?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A total of 525 catfish samples were collected with various fishing equipment, including hooks, longlines, and different mesh sizes of gillnets (4–12 cm). Out of these specimens, 379 (72.2%) stomachs contained prey items, whereas 146 (27.8%) were empty stomachs. Overall, fish prey, zooplankton, detritus and phytoplankton were the four most important food items, accounting for 48.1%, 21.8%, 17.1% and 5.9% of C. gariepinus diets by volume respectively. Diet composition varied across different size classes of the fish. The smallest fish (<37 cm standard length) mainly consumed detritus, mud (sediment), and zooplankton which comprise 41.2%, 29.3%, and 19.8% of the total volume, respectively. The larger fish (>37 cm SL) primarily fed on fish prey (14.0–74.1%) followed by zooplankton (11.2–21.3%) by volume. The relative importance of detritus, mud, zooplankton, phytoplankton, and insects decreased with increasing fish size from the Ribb Reservoir. The food and feeding habits of C. gariepinus significantly differed between dry and wet seasons. Fish prey and detritus were mainly consumed in the dry season and contributed to 63.6% and 21.9% of the total volume respectively. Zooplankton and phytoplankton were the most preferred food items during the wet season, contributing 71.8% and 22.2% of the total volume. Generally, C. gariepinus appears to be an omnivore, the species exhibits ontogenetic dietary shifts with larger specimens being more carnivorous, and the species exhibits dietary plasticity across wet and dry seasons, which may be linked to food availability from the Ribb Reservoir.
PUBLIC INTEREST STATEMENT
Ethiopia’s agricultural sector is still insufficient to supply food to this fast growing population. Clarias gariepinus African sharptooth mudcatfish is one of the most commercially important fish species in Ethiopia. Fisheries and aquaculture are alternatives to meet the high protein demand. The African catfish Clarias gariepinus (family Clariidae) is one of Ethiopia’s most important commercial fisheries. Understanding fish feeding habits and their trophic interactions within food webs is important for fisheries management and establishing sound aquaculture practices. The food and feeding habits of this fish species is impacted by the expansion of irrigation practices such as high sedimentation load, poor land use practice, lack of vegetation cover, and the construction of dams and weirs within the rivers. This fish resource is not well utilized due to the scientific knowledge gap. Therefore, water buffer zone management is needed to improve food and feeding habits of this fish species for better sustainable utilization.
1. Introduction
The African catfish Clarias gariepinus (1822) is extremely widespread throughout most of Africa (Spataru et al., Citation1987; Willoughby & Tweddle, Citation1978). In Ethiopia, it is widespread in almost all water bodies (lotic and lentic systems) such as in the rift valley, Abay, Awash, Baro-Akobo, Omo-Gibe, Tekeze, and Wabishebele-Genale basins (Awoke, Citation2015; Golubtsov & Mina, Citation2003). In many regions of Africa, it is one of the most important commercial freshwater fish species (Dadebo et al., Citation2014; Yesuf et al., Citation2023). Consequently, it is also the second commercially important fish species from the newly established Ribb reservoir, the Lake Tana Basin (Alebachew et al., Citation2022; Tesfahun & Alebachew, Citation2023). Understanding fish feeding habits and their trophic interactions within food webs is important for fisheries management and establishing sound aquaculture practices (Tesfahun & Temesgen, Citation2018). According to Adeyemi et al. (Citation2009), the food and feeding habits of fish provides vital evidence for management in a controlled environment and for the formulation of suitable nutrition provided for the fishin aquaculture. C. gariepinus feeds on a wide spectrum of prey items such as phytoplankton, macrophytes, zooplankton, insects, fish, detritus, amphibians, molluscs, birds, and sediment (Admassu et al., Citation2015; Dadebo et al., Citation2014; Eyayu, Citation(2019); Teka & Admassu, Citation2016; Tekle-Giorgis et al., Citation2016). The food and feeding habits of this catfish species depend on the season of the year and the spatial differences (Dereba, Citation2019; Houlihan et al., Citation2001; Kamal et al., Citation2010). Several studies were conducted with respect to the food and feeding habits of the C. gariepinus in Ethiopian water bodies (Abera, Citation2007; Admassu et al., Citation2015; Dadebo, Citation2000, Citation2009; Dadebo et al., Citation2014; Eyayu, Citation(2019); Teka & Admassu, Citation2016; Tekle-Giorgis et al., Citation2016). In the Ribb Reservoir, while a few studies on fish diversity and abundance (Mequanent et al., Citation2022) and the feeding ecology of the Nile tilapia (Oreochromis niloticus) (Tesfahun & Alebachew, Citation2023) have been conducted, there has so far been no study on the diet and feeding habits of C. gariepinus, despite its importance to the commercial fishery of the area. Such area-specific information on the food and feeding habits of this commercially and ecologically important species is significant for management (protecting its natural prey types and understanding its feed demand in fish culture systems) to sustain fish resources. Therefore, the current study was conducted to study diet composition and feeding habits of the African sharptooth catfish Clarias gariepinus (Burchell, 1822) from Ribb Reservoir, South Gondar, Ethiopia.
