=SA+AA, T6=SA+MLE, T7=AA+MLE and T8= SA+AA+MLE.
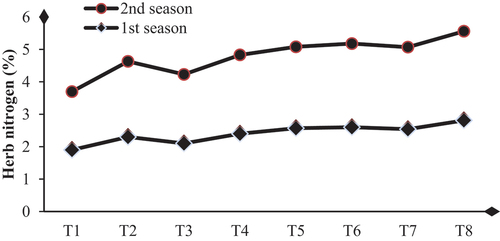
Figure 4. Influence of biological substances on phosphorus content (%) (mg/g D. W.) of D. pinnata under sandy soils during two seasons of 2022 and 2023. T1=control, T2=salicylic acid (SA) at a rate of 300 mg/L., T3= AA at a rate of 300 mg/L, T4=moringa leaf extract at a rate of 10 g/l MLE, T5=SA+AA, T6=SA+MLE, T7=AA+MLE and T8= SA+AA+MLE.
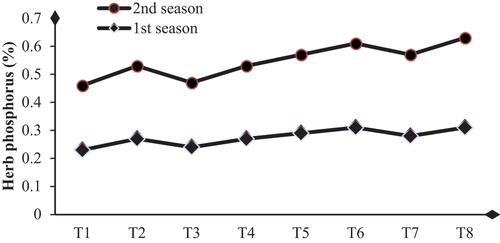
Figure 5. Influence of biological substances on potassium content (%) (mg/g D. W.) of D. pinnata under sandy soils during two seasons of 2022 and 2023. T1=control, T2=salicylic acid (SA) at a rate of 300 mg/L., T3= AA at a rate of 300 mg/L, T4=moringa leaf extract at a rate of 10 g/l MLE, T5=SA+AA, T6=SA+MLE, T7=AA+MLE and T8= SA+AA+MLE.
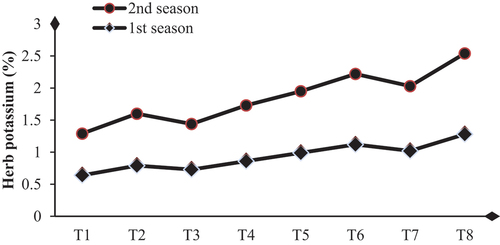
Table 10. Influence of biostimulants on total carbohydrate content (mg/g D.W.) and total chlorophylls (mg/g F.W.) of Dahlia pinnata under newly reclaimed soils during the two seasons of 2021 and 2022
Table 11. Influence of biostimulants on nitrogen, phosphorus, and potassium content (%) of Dahlia pinnata herb under newly reclaimed soils during the two seasons of 2021 and 2022
The growth parameters of Triticum aestivum, including tiller and spike count, chlorophyll content, and 1000-grain weight, exhibited an increase when treated with a concentration of 100 mg/L of AA. According to the study conducted by Rehman and Wei (Citation2022), it has been suggested that the application of AA has a regulatory effect on the hormonal conditions of plants, leading to an increase in auxin levels, specifically in basil plants when they are not subjected to any form of stress (Hamidi et al., Citation2022). The presence of AA in wheat plants has been found to enhance the levels of auxin and ABA while also preventing a reduction in cytokinin levels during periods of drought-induced stress (Shamsipur et al., Citation2012). The positive impact of AA on plant growth can be attributed to its essential function as a significant cofactor in the biosynthesis of plant hormones that regulate cell growth and division (e.g., gibberellin & auxin) and the maintenance of pigment stability in the photosynthetic apparatus. It acts efficiently in plants as an immunomodulator when applied at an appropriate rate and at the current stage of plant development (Khan et al., Citation2011).
4. Conclusions
Based on the findings of our study, the utilization of certain biostimulants, such as Moringa oleifera leaves extract at a concentration of 10 g/L, salicylic acid at 300 mg/L, and AA at 300 mg/L, as well as their combinations, exhibited a positive impact on the growth, flowering, yield, and mineral composition of dahlia plants cultivated in sandy soils. However, dahlia plants’ vegetative and reproductive growth was significantly boosted when treated with the combination of the three substances, followed by SA + Moringa leaves extract. As a result, the different biostimulants are thought to be primarily responsible for dahlia growth and yield under sandy soil conditions.
