Abstract
Kimchi, a traditional fermented food in Korea, has gained global attention owing to its health benefits. Herein, we determined the fermentation and quality characteristics of radish kimchi containing different sweeteners using physicochemical and sensory evaluations. The characteristics of radish kimchi fermented with sucrose, saccharin, stevioside, or sorbitol were evaluated. Kimchi without the addition of any sweetener was used as a control. The kimchi samples were stored for 7 weeks at 4°C; thereafter, their physicochemical properties and sensory characteristics were measured. Kimchi containing sweeteners was found to have higher acidity and lower pH than the control during fermentation, except for kimchi with saccharin, which had a lower acetic acid content and higher hardness than kimchi with other sweeteners. Based on the results of sensory analysis, the preferences for kimchi with saccharin, sucrose, and sorbitol were similar. These results suggest that different types of sweeteners significantly influence kimchi fermentation and sensory characteristics.
1. Introduction
Kimchi is one of the best-known traditional fermented foods in Korea and is fermented by adding seasonings such as salted seafood, red-pepper powder, garlic, and ginger to salted kimchi cabbage or vegetables (Cheigh et al., Citation1994; Jung et al., Citation2014). It is a storable food that can be consumed during winter when vegetables are not produced. In recent years, it has become a commercialized food that can be consumed anytime owing to the general availability of refrigerated storage facilities. In 2006, Health (Raymond, Citation2013), an American health-related magazine, selected kimchi as one of the top five health foods worldwide, garnering considerable global interest. Kimchi is low in calories, high in vitamins, and has an abundance of functional substances such as dietary fiber, capsaicin, allyl compounds, gingerol, and isothiocyanate (Park et al., Citation2014). Furthermore, it has an intestinal regulatory effect, antibacterial action owing to the presence of lactic acid bacteria, obesity prevention effects owing to capsaicin, and anticancer and atherosclerosis prevention effects owing to the metabolites of lactic acid bacteria, as well as other health benefits (Choi et al., Citation2015; Jang et al., Citation2015). Kkakdugi, commonly known as cubed radish kimchi, is a type of kimchi that uses radish (Raphanus sativus) as its main ingredient. Its production method is simple, and it is highly preferred (Park & Lee, Citation2000), with the second highest consumption rate after cabbage kimchi. During Kkakdugi preparation, sugar may be added to impart a sweet flavor and accelerate fermentation; however, accelerated fermentation results in rapid quality degradation and a soft texture. Thus, some commercial manufacturers of Kkakdugi and restaurants use sodium saccharin instead of sugar (Park & Sohn, Citation2009).
Saccharin, the most economical alternative sweetener, is 300 times (per measure) sweeter than sucrose (Chattopadhyay et al., Citation2014). Moreover, because it is stable even at high temperatures of ≥ 300°C and does not lose its sweetness under conventional processing conditions, it is widely used worldwide in processed foods (Bruno et al., Citation2014; Qurrat-Ul-Ain & Khan, Citation2015). However, negative social perceptions still exist in South Korea owing to the improper use of sodium saccharin by a small number of food manufacturers and erroneous studies in the 1970s and 1980s that reported that it potentially causes bladder cancer (Price et al., Citation1970; Weihrauch & Diehl, Citation2004). Sweeteners are food additives that mimic the effects of sugars on taste buds. Therefore, they are referred to as sugar substitutes. Consumers often choose foods containing low-calorie sweeteners because they want a sweet taste without the addition of calories (Chattopadhyay et al., Citation2014; Ozdemir et al., Citation2015). Although various sugars and sweeteners are added to kimchi in the food industry, research on objective indicators based on the fermentation period, including physicochemical and microbiological studies, is lacking. Additionally, Kkakdugi is considered to be more suitable than cabbage kimchi for such studies because it exhibits a greater effect on the difference in texture depending on the type of sweetener used. Therefore, in the present study, we elucidated the effects of sweeteners on the quality characteristics of radish kimchi during fermentation. We believe that the findings of this study may serve as a basis for further studies on the influence of sweeteners on kimchi and other salted vegetables.