2. Materials and methods
2.1. Study area
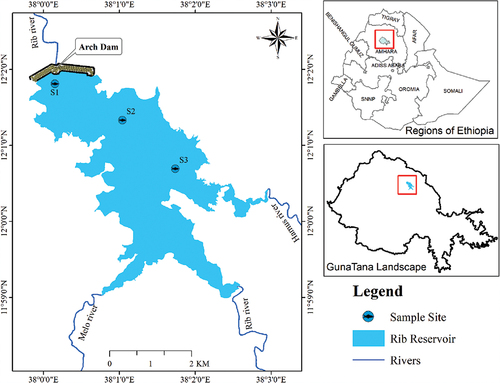
The Ribb Reservoir was built at the midpoint of the two districts namely Farta and Ebinat for the purposes of irrigation (Bezabih, Citation2021; Mequanent et al., Citation2022) (Figure ). The Ribb Reservoir is located in the northeastern part of the Lake Tana sub-basin, and was formed by the damming of the Ribb River in 2018. The longitudinal catchment of the Ribb River covers about 130 km and comprises a drainage area of approximately 1790 km2 with an annual average discharge of 14.6331 m3s−1. The four administrative kebeles such as Medeb Gubda, Jarashikra, and Ayvaniva (Farta district), and Amstya (Ebnat district) neighbored the Ribb Reservoir. It is located at 11°59’0” N to 12°2’0“N Latitude and 38°0’0“E to 38°2’59“E Longitude (Figure ). The Ribb watershed is experienced with a higher rate of irrigation practice particularly at Ribb Reservoir which increasing time to time at an alarming rate (Mequanent et al., Citation2021). Concerning the climate condition May to July is characterized as the rainy season. The monthly rainfall fluctuated from 65 mm in May to 411 mm in July. The mean precipitation was (1,400 mm annum−1) and the minimum (1,200 mm annum−1). The weather condition of Ribb Reservoir is categorized as Woina Dega (moderate climate) (Ezezew, Citation(2019)). The annual temperature ranges from 22.5°C to 26.2°C (Mequanent et al., Citation2022). The Microcystis (blue-green algae were found along the littoral side of the reservoir (Tesfahun & Alebachew, Citation2023). Fourteen zooplankton species were found in the reservoir. The Nile tilapia O. niloticus, the African catfish C. gariepinus, and Labeobarbus species were commercially important in the reservoir (Mequanent et al., Citation2022). Fishing in Ribb reservoirs is a common practice. Fishers established an association with the support of the government and began fishing in 2018. Thus, annual production in 2018 was 148.5 tons, 1028.3 tons and 1034.1 tons, respectively, and shows an increasing trend (Mequanent et al., Citation2022).
2.2. Fish sampling and extraction of stomach contents
The fish sampling sites (S1, S2 and S3) were selected based on the availability of fish species. Fish specimen collection was conducted between February and April 2021 and between June and July 2021 in dry and wet seasons respectively. Handlines, longlines, and single filament gillnets were used with a mesh size of 4, 5, 6, 8, 10 and 12 cm and 25 m in length. Gillnets and longlines were secured in the evening time (5:30 p.m.) and inspected the following morning (7:30 a.m.). Length of fishes, such as total length (TL) and standard length (SL), were measured to the nearest millimeter using a measuring board. The weights of the smallest fish (TW) were measured using a sensitive balance with a sensitivity of 0.1 g while the larger fish were measured by non-digital balance. Following the morphological and weight measurements, the fish were immediately dissected by a dissection kit. Subsequently, the entire stomach of C. gariepinus was isolated and exerted pressure to extract the contents of the stomach. In addition, the contents of the stomach (food) were immediately stored in a 5% formalin solution for further analysis. Finally, all samples were marked (date of sampling, measurement of length and weight, location of sampling, fish species, etc.) and transferred to the University of Debre Tabor for further analysis.
2.3. Stomach content analysis
During laboratory analysis, the stomach contents were placed into a Petri dish and investigated (Hyslop, Citation1980). Therefore, the smallest food is analyzed using the XSZ-70DN dissection microscope model and the ST-30-2 L stereo microscope (100 to 400 magnification). The identified food items were classified to the lowest taxonomic level by using descriptions, keys, and literature (Vuuren et al., Citation2006, Carling et al., Citation2004). The contents of fish’s stomachs were analyzed by relative measures of prey quantities (RMPQ) (Hyslop, Citation1980). The relative importance of each food item in the total food content of the stomach was analyzed based on percentage frequency (%Q) and percentage volume (%V) (Assis, Citation1996).
In the frequency of occurrence, the number of stomach samples containing one or more of a given food item was expressed as a percentage of all nonempty stomachs examined (Hyslop, Citation1980).
The proportion of fish that consumed certain foods is estimated and the frequency of occurrence is calculated (Bowen, Citation1983; Hyslop, Citation1980). The proportion of the African sharptooth catfish that feed on certain food items was estimated by this method.
The frequency of occurrence was calculated as:
where %Oi = frequency of occurrence of the i food item in the sample; ni = number of stomachs in which the i item is found; n = total number of food contained stomachs in the sample (Bowen, Citation1983).
Food items were sorted into different taxonomic categories, and the water displaced by a group of items in each category was measured in a partially filled graduated cylinder (Bowen, Citation1983). The volume of water displaced by each category of food items was expressed as a percentage of the total volume of the stomach contents (Bowen, Citation1983).
The volumetric measurement (%Vi) was estimated as:
Where %Vi is the percentage of volume.
The stomach analysis parameters such as frequency of occurrence (%Qi) and volumetric contribution (%Vi, ml) were used to estimate the index of food preponderance (PIi) and geometric importance of index (GIIi). To determine the importance of each food item the index of preponderance (PIi) (Tomojiri et al., Citation2019) was calculated as:
Where Qi is the frequency of occurrence of species i and %Vi is the percent composition by volume of species i. To facilitate comparisons among species, PIi was converted into percent PIi (%PIi). Furthermore, to estimate the relative importance of food items and species-level dietary differences, the Geometric Index of Importance (GIIi) (Assis, Citation1996) was estimated as:
Where RMPQi = percentage of volume and frequency of occurrence (as a percentage of total occurrences) and n = total number of RMPQ. The index of GIIi value ranges from 0 to 1 (1–100%), with values close to 0 indicating feeding specialization and values close to 1.0 representing generalization (Hurlbert, Citation1978).
2.4. Data analysis
Food and feeding habits in relation to season and size class of C. gariepinus in the Ribb Reservoir were studied using the percentage of volumetric contribution, frequency occurrence, preponderance importance index (PIi), and geometric importance of index (GIIi) of the five size classes (<37.0, 37.0–42.0, 42.5–47.0, 47.5–52.0 and ≥52.5 cm SL), and seasons (the dry and the wet) of the year (Tomojiri et al., Citation2019).
Descriptive statistics were interpreted and analyzed by Microsoft Excel (Windows 10). IBM SPSS version 20 was also used to analyze the diet composition and feeding habits differences within the size classes of the C. gariepinus through analysis of variance (ANOVA) at 95% confidence level. Whereas, the t-test was used to compute the seasonal variation in the diet of the fish.