Author contributions
Conceptualization, E.A.S.; methodology, E.A.S., M.A.A.A. and R.G.E.; software, E.A.S., M.A.A.A. and R.G.E.; formal analysis, E.A.S., M.A.A.A. and R.G.E.; investigation, E.A.S., R.J.B. and R.G.E.; resources, E.A.S., M.A.A.A. and R.G.E.; writing—original draft preparation, E.A.S., M.A.A.A. and R.G.E.; writing—review and editing, M.A.A.A. and R.J.B. All authors provided critical feedback and helped shape the research, analysis, and manuscript. Also, all authors discussed the results and contributed to the final manuscript. All authors read and approved the final manuscript.
Conflicts of interest
All the authors declared that they have no competing interests.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article.
Additional information
Funding
References
- Ahmad, A., Blasco, B., & Martos, V. (2022). Combating salinity through natural plant extracts based biostimulants: A review. Frontiers in Plant Science, 13, 862034. https://doi.org/10.3389/fpls.2022.862034
- Ahmed, T., Abou Elezz, A., & Khalid, M. F. (2021). Hydropriming with moringa leaf extract mitigates salt stress in wheat seedlings. Agriculture, 11(12), 1254. https://doi.org/10.3390/agriculture11121254
- Alkuwayti, M., El-Sherif, F., Yap, Y.-K., & Khattab, S. (2020). Foliar application of Moringa oleifera leaves extract altered stress-responsive gene expression and enhanced bioactive compounds composition in Ocimum basilicum. South African Journal of Botany, 129, 291–13. https://doi.org/10.1016/j.sajb.2019.08.001
- Alves, R., Rossatto, D. R., da Silva, J., MV, C., de Oliveira, K. R., Oliveira, F., de Queiroz, S. F., da Cruz, M. C. P., & Gratão, P. L. (2021). Seed priming with ascorbic acid enhances salt tolerance in micro-tom tomato plants by modifying the antioxidant defense system components. Biocatalysis and Agricultural Biotechnology, 31, 101927. https://doi.org/10.1016/j.bcab.2021.101927
- Amin, A., Rashad, M., & El-Abagy, H. (2007). Physiological effect of indole-3-butyric acid and salicylic acid on growth, yield and chemical constituents of onion plants. Journal of Applied Sciences Research, 3, 1554–1563.
- Bakry, A. B., El-Hariri, D. M., Sadak, M. S., & El-Bassiouny, H. M. S. (2012). Drought stress mitigation by foliar application of salicylic acid in two linseed varieties grown under newly reclaimed sandy soils. The Journal of Applied Sciences Research, 8, 3503–3514.
- Barkosky, R. R., & Einhellig, F. A. (1993). Effects of salicylic acid on plant-water relationships. Journal of Chemical Ecology, 19(2), 237–247. https://doi.org/10.1007/BF00993692
- Blokhina, O., Virolainen, E., & Fagerstedt, K. V. (2003). Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany, 91(2), 179–194. https://doi.org/10.1093/aob/mcf118
- Dubious, M. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–366. https://doi.org/10.1021/ac60111a017
- El-Khalifaa, Z. S., El-Gamalb, E. H., & Zahran, H. F. (2022). Evaluation of barley cultivated areas’actual status in Egyptian newly reclaimed lands. Asian Journal of Agriculture and Rural Development, 12(3), 164–172. https://doi.org/10.55493/5005.v12i3.4532
- El-Naggar, A. H. (2010). Effect of biofertilizer, organic compost and mineral fertilizers on the growth, flowering and bulbs production of Narcissus tazetta. Journal of Agricultural and Environmental Sciences, 9, 24–52.
- Fuglie, L. (2000). The miracle tree: Moringa oleifera: Natural nutrition for the tropics. The multiple attributes of moringa. International Journal of Advance Research, Ideas and Innovations in Technology, 3, 172.
