?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Climate-resilient wheat genotype plays a crucial role in food and nutritional security of the world. The production of wheat is mainly limited by abiotic stresses such as heat stress and drought. To identify the most appropriate trait for the selection of high yielding genotypes under heat stress (HS) and heat drought (HD) environment, a field experiment was conducted in the western region of Nepal during the wheat growing season of 2021–22 to 2022–23 comprising 20 wheat genotypes under alpha lattice design. The additive main-effect multiplicative interaction (AMMI) and combined analysis of variance (ANOVA) showed environment had the most substantial effect on the expression of the quantitative traits studied with 24 and 48% yield reduction under HS and HD environments, as compared to irrigated. PCA extracted, days to booting (DTB), days to heading (DTH), days to anthesis (DTA), booting-anthesis duration (BtoA), spikelets per spike (NSPS) under irrigated; DTB, DTH, DTA, 10 spike weight (TSW), thousand kernel weight (TKW) under HS; and DTB, BtoA, spike length (SL), TSW under HD environment. Phenotypic correlation and path analysis revealed, to get high yield, selection can be done with DTB, DTH, DTA, booting-heading duration (BtoH), BtoA, plant height (Ph), spikes per meter square (NSPMS), SL, and TKW. Early booting and heading genotypes with longer spikes, more tillers, grains per spike, and seed weight benefits all environments. Extended booting-heading and booting-anthesis duration with shorter heading-anthesis duration are advantageous as well. Taller genotypes yield more under HS and HD environment whereas shorter genotypes are productive under irrigated.
1. Introduction
Hunger and malnutrition continue to be pressing global challenges, with significant implications for food security and human health. According to the FAO and United Nations, approximately 12.5% (1 billion) of the world’s population suffer from hunger each day, with 6.1% (488 million) suffering from extreme malnutrition, 5.77% (462 million) suffering from underweight problems, and 0.56% (45 million) from wasting. Furthermore, severe micronutrient deficiency affects around 17% (1.36 billion) of the global population (FAOSTAT, Citation2022). UN report shows, around 22% (149 million) of children below age five are stunted and 45% of child death below age five are associated with undernutrition in the world (FAOSTAT, Citation2022; Farcas et al., Citation2021; FAO, Citation2018). The Sustainable Development Goals (SDGs) established by the United Nations aim to address these issues by promoting food security, ending hunger, and improving human nutrition (Ferrari et al., Citation2020; Sachs et al., Citation2022). To achieve these goals, it is crucial to enhance the production and productivity of the most important cereal crop in the world, wheat (Shewry & Hey, Citation2015).
A steady trend of rising air temperatures from year to year leads to more extreme climate events. Drought is a steadily developing climatological phenomenon that covers entire countries, turning into an environmental problem not only of regional significance, but also of global scale. According to (IPCC, Citation2021), in 2020, the average annual temperature change in more than 150 countries was at least 1.0°C higher than the 1951–1980 average. A pronounced trend in temperature and precipitation changes, a decrease in moisture, and an increase in climate aridity invariably entail changes in ecosystems. Climate change will have an extremely adverse impact on agricultural yields, which will require increased adaptability, stability, diversification of crop production and adaptation to new changing requirements of agronomy, population nutrition and climate (Khan et al., Citation2021). Global climate change has recently greatly affected the productivity of many agricultural crops, including the productivity of an important grain crop—wheat worldwide, where it is cultivated on an area of 220 million hectares, with a gross production of 770 million tons (FAOSTAT, Citation2022).
Wheat (Triticum aestivum L.) plays a vital role in global food and nutritional security. Almost 35% of the world’s population consumes wheat as a staple food, and more than two-thirds of global wheat production is used for food, while one-fifth is used for livestock feed. Wheat is a vital source of food as it contains carbohydrates, fats, proteins, fiber, zinc, calcium, vitamin E etc. and contributes approximately 25% of the total calorie intake and 22% of total dietary protein (Bhandari et al., Citation2021; Shewry & Hey, Citation2015). Despite its significance, global wheat production still falls short of meeting the needs of the growing population (Djanaguiraman et al., Citation2020; Kamrani et al., Citation2017). In 2021/22, the global production of wheat reached 770 million metric tons (FAOSTAT, Citation2022), but it remains insufficient to feed the around 889 million people in the world (FAOSTAT, Citation2022). To meet the demand, the production of wheat should be increased by 15% (111.1 million metric tons). Furthermore, the demand is projected to increase by 25% by 2050, while climate-induced factors such as drought and heat stress are predicted to reduce wheat yield by 44–47% in South Asia (Lesk et al., Citation2016; Liu et al., Citation2016). As the leading crop, enhancing wheat production would play a vital role in addressing hunger and malnutrition. However, limited land availability and inadequate irrigation infrastructure hinder yield improvements through expansion. Despite a 14% increase in net cropping area from 1.36 billion ha to 1.5 billion ha, wheat production has had to accommodate a population rise of 200% from 3.5 billion to 7 billion between 1961 and 2011 (Bhanu, Citation2018; FAOSTAT, Citation2022).
The projected temperature increase of 0.92–1.07°C from 2016 to 2045 posed a significant threat to wheat production (WBG, Citation2022). Elevated temperature and low moisture stress induces physiological changes in the cell influencing source-sink transport in wheat (Zandalinas et al., Citation2018). Wheat has specific temperature requirements for growth, anthesis, and ripening, with optimal ranges of 16–22°C, 12–22°C, and 21–25°C, respectively (Akter & Rafiqul Islam, Citation2017a; Djanaguiraman et al., Citation2020). Temperatures exceeding 25°C causes heat stress on wheat, which negatively imparts wheat physiology and the biochemical process leading to poor wheat production (Akter & Rafiqul Islam, Citation2017b; Pan et al., Citation2018). Such temperature fluctuations, along with altered precipitation patterns and increased extreme weather events, had compounded the challenges of global wheat production (Djanaguiraman et al., Citation2020; Kamrani et al., Citation2017; WBG, Citation2022).
To mitigate the impact of climate change on wheat production and to address the challenges of hunger and malnutrition, it is crucial to develop climate-resilient wheat genotypes (Bhandari et al., Citation2021; Chaves et al., Citation2013; Shiferaw et al., Citation2011). Selection of climate-resilient genotypes should not just be focused on yield as yield is influenced by a variety of factors such as environments, genotype, genotype * environment interaction. Hence, climate-resilient genotypes should be selected by considering various yield-attributing parameters (Mahmudul et al., Citation2022). Addressing the challenge of low wheat productivity and increasing demand requires innovative strategies. Traditional methods of expanding agricultural land or improving irrigation infrastructure have limitations, making the breeding of stress-tolerant wheat genotypes a promising avenue for sustainable wheat production (Guzmán et al., Citation2017). The trait-based selection offers a promising approach to achieving this goal. Trait-based selection refers to the identification and selection of wheat genotypes with specific agronomic traits that contribute to higher yield potential and stress tolerance (Mwadzingeni et al., Citation2017; Tshikunde et al., Citation2019). Identification of the traits having direct and indirect effect on grain yield, breeders can develop improved wheat varieties that are better equipped to withstand heat stress and drought environments prevalent in the world (Crespo-Herrera et al., Citation2018; Joudi et al., Citation2014; Lopes et al., Citation2012).
In this study, we aim to evaluate the importance of trait-based selection in improving wheat yield under heat stress and heat drought environment. The findings would help the wheat improvement program of Nepal to develop climate-resilient wheat genotypes, thereby addressing the challenges of low productivity and the increasing demand for wheat. That could potentially help to eradicate the yield gap and combat the hunger and malnutrition (IPCC, Citation2021; Mwadzingeni et al., Citation2017; Tshikunde et al., Citation2019). Ultimately, our research aims to support the varietal improvement program in Nepal and contribute to global efforts in achieving food security and reducing hunger and malnutrition (IPCC, Citation2021; Mwadzingeni et al., Citation2017; Tshikunde et al., Citation2019).