3. Results
3.1. Overall diet composition and feeding habits of Clarias gariepinus
From this study, 525 specimens of Clarias gariepinus were sampled. Out of these specimens, 379 (72.2%) consisted of different foods, whereas 146 (27.8%) had empty stomachs. The diet composition of C. gariepinus was comprised of numerous prey items such as zooplankton, fish, phytoplankton, plant detritus, insects, ostracods, sand particles, macrophytes and plant seeds (Table ). Zooplankton was the most consumed food type followed by fish prey, phytoplankton, and detritus in the diet of C. gariepinus. However, insects, sand particles, ostracods, and macrophytes contributed less to the diet of the fish. The percentage of the geometric importance index (%GIIi) revealed that zooplankton was the main prey type fed by the fish (Figure ). Based on this index, fish prey, phytoplankton, and detritus were the second, third and fourth most important preys in C. gariepinus diet respectively, whereas sand grains, ostracods, and insects are occasionally ingested.
Figure 2. The percentage contribution of geometric index importance (%GIIi) in the diet of C. gariepinus (n = 379) from Ribb Reservoir.
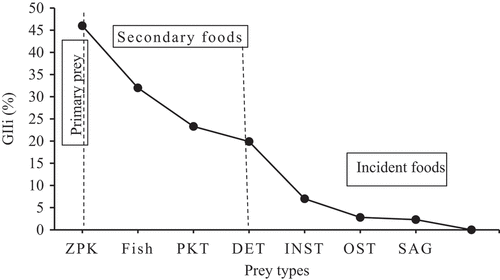
Table 1. Proportions of different food items in the diet of C. gariepinus (n = 379) in Ribb Reservoir, Ethiopia
Zooplankton (Rotifers, Copepods, and Cladocera) collectively occurred in 100% of all stomachs analyzed and comprised 21.8% of the stomach contents by volume. Fish prey occurred in 36.1% of all stomachs analyzed in the diet and comprised 48.8% of the stomach contents by volume. Phytoplankton (blue-green algae, green algae, and euglenoids) together consisted of 55.7% of the total occurrences and accounted for 5.9% of the total volume. The index of preponderance (%PI) of food types such as zooplankton, fish prey, phytoplankton, and detritus were 32.95, 26.6, 4.96, and 9.2 respectively. The index of preponderance (%PI = 32.95%) and geometric importance index (%GIIi = 46.0%) revealed that zooplankton was the dominant food type in the diet of C. gariepinus. Fish prey (%GIIi = 32.0%), phytoplankton (%GIIi = 23.0%), and detritus (%GIIi = 19.8%) exhibited the second favorite food types in the diet. However, the remaining food items such as insects (%PI = 0.4%; %GIIi = 7.0%), ostracods (%PI = 0.02%; %GIIi = 2.8%), sand particles (%PI = 0.02%; %GIIi = 2.3%), and macrophytes (%PI = 0.01%; %GIIi = 1.5%) contributed less in the diet of C. gariepinus (Table and Figure ).
3.2. Food and feeding habits in relation to the size class
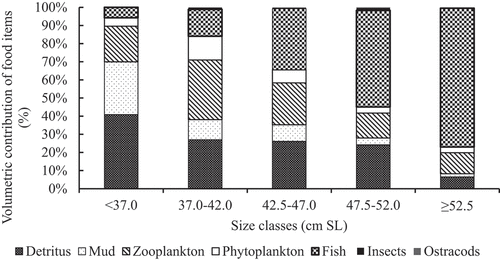
The food and feeding habit of C. gariepinus was significantly varied in relation to the size classes (ANOVA, p < 0.05). Detritus was the foremost important food item followed by mud and zooplankton for the size class below 37.0 cm (SL) (Figure ). Their volumetric contributions were 41.2%, 29.3%, and 19.8% of the total volume in the diet of this size class respectively. But, other food items such as phytoplankton (%V = 4.7) and fish prey (%V = 5.8) had a slight contribution to the diet of the fish this size class. According to the index of preponderance (PIi%) and Geometric Index of importance (GIIi%), zooplankton was the most preferred food item (PIi% = 29.3 and GIIi% = 76.2) followed by detritus (PIi% = 44.34 and GIIi% = 65.5), phytoplankton (PIi% = 5.06 and GIIi% = 51.8), and mud (PIi% = 19.7 and GIIi% = 42.4) to the diet of this size class (Figure ).
Figure 4. The relative importance of prey items (PIi% and GIIi%) with respect to size classes in the diet of C. gariepinus.
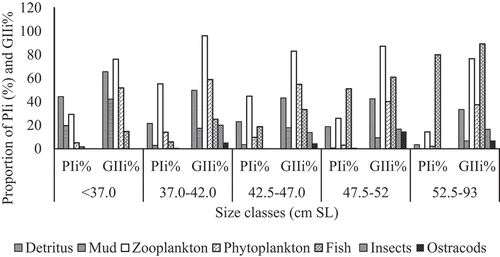
Zooplankton was the most important food type for size interval between 37.0 and 42.0 cm (SL). It accounted for 31.2% of the total volume of the stomachs. In addition, detritus, fish prey, phytoplankton, and mud were the following most preferred food items in this size class and their volumetric contributions were 25.6%, 14.0%, 12.4%, and 10.5% respectively. While, remaining food items such as ostracods, and insects had the least importance in the diet of the fish. Considering the index of preponderance (PIi%) and Geometric Index of importance (GIIi%), zooplankton was the most preferred food item (PIi% = 55.2 and GIIi% = 96.2) followed by detritus (PIi% = 21.5 and GIIi% = 49.76), phytoplankton (PIi% = 14.1 and GIIi% = 58.9), and fish prey (PIi% = 5.8 and GIIi% = 25.1) to the diet of this size class (Figure ).