- Gunes, A., Inal, A., Alpaslan, M., Cicek, N., Guneri, E., Eraslan, F., & Guzelordu, T. (2005). Effects of exogenously applied salicylic acid on the induction of multiple stress tolerance and mineral nutrition in maize (zea mays L.). Archives of Agronomy and Soil Science, 51(6), 687–695. https://doi.org/10.1080/03650340500336075
- Hamidi, M., Moghadam, H. T., Nasri, M., Kasraie, P., & Larijani, H. (2022). The effect of ascorbic acid and bio fertilizers on basil under drought stress. Brazilian Journal of Biology, 84, 262459. https://doi.org/10.1590/1519-6984.262459
- Hassan, F., Al-Yasi, H., Ali, E., Alamer, K., Hessini, K., Attia, H., & El-Shazly, S. (2020). Mitigation of salt-stress effects by moringa leaf extract or salicylic acid through motivating antioxidant machinery in damask rose. Canadian Journal of Plant Science, 101(2), 157–165. https://doi.org/10.1139/cjps-2020-0127
- Hassanein, R. A., Abdelkader, A. F., & Faramawy, H. M. (2019). Moringa leaf extracts as biostimulants-inducing salinity tolerance in the sweet basil plant. Egyptian Journal of Botany, 59, 303–318. https://doi.org/10.21608/ejbo.2019.5989.1242
- Hayat, Q., Hayat, S., Irfan, M., & Ahmad, A. (2010). Effect of exogenous salicylic acid under changing environment: A review. Environmental and Experimental Botany, 68(1), 14–25. https://doi.org/10.1016/j.envexpbot.2009.08.005
- Khan, T. A., Mazid, M., & Mohammad, F. (2011). A review of ascorbic acid potentialities against oxidative stress induced in plants. Journal of Agrobiology, 28(2), 97–111. https://doi.org/10.2478/v10146-011-0011-x
- Khazaei, Z., & Estaji, A. (2020). Effect of foliar application of ascorbic acid on sweet pepper (capsicum annuum) plants under drought stress. Acta Physiologiae Plantarum, 42(7), 1–12. https://doi.org/10.1007/s11738-020-03106-z
- Khodary, S. E. A. (2004). Effect of salicylic acid on growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. International Journal of Agriculture & Biology, 6, 5–6.
- Meena, B., Marimuthu, T., & Velazhahan, R. (2001). Salicylic acid induces systemic resistance in groundnut against late leaf spot caused by cercosporidium personatum. Journal of Mycology and Plant Pathology, 31, 139–145.
- Mohamed, R., Abdelbaset, A.-K., & Abd-Elkader, D. (2017). Salicylic acid effects on growth, yield, and fruit quality of strawberry cultivars. Journal of Medicinally Active Plants, 6, 1–11.
- Moran, R., & Porath, D. (1980). Chlorophyll determination in intact tissues using N, N-dimethylformamide. Plant Physiology, 65(3), 478–479. https://doi.org/10.1104/pp.65.3.478
- Muthulakshmi, S., & Lingakumar, K. (2016). Salicylic acid induced responses on growth and biochemical constituents in (Vigna mungo L.) Hepper. European Journal of Experimental Biology, 6, 9–14.
- Paciolla, C., Fortunato, S., Dipierro, N., Paradiso, A., De Leonardis, S., Mastropasqua, L., & de Pinto, M. C. (2019). Vitamin C in plants: From functions to biofortification. Antioxidants, 8(11), 519. https://doi.org/10.3390/antiox8110519
- Pazuky, A., Rezaei, H., Habibi, D., & Pak-Nejad, F. (2013). Effects of drought stress, ascorbate and gibberellin spray on some morphological traits, relative water content and stability of the cytoplasmic membrane thyme (thymus vulgaris L.). Journal of Agronomy and Plant Breeding, 8(1), 1–13. in persian.
- Ramadan, H., Gaber, H., & El-Fayoumy, M. (2002). Ssessment and comparison of bio and mineral fertilization on farm profitability in different newly-reclaimed soils. Alexandria Journal of Agricultural Research (Egypt).