2. Materials and methods
The field experiment was conducted in the agronomy farm of Institute of Agriculture and Animal Science (IAAS), Paklihawa Campus situated in the western region of Nepal at Bhairahawa, Rupandehi. The experiment comprises three wheat growing environments, irrigated (I), heat stress (HS), and heat drought (HD) laid during the wheat growing seasons of 2021–22 and 2022–23. The site of the experiment lies in the tropical Terai region of Nepal at the geographic location of 27°29’02“N and 83°27’17” E and an altitude of 104 meters above sea level. The experiment was conducted in loamy textured soil with pH 5.9 with sand, silt, and clay percentage of 31.3%, 48%, and 20.7%, respectively. The total Nitrogen (N), phosphorus (P2O5), and potassium (K2O) content present in the soil was 0.07%, 13.53 kg ha−1, and 160.8 kg ha−1 whereas total organic matter, Boron (B), Sulphur (S), and Zinc (Zn) content were 2.13%, 0.18% ppm, 1.51 ppm, and 0.88 ppm, respectively.
Twenty elite wheat lines were used in the experiment as treatments 15 Nepal lines, three Bhairahawa lines, and two commercial checks, i.e., Bhrikuti and Gautam. The list of genetic materials used in the experiment is presented in (Table ).
Table 1. Plant materials used in the experiment
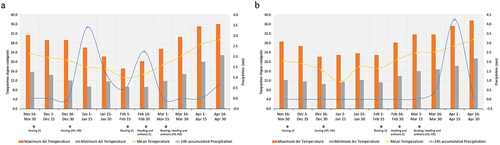
The field experiment was conducted using a serpentine alpha lattice design having two replications and five blocks for all tested environments. Each genotype was planted in a 10 m2 plot with a plot dimension of 4m *2.5 m. The gap between blocks and replications was maintained at one meter. A row-to-row spacing of 25 cm was provided for a genotype in a plot whereas continuous sowing of plant to plant was done in an east-west direction. The seed rate was kept at 120 kg ha −1 and the fertilizer dose was provided at the rate of 100:60:40 NPK kg ha−1. Fertilizers were applied by line placement method. Standard agronomic practices were conducted for wheat. Weeding was done manually two times at the jointing and booting stage. A full dose of irrigation was provided for irrigated and heat stress environment in six splits during pre-sowing, crown root initiation stage (CRI), jointing, booting, heading, and at the soft dough stage whereas, no artificial irrigation was provided for heat drought environment. The sowing time was maintained according to the environment. Sowing was done on 25th November for irrigated environments, and 25th December for heat stress and heat drought environment. The sowing of wheat under stress environments was late to coincide the reproductive and ripening stage of wheat with the terminal heat wave of March–April (Figure ). Along with that, the temperature of the field during the reproductive and ripening stage of wheat sown under HS and HD environment was above 24 °C which created heat stress on wheat (Figure ).
Figure 1. Maximum, minimum, and mean temperature along with 24h accumulated precipitation during the wheat growing season of 2021–22 (1a) and 2022–23 (1b).
A sampling of 10 random samples was done on each plot except from the border line. The phenological data of days to booting (DTB), days to heading (DTH), and days to anthesis (DTA) were taken when 50% of the population achieved their respective stage. Inter-phenological duration such as booting to heading duration (BtoH), booting to anthesis duration (BtoA), and heading to anthesis duration (HtoA) were collected. Plant height (Ph) was measured from the bottom to the top of the spike. Spike length (SL) was measured from the base of the spike to the top of the uppermost floret. The number of spikes per meter square, spikelets per spike (SPS), and grains per spike (GPS) were measured by counting them manually whereas 10 spike weights (TSW) and thousand kernel weights (TKW) were determined by weighing 10 spikes and 1000 grains on a weighing balance. The grain yield was determined by harvesting two quadrants of 1 m2 plots, averaged, and converted to tons per hectare.
Characterization of the environments was done with daily maximum, minimum temperature and mean precipitation. The weather report of the experimental site was obtained from the Department of Hydrology and Meteorology (DTM), Bhairahawa (Figure ).
The study employed additive main effects and multiplicative interaction (AMMI), combined analysis of variance (ANOVA), correlation, and path analysis to identify the most appropriate trait for abiotic stress tolerance in wheat.
The AMMI model ANOVA was conducted to assess the genotype-by-environment interactions. The AMMI model is represented by the formula.
(Purchase et al., Citation2000)
where Yij is the observed value of the ith genotype in the jth environment, μ is the overall mean, gi is the ith genotype effect, ej is the jth environment effect, λk is the singular value of the kth principal component, tki is the score of the ith genotype on the kth principal component, and εij is the residual error.
A combined ANOVA was performed to quantify the effect of the tested wheat growing environment on the quantitative traits of wheat.
Correlation analysis was conducted to assess the degree of association between various quantitative traits and grain yield under tested environments. The Pearson correlation coefficient (r) was calculated using the formula,
where Xi and Yi are the values of variables X and Y, respectively.
Path analysis was employed to determine the direct and indirect effects of different quantitative traits on grain yield. Principal Component Analysis (PCA) was conducted to identify the principal components and assess the contribution of different variables to the overall variability in the dataset. This involved calculating the eigenvalues and eigenvectors of the correlation matrix
Data entry and descriptive statistics were carried out in Microsoft Excel 2021. The combined analysis of variance (ANOVA), Pearson’s correlation coefficients, and principal component analysis (PCA) were conducted on IBM SPSS statistics V. 26. Additive main effect multiplicative interaction (AMMI), ANOVA of AMMI model and genotype (G) and genotype*environment (G*E) interaction was done from GEA-R Version 4.0 software provided by CIMMYT, Mexico, and the correlation plots were created using R-4.3.1.
3. Results and discussion
3.1. Effect of environment, genotype, and G*E interaction
The additive main effects and multiplicative interaction (AMMI) model analysis of variance (ANOVA) showed that the environment had a significant effect on the performance of all quantitative traits studied (p ≤ 0.05) (Table ) i.e., DTB, DTH, DTA, BtoH, BtoA, HtoA, Ph, SL, NSPMS, NSPS, NGPS, TSW, TKW, and GY. The variation in DTB, DTH, DTA, BtoH, BtoA, Ph, SL, NSPM, NSPS, NGPS, TSW, and GY was mainly explained by environment whereas the majority of the variation on TKW and HtoA was mainly explained by genotype and genotype *environment interaction, respectively (Table ). The environmental percentage variation explained on different traits evaluated in the experiment ranged from 31.99% for TKW to 94.29% for DTA. Whereas, the genetic percentage variation ranged from 3.2% for GY to 36.19% for TKW and the genetic*environmental percentage variation explained ranged from 2.18% for DTA to 31.85% for TKW (Table ). The result implies environment (E), genotype (G), and G* E interaction have a substantial heterogenic effect across the traits evaluated. The result showed that DTB, DTH, DTA, BtoH, BtoA, Ph, SL, NSPM, NSPS, NGPS, TSW, and GY are mainly affected by environments, TKW by genetic variations among genotypes, and HtoA by genetic and environmental interactions. Hence, a trait performing well in one environment might not perform well in another environment. Understanding the effect of different wheat growing environments on quantitative traits is necessary for the trait-based selection of high-yielding stable genotypes. Along with the environmental effect, understanding genetic variation is necessary for effective breeding. The significant genotype explained percentage variation on different quantitative traits implies that there are considerable genetic variations across the genotypes evaluated (df = 19) for all traits except grain yield (Table ). The genetic materials with high genetic variation provide a better chance of getting desirable traits that can be effective in heterosis breeding.