Fish prey was the most frequent food item for the size class between 42.5 and 47.0 cm (SL). It consisted of 31.1% of the total volume in the diet of this size class. Detritus and zooplankton were the second most important food items in this size class and their volumetric contributions were 24.1%, and 21.3% respectively. However, other food items such as ostracods, phytoplankton, and insects had less contribution to the diet of the fish this size class. As per the index of preponderance (PIi%) and Geometric Index of importance (GIIi%), zooplankton was the most preferred food item (PIi% = 44.8 and GIIi% = 83.1) followed by phytoplankton (PIi% = 9.7 and GIIi% = 54.8), detritus (PIi% = 23.0 and GIIi% = 43.2), and fish prey (PIi% = 18.8 and GIIi% = 33.4) to the diet of this size class (Figure ).
Fish prey was the most important food item for the size class between 47.5 and 52.0 cm (SL). It comprised 50.3% of the total volume of the diet. Detritus was the second most favored food item in this size class and its corresponding volumetric contribution was 22.9%. While, the remaining food items such as ostracods, phytoplankton, insects, mud, and zooplankton contributed less importance to the diet of the fish this size class. As per the index of preponderance (PIi%) and Geometric Index of importance (GIIi%), zooplankton was the most preferred food item (PIi% = 25.9 and GIIi% = 87.2) followed by fish prey (PIi% = 51.0 and GIIi% = 60.9), detritus (PIi% = 18.8 and GIIi% = 42.5) and phytoplankton (PIi% = 3.0 and GIIi% = 40.1) to the diet of this size class (Figure ).
Fish prey was by far the most important food item for the size class ≥52.5 cm (SL). It accounted for 74.1% of the total volume of diets. Zooplankton was the following chosen food item in this size class and its volumetric contribution was 11.2%. However, the remaining food items such as detritus, mud, ostracods, phytoplankton, and insects were ingested in the least amount in the diet of the fish in this size class. The volumetric contribution of fish prey revealed an increasing trend with the size of the fish increases. However, the volumetric contribution of detritus, mud, phytoplankton, and zooplankton exhibited decreasing trend as the fish size increased (ANOVA, p < 0.05 (Figure ). Furthermore, as per the index of preponderance (PIi%) and Geometric Index of importance (GIIi%), fish prey (PIi% = 79.9 and GIIi% = 89.2) was the most favorite food item followed by zooplankton (PIi% = 14.3 and GIIi% = 76.6), phytoplankton (PIi% = 1.9 and GIIi% = 37.4) and detritus (PIi% = 3.4 and GIIi% = 33.4) to the diet of this size class (Figure ).
3.3. Seasonal variation of the diet
The seasonal differences in the diet of C. gariepinus were presented in (Table ) from Ribb Reservoir. In this study, the results revealed that seasonal variations in the diet of the fish were observed (t-test, p < 0.05). During the dry season, fish prey (%IP = 64.5 and %GIIi = 40.5) was the most important food item accounted for 66.1% of the total stomachs, and comprised 63.6% of the total volume. Zooplankton (%IP = 7.3 and %GIIi = 32.6), and phytoplankton (%IP = 1.2 and %GIIi = 20.6) also secondly consumed food items occurred in 99.5%, and 64.6% of the total stomach contents and their volumetric contributions were 4.8%, and 1.2% of the total volume in the diet of fish during the dry season respectively (Table ). The remaining food types such as mud (%IP = 2.4 and %GIIi = 8.8), insect (%IP = 0.1 and %GIIi = 7.9), ostracod (%IP = 0.04 and %GIIi = 4.3), sand particles (%IP = 0.04 and %GIIi = 3.7), macrophytes (%IP = 0.01 and %GIIi = 2.4), and plant seeds (%IP = 0.01 and %GIIi = 0.5) had less contribution to the diet of the fish.
Table 2. Relative contribution percentage frequency of occurrence (%Qi), volumetric contribution (%Vi), index of preponderance (%PIi) and geometric index of importance (%GIIi) of different food items in the diet of Clarias gariepinus in Ribb Reservoir during the dry and wet seasons
In the wet season, zooplankton and phytoplankton were by far the most important prey type in the stomachs of C. gariepinus. These prey types occurred in 100% and 96.8% of the total stomachs and their respective volumetric contributions were 71.8% and 22.2% of the total volume respectively. The %IP and %GIIi indexes also exhibited that zooplankton (%IP = 40.3 and %GIIi = 71.6) was by far the most important food items in the diet during the wet season. Phytoplankton (%IP = 12.1 and %GIIi = 49.6) was the second most important food item in the diet of the fish. However, detritus (%IP = 0.08 and %GIIi = 4.0), insects (%IP = 0.08 and %GIIi = 3.9), fish prey (%IP = 0.11 and %GIIi = 3.8), and mud (%IP = 0.003 and %GIIi = 0.8) had less contribution to the diet of the fish during the wet season (Table ).
4. Discussion
4.1. Diet composition and feeding habits
In this study, 525 specimens of Clarias gariepinus were sampled. Out of these specimens, 379 (72.2%) were found with prey items in their stomachs, whereas 146 (27.8%) had empty stomachs. Most empty stomachs may be related to the digestive process after the fish has been caught. Similarly, inappropriate sampling of fish also leads to an accelerated digestion process (Tesfahun & Alebachew, Citation2023). The authors confirmed that a great proportion of fishes with empty stomachs were recorded in various Ethiopian water bodies when fishes were sampled with gillnets (Dadebo et al., Citation2014; Temesgen et al., Citation2022; Wagaw et al., Citation2022). This can be due to the fact that food in their stomach may be vomited or assimilated as fish fight to escape the fishing gear during fishing.
In this study, different food types were documented in the stomachs of C. gariepinus including zooplankton, fish prey (the Nile tilapia and Labeobarbus spp.), phytoplankton, detritus, insects, ostracods, macrophyte, and sand particles in Ribb Reservoir. Several studies also confirmed the presence of the aforementioned prey types in the diet of C. gariepinus in Lake Hawassa (Dadebo, Citation2000); Lake Langano (Teka & Admassu, Citation2016); Lake Babogaya (Admassu et al., Citation2015); Lake Hayq (Dereba, Citation2019); Lake Koka (Dadebo et al., Citation2014), Lake Chamo (Dadebo, Citation2009), Egbe Reservoir (Adewumi et al., Citation2014), Lake Alau (Wakil et al., Citation2014), Tungan Kawo Reservoir (Habiba, Citation2021) and Lake Hawassa and Shallo swamp (Tekle-Giorgis et al., Citation2016).