2. Materials and methods
2.1. Sample preparation and storage method
Radishes, leeks, red pepper powder, ginger, garlic, salted shrimp, and salt were purchased from Seobu Market (Maewol-dong, Gwangju, Korea). Sweeteners purchased as additives for radish kimchi were sucrose (CJ, Seoul, Korea), sodium saccharin (JMC, Seoul, Korea), stevioside (Daepyung, Seongnam, Korea), and sorbitol (Rocket Korea, Seoul, Korea). Radishes were washed and the head portion and bottom portion were discarded after removing the fine roots. From the remaining middle portion, excluding the core, the nonedible parts were removed and the rest was cut into cubes of 2 × 2 × 1.5 cm. The samples were salted using a mixture of 20 g of salt per 1 kg of radish via the dry processing method. The mixture ratio of the main ingredients and sub-ingredients was as follows: 91.2% (w/w) salted radish, 2.9% (w/w) red pepper, 2.7% (w/w) green onion, 1.2% (w/w) garlic, 0.5% (w/w) ginger, and 1.5% (w/w) salted shrimp. Radish kimchi was prepared by mixing the salted radish with spices by adjusting the amount of sweeteners added according to their sweetness. The experiment was conducted using four separate sweeteners. The control lot did not have any sweeteners and was labeled as CON. Radish kimchi containing sucrose, saccharin, stevioside, and sorbitol were labeled as SUC, SAC, STE, and SOR, respectively. Sucrose, sodium saccharin, sorbitol, and stevioside have different degrees of sweetness at the same weight. Saccharin is 300 times sweeter, stevioside is 100 times sweeter, and sorbitol is 0.7 times sweeter than sucrose. Therefore, to prepare kimchi with the same sweetness, 0.03% sodium saccharin, 0.01% stevioside, and 1.67% sorbitol were used to obtain the sweetness of 1% sucrose, which was confirmed using an electronic tongue (Astree2, ALPHA M.O.S., Ltd., Toulouse, France). As shown in Table , it was confirmed that the SWS sensor values, which are indicators of sweetness, were similar. Therefore, according to the reference sweetener, the sweetener ratios of the radish kimchi samples (SUC, SAC, SOR, and STE) used in this study were as follows: 0.6% (w/w) sucrose (SUC), 0.002% (w/w) sodium saccharin (SAC), 1% (w/w) sorbitol (SOR), and 0.006% (w/w) stevioside (STE). After their preparation, the kimchi samples were stored and fermented for 49 days at 4°C. The quality characteristics of the kimchi samples were elucidated at 0, 1, 3, 5, and 7 weeks.
Table 1. Sensory values of sweeteners, as detected by the seven sensors of the electronic tongue
2.2. Measurement of pH and titratable acidity
Samples were pureed using a blender. Then, pH measurements were performed by directly placing a pH electrode into these pureed samples. To measure the titratable acidity of the samples, 1 g of each sample was pureed using a blender. This puree was then diluted (approximately 100×) and filtered using the Advantec filter paper No. 1 (Toyo Roshi Kaisha, Ltd., Tokyo, Japan). Twenty milliliters of the filtrate were titrated against a 0.01 N NaOH solution until the pH value reached 8.3. Titratable acidity was determined as the percentage of lactic acid produced.
2.3. Measurement of reducing sugar
The 3,5-dinitrosalicylic acid (DNS) method was used to measure the reducing sugar content of the kimchi samples (Miler, Citation1959). Samples were made into a paste form using a blender. Then, 1 g of the paste was precisely weighed, appropriately diluted (approximately 50×), and filtered through the Advantec filter paper No. 1. Thereafter, 3 mL of the DNS reagent was added to 1 mL of each sample filtrate. The mixtures were thoroughly mixed using a vortex mixer and then heated in a boiling water bath for 5 min. After cooling to 25°C, the sample mixture was diluted with 16 mL of distilled water. The absorbance of the sample mixture was measured at 550 nm using the UV1800 UV—VIS spectrophotometer (Shimadzu, Kyoto, Japan). The reducing sugar content of the samples was calculated using a predetermined glucose standard curve.
2.4. Measurement of hardness
A texture analyzer (TAXT-2; Stable Micro System, Ltd., Surrey, England) was used to measure the texture 10 times; the mean value was derived. The p5 cylinder probe (diameter: 5 mm) was used to determine the maximum intensity received from 100% penetration originating from the center of the cube-shaped kimchi surface (2 × 2 × 1.5 cm). The operating conditions of the texture analyzer were as follows: pretest speed, 5.0 mm/s; test speed, 0.5 mm/s; posttest speed, 10.0 mm/s; and distance, 20 mm. The texture was assessed in terms of force (kg).
2.5. Measurement of free sugar content
Pulverized kimchi samples (~10 g) were added to 25 mL of deionized water and sonicated for 30 min. Then, the samples were diluted to 50 mL in a measuring flask using acetonitrile. The prepared solution was filtered using a 0.45-µm membrane filter and used as the sample solution for measuring free sugar content. Standard products of fructose, glucose, sucrose, and mannitol were purchased from Sigma-Aldrich (St. Louis, MO, USA). Twenty microliters of the test and standard solutions was injected, and the calibration curve was prepared. Using the prepared calibration curves, the free sugar content in the test solution was calculated. Free sugar content was measured using an HPLC system (LC-Net II/ADC, JASCO, Japan) equipped with a refractive index detector. A carbohydrate column (Asahipak NH2P-504E, Shodex, Tokyo, Japan) was used, and the oven temperature was set at 40°C. The mobile phase was 75% acetonitrile and the flow rate was 1 mL/min.