Table 2. Additive main effects and multiplicative interaction (AMMI) model analysis of variance (ANOVA) of quantitative traits studied
3.2. Combined Analysis of Variance (ANOVA)
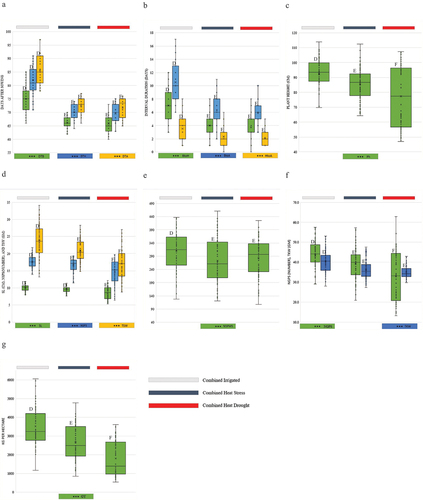
The combined analysis of variance (ANOVA) revealed that there was a significant reduction in the expression of yield and yield-attributing parameters of bread wheat under combined HS and combined HD environments as compared to combined irrigated environment (p ≤ 0.01). DTB, DTH, DTA, BtoH, BtoA, Ph, SL, NSPMS, NSPS, NGPS, TSW, TKW, and GY was reduced by (11.8, 12.3%), (14.5, 15.2%), (15.3, 16.2%), (42.8, 46.9%), (40, 44.4%), (34.3, 39.2%), (7.5, 16.3%), (4, 16.1%), (9.5, 23.7%), (12.3, 28.6%), (10.5, 13.1%), and (24.4, 48.7%) under combined HS and combined HD environments, respectively, as compared to the combined irrigated environment (Table ). HD was the least suitable environment for the growth and yield performance of wheat.
Table 3. Combined analysis of variance (ANOVA) showing percentage reduction in yield and yield attributing parameters of bread wheat under HS and HD environments
The performance of wheat under combined heat stress and heat drought environment was poor as compared to irrigated environments. The poor performance of wheat under stress conditions were brought by a variety of factors. Moisture and heat stress inhibits the process of germination, growth, maturity, grain formation and yield (Akter & Rafiqul Islam, Citation2017a; Asseng et al., Citation2015; Mukherjee et al., Citation2019; Pask et al., Citation2014; Qaseem et al., Citation2019; Whittal et al., Citation2018). A drought environment retards the imbibition process of wheat seeds resulting in poor germination whereas heat stress induces tiller abortion and tiller mortality in developing tillers due to which the NSPMS under heat stress and heat drought environment are poor (Table ). The optimum temperature for growth, spike initiation, booting, anthesis, and ripening of wheat is 16–20 °C 16–28 °C, 16–22 °C,12–22°C, and 21–25°C, respectively (Asseng et al., Citation2015; Mukherjee et al., Citation2019; Pask et al., Citation2014; Tack et al., Citation2015). The temperature of the field at the booting, heading, and anthesis stage were below 24°C under irrigated environment whereas it was above 24°C under heat stress and heat drought environment (Figure ). Temperature above 18 °C during spike initiation, 22°C during booting-heading, and 24 °C during the anthesis-grain filling period is considered heat stress in wheat (Batool et al., Citation2019; Khan et al., Citation2020; Mathur et al., Citation2014). Temperature above optimum during growth accelerates the production of reactive oxygen species (ROS) (Lal et al., Citation2022; Posch et al., Citation2019). The biochemical changes due to high temperature induce the production of ethylene and abscisic acid (ABA) as well. Ethylene, ABA along with ROS induces senescence-related metabolic reaction (SRMR) causing oxidative damage to wheat (Christopher et al., Citation2018; Shirdelmoghanloo et al., Citation2016). As a result, the vegetative growth of wheat is accelerated, and wheat reaches to booting, heading, anthesis, ripening, and maturity stage earlier when sown under heat stress and heat drought environments (Akter & Rafiqul Islam, Citation2017a; Khan et al., Citation2020). Accelerated growth of wheat creates a shortage of time for tiller elongation, spike formation inside the stems, and photosynthate accumulation and as a consequence spike length (SL), numbers of spikelet per spike (NSPS), and spike weight (SW) reduces (Table ). Temperature above 24 °C, during anthesis causes pollen abortion (Joshi et al., Citation2016), pollen mortality (Dwivedi et al., Citation2017a), and induces inefficient pollination on wheat as a result the number of fertile embryos decreases (Oyewole, Citation2016). The result is seen in NGPS with (9.46%, and 23.75%) reduction under heat stress and heat drought environment (Table ). Due to a shorter graining filling period, and accelerated temperature, the seed gets shrunken, resulting in lower seed weight and lower 10 spike weight (Ni et al., Citation2017; Prasad & Djanaguiraman, Citation2014). The effect of heat stress and heat drought environments on seed weight was observed with (10.52%, and 13.14%) reduction in thousand-kernel weight (TKW) of wheat seeds under heat stress and heat drought environments, respectively (Table ). The effect of wheat growing environments on yield attributing parameters is cumulated to grain yield (Figure ) The yield of wheat is reduced by 25.37% and 48.72% under heat stress and heat drought environments, respectively (Table ). Heat drought causes more severe damage on wheat growth and yield as the addition of moisture stress generally imbalances the cell water potential, the accent of sap, and the source-sink transport processes of wheat leading poor seed growth and yield (Shirdelmoghanloo et al., Citation2016).
Figure 2. Boxplot showing mean performance and the impact of heat stress and heat drought environment on days to booting (DTB), days to heading (DTH) and days to anthesis (DTA) (2a); booting to heading duration BtoH), booting to anthesis duration (BtoA), heading to anthesis duration (BtoA) (2b); plant height (Ph) (2c); spike length (SL); number of spikes per spikelets (NSPS), 10 spike weight (TSW) (2d); number of spikes per meter square (2e); number grains per spike, thousand kernel weight (TKW) (2f); and grain yield (GY)(2g).
The grain yield of wheat ranged from 4.61 t ha−1 (BL 4919) to 3.09 t ha−1 (NL 1179) under combined irrigated environment whereas from 3.04 t ha−1 (BL 4919) to 1.8 t ha−1 (NL 1387) under combined heat stress environment. Similarly, the wheat yield ranged from 2.23 t ha−1 (Bhrikuti) to 1.52 t ha−1 (NL 1179) under heat drought environment. The yield loss of wheat ranged from 6.2% (NL 1368) to 47.4% (NL 1387) under combined heat stress environments as compared to combined irrigated environment. Similarly, the yield loss ranged from 32% (Bhrikuti) to 56.3% (BL 4919) under combined heat drought environments as compared to combined irrigated environment (Table .
Table 4. Yield performance, percentage yield reduction, coefficient of variation (CV), and F-Value (df = 5) of elite wheat genotypes (df = 19) across tested environments (df = 5)
The coefficient of variation (CV) ranged from 29.17 for NL 1420 to 52.79 for BL 4919. Variation in CV signifies, the environment had a variable effect on the yield performance of wheat genotypes and there is substantial heterogeneity among the genotypes evaluated (Table ).
3.3. Correlation and path coefficient analysis
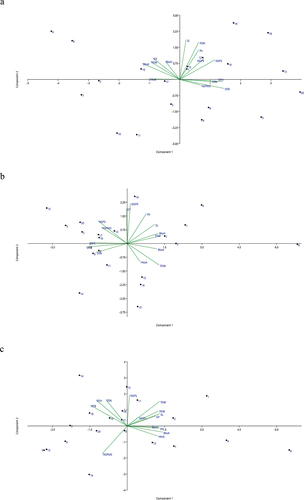
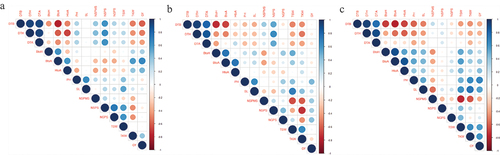
The grain yield of wheat was found to have a significant positive correlation with BtoA and HtoA duration and a significant negative correlation with DTB and DTH under irrigated environments. Under HS environments, GY had a significant positive correlation with Ph, and no significant negative correlation was observed with other attributes studied. Whereas under HD environments, GY had a significant positive correlation with Ph, SL, TSW, and TKW, and no significant negative correlation was observed. SL and NGPS had a positive association with grain yield under all wheat growing environments whereas, DTB, DTH, and DTA had a negative association in all tested environments (Figure ).