Zooplankton was by far the most important food type in the diet of C. gariepinus from Ribb Reservoir. This may be a result of an abundance of zooplankton owing to suitable physico-chemical conditions in the Ribb Reservoir (Bhuyan et al., Citation2020; Mequanent et al., Citation2022). The diversity of zooplankton also varies depending on many factors, including geographical regions, feeding type, and morphometric characteristics of water (Vijayakumari et al., Citation2018). The abundance and diversity of zooplankton also varied with seasonal fluctuations. The average zooplankton biodiversity index values of the Ribb reservoir, such as the dominance index (0.902) and the Shannon diversity index (H′) (2.47) indicate that the reservoir is in a good state (Mequanent et al., Citation2022). Besides, the abundance of zooplankton in the diet of C. gariepinus might be associated with a high production of zooplankton due to high temperature and nutrient availability this makes C. gariepinus consumed high bulk of zooplankton (Wakil et al., Citation2014). In agreement with the present study, Lakes Langano (Teka & Admassu, Citation2016) and Chamo (Dadebo, Citation2009) conveyed zooplankton as the main food source in the diet of C. gariepinus. However, the food items such as detritus, insects, macrophytes, and fish prey were reported as the main food component from Lakes Koka (Dadebo et al., Citation2014), Hayq (Dereba, Citation2019), Hawassa and Shallo swamp (Tekle-Giorgis et al., Citation2016), Alau Nigeria (Wakil et al., Citation2014), Babogaya (Admassu et al., Citation2015) and Tungan Kawo reservoir Nigeria (Habiba, Citation2021). Fish preys (O. niloticus and Labeobarbus spp.) were the second most important food items in the diet of the fish. The piscivorous feeding behavior of the C. gariepinus was also reported in Lakes Koka (Dadebo et al., Citation2014), Alau Nigeria (Wakil et al., Citation2014), Hayq (Dereba, Citation2019), Langano (Teka & Admassu, Citation2016), Hawassa (Desta et al., Citation2006; Dadebo, Citation2000). However, phytoplankton was reported as the major food type in Egbe reservoir (Adewumi et al., Citation2014). The variability of food and feeding habits of fish may be due to the different prey availability in different waters. The variations in prey availability is likewise linked with nutrient availability and the characteristics of the physicochemical of waters. Moreover, variations in prey availability may be linked to changes in the environment and biological factors of the freshwater, which impact the food type in the diet of this fish (Temesgen et al., Citation2022). Information on food and feeding habits of freshwater fish species is a subject of continuous research. Because it makes up a basis for the development of a successful management program on fish culture and capture (Temesgen et al., Citation2022). Furthermore, studies on the natural feeding habit of fish enable us to identify the trophic relationships present in aquatic ecosystems, identifying feeding composition, structure and stability of food webs (Teka & Admassu, Citation2016). Now a days the natural water bodies of Ethiopia experiencing pollution at alarming rate. This phenomenon makes declining the wild capture fishery. The aquaculture is the alternative option of fish production for this fast-growing population. Therefore, identifying the natural food and feeding habits of this fish species is important for in view of aquaculture applications as well as climate change.
4.2. Food and feeding habits in relation to the size class
In this study, size class variations in the diet C. gariepinus were observed. The smallest size class (<37 cm SL) primarily fed on detritus, zooplankton, and mud. Dadebo et al. (Citation2014) also reported detritus and insects as the main food component for the smallest fish in Lake Koka. Admassu et al. (Citation2015) and Tekle-Giorgis et al. (Citation2016) also reported that the smallest fish consumed more insects and zooplankton whereas, adult fish ingested fish prey in Lakes Babogaya and Hawassa and Shallo swamp respectively. Tesfahun and Temesgen (Citation2018) also reported the dominance of insects and zooplankton in the diet of the smallest fish while the larger fish had more fish prey and zooplankton as well in Ethiopian waters. This might be due to, the smallest fish requiring a high amount of protein to improve their growth rate and metabolism (Temesgen et al., Citation2022). Other researchers also confirmed that C. gariepinus exhibited diet shifts because of the change in habitat use in different water bodies (Dadebo, Citation2000, Citation2009). Dadebo (Citation2000) studied size-based differences in feeding habits of the smallest C. gariepinus fed more on insects. In the present study, larger C. gariepinus with size classes 37.0–42.0 cm SL, 42.5–47.0 cm SL, 47.5–52.0 cm SL and ≥52.5 cm SL primarily fed on zooplankton and fish prey. The importance of fish prey progressively increased, while the contributions of detritus, zooplankton, and mud decreased with fish size. The fish shifted its feeding habit from detritus, zooplankton, and mud to fish prey from Ribb reservoir as the fish length increased. The ontogenetic dietary shift may be due to the productivity of the water and habitat differences of the fish (Tekle-Giorgis et al., Citation2016)). Furthermore, this could be because large-sized C. gariepinus living deeper waters, whereas small ones exist in shallow waters among macrophytes as a refuge where bulks of benthic macroinvertebrates are usually high. In addition, the life history of fish depends on habitat use, and their feeding habits differ significantly (Njiru et al., Citation2004; Temesgen et al., Citation2022; Wagaw et al., Citation2022).