2.6. Analysis of organic acids
One gram of the pulverized kimchi sample was precisely weighed and mixed with 1 mL of HPLC-grade water. This sample mixture was sonicated for 30 min and then diluted to 25 times the extract solution. This solution was filtered through a 0.2-µm membrane filter and used as the sample solution for organic acid analysis. Standard products of acetic acid, citric acid, malic acid, oxalic acid, lactic acid, and fumaric acid were purchased from Sigma-Aldrich. The calibration curve was constructed by deriving the peaks after injecting 20 μL of the test and standard solutions, followed by calculating the organic acid content in the test solution. Organic acid analysis was performed using an HPLC system (LaChromUltra L-2000 U, Hitachi, Tokyo, Japan) equipped with a UV detector. An organic acid analysis column (Bio-rad, Aminex HPX-87 H column, Hercules, CA, USA) was used for separation. The oven temperature was set at 50°C. The mobile phase was 75% acetonitrile, and the flow rate was 0.6 mL/min. Absorbance was measured at a wavelength of 210 nm.
2.7. Analysis of microbial properties
Samples were aseptically collected and ground in a blender. Then, the sample paste was diluted 10-fold using 0.85% NaCl in a sterile filter bag and homogenized in a stomacher (Bagmixer R400, Interscience, Saint Nom, France) for 1 min. To differentially count the number of lactic acid bacteria based on their morphological characteristics, samples were serially diluted and inoculated onto MRS agar (Lactobacilli MRS agar, Difco, Maryland, USA) supplemented with 0.2% bromophenol blue (BPB). Thereafter, the samples were incubated for 72 h at 30°C. Colonies without a ring or showing dark blue coloration were counted as Leuconostoc spp., whereas those with a dark blue ring in the center or having a light blue color throughout were counted as Lactobacillus spp (Lee & Lee, Citation2008).
2.8. Sensory evaluation
Sensory evaluation was conducted when the prepared kimchi was being stored at 4°C. For the sensory test panel, those with high taste acuity at our institute were selected. Twenty trained panel members participated in the study. To prevent the sensory properties of the previous sample from affecting the next sample, panel members were instructed to rinse their mouths with water and a cracker before the sample test. For sensory evaluation, the SensMine program (Sensometrics Co., LTD., Seoul, Korea) was set up in the sensory evaluation room. A 9-point scale was used to indicate the intensity sensed for each characteristic. The assessment items were ripeness flavor for flavor; ripeness taste, sweetness, and carbonation taste for taste; and crispness and softness for texture. Finally, overall preferences were also assessed.
2.9. Statistical analysis
IBM SPSS Statistics 19 (SPSS Inc., Chicago, USA) was used for performing statistical analysis. The mean values and standard deviation were derived. Two-way analysis of variance and Duncan’s multiple range test for post-hoc testing were used. All analyses were performed at a significance level of P < 0.05. XLSTAT Premium v19.4 (Addinsoft, New York, NY, USA) was used to perform correlation analysis between principal component analysis (PCA) and the variables.
3. Results and discussion
3.1. Changes in chemical properties during fermentation
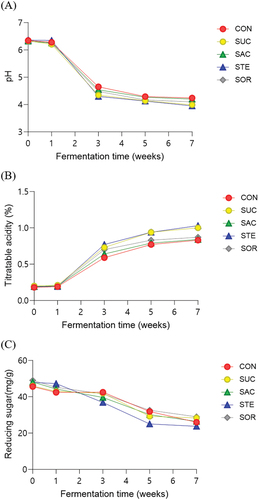
The results of the pH, acidity, and reducing sugar analyses for the five types of radish kimchi (CON, SAC, STE, SOR, and SUC) during fermentation for 49 days at 4°C are shown in Figure . After kimchi preparation, the pH range was 6.32–6.36, which was not very different from the pH at initial storages. Changes in pH and acidity were the most significant during weeks 1–3 of fermentation, with pH decreasing and acidity increasing as the fermentation progressed; this is consistent with the fermentation patterns of other types of kimchi. In general, the optimal pH for the optimum ripening of kimchi is pH 4.2–4.4, and because the pH during weeks 3 and 5 of fermentation showed similar values, this period was determined to be the optimum ripening period. At week 7 of fermentation, STE had a pH of 3.95, which was lower than that of kimchi with other additives, whereas the acidity was high at 1.03%. SAC had a pH of 4.20 and maintained a significantly higher pH and lower acidity than other kimchi with additives during the storage period. Reducing sugars showed a decreasing pattern as fermentation progressed in all kimchi with additives, with a drastic decrease starting in week 3 of fermentation. The reducing sugar content was similar among SAC, CON, and SUC. These findings corroborate the results of a previous study that reported that microbes use reducing sugars in kimchi as a carbon source, which subsequently form organic acids to reduce pH and increase acidity (Kim et al., Citation2009).
Figure 1. Changes in the chemical properties of titratable acidity (a) pH (b) and reducing sugar (c) of radish kimchi with or without sweeteners during fermentation. CON, radish kimchi with no added sweetener; SUC, radish kimchi with sucrose; SAC, radish kimchi with sodium saccharin; STE, radish kimchi with stevioside; and SOR, radish kimchi with sorbitol.