Figure 3. Combined phenotypic correlation across 14 quantitative traits of wheat genotypes under irrigated (3a), heat stress (3b), and heat drought (3c) environment.
Path analysis revealed that DTA, BtoA, SL NSPMS, NGPS, and TKW had a direct positive effect and DTB, DTH, BtoA, HtoA, Ph, NSPS, TSW had a direct negative effect on grain yield of wheat under irrigated environment. Under heat stress environment, DTB, DTA, BtoH, Ph, NSPMS, NSPS, NGPS, TSW, and TKW had a direct positive contribution to yield and DTH, BtoA, HtoA, and SL had a direct negative effect on grain yield of wheat. Under heat drought environment, DTH, BtoA, Ph, SL, NSPMS, NGPS, TSW, and TKW had a direct positive effect on the grain yield of wheat, and DTB, DTA, BtoH, HtoA had a direct negative effect on grain yield of wheat. NSPMS, NGPS, and TKW had a direct positive effect on yield under all environments whereas, HtoA duration had a direct negative in all environmental environments (Table ).
Table 5. Combined path analysis among 14 quantitative traits studied under irrigated, heat stress, and heat drought environment under two years
Selection of genotypes that are earlier in booting, heading, and anthesis with longer spike length, NGPS, higher tillering capacity, and TKW would be beneficial for all environments. Whereas, the selection of genotype with longer booting to heading duration and booting to anthesis duration with shorter heading to anthesis duration would be beneficial across all tested environments. The taller genotype would give a higher yield under heat stress and heat drought environments whereas, the shorter genotype would be productive under irrigated environments. Late sowing has been a major reasons for terminal heat stress in wheat in south Asian countries including Nepal (Poudel et al., Citation2017; Shrestha et al., Citation2020). Generally, wheat sowing starts from 25th November but due to climatic, geographic, input-related, and socioeconomic constraints, it is extended up to 25th December in Nepalese context (Dhakal et al., Citation2020; Puri et al., Citation2020). The temperature of the field during booting, heading, and anthesis during early sowing stages (25th November) is generally below 24 °C whereas, the temperature exceeds 24 °C during the booting, heading, and anthesis under late sown conditions (December 25th) under HS and HD environment. (Figure ). This signifies the influence of heat stress on wheat due to late sowing. Since, wheat sowing in Nepal is closely associated with rice-wheat cropping pattern, the sowing time of wheat would get influenced by the growth cycle of rice varieties as well. Hence, it becomes crucial for breeding early maturing wheat cultivars (Asseng et al., Citation2015; Krupnik et al., Citation2021; Peña-Bautista et al., Citation2017). Hence, a genotype with earlier booting, heading and anthesis could significantly contribute to produce more under stress environments. Earliness in wheat is an avoidance mechanism against terminal heat stress (Mondal et al., Citation2013). The phenomena have been considered a key factor for stress tolerance and had extensively been used in climate-resilient wheat improvement programs (Chen et al., Citation2016a, Citation2016b; Gomez et al., Citation2014; Mondal et al., Citation2013, Citation2016; Pandey et al., Citation2015; Puri & Gautam, Citation2015; Yu et al., Citation2017).
Besides, earliness, plant breeders shall focus on attributes such as NSPMS, SL, NGPS, and TKW to produce more (Poudel et al., Citation2020; Puri et al., Citation2020). Genotypes with high tillering capacity are often associated with higher yield in wheat (Chavan et al., Citation2019; Dias de Oliveira et al., Citation2015). Wheat has a tillering ability to produce up to 500 tillers per meter square which can reduce up to 8–15% under HS and HD environments (Pandey et al., Citation2021; Tiwari et al., Citation2019). System of wheat intensification (SWI) also focuses on improving yield by improving the tillering ability of wheat (Rana et al., Citation2017). Since, under heat stress and heat drought environments, tiller suffers from tiller mortality and tiller abortion at rapid tiller growing stages, genotypes with a higher tillering ability and higher tiller survival rate could help to produce more under stress environments (Dwivedi et al., Citation2017b). Hence, heat stress and heat drought breeding shall focus on identification and selection of wheat genotypes having higher tillering capacity. Since, HS and HD environment severely affects SL (Zhang et al., Citation2020), GPS (Zhang et al., Citation2017), and TKW, it is crucial to consider these attributes as well. High wheat yields are often associated with longer spike lengths in wheat, increment in the spike length increases net spikelet per spike which upon successful pollination could produce higher number of grains per spike (Barber et al., Citation2015; Bheemanahalli et al., Citation2019; Mirosavljević et al., Citation2021). Spike itself performs photosynthesis and contributes around 9.8% − 39% to the yield of wheat (Zhang et al., Citation2020). Higher GPS on wheat causes competition among developing grains (Ullah et al., Citation2022) hence, selection along with TKW would enhance the success of wheat breeding (Mondal et al., Citation2013). reported, a genotype producing TKW of 34.5 under heat stress environment could produce a yield of 5 tons per hectare under irrigated environment.
Plant height has a crucial role in yield improvement program of wheat (Chairi et al., Citation2018; Chen et al., Citation2016a; Du et al., Citation2018). Plant height is associated with biomass accumulation, photosynthesis, and canopy temperature maintenance. Under irrigated environment, dwarf genotype resists lodging, which reduces yield by up to 40% (Berry & Spink, Citation2012; Berry et al., Citation2015; Chen et al., Citation2016a). Shorter height also improves source-sink transport by facilitating proper nutrition for developing grains. Hence, there is continuous work being done to incorporate dwarfish genes Rht1 (Rht-B1b), Rht2 (Rht-D1b), Rht1-D1c, and Rht8 to produce more under-irrigated environments (Chairi et al., Citation2018; Divashuk et al., Citation2013; Grover et al., Citation2018; Joudi et al., Citation2014; Lopes et al., Citation2013; S Kumar et al., Citation2013; Zhang et al., Citation2016). In contrast temperature plays a critical role under heat stress and heat drought environment. Maintaining a cooler canopy temperature is necessary to facilitate proper photosynthesis and adequate growth of grains. Taller plants have larger canopy coverage that reduces net canopy temperature as compared to dwarf genotypes. Larger biomass also increases net leaf area for photosynthesis thus producing more photosynthates to the grains (Acuña-Galindo et al., Citation2015; Balota et al., Citation2017; Cossani & Reynolds, Citation2015; Gao et al., Citation2017; Sharma et al., Citation2015). Hence, dwarf genotypes are promoted under irrigated environments whereas taller genotypes are promoted under heat stress and heat drought environments.
3.4. Principal Component Analysis (PCA)
Principal Component Analysis extracted five components for irrigated and heat stress environments whereas four components for heat drought environments. The first five principal components described 86.02%, and 79.84% of the total variation in yield under irrigated and heat-stress environments, respectively. Whereas the first four PC described 80.91% of the total variation in grain yield under heat drought conditions.
The majority of the variation on the data yield was governed by PC1 and PC2 under all tested environments. PC1 and PC2 explained a cumulative of 57.44%, 56.66%, and 61.95% of the total variation under irrigated, heat stress, and heat drought environment, respectively. Under Irrigated and heat stress environment, PC1 and PC2 showed negative and positive correlation with GY whereas PC1 and PC2 showed positive correlation with GY under HD environment (Table ). Hence, a genotype with lower PC1 score and higher PC2 score would yield higher under irrigated and heat-stress environment. Whereas, a genotype with higher PC1 and PC2 score would yield more under heat-drought environment. Based on PCA biplot BL 4919 and NL 1350 were the highest yielding wheat genotype under irrigated environment. Whereas, NL 1384 and Bhrikuti were the most suitable wheat genotype under heat-stress and heat-drought environments.