4.3. Seasonal variations in the diet of Clarias gariepinus
There was a seasonal variation in the diet of C. gariepinus from Ribb Reservoir. In the present study, fish prey was by far the most important food item followed by zooplankton in the diet of C. gariepinus during the dry season. Many of the larger fish species consumed small fishes as source of food. This might be due to C. gariepinus is the top predator in the reservoir it feeds on small fishes. The other reason may be the piscivory nature of C. gariepinus associate with water level fluctuation. For instance, when the water level decreases the prey fishes aggregate somewhere this creates good opportunity for the predator fish (Tekle-Giorgis et al., Citation2016). Alebachew et al. (Citation2022) also confirmed that the water level of the Ribb Reservoir decreases in the dry season when the water needed for irrigation purposes. According to Spataru et al. (Citation1987) piscivory is common in fishes with presence of oral teeth, tough stomach and stomach acidity for digesting of large preys. During the dry season, zooplankton was the second most important food item in the diet of C. gariepinus. This might be due to high nutrient availability and good water quality that supports production of zooplankton biomass in the reservoir. During the dry season, the contribution of phytoplankton was less in the diet of C. gariepinus. This may be due to the turbidity that might have cause a low light penetration in the reservoir and reduced photosynthetic rate. The high turbidity of the reservoir may be due to the water abstraction and water release for irrigation purposes (personal observation). The seasonal variation in the diet of the fish may be due to the opportunistic habits of the fish which has the ability of shifting from one food item to another food item. Besides, the seasonal variation of the food and feeding habits of C. gariepinus also may be due to the seasonal production difference of the preys in the reservoir. The food items such as mud, insects, ostracods, sand particles, macrophytes and plant seeds had minor contribution in the diet of the fish. This is may be due to the food and feeding habits of the fish depending on the availability of preys in the reservoir. For instance, the contribution of macrophyte in the diet of the fish was minor, this may be due to the less importance of macrophytes in the diet of C. gariepinus during the dry season in Ribb Reservoir might be due to decrease of nutrients that facilitate the growth of macrophytes. The most important factor that determine the availability and emergence of various food items in Ribb Reservoir could be the seasonal changes in water level.
On the other hand, zooplankton and phytoplankton were the main prey type in the wet season. During the wet season the contribution of zooplankton in the diet of C. gariepinus was high in reservoir. According to Dadebo (Citation2009) high productivity of the water bodies in terms of zooplankton biomass that leads to shift the feeding habits of C. gariepinus to zooplankton filter feeding. In the wet season, the high zooplankton production may be due to high water temperature in the Ribb Reservoir. In addition, some reasons could be mentioned to elaborate this high consumption of zooplankton. It is known that the Ribb Reservoir is tropical, may be eutrophic and have high water temperature that promote high level of phytoplankton, and thus high zooplankton production. As the biomass of zooplankton increases, the fish may use ram feeding by opening its mouth. According to Dadebo (Citation2009) C. gariepinus has the anatomical adaptations for filter feeding, which allows the fish to feed on prey ranging from zooplankton to a fish half its own length. This may be the reason it shifted from piscivorous feeding habit to ram feeding. The other reason may be the increment of water level when the water level increases the prey fishes may be unevenly distributed which makes C. gariepinus may not be able to compete for the available prey fish in such an environment. Phytoplankton was also the second most important food item in the wet season. The high contributions of phytoplankton in the diet of C. gariepinus during the wet season in reservoir might be due to increase of nutrients during the floods that promote the growth of phytoplankton. The remaining food items such as insects, fish, macrophytes and mud had minor contribution in the diet of C. gariepinus in the reservoir.
Many investigators also documented a seasonal variation of food items in the diet composition of C. gariepinus in various water bodies (Adewumi et al., Citation2014; Admassu et al., Citation2015; Dadebo et al., Citation2014; Dereba, Citation2019; Eyayu, Citation(2019); Habiba, Citation2021; Teka & Admassu, Citation2016; Tekle-Giorgis et al., Citation2016; Tesfahun & Temesgen, Citation2018; Wakil et al., Citation2014). However, phytoplankton, mud, insects, ostracods, sand grains, macrophytes, and plant seeds have insignificant contributions to the bulk of the fish in the dry season. Comparable studies were also conducted on Lakes Koka (Dadebo et al., Citation2014) and Hawassa (Tekle-Giorgis et al., Citation2016) fish prey and zooplankton as the main food sources in the dry season. From this study, zooplankton and phytoplankton were important prey types in the wet season. In contrast, detritus, macrophytes, gastropods, and insects were reported as the main consumed food types in Lake Koka (Dadebo et al., Citation2014). Other investigators also found different food items in the wet season from different lakes in Ethiopia. For instance, Teka and Admassu (Citation2016) investigated that fish prey was the most preferred food item from Lake Langano in the wet season. Moreover, in Lake Hawassa (insects and fish eggs) and Shallo swamp (macrophyte and detritus) were the most ingested food items in the wet season (Tekle-Giorgis et al., Citation2016). The most important factors influencing the availability and appearance of different food in Ribb reservoirs may be seasonal changes in water levels. The high contribution of zooplankton and phytoplankton to the diet of C. gariepinus during the humid months of the Ribb Reservoir could be due to increased nutrients during floods promoting phytoplankton.
5. Conclusions
This finding established zooplankton as the main food item, followed by fish prey, phytoplankton, and detritus. The smallest-sized fish mainly fed on detritus followed by zooplankton and mud whereas the adult fish fed majorly on fish prey followed by zooplankton. The prey types such as fish prey followed by zooplankton and phytoplankton were the most favored food types during the dry season. Whereas zooplankton followed by phytoplankton and detritus were the most significant food items in the diet C. gariepinus in the wet season. C. gariepinus revealed omnivorous feeding habits in its diet from the Ribb Reservoir. Generally, the diet composition and feeding habits of fish are affected by habitat differences, seasons, and the size class of fish in the reservoir. Therefore, watershed management is required to improve the food and feeding habits of this fish species for better sustainable use.