3.2. Changes in texture properties during fermentation
The texture properties of the five types of radish kimchi (CON and SAC, STE, SOR, and SUC) during fermentation are shown in Table . Results of hardness measurements, which indicate kimchi crispiness, showed a significantly higher hardness value in SAC than in kimchi with other additives. Moreover, hardness decreased as fermentation progressed; however, CON and SAC showed significantly higher hardness values than kimchi with other additives. In contrast, SUC and STE showed significantly lower hardness values than CON and SAC. These findings are contrary to the results of an experiment by Park and Sohn (Citation2009), who reported no significant difference in hardness with the addition of sodium saccharin or stevioside to kimchi; additional studies are needed to clarify this inconsistency. The addition of saccharin instead of simple sugars as a carbon source may delay kimchi fermentation, thereby maintaining its hardness value and thus its crispness.
Table 2. Changes in the hardness of radish kimchi with or without sweeteners during fermentation
3.3. Changes in the contents of free sugars and organic acids during fermentation
Tables present the free sugar and organic acid contents in radish kimchi during the fermentation period, respectively. Among the free sugars, fructose, mannitol, glucose, and sucrose contents were analyzed (Table ). Fructose showed a decreasing trend as fermentation progressed in all kimchi types, including CON. Mannitol, a known product of lactic acid bacteria, is produced during kimchi fermentation (Kwon et al., Citation1999). Although mannitol was not detected at weeks 0, 1, and 3 of fermentation, 0.36–1.48% of mannitol was detected after week 5; its content was higher in SUC. Mannitol content in the present study showed a pattern similar to that observed in a previous study (Ku et al., Citation1999). Glucose showed a decreasing trend with fermentation progression in all kimchi with additives; however, an increasing trend was observed from week 7 onwards in all kimchi with additives, except SUC and SAC. Sucrose content was significantly higher in SUC than in SAC, STE, and SOR, which showed a decreasing trend as fermentation progressed, and no sucrose was detected after 5 weeks of fermentation. Mannitol content increased as fermentation time increased and showed an opposite trend compared to free sugars. Mannitol is produced from mannose via enzymatic reaction and from fructose via fermentation (Ha et al., Citation1989). Notably, homofermentative LAB does not produce mannitol, whereas heterofermentative LAB can produce mannitol from fructose and sucrose (Ghoreishi & Gholami Shahrestani, Citation2009). In this study, mannitol was produced in kimchi with added sugar. Among organic acids, oxalic, citric, malic, lactic, fumaric, and acetic acid contents were analyzed throughout the fermentation period (Table ). Oxalic, malic, and fumaric acid content showed an overall decrease, with oxalic acid and fumaric acid showing a gradual decrease up to week 7 of fermentation. Citric acid showed a slight increase at week 1 in all kimchi with additives compared to week 0, but decreased in all types, except CON, from week 3 onwards. In SOR, it was undetectable from week 3 of fermentation. Both citric acid and malic acid were undetectable in the later periods of fermentation. However, lactic acid was detected in all types of kimchi after 5 weeks of fermentation, and its content increased after 7 weeks compared with those after 5 weeks for SAC, STE, and SOR. SUC and CON showed a slightly decreased content after 7 weeks than after 5 weeks. Acetic acid was detected in all kimchi with additives after 5 weeks of fermentation; the acetic acid content was the highest in SUC and STE and significantly lower in SAC than in kimchi with other additives.
Table 3. Changes in the freesugar content of radish kimchi with or without sweeteners during fermentation
Table 4. Changes in the organic acid content of radish kimchi with or without sweeteners during fermentation
Changes in pH and total acid content during kimchi storage are correlated with microbial activity and are associated with flavor, organic acid, and salt content. Furthermore, lactic acid bacteria produce organic acids, decreasing pH and increasing acidity (Park et al., Citation2010). In the present study, the pattern of decreased pH and free sugar content was observed in all types of kimchi during fermentation. Reducing sugars in kimchi are used as a carbon source by microbes, and the subsequent breakdown of lactic acid, alcohol, and CO2 produces various components or metabolites that impart kimchi its unique taste and flavor (Choi et al., Citation2019; Park et al., Citation2019). Furthermore, their content is used as a scale for assessing the degree of aging, growth level of microbes, and flavor changes in kimchi. Subsequently, we observed a decrease in reduced sugar content owing to lactic acid formation and an increase in the acidity of kimchi. The acidity was low during the early periods of fermentation but showed a pattern of gradual increase over time in all kimchi types. In particular, the total organic acid yield was lower in SAC than in kimchi with other additives.