Table 6. Correlation among PC1-PC5 with yield and yield attributing parameters of bread wheat under irrigated, heat stress and heat drought environment
Based on the ranking of correlation of PC1 with morphological traits studied DTB, DTH, DTA, BtoH, and NSPS were extracted under irrigated environment whereas, DTB, DTH, TSW, TKW, and DTA under heat stress environment and DTB, BtoA, SL, and TSW under heat drought environment (Table ).
PCA analysis suggested, trait-based selection should be focused on phenological stages especially, DTB, DTH, and DTA, besides that, inter-phenological interval also creates variation in yield of wheat. Along with that, selection based on spike-related parameters such as NSPS under irrigated, TSW under heat stress, and SL, TSW under heat drought environment can be considered (Figure ).
4. Conclusion
Production of wheat in world is mainly limited by abiotic stresses such as heat stress and drought. The majority of wheat is grown in winter season in South Asian region where it suffers from terminal heat stress during the reproductive stage. Furthermore, uneven rainfall patterns and poor infrastructure development had also added drought stress to wheat. Hence, it is crucial to develop climate-resilient wheat genotypes for the food and nutritional security of the world. To identify the most appropriate for the selection of high-yielding wheat genotype, a field experiment was conducted in the wheat growing season of 2021–22 to 2022–23. The AMMI model ANOVA showed, the environment had the most substantial effect on the expression of the quantitative traits of wheat genotypes. The grain yield of wheat was reduced by an average of 24 and 48% under the HS and HD environment, respectively. To get a high-yielding genotype, selection should be done with DTB, DTH, BtoH, BtoA, Ph, NSPMS, SL, NGPS and TKW. Genotypes that are earlier in booting, heading, and anthesis with longer spike length, NGPS, higher tillering capacity, and TKW would be beneficial for all environments. Along with that, the genotype with longer booting to heading duration and booting to anthesis duration with shorter heading to anthesis duration would be beneficial across all tested environments. The taller genotype would give a higher yield under heat stress and heat drought environments whereas, the shorter genotype would be productive under irrigated environments.
Conflicts of interest
The authors declare they have no conflicts of interest.
Authors contribution
Radha Krishna Bhandari and Mukti Ram Poudel conceptualized the research. Radha Krishna Bhandari and Shivalal Nyaupane conducted the first year field experiment (2021-22) while Radha Krishna Bhandari conducted the second year field experiment (2022-23). Data collection, data analysis, visualization, manuscript preparation, review and editing were done by Radha Krishna Bhandari. The research was supervised by Mukti Ram Poudel. The final version of the manuscript has been approved by all the authors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Acuña-Galindo, M. A., Mason, R. E., Subramanian, N. K., & Hays, D. B. (2015). Meta-analysis of wheat QTL regions associated with adaptation to drought and heat stress. Crop Science, 55(2), 477–21. https://doi.org/10.2135/CROPSCI2013.11.0793
- Akter, N., & Rafiqul Islam, M. (2017a). Heat stress effects and management in wheat. A review. Agronomy for Sustainable Development, 37(5). https://doi.org/10.1007/s13593-017-0443-9
- Akter, N., & Rafiqul Islam, M. (2017b). Heat stress effects and management in wheat. A review. Agronomy for Sustainable Development, 37(5). https://doi.org/10.1007/S13593-017-0443-9
- Asseng, S., Ewert, F., Martre, P., Rötter, R. P., Lobell, D. B., Cammarano, D., Kimball, B. A., Ottman, M. J., Wall, G. W., White, J. W., Reynolds, M. P., Alderman, P. D., Prasad, P. V. V., Aggarwal, P. K., Anothai, J., Basso, B., Biernath, C., Challinor, A. J. … Zhu, Y. (2015). Rising temperatures reduce global wheat production. Nature Climate Change, 5(2), 143–147. https://doi.org/10.1038/NCLIMATE2470
- Balota, M., Green, A. J., Griffey, C. A., Pitman, R., & Thomason, W. (2017). Genetic gains for physiological traits associated with yield in soft red winter wheat in the Eastern United States from 1919 to 2009. The European Journal of Agronomy, 84, 76–83. https://doi.org/10.1016/J.EJA.2016.11.008
- Barber, H. M., Carney, J., Alghabari, F., & Gooding, M. J. (2015). Decimal growth stages for precision wheat production in changing environments? The Annals of Applied Biology, 166(3), 355–371. https://doi.org/10.1111/AAB.12207
- Batool, A., Akram, N. A., Cheng, Z. G., Lv, G. C., Ashraf, M., Afzal, M., Xiong, J. L., Wang, J. Y., & Xiong, Y. C. (2019). Physiological and biochemical responses of two spring wheat genotypes to non-hydraulic root-to-shoot signalling of partial and full root-zone drought stress. Plant Physiology & Biochemistry: PPB / Societe Francaise de Physiologie Vegetale, 139, 11–20. https://doi.org/10.1016/J.PLAPHY.2019.03.001
- Berry, P. M., Kendall, S., Rutterford, Z., Orford, S., & Griffiths, S. (2015). Historical analysis of the effects of breeding on the height of winter wheat (triticum aestivum) and consequences for lodging. Euphytica, 203(2), 375–383. https://doi.org/10.1007/S10681-014-1286-Y
- Berry, P. M., & Spink, J. (2012). Predicting yield losses caused by lodging in wheat. Field Crops Research, 137, 19–26. https://doi.org/10.1016/J.FCR.2012.07.019
- Bhandari, R., Gnawali, S., Nyaupane, S., Kharel, S., Poudel, M., & Panth, P. (2021). Effect of drought & irrigated environmental condition on yield & yield attributing characteristic of bread wheat-a review. Reviews in Food and Agriculture, 2(2), 59–62. https://doi.org/10.26480/rfna.02.2021.59.62
- Bhanu, A. N. (2018). Genetic variability, heritability and correlation study of physiological and yield traits in relation to heat tolerance in wheat (triticum aestivum L.). Biomedical Journal of Scientific & Technical Research, 2(1), Res. 2. https://doi.org/10.26717/bjstr.2017.01.000636
- Bheemanahalli, R., Sunoj, V. S. J., Saripalli, G., Prasad, P. V. V., Balyan, H. S., Gupta, P. K., Grant, N., Gill, K. S., & Jagadish, S. V. K. (2019). Quantifying the impact of heat stress on pollen germination, seed set, and grain filling in spring wheat. Crop Science, 59(2), 684–696. https://doi.org/10.2135/CROPSCI2018.05.0292
- Chairi, F., Vergara-Diaz, O., Vatter, T., Aparicio, N., Nieto-Taladriz, M. T., Kefauver, S. C., Bort, J., Serret, M. D., & Araus, J. L. (2018). Post-green revolution genetic advance in durum wheat: The case of Spain. Field Crops Research, 228, 158–169. https://doi.org/10.1016/J.FCR.2018.09.003