- Raskin, I. (1992). Salicylate, a new plant hormone. Plant Physiology, 99(3), 799. https://doi.org/10.1104/pp.99.3.799
- Rehman, Z., Hussain, A., Saleem, S., Khilji, S. A., & Sajid, Z. A. (2022). Exogenous application of salicylic acid enhances salt stress tolerance in lemongrass (Cymbopogon flexuosus steud. Wats). Pakistan Journal of Botany, 54(2), 371–378. https://doi.org/10.30848/PJB2022-2(13)
- Rehman, S., & Wei, X. (2022). To study the effect of ascorbic acid as a foliar spray on the growth of Triticum aestivum L. European Journal of Botany, Plant Sciences and Phytology, 7(1), 62–72.
- Rizk, G. W., & Elngar, M. A. (2020). Effect of soil Conditioners,Seaweed extracts and chemical fertilizers: onGrowth, flowering and bulbs production ofNarcissus tazettaL. subsp. Italicus Plant. Egyptian Academic Journal of Biological Sciences, H Botany, 11(2), 69–79. https://doi.org/10.21608/eajbsh.2020.153534
- SAS Institute. (1999). SAS for windows. version 802SAS inst.
- Shamsipur, M., Miran Beigi, A. A., Teymouri, M., Poursaberi, T., Mostafavi, S. M., Soleimani, P., Chitsazian, F., & Tash, S. A. (2012). Biotransformation of methyl tert-butyl ether by human cytochrome P450 2A6. Biodegradation, 23(2), 311–318. https://doi.org/10.1007/s10532-011-9510-0
- Siddhuraju, P., & Becker, K. (2003). Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (moringa oleifera Lam.) leaves. Journal of Agricultural and Food Chemistry, 2144–2155.
- Stevens, J., Senaratna, T., & Sivasithamparam, K. (2006). Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): Associated changes in gas exchange, water relations and membrane stabilization. Plant Growth Regulation, 49, 77–83.
- Szepesi, Á. (2005). Role of salicylic acid pre-treatment on the acclimation of tomato plants to salt-and osmotic stress. Acta Biologica Szegediensis, 49, 123–125.
- Taiz, L., & Zeiger, E. (2010). Responses and adaptations to abiotic stress. In Plant physiology (Fifth ed., pp. 755–778). Sinauer Associates, Inc.
- Wang, K., Shen, Y., Wang, H., He, S., Kim, W. S., Shang, W., Wang, Z., & Shi, L. (2022). Effects of exogenous salicylic acid (SA), 6-benzylaminopurine (6-BA), or abscisic acid (ABA) on the physiology of Rosa hybrida ‘Carolla’under high-temperature stress. Horticulturae, 8(9), 851. https://doi.org/10.3390/horticulturae8090851
- Westerman, R. L. (1990). Soil testing and plant analysis. American Society of Agronomy.
- Yang, R.-Y., Tsou, S. C., Lee, T.-C., Chang, L.-C., Kuo, G., & Lai, P.-Y. (2006). Moringa, a novel plant rich in antioxidants, bioavailable iron, and nutrients. ACS Publications, 925, 224–239.
- Yap, Y.-K., El-Sherif, F., Habib, E. S., & Khattab, S. (2021). Moringa oleifera leaf extract enhanced growth, yield, and silybin content while mitigating salt-induced adverse effects on the growth of Silybum marianum. Agronomy, 11(12), 2500. https://doi.org/10.3390/agronomy11122500
- Yasmeen, A., Basra, S., Farooq, M., Rehman, H. U., Hussain, N., & Athar, H. U. R. (2013). Exogenous application of moringa leaf extract modulates the antioxidant enzyme system to improve wheat performance under saline conditions. Plant Growth Regulation, 69(3), 225–233. https://doi.org/10.1007/s10725-012-9764-5
- Yildirim, E., Turan, M., & Guvenc, I. (2008). Effect of foliar salicylic acid applications on growth, chlorophyll, and mineral content of cucumber grown under salt stress. Journal of Plant Nutrition, 31(3), 593–612. https://doi.org/10.1080/01904160801895118
- Zahra, N., Wahid, A., Hafeez, M. B., Lalarukh, I., Batool, A., Uzair, M., El-Sheikh, M. A., Alansi, S., & Kaushik, P. (2022). Effect of salinity and plant growth promoters on secondary metabolism and growth of milk thistle ecotypes. Life, 12(10), 1530. https://doi.org/10.3390/life12101530