3.4. Multivariate statistical analysis
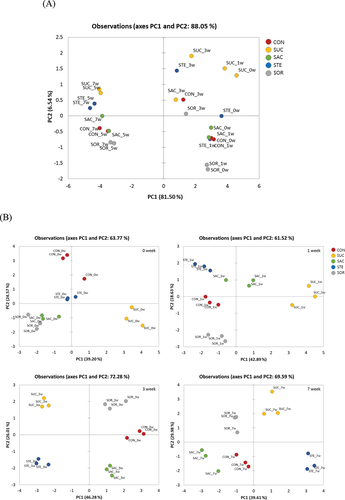
PCA, a popular multivariate statistical analysis technique, is used to simplify the data in multidimensional datasets; interpretations can be derived via graphical visualization (Giuliani, Citation2017). As shown in Figure , sample (dots) movement from right to left in the PCA score plot indicates ongoing metabolic changes during fermentation. From week 0 to 3 of fermentation, sample movement proceeded slowly, and the change in metabolites during this period was slow, consistent with the findings of previous studies on kimchi fermentation (Seo et al., Citation2018; Song et al., Citation2020). To determine the differences in the metabolite profiles of kimchi with different additives, PCA was performed using variables obtained from pH, acidity, reducing sugar, free sugar, and organic acid data at weeks 0, 1, 3, and 7; thereafter, a model was constructed. During the initial stages of fermentation, based on the X-axis in the PCA score plot, SUC clearly showed positive values and STE, SOR, and SAC showed negative values, which were clearly distinguished. However, in the first week of fermentation, SUC and artificial sweeteners could be distinguished based on PCA analysis. Figure demonstrates that the metabolite profiles of kimchi with artificial sweeteners were completely distinguishable from that of SUC in the first week of fermentation. However, during the latter half of the fermentation period, SUC and STE exhibited similar patterns on the X-axis, whereas SAC and CON exhibited similar patterns. Differences in the metabolite profiles of kimchi are attributed to differences in raw materials (Lee et al., Citation2023). In particular, metabolite differences were caused by minor ingredients (radish, glutinous rice paste, and seaweed) rather than by major ingredients. Even if the salt concentration is the same, the type of salt added may affect the metabolite profiles of kimchi (Lee et al., Citation2021). Furthermore, even when the same starch is added, the metabolites of kimchi are affected by amylose content (Park et al., Citation2023). Therefore, the sweetener used has a considerable effect on the metabolite profiles of kimchi. Furthermore, the type of sweetener used has a considerable effect on kimchi quality.
Figure 2. Principal component analysis (PCA) score plots derived from the metabolite data of kimchi samples fermented with or without sweeteners. (a) PCA score plots derived from the metabolite data for each day of fermentation. (b) PCA score plots derived from the metabolite data of kimchi with or without sweeteners at 0, 1, 3, and 7 weeks of fermentation. PC1, principal component 1; PC2, principal component 2; CON, radish kimchi with no added sweetener; SUC, radish kimchi with sucrose; SAC, radish kimchi with sodium saccharin; STE, radish kimchi with stevioside; and SOR, radish kimchi with sorbitol.
3.5. Changes in microbial properties during fermentation
Figure presents the changes in the microbiological characteristics of lactic acid bacteria during the fermentation of SUC, SAC, SOR, STE, and CON. The total count of lactic acid bacteria increased in all kimchi samples as fermentation progressed. However, a decreasing trend was observed in all types, except SAC, in week 7 (data not shown). The color of MRS agar (with BPB) changes at pH values of 3–5; this is useful for detecting pH changes during fermentation with lactic acid bacteria. Lactic acid bacteria produced several colonies, including light blue, small, and flat colonies of Leuconostoc spp.; white colonies with blue centers; and convex colonies of Lactobacillus spp., during fermentation (Lee & Lee, Citation2010) Leuconostoc spp., which proliferate during the early fermentation period, typically drive the fermentation process and are more abundant than other lactic acid bacteria during this period owing to their faster growth rate. Lactobacillus spp., a homofermentative lactic acid bacteria, exhibits strong proliferation rates in the late fermentation periods. Therefore, the fermentation patterns of Leuconostoc spp. and Lactobacillus spp. were compared. Leuconostoc spp. and Lactobacillus spp. exhibited an increased number of colonies after the longest fermentation period. The number of Leuconostoc spp. colonies rapidly increased in SUC during week 1 of fermentation. In week 3, the count was 7.12 log CFU/g, exhibiting a gradually decreasing trend in all kimchi with additives from week 5 onwards. The number of Lactobacillus spp. colonies decreased only in CON and STE in week 7 of fermentation, with kimchi with all other additives showing an increasing trend up to week 7. These findings are somewhat similar to those of studies that reported the lactic acid bacteria count, which has the biggest influence on kimchi fermentation, rapidly increases during the early fermentation period owing to a decrease in pH but gradually decreases thereafter as the acidity increases; on the other hand, as pH decreases, the Leuconostoc strains dominant in the early fermentation period decrease, leading to an increase in the acid-resistant Lactobacillus strains (Cho & Rhee, Citation1991; Lee et al., Citation1992). Moreover, because a high number of lactic acid bacteria was detected in SAC, sodium saccharin may act as a prebiotic for Lactobacillus strains during kimchi fermentation, similar to the findings of Daly et al. (Citation2014).