- Chavan, S. G., Duursma, R. A., Tausz, M., Ghannoum, O., & Hancock, R. (2019). Elevated CO2 alleviates the negative impact of heat stress on wheat physiology but not on grain yield. Journal of Experimental Botany, 70(21), 6447–6459. https://doi.org/10.1093/JXB/ERZ386
- Chaves, M. S., Martinelli, J. A., Wesp-Guterres, C., Graichen, F. A. S., Brammer, S. P., Scagliusi, S. M., da Silva, P. R., Wiethölter, P., Torres, G. A. M., Lau, E. Y., Consoli, L., & Chaves, A. L. S. (2013). The importance for food security of maintaining rust resistance in wheat. Food Security, 5(2), 157–176. https://doi.org/10.1007/S12571-013-0248-X
- Chen, H., Moakhar, N. P., Iqbal, M., Pozniak, C., Hucl, P., & Spaner, D. (2016a). Genetic variation for flowering time and height reducing genes and important traits in western Canadian spring wheat. Euphytica, 208(2), 377–390. https://doi.org/10.1007/S10681-015-1615-9
- Chen, H., Moakhar, N. P., Iqbal, M., Pozniak, C., Hucl, P., & Spaner, D. (2016b). Genetic variation for flowering time and height reducing genes and important traits in western Canadian spring wheat. Euphytica, 208(2), 377–390. https://doi.org/10.1007/s10681-015-1615-9
- Christopher, M., Chenu, K., Jennings, R., Fletcher, S., Butler, D., Borrell, A., & Christopher, J. (2018). QTL for stay-green traits in wheat in well-watered and water-limited environments. Field Crops Research, 217, 32–44. https://doi.org/10.1016/J.FCR.2017.11.003
- Cossani, C. M., & Reynolds, M. P. (2015). Heat stress adaptation in elite lines derived from synthetic hexaploid wheat. Crop Science, 55(6), 2719–2735. https://doi.org/10.2135/CROPSCI2015.02.0092
- Crespo-Herrera, L. A., Crossa, J., Huerta-Espino, J., Vargas, M., Mondal, S., Velu, G., Payne, T. S., Braun, H., & Singh, R. P. (2018). Genetic gains for grain yield in cimmyt’s semi-arid wheat yield trials grown in suboptimal environments. Crop Science, 58(5), 1890–1898. https://doi.org/10.2135/CROPSCI2018.01.0017
- Dhakal, N., Shrestha, S., Manandhar, H., Aryal, L., C, S., & Pant, K. (2020). Identification of resistant wheat genotypes against spot blotch (bipolaris sorokininana) for different sowing time and assessing their seed infection after harvest at rampur, Chitwan, Nepal. Fundamental and Applied Agriculture, 5, 1. https://doi.org/10.5455/faa.97888
- Dias de Oliveira, E. A., Siddique, K. H. M., Bramley, H., Stefanova, K., & Palta, J. A. (2015). Response of wheat restricted-tillering and vigorous growth traits to variables of climate change. Global Change Biology, 21(2), 857–873. https://doi.org/10.1111/GCB.12769
- Divashuk, M. G., Bespalova, L. A., Vasilyev, A. V., Fesenko, I. A., Puzyrnaya, O. Y., & Karlov, G. I. (2013). Reduced height genes and their importance in winter wheat cultivars grown in southern Russia. Euphytica, 190(1), 137–144. https://doi.org/10.1007/S10681-012-0789-7
- Djanaguiraman, M., Narayanan, S., Erdayani, E., & Prasad, P. V. V. (2020). Effects of high temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC Plant Biology, 20(1), 1–12. https://doi.org/10.1186/s12870-020-02479-0
- Du, Y., Chen, L., Wang, Y., Yang, Z., Saeed, I., Daoura, B. G., & Hu, Y. G. (2018). The combination of dwarfing genes Rht4 and Rht8 reduced plant height, improved yield traits of rainfed bread wheat (triticum aestivum L.). Field Crops Research, 215, 149–155. https://doi.org/10.1016/J.FCR.2017.10.015
- Dwivedi, S. K., Basu, S., Kumar, S., Kumar, G., Prakash, V., Kumar, S., Mishra, J. S., Bhatt, B. P., Malviya, N., Singh, G. P., & Arora, A. (2017a). Heat stress induced impairment of starch mobilisation regulates pollen viability and grain yield in wheat: Study in Eastern Indo-Gangetic Plains. Field Crops Research, 206, 106–114. https://doi.org/10.1016/J.FCR.2017.03.006
- Dwivedi, S. K., Basu, S., Kumar, S., Kumar, G., Prakash, V., Kumar, S., Mishra, J. S., Bhatt, B. P., Malviya, N., Singh, G. P., & Arora, A. (2017b). Heat stress induced impairment of starch mobilisation regulates pollen viability and grain yield in wheat: Study in Eastern Indo-Gangetic Plains. Field Crops Research, 206, 106–114. https://doi.org/10.1016/j.fcr.2017.03.006
- FAO. (2018). World food and agriculture-statistical pocketbook. FAO. https://www.fao.org/documents/card/en/c/ca1796en
- FAOSTAT. 2022. FAOSTAT [WWW Document]. (Retrieved March 23, 2021). http://www.fao.org/faostat/en/#data/QC
- Farcas, A. C., Galanakis, C. M., Socaciu, C., Pop, O. L., Tibulca, D., Paucean, A., Jimborean, M. A., Fogarasi, M., Salanta, L. C., Tofana, M., & Socaci, S. A. (2021). Food security during the pandemic and the importance of the bioeconomy in the new era. Sustainability, 13(1), 1–11. https://doi.org/10.3390/SU13010150
- Ferrari, M., Benvenuti, L., Rossi, L., De Santis, A., Sette, S., Martone, D., Piccinelli, R., Le Donne, C., Leclercq, C., & Turrini, A. (2020). Could dietary goals and climate change mitigation be achieved through optimized diet? The experience of modeling the National food Consumption data in Italy. Frontiers in Nutrition, 7. https://doi.org/10.3389/FNUT.2020.00048
- Gao, F., Ma, D., Yin, G., Rasheed, A., Dong, Y., Xiao, Y., Xia, X., Wu, X., & He, Z. (2017). Genetic progress in grain yield and physiological traits in Chinese wheat cultivars of southern yellow and Huai Valley since 1950. Crop Science, 57(2), 760–773. https://doi.org/10.2135/CROPSCI2016.05.0362
- Gomez, D., Vanzetti, L., Helguera, M., Lombardo, L., Fraschina, J., & Miralles, D. J. (2014). Effect of vrn-1, ppd-1 genes and earliness per se on heading time in Argentinean bread wheat cultivars. Field Crops Research, 158, 73–81. https://doi.org/10.1016/J.FCR.2013.12.023
- Grover, G., Sharma, A., Gill, H. S., Srivastava, P., Bains, N. S., & Zhang, A. (2018). Rht8 gene as an alternate dwarfing gene in elite Indian spring wheat cultivars. PLoS One, 13(6), e0199330. https://doi.org/10.1371/JOURNAL.PONE.0199330
- Guzmán, C., Autrique, E., Mondal, S., Huerta-Espino, J., Singh, R. P., Vargas, M., Crossa, J., Amaya, A., & Peña, R. J. (2017). Genetic improvement of grain quality traits for CIMMYT semi-dwarf spring bread wheat varieties developed during 1965–2015: 50 years of breeding. Field Crops Research, 210, 192–196. https://doi.org/10.1016/J.FCR.2017.06.002
- IPCC. (2021). Climate change 2021: The physical Science Basis - summary for the policymakers (working group I). https://www.ipcc.ch/report/ar6/wg1/
- Joshi, M. A., Faridullah, S., & Kumar, A. (2016). Effect of heat stress on crop phenology, yield and seed quality attributes of wheat (triticumaestivum L.). Journal of Agrometeorology, 18(2), 206–215. https://doi.org/10.54386/jam.v18i2.937
- Joudi, M., Ahmadi, A., Mohammadi, V., Abbasi, A., & Mohammadi, H. (2014). Genetic changes in agronomic and phenologic traits of Iranian wheat cultivars grown in different environmental conditions. Euphytica, 196(2), 237–249. https://doi.org/10.1007/S10681-013-1027-7