Author’s contribution
Agumassie Tesfahun: Conceptualization, Methodology, Software: Agumassie Tesfahun. Data curation, writing-original draft preparation. Agumassie Tesfahun Visualization and Investigation. Sale Alebachew, Supervision.: Agumassie Tesfahun Software, Validation.: Sale Alebachew and Agumassie Tesfahun: Writing- Reviewing and Editing
Availability of data
All the data sets used in this manuscript are accessible in the corresponding author via upon a reasonable claim
Acknowledgments
We thank the Guna Tana Integrated Field Research Development Centre Debre Tabor University for helping financial provision and vehicle support. We also thank the fishermen of Ribb Reservoir for the helping with the sampling of fish specimens.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors

Agumassie Tesfahun
Agumassie Tesfahun received his MSc Degree in Fisheries, Limnology, and Aquatic-eco-toxicology from Hawassa University, Ethiopia. Currently, he is the Assistant Professor at the Department of Biology, Debre Tabor University, Ethiopia. Besides, he has been working in community service and research on fisheries biology of the most commercially important fish species from Ribb Reservoir Tana basin, Ethiopia, and aquaculture establishment (earthen pond system) from Fogera District, South Gondar, Ethiopia.
Sale Alebachew
Sale Alebachew was awarded his MSc Degree in Animal Production from Debre Markos University, Ethiopia. Now, he is a lecturer and researcher at the Department of Animal Sciences, Debre Tabor University, Ethiopia. Moreover, he has been working on fisheries biology of the most commercially important fish species from Ribb Reservoir Tana basin, Ethiopia, and aquaculture establishment (earthen pond system) from Fogera District, South Gondar, Ethiopia.
References
- Abera, L. (2007). Reproduction, food, length-weight relationship, and condition factor of the African catfish Clarias gariepinus (Burchell) in Lake Babogaya, Ethiopia [ MSc. thesis]. Addis Ababa University, pp. 65.
- Adewumi, A. A., Idowu, O. E., & Tolu, B. S. (2014). Food and feeding habits of clarias gariepinus (Burchell 1822) in Egbe reservoir, Ekiti state, Nigeria. Animal Research International, 11(3), 2041–14.
- Adeyemi, S. O., Bankole, N. O., Adikwu, I. A., & Akombu, P. M. (2009). Food and feeding habits of some commercially important fish species in Gbedikere Lake, Bassa, Nigeria. International Journal of Lakes and Rivers, 2(1), 31–36. https://scholar.google.com/citations?view_op=view_citation&hl=en&user=uL2_MQgAAAAJ&citation_for_view=uL2_MQgAAAAJ:WF5omc3nYNoC
- Admassu, D., Abera, L., & Tadesse, Z. (2015). The food and feeding habits of the African catfish, Clarias gariepinus (Burchell), in Lake Babogaya, Ethiopia. Global Journal of Fish Aquaculture, 3(4), 211–220.
- Alebachew, S., Tesfahun, A., & Kebtieneh, N. (2022). Abundance, distribution, and diversity of fishes in Ribb Reservoir, Lake Tana basin, Ethiopia. Cogent Food & Agriculture, 8(1), 2105934. https://doi.org/10.1080/23311932.2022.2105934
- Assis, C. A. (1996). A generalized index for stomach contents analysis in fish. Scientia Marina, 60, 385–389. https://cir.nii.ac.jp/crid/1570291224352920320
- Awoke, T. (2015). Fish species diversity in major river basins of Ethiopia. World Journal of Fish & Marine Sciences, 7(5), 365–374.
- Bezabih, A. W. (2021). Evaluation of small hydropower plant at Ribb irrigation dam in Amhara regional state, Ethiopia. Environmental Systems Research, 10(1), 1. https://doi.org/10.1186/s40068-020-00196-z
- Bhuyan, M. S., Sharif, M. A. S., Islam, M. M., Mojumder, I. A., Das, M., & Islam, S. M. (2020). Fresh/River water zooplankton in Bangladesh: A critical review. Environmental Analysis & Ecology Studies, 7(2), 716–749. https://doi.org/10.31031/EAES.2020.07.000658
- Bowen, S. H. (1983). Quantitative description of the diet. In L. A. Nielsen & D. L. Johnson (Eds.), Fisheries techniques (pp. 325–336). Bethesda.
- Carling, K. J., Ater, I. M., Pellam, M. R., Bouchard, A. M., & Mihuc, T. B. (2004). A guide to the zooplankton of Lake Champlain. Plattsburgh State University of New York, 1, 33–66. https://soar.suny.edu/bitstream/handle/20.500.12648/1251/fulltext.pdf?sequence=1&isAllowed=y
- Dadebo, E. (2000). Reproductive biology and feeding habits of the catfish Clarias gariepinus (Burchell) (Pisces: Clariidae) in lake Awassa, Ethiopia. SINET: Ethiopian Journal of Science, 23(2), 231–246. https://doi.org/10.4314/sinet.v23i2.18168
- Dadebo, E. (2009). Filter-feeding habits of the African catfish Clarias gariepinus Burchell (Pisces: Clariidae) in Lake Chamo, Ethiopia. Journal of Biology Science, 8(1), 15–30.
- Dadebo, E., Aemro, D., & Tekle-Giorgis, Y. (2014). Food and feeding habits of the African catfish Clarias gariepinus (Burchell, 1822) (Pisces: Clariidae) in Lake Koka, Ethiopia. African Journal of Ecology, 52(4), 471–478. https://doi.org/10.1111/aje.12146
- Dereba, A. (2019). Reproductive biology and feeding habits of African catfish Clarias gariepinus (Burchell, 1822) in Lake Hayk, Ethiopia [ A thesis submitted to the school of graduate studies of Hawassa University in partial fulfillment of the requirements for the degree of Master of Science in Biology]. Aquaculture and fishery management, pp. 84.
- Desta, Z., Borgstrøm, R., Rosseland, B. O. & Gebre-Mariam, Z. (2006). Major difference in mercury concentrations of the African big barb, barbus intermedius (R.) due to shifts in trophic position. Ecology of Freshwater Fish, 15.
- Eyayu, A. (2019). Fish biology and fisheries of the floodplain rivers in the Alitash national park, northwestern Ethiopia, [ Ph.D. dissertation]. Addis Ababa University, pp. 222.
- Ezezew, G. (2019). Estimation of Ribb dam catchment sediment yield and reservoir effective life using swat model and empirical methods. MSc. thesis, Bahir Dar University, 79pp.
- Golubtsov, A. S., & Mina, M. V. (2003). Fish species diversity in the main drainage systems of Ethiopia: Current knowledge and research perspectives. Ethiopian Journal of Natural Resources, 5(2), 281–318.