Figure 3. Changes in the microbiological properties including Leuconostoc species (a) and Lactobacillus species (b) growth in radish kimchi with or without sweeteners during fermentation. CFU, colony-forming unit; CON, radish kimchi with no added sweetener; SUC, radish kimchi with sucrose; SAC, radish kimchi with sodium saccharin; STE, radish kimchi with stevioside; and SOR, radish kimchi with sorbitol.
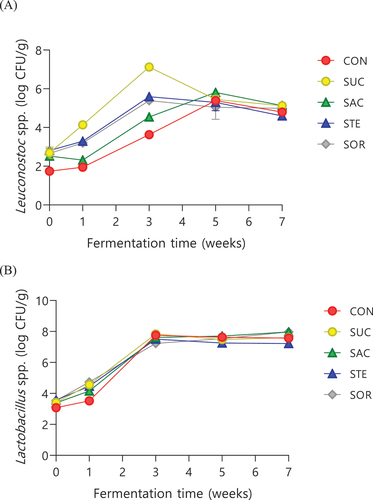
With a decrease in pH and increase in acidity, lactic acid bacteria count in kimchi showed an increasing trend; this result was similar to that reported by Cho et al. (Citation2005) Because the period with maximum Leuconostoc spp. count is considered the optimum ripening period, week 3 of fermentation appeared to be the optimum ripening period, except for CON and SAC, for which, week 5 was considered the optimum ripening period.
3.6. Changes in sensory evaluation during fermentation
Results of the analysis of the sensory qualities of SUC, SAC, SOR, STE, and CON during the fermentation are shown in Table . Regarding flavor characteristics, SUC received a significantly higher score than SAC, SOR, and STE for well-ripe flavor, and the scores for well-ripe flavor increased in all kimchi samples as the fermentation progressed. Regarding ripeness, no significant differences were observed between the kimchi types during the early fermentation period; however, significant increases were observed with increasing fermentation duration, with high values observed in SUC and STE in week 7 of fermentation. The sweetness score of the CON was the lowest, which was significantly different from that of kimchi with other additives. Nevertheless, no significant differences were observed during the fermentation period. The carbonation taste was high in STE, which was attributed to the carbonation taste of stevioside itself; however, it also made the taste something other than kimchi. Among the texture characteristics, SAC had the highest score for the degree of crispness compared with kimchi with any other additive throughout the fermentation period; however, the differences were not statistically significant. The degree of softness was high in SUC and STE, but the difference was not statistically significant. Although SAC did not show statistically significant differences from kimchi with other additives, it received the lowest score. In the comprehensive preference category, SUC received a high score in week 0 of fermentation; however, in week 1, CON and SAC received high scores. In later fermentation periods, SUC, SAC, and SOR received significantly higher comprehensive preference scores. SAC showed excellent assessment results, similar to SUC.
Table 5. Comparison of sensory properties of radish kimchi with or without sweeteners during fermentation
4. Conclusion
In the present study, we elucidated the effects of different sweeteners on the quality characteristics of radish kimchi (Kkakdugi) during fermentation. SUC and STE exhibited lower pH values and higher acidity than kimchi fermented with other sweeteners, whereas SAC showed higher pH and lower acidity. Concerning texture, SAC was harder than SUC, STE, and SOR. The colony count of Leuconostoc spp. rapidly increased in SUC during the early stages of fermentation. Heterofermentation was the best in SUC for producing mannitol, but the kimchi was not sufficiently hard and tended to fall. However, when artificial sweeteners were used, the production of mannitol was less, but the hardness of kimchi was maintained until the end of fermentation. Thus, the advantages and disadvantages of alternative sugars used in kimchi are reported in this study. Our study findings can be used as a basis for establishing an objective indicator for the addition of artificial sweeteners, thereby creating a healthy and reliable consumer culture.