- Kamrani, M., Hoseini, Y., & Ebadollahi, A. (2017). Evaluation for heat stress tolerance in durum wheat genotypes using stress tolerance indices. Archives of Agronomy and Soil Science, 64(1), 38–45. https://doi.org/10.1080/03650340.2017.1326104
- Khan, A., Ahmad, M., Ahmed, M., & Iftikhar Hussain, M. (2020). Rising atmospheric temperature impact on wheat and thermotolerance strategies. Plants (Vol. 10). https://doi.org/10.3390/PLANTS10010043
- Khan, A., Ahmad, M., Ahmed, M., & Iftikhar Hussain, M. (2021). Rising atmospheric temperature impact on wheat and thermotolerance strategies. Plants, 10(1), 1–24. https://doi.org/10.3390/PLANTS10010043
- Krupnik, T. J., Timsina, J., Devkota, K. P., Tripathi, B. P., Karki, T. B., Urfels, A., Gaihre, Y. K., Choudhary, D., Beshir, A. R., Pandey, V. P., Brown, B., Gartaula, H., Shahrin, S., & Ghimire, Y. N. (2021). Agronomic, socio-economic, and environmental challenges and opportunities in Nepal’s cereal-based farming systems. Advances in Agronomy, 170, 155–287. https://doi.org/10.1016/bs.agron.2021.06.004
- Lal, M. K., Tiwari, R. K., Gahlaut, V., Mangal, V., Kumar, A., Singh, M. P., Paul, V., Kumar, S., Singh, B., & Zinta, G. (2022). Physiological and molecular insights on wheat responses to heat stress. Plant Cell Reports, 41(3), 501–518. https://doi.org/10.1007/s00299-021-02784-4
- Lesk, C., Rowhani, P., & Ramankutty, N. (2016). Influence of extreme weather disasters on global crop production. Nature, 529(7584), 84–87. https://doi.org/10.1038/nature16467
- Liu, B., Asseng, S., Müller, C., Ewert, F., Elliott, J., Lobell, D. B., Martre, P., Ruane, A. C., Wallach, D., Jones, J. W., Rosenzweig, C., Aggarwal, P. K., Alderman, P. D., Anothai, J., Basso, B., Biernath, C., Cammarano, D., Challinor, A. … Zhu, Y. (2016). Similar estimates of temperature impacts on global wheat yield by three independent methods. Nature Climate Change, 6(12), 1130–1136. https://doi.org/10.1038/nclimate3115
- Lopes, M. S., Reynolds, M. P., Manes, Y., Singh, R. P., Crossa, J., & Braun, H. J. (2012). Genetic yield gains and changes in associated traits of CIMMYT spring bread wheat in a “historic” set representing 30 years of breeding. Crop Science, 52(3), 1123–1131. https://doi.org/10.2135/CROPSCI2011.09.0467
- Lopes, M. S., Reynolds, M. P., McIntyre, C. L., Mathews, K. L., Jalal Kamali, M. R., Mossad, M., Feltaous, Y., Tahir, I. S. A., Chatrath, R., Ogbonnaya, F., & Baum, M. (2013). QTL for yield and associated traits in the Seri/Babax population grown across several environments in Mexico, in the West Asia, North Africa, and South Asia regions. TAG Theoretical and Applied Genetics Theoretische Und Angewandte Genetik, 126(4), 971–984. https://doi.org/10.1007/S00122-012-2030-4
- Mahmudul, M., Khan, H., Rafii, M. Y., Ramlee, S. I., Jusoh, M., & Al Mamun, M. (2022). Path-coefficient and correlation analysis in Bambara groundnut (Vigna subterranea [L.] verdc.) accessions over environments. Scientific Reports. https://doi.org/10.1038/s41598-021-03692-z
- Mathur, S., Agrawal, D., & Jajoo, A. (2014). Photosynthesis: Response to high temperature stress. Journal of Photochemistry and Photobiology B, Biology, 137, 116–126. https://doi.org/10.1016/j.jphotobiol.2014.01.010
- Mirosavljević, M., Mikić, S., Župunski, V., Kondić Špika, A., Trkulja, D., Ottosen, C. O., Zhou, R., & Abdelhakim, L. (2021). Effects of high temperature during anthesis and grain filling on physiological characteristics of winter wheat cultivars. Journal of Agronomy and Crop Science, 207(5), 823–832. https://doi.org/10.1111/JAC.12546
- Mondal, S., Singh, R. P., Crossa, J., Huerta-Espino, J., Sharma, I., Chatrath, R., Singh, G. P., Sohu, V. S., Mavi, G. S., Sukaru, V. S. P., Kalappanavarg, I. K., Mishra, V. K., Hussain, M., Gautam, N. R., Uddin, J., Barma, N. C. D., Hakim, A., & Joshi, A. K. (2013). Earliness in wheat: A key to adaptation under terminal and continual high temperature stress in South Asia. Field Crops Research, 151, 19–26. https://doi.org/10.1016/j.fcr.2013.06.015
- Mondal, S., Singh, R. P., Mason, E. R., Huerta-Espino, J., Autrique, E., & Joshi, A. K. (2016). Grain yield, adaptation and progress in breeding for early-maturing and heat-tolerant wheat lines in South Asia. Field Crops Research, 192, 78–85. https://doi.org/10.1016/j.fcr.2016.04.017
- Mukherjee, A., Wang, S. Y. S., & Promchote, P. (2019). Examination of the climate factors that reduced wheat yield in northwest India during the 2000s. Water (Switzerland), 11(2), 11. https://doi.org/10.3390/W11020343
- Mwadzingeni, L., Shimelis, H., Rees, D. J. G., Tsilo, T. J., & Yadav, R. S. (2017). Genome-wide association analysis of agronomic traits in wheat under drought-stressed and non-stressed conditions. PLoS One, 12(2), 12. https://doi.org/10.1371/journal.pone.0171692
- Ni, Z., Li, H., Zhao, Y., Peng, H., Hu, Z., Xin, M., & Sun, Q. (2017). Genetic improvement of heat tolerance in wheat: Recent progress in understanding the under lying molecular mechanisms. The Crop Journal, 6(1), 32–41. https://doi.org/10.1016/j.cj.2017.09.005
- Oyewole, C. (2016). The wheat Crop. Science, 93(2423), 12–13. https://doi.org/10.1126/science.93.2423.12.t
- Pan, C., Ahammed, G. J., Li, X., & Shi, K. (2018). Elevated CO2 improves photosynthesis under high temperature by attenuating the Functional limitations to Energy Fluxes, Electron transport and Redox Homeostasis in tomato leaves. Frontiers in Plant Science, 9, 871. https://doi.org/10.3389/FPLS.2018.01739
- Pandey, G. C., Mamrutha, H. M., Tiwari, R., Sareen, S., Bhatia, S., Siwach, P., Tiwari, V., & Sharma, I. (2015). Physiological traits associated with heat tolerance in bread wheat (triticum aestivum L.). Physiology and Molecular Biology of Plants: An International Journal of Functional Plant Biology, 21(1), 93–99. https://doi.org/10.1007/s12298-014-0267-x
- Pandey, D., Pant, K. R., Bastola, B. R., Giri, R., Bohara, S., Shrestha, S., Hamal, G. B., & Shrestha, J. (2021). Evaluation of bread wheat genotypes under rain-fed conditions in Terai districts of Nepal. Journal of Agriculture and Natural Resources, 4(2), 303–315. https://doi.org/10.3126/JANR.V4I2.33946
- Pask, A., Joshi, A. K., Manès, Y., Sharma, I., Chatrath, R., Singh, G. P., Sohu, V. S., Mavi, G. S., Sakuru, V. S. P., Kalappanavar, I. K., Mishra, V. K., Arun, B., Mujahid, M. Y., Hussain, M., Gautam, N. R., Barma, N. C. D., Hakim, A., Hoppitt, W., Trethowan, R., & Reynolds, M. P. (2014). A wheat phenotyping network to incorporate physiological traits for climate change in South Asia. Field Crops Research, 168, 156–167. https://doi.org/10.1016/J.FCR.2014.07.004