- Habiba, U. (2021). Feeding ecology and nutritional status of some fish species from Tungan Kawo reservoir, Kontagora, Niger state, Nigeria department of animal biology the federal. University of Technology.
- Houlihan, D., Boujard, T., & Jobling, M. (2001). Food intake in fish. Blackwell Science publisher.
- Hurlbert, S. H. (1978). The measurement of niche overlap and some relatives. Ecology, 59(1), 67–77. https://doi.org/10.2307/1936632
- Hyslop, E. J. (1980). Stomach contents analysis—a review of methods and their application. Journal of Fish Biology, 17(4), 411–429. https://doi.org/10.1111/j.1095-8649.1980.tb02775.x
- Kamal, M., Kurt, R., & Michael, L. B. (2010). Tilapia profile and economic importance South Dakota cooperative extension service USDA Doc. AgBio/FS, 963(1), 2–5. 108.
- Mequanent, D., Mingist, M., Getahun, A., and Anteneh, W. (2021). Impact of irrigation practices on Gilgel Abay, Ribb and Gumara fisheries, Tana Sub-Basin, Ethiopia. Heliyon, 7(3), e06523. https://doi.org/10.1016/j.heliyon.2021.e06523
- Mequanent, D., Mingist, M., Getahun, A., Anteneh, W., Getnet, B., & Birie, S. (2022). The investigation of the zooplankton community in the newly formed Ribb Reservoir, Ethiopia: The tropical highland reservoir. Heliyon, 8(9), e10533. https://doi.org/10.1016/j.heliyon.2022.e10533
- Njiru, M., Okeyo-Owuor, J. B., Muchiri, M., & Cowx, I. G. (2004). Shifts in the food of nile tilapia, oreochromis niloticus (L.) in Lake Victoria, Kenya. African Journal of Ecology, 42(3), 163–170. https://doi.org/10.1111/j.1365-2028.2004.00503.x
- Spataru, P., Viveen, W., & Gophen, M. (1987). Food composition of Clarias gariepinus (Clarias lazera) (Cypriniformes, Clariidae) in Lake Kinneret (Israel). Hydrobiologia, 144(1), 77–82. https://doi.org/10.1007/BF00008053
- Teka, L., & Admassu, D. (2016). The food and feeding habits of the African catfish, Clarias gariepinus (Burchell, 1822), in Lake Langeno, Ethiopian rift valley Ethiop. The Journal of Biological Sciences, 15(2), 141–161.
- Tekle-Giorgis, Y., Wagaw, S., & Dadebo, E. (2016). The food and feeding habits of the African catfish, Clarias gariepinus (Burchell, 1822) (Pisces: Clariidae) in Lake Hawassa and Shallo swamp, Ethiopia. Journal of Biology Science, 15(1), 1–18.
- Temesgen, M., Getahun, A., Lemma, B., & Geert, P. J. (2022). Food and feeding biology of Nile tilapia (oreochromis niloticus) in Lake Langeno, Ethiopia. Sustainability, 14(2), 974. https://doi.org/10.3390/su14020974
- Tesfahun, A., & Alebachew, S. (2023). Food and feeding habits of the Nile tilapia Oreochromis niloticus (Linnaeus, 1758) from Ribb reservoir, Lake Tana sub-basin, Ethiopia. Cogent Food & Agriculture, 9(1), 2212457. https://doi.org/10.1080/23311932.2023.2212457
- Tesfahun, A., & Temesgen, M. (2018). Food and feeding habits of Nile tilapia Oreochromis niloticus (L.) in Ethiopian water bodies: A review. International Journal of Fisheries and Aquatic Studies, 6(1), 43–47.
- Tomojiri, D., Musikasinthorn, P., & Iwata, A. (2019). Food habits of three non-native cichlid fishes in the lowermost Chao Phraya River basin, Thailand. Journal of Freshwater Ecology, 34(1), 419–432. https://doi.org/10.1080/02705060.2019.1585392
- Vijayakumari, V., Prasad, G., & Moses, S. A. (2018). Selecting a suitable diversity index for a tropical ramsar wetland site. Lakes & Reservoirs: Science, Policy and Management for Sustainable Use, 23(2), 139–151. https://doi.org/10.1111/lre.12219
- Vuuren, S. J., Taylor, J., Ginkel, C., & Gerber, A. (2006). Easy identification of the most common FRESHWATER ALGAE; a guide for the identification of microscopic algae in South African freshwaters. Department of Water and Forestry, North-West University. https://www.dws.gov.za/iwqs/eutrophication/NEMP/Janse_van_Vuuren_2006_Easy_identification_of_the_most_common_freshwater_alga
- Wagaw, S., Mengistou, S., and Getahun, A. (2022). Diet composition and feeding habits of oreochromis niloticus (Linnaeus, 1758) in Lake Shala, Ethiopia. Fisheries and Aquatic Sciences, 25(1), 20–30. https://doi.org/10.47853/FAS.2022.e3
- Wakil, U., Haruna, A., Mohammed, G., Ndirmbita, W., Yachilla, B., & Kumai, M. (2014). Examinations of the stomach contents of two fish species (C. gariepinus and O. niloticus) in Lake 36 Alau, North-Eastern Nigeria. Agriculture, Forestry and Fisheries, 3(5), 405–409. https://doi.org/10.11648/j.aff.20140305.23
- Willoughby, N. G., & Tweddle, D. (1978). The ecology of the catfish Clarias gariepinus, and Clarias ngamensis in the Shire Valley. Journal of Zoology, 186(4), 507–534. https://doi.org/10.1111/j.1469-7998.1978.tb03936.x
- Yesuf, B. M., Getahun, A., Mengistou, S., Wilson, G., Anteneh, W., & Abera, A. (2023). Time-series ARIMA modelling of the Labeobarbus spp (cyprinidae) fishery in water hyacinth-infested and non-infested sites in Lake Tana, Ethiopia. Journal of Freshwater Ecology, 38(1), 2216218. https://doi.org/10.1080/02705060.2023.2216218