Author contributions
Yun-Jeong Choi: Data curation, Formal analysis, and Writing-original draft; Hae-Won Lee: Formal analysis and Writing-original draft; Ji-Hee Yang: Validation and Methodology; Sung-Hee Park: Conceptualization and Project administration; and Mi-Ai Lee: Conceptualization, Writing-review and editing, and Supervision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Ain, Q., & Khan, S. A. (2015). Artificial sweeteners: safe or unsafe? JPMA: The Journal of the Pakistan Medical Association, 65(2), 225–17. https://pubmed.ncbi.nlm.nih.gov/25842566/
- Bruno, S. N., Cardoso, C. R., Maciel, M. M., Vokac, L., & da Silva Junior, A. I. (2014). Selective identification and quantification of saccharin by liquid chromatography and fluorescence detection. Food Chemistry, 159, 309–315. https://doi.org/10.1016/j.foodchem.2014.03.001
- Chattopadhyay, S., Raychaudhuri, U., & Chakraborty, U. (2014). Artificial sweeteners - a review. Journal of Food Science and Technology, 51(4), 611–621. https://doi.org/10.1007/s13197-011-0571-1
- Cheigh, H. S., Park, K. Y., & Lee, C. Y. (1994). Biochemical, microbiological, and nutritional aspects of kimchi (Korean fermented vegetable products). Critical Reviews in Food Science and Nutrition, 34(2), 175–203. https://doi.org/10.1080/10408399409527656
- Choi, H. J., Lee, N. K., & Paik, H. D. (2015). Health benefits of lactic acid bacteria isolated from kimchi, with respect to immunomodulatory effects. Food Science & Biotechnology, 24(3), 783–789. https://doi.org/10.1007/s10068-015-0102-3
- Choi, Y. J., Yong, S. J., Lee, M. J., Park, S. J., Yun, Y. R., Park, S. H., & Lee, M. A. (2019). Changes in volatiles and non-volatiles compounds of model kimchi through fermentation by lactic acid bacteria. LWT - Food Science & Technology, 105, 118–126. https://doi.org/10.1016/j.lwt.2019.02.001
- Cho, I. Y., Lee, H. R., & Lee, J. M. (2005). The quality changes of less salty kimchi prepared with extract powder of fine root of ginseng and Schinzandra chinensis juice. Journal of the Korean Society of Food Culture, 20, 305–314. http://www.koreascience.or.kr/article/JAKO200504637321357.page
- Cho, Y., & Rhee, H. S. (1991). Effect of lactic acid bacteria and temperature of kimchi fermentation(II). Korean Journal of Food & Cookery Science, 7, 8–16. http://www.koreascience.or.kr/article/JAKO199111921377968.page
- Daly, K., Darby, A. C., Hall, N., Nau, A., Bravo, D., & Shirazi-Beechey, S. P. (2014). Dietary supplementation with lactose or artificial sweetener enhances swine gut Lactobacillus population abundance. The British Journal of Nutrition, Suppl 111(S1), S30–S35. https://doi.org/10.1017/S0007114513002274
- Ghoreishi, S. M., & Gholami Shahrestani, R. (2009). Innovative strategies for engineering mannitol production. Trends in Food Science & Technology, 20(6–7), 263–270. https://doi.org/10.1016/j.tifs.2009.03.006
- Giuliani, A. (2017). The application of principal component analysis to drug discovery and biomedical data. Drug Discovery Today, 22(7), 1069–1076. https://doi.org/10.1016/j.drudis.2017.01.005
- Ha, J. H., Hawer, W. S., Kim, Y. J., & Nam, Y. J. (1989). Changes of free sugars in kimchi during fermentation. Korean Journal of Food Science & Technology, 21, 633–638. http://www.koreascience.or.kr/article/JAKO198903041949189.page
- Jang, J. Y., Lee, M. E., Lee, H. W., Lee, J. H., Park, H. W., Choi, H. J., Pyun, Y. R., & Kim, T. W. (2015). Extending the shelf life of kimchi with Lactococcus lactis strain as a starter culture. Food Science & Biotechnology, 24(3), 1049–1053. https://doi.org/10.1007/s10068-015-0134-8
- Jung, J. Y., Lee, S. H., & Jeon, C. O. (2014). Kimchi microflora: history, current status, and perspectives for industrial kimchi production. Applied Microbiology and Biotechnology, 98(6), 2385–2393. https://doi.org/10.1007/s00253-014-5513-1
- Kim, Y. W., Kung, J. K., Cho, Y. J., Lee, S. J., Kim, S. H., Park, K. Y., & Kang, S. A. (2009). Quality changes in brined Baechu cabbage using different types of polyethylene film, and salt content during storage. Korean Journal of Food Preservation, 16, 605–611. http://www.koreascience.or.kr/article/JAKO200908856869701.page
- Ku, K. H., Cho, J. S., Park, W. S., & Nam, Y. J. (1999). Effects of sorbitol and sugar sources on the fermentation and sensory properties of baechu kimchi. Korean Journal of Food Science & Technology, 31, 794–801. http://www.koreascience.or.kr/article/JAKO199903042096565.page
- Kwon, D. J., Chang, Y. S., Jo, K. S., & Kang, Y. H. (1999). Effects of sugars addition on physiochemical characteristics and sensory evaluation of kimchi. The Korean Journal of Food and Nutrition, 12, 608–614. http://www.koreascience.or.kr/article/JAKO199911920138436.page
- Lee, M. A., Choi, Y. J., Lee, H., Hwang, S., Lee, H. J., Park, S. J., Chung, Y. B., Yun, Y. R., Park, S. H., Min, S., Kwon, L. S., & Seo, H. Y. (2021). Influence of salinity on the microbial community composition and metabolite profile in kimchi. Fermentation, 7(4), 308. https://doi.org/10.3390/fermentation7040308
- Lee, C. W., Ko, C. Y., & Ha, D. M. (1992). Microfloral changes of the lactic acid bacteria during kimchi fermentation and identification of the isolates. Korean Journal of Applied Microbiology and Biotechnology, 20, 102–109. http://www.koreascience.or.kr/article/JAKO199211920012728.page
- Lee, H. M., & Lee, Y. A. (2008). A differential medium for lactic acid-producing bacteria in a mixed culture. Letters in Applied Microbiology, 46(6), 676–681. https://doi.org/10.1111/j.1472-765X.2008.02371.x
- Lee, K. E., & Lee, Y. H. (2010). Effect of Lactobacillus plantarum as a starter on the food quality and microbiota of kimchi. Food Science & Biotechnology, 19(3), 641–646. https://doi.org/10.1007/s10068-010-0090-2
- Lee, D. Y., Park, S. H., Park, S. E., Kim, E. J., Kim, H. W., Seo, S. H., Cho, K. M., Kwon, S. J., Whon, T. W., Min, S. G., Choi, Y. J., Roh, S. W., Seo, H. Y., & Son, H. S. (2023). Comprehensive elucidation of the terroir of Korean kimchi through the study of recipes, metabolites, microbiota, and sensory characteristics. Food Research International, 166, 112614. https://doi.org/10.1016/j.foodres.2023.112614
- Miler, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428. https://doi.org/10.1021/ac60147a030
- Ozdemir, C., Arslaner, A., Ozdemir, S., & Allahyari, M. (2015). The production of ice cream using stevia as a sweetener. Journal of Food Science and Technology, 52(11), 7545–7548. https://doi.org/10.1007/s13197-015-1784-5
- Park, S. E., Cho, K. M., Kwon, S. J., Kim, E. J., Seo, S. H., Jeong, D., Chung, H. J., & Son, H. S. (2023). Effects of the addition of starches with different amylose contents on kimchi microbiota and metabolites. LWT - Food Science & Technology, 175, 114475. https://doi.org/10.1016/j.lwt.2023.114475
- Park, K. Y., Jeong, J. K., Lee, Y. E., & Daily, J. W. (2014). Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. Journal of Medicinal Food, 17(1), 6–20. https://doi.org/10.1089/jmf.2013.3083
- Park, E. S., & Lee, K. H. (2000). The intake, preference, and utilization of kimchi in female high school students. Korean Journal of Community Nutrition, 5, 598–607. http://www.koreascience.or.kr/article/JAKO200011921112798.page
- Park, S. E., Seo, S. H., Kim, E. J., Byun, S., Na, C. S., & Son, H. S. (2019). Changes of microbial community and metabolite in kimchi inoculated with 567 different microbial community starters. Food Chemistry, 274, 558–565. https://doi.org/10.1016/j.foodchem.2018.09.032
- Park, J. M., Shin, J. H., Lee, D. W., Song, J. C., Suh, H. J., Chang, U. J., & Kim, J. M. (2010). Identification of the lactic acid bacteria in kimchi according to initial and over-ripened fermentation using PCR and 16S rRNA gene sequence analysis. Food Science & Biotechnology, 19(2), 541–546. https://doi.org/10.1007/s10068-010-0075-1
- Park, H. O., & Sohn, C. Y. (2009). Effect of sweeteners on the quality properties of kakdugi. The Korean Journal of Food and Nutrition, 22(3), 443–448. http://www.koreascience.or.kr/article/JAKO200903263798008.page
- Price, J. M., Biava, C. G., Oser, B. L., Vogin, E. E., Steinfeld, J., & Ley, H. L. (1970). Bladder tumors in rats fed cyclohexylamine or high doses of a mixture of cyclamate and saccharin. Science (New York, NY), 167(3921), 1131–1132. https://doi.org/10.1126/science.167.3921.1131
- Raymond, J. (2013). World’s Healthiest Foods: Kimchi (Korea). Health. https://www.health.com/condition/digestive-health/worlds-healthiest-foods-kimchi-korea
- Seo, S. H., Park, S. E., Kim, E. J., Lee, K. I., Na, C. S., & Son, H. S. (2018). A GC-MS based metabolomics approach to determine the effect of salinity on kimchi. Food Research International, 105, 492–498. https://doi.org/10.1016/j.foodres.2017.11.069
- Song, H. S., Whon, T. W., Kim, J., Lee, S. H., Kim, J. Y., Kim, Y. B., Choi, H. J., Rhee, J. K., & Roh, S. W. (2020). Microbial niches in raw ingredients determine microbial community assembly during kimchi fermentation. Food Chemistry, 318, 126481. https://doi.org/10.1016/j.foodchem.2020.126481
- Weihrauch, M. R., & Diehl, V. (2004). Artificial sweeteners–do they bear a carcinogenic risk? Annals of Oncology: Official Journal of the European Society for Medical Oncology, 15(10), 1460–1465. https://doi.org/10.1093/annonc/mdh256