- Peña-Bautista, R. J., Hernandez-Espinosa, N., Jones, J. M., Guzmán, C., & Braun, H. J. (2017). CIMMYT series on Carbohydrates, wheat, grains, and health: Wheat-based foods: Their global and regional importance in the food supply, nutrition, and health. Cereal Foods World, 62(5), 231–249. https://doi.org/10.1094/CFW-62-5-0231
- Posch, B. C., Kariyawasam, B. C., Bramley, H., Coast, O., Richards, R. A., Reynolds, M. P., Trethowan, R., Atkin, O. K., & Raines, C. (2019). Exploring high temperature responses of photosynthesis and respiration to improve heat tolerance in wheat. Journal of Experimental Botany, 70(19), 5051–5069. https://doi.org/10.1093/JXB/ERZ257
- Poudel, M. R., Ghimire, S., Prasad, P., Dhakal, K. H., Thapa, D. B., & Poudel, H. K. (2020). Evaluation of wheat genotypes under irrigated,Heat stress and drought conditions. Journal of Biological Today’s World, 9, 212. https://doi.org/10.35248/2322-3308.20.9.212
- Poudel, A., Thapa, D. B., & Sapkota, M. (2017). Cluster analysis of wheat (triticum aestivum L.) genotypes based upon response to terminal heat stress. International Journal of Applied Sciences and Biotechnology, 5(2), 188–193. https://doi.org/10.3126/IJASBT.V5I2.17614
- Prasad, P. V. V., & Djanaguiraman, M. (2014). Response of floret fertility and individual grain weight of wheat to high temperature stress: Sensitive stages and thresholds for temperature and duration. Functional Plant Biology: FPB, 41(12), 1261–1269. https://doi.org/10.1071/fp14061
- Purchase, J. L., Hatting, H., & van Deventer, C. S. (2000). Genotype × environment interaction of winter wheat (triticum aestivum L.) in South Africa: II. Stability analysis of yield performance. South African Journal of Plant and Soil, 17(3), 101–107. https://doi.org/10.1080/02571862.2000.10634878
- Puri, R. R., & Gautam, N. R. (2015). Performance analysis of spring wheat genotypes under rain-fed conditions in warm humid environment of Nepal. International Journal of Environment, 4(2), 289–295. https://doi.org/10.3126/ije.v4i2.12649
- Puri, R. R., Tripathi, S., Bhattarai, R., Dangi, S. R., & Pandey, D. (2020). Wheat Variety Improvement for Climate Resilience. Asian Journal of Research in Agriculture and Forestry, 6, 21–27. https://doi.org/10.9734/AJRAF/2020/V6I230101
- Qaseem, M. F., Qureshi, R., Shaheen, H., Shafqat, N., & Zhang, A. (2019). Genome-wide association analyses for yield and yield-related traits in bread wheat (triticum aestivum L.) under pre-anthesis combined heat and drought stress in field conditions. PLoS One, 14(3), e0213407. https://doi.org/10.1371/journal.pone.0213407
- Rana, L., Banerjee, H., Ray, K., Sarkar, S., Krishi Viswavidyalaya, C., & Shyamala Krishi Vigyan Kendra, S. (2017). System of wheat intensification (SWI) – a new approach for increasing wheat yield in small holder farming system. Journal of Applied and Natural Science, 9(3), 1453–1464. https://doi.org/10.31018/jans.v9i3.1384
- Sachs, J., Kroll, C., Lafortune, G., Fuller, G., & Woelm, F. (2022). Sustainable Development report 2022. Cambridge University Press. https://doi.org/10.1017/9781009210058
- Sharma, D., Mamrutha, H. M., Gupta, V. K., Tiwari, R., & Singh, R. (2015). Association of SSCP variants of HSP genes with physiological and yield traits under heat stress in wheat. Research on Crops, 16(1), 139–146. https://doi.org/10.5958/2348-7542.2015.00020.0
- Shewry, P. R., & Hey, S. J. (2015). The contribution of wheat to human diet and health. Food and Energy Security, 4(3), 178–202. https://doi.org/10.1002/FES3.64
- Shiferaw, B., Prasanna, B. M., Hellin, J., & Bänziger, M. (2011). Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Security, 3(3), 307–327. https://doi.org/10.1007/S12571-011-0140-5
- Shirdelmoghanloo, H., Cozzolino, D., Lohraseb, I., & Collins, N. C. (2016). Truncation of grain filling in wheat (triticum aestivum) triggered by brief heat stress during early grain filling: Association with senescence responses and reductions in stem reserves. Functional Plant Biology: FPB, 43(10), 919–930. https://doi.org/10.1071/FP15384
- Shirdelmoghanloo, H., Taylor, J. D., Lohraseb, I., Rabie, H., Brien, C., Timmins, A., Martin, P., Mather, D. E., Emebiri, L., & Collins, N. C. (2016). A QTL on the short arm of wheat (triticum aestivum L.) chromosome 3B affects the stability of grain weight in plants exposed to a brief heat shock early in grain filling. BMC Plant Biology, 16(1). https://doi.org/10.1186/s12870-016-0784-6
- Shrestha, S. M., Manadhar, H. K., & Yadav, R. K. (2020). Field response of wheat genotypes to spot blotch under different sowing dates at Rampur, Chitwan, Nepal. Journal of Agriculture and Forestry University, 4, 83–90. https://doi.org/10.3126/jafu.v4i1.47050
- S Kumar, P. K. U. K. M. G. A. S. R. S. R. S., Kumari, P., Kumar, U., Grover, M., Singh, A. K., Singh, R., & Sengar, R. S. (2013). Molecular approaches for designing heat tolerant wheat. Journal of Plant Biochemistry and Biotechnology, 22(4), 359–371. https://doi.org/10.1007/s13562-013-0229-3
- Tack, J., Barkley, A., & Nalley, L. L. (2015). Effect of warming temperatures on US wheat yields. Proceedings of the National Academy of Sciences of the United States of America, 112(22), 6931–6936. https://doi.org/10.1073/PNAS.1415181112
- Tiwari, D. N., Tripathi, S. R., Tripathi, M. P., Khatri, N., & Bastola, B. R. (2019). Genetic variability and correlation coefficients of Major traits in early maturing rice under rainfed lowland environments of Nepal. Advanced Agriculture, 2019. https://doi.org/10.1155/2019/5975901
- Tshikunde, N. M., Mashilo, J., Shimelis, H., & Odindo, A. (2019). Agronomic and physiological traits, and associated quantitative trait Loci (QTL) affecting yield response in wheat (triticum aestivum L.): A review. Frontiers in Plant Science, 10, 1428. https://doi.org/10.3389/fpls.2019.01428
- Ullah, A., Nadeem, F., Nawaz, A., Siddique, K. H. M., & Farooq, M. (2022). Heat stress effects on the reproductive physiology and yield of wheat. Journal of Agronomy and Crop Science, 208(1), 1–17. https://doi.org/10.1111/jac.12572
- WBG. (2022). September 2022. International Journal for Modern Trends in Science and Technology, 8. https://doi.org/10.46501/ijmtst0809
- Whittal, A., Kaviani, M., Graf, R., Humphreys, G., Navabi, A., & Zhang, A. (2018). Allelic variation of vernalization and photoperiod response genes in a diverse set of North American high latitude winter wheat genotypes. PLoS One, 13(8), e0203068. https://doi.org/10.1371/JOURNAL.PONE.0203068
- Yu, T. F., Xu, Z. S., Guo, J. K., Wang, Y. X., Abernathy, B., Fu, J. D., Chen, X., Zhou, Y. B., Chen, M., Ye, X. G., & Ma, Y. Z. (2017). Improved drought tolerance in wheat plants overexpressing a synthetic bacterial cold shock protein gene SeCspA. Scientific Reports, 7(1), 1–14. https://doi.org/10.1038/srep44050
- Zandalinas, S. I., Mittler, R., Balfagón, D., Arbona, V., & Gómez‐Cadenas, A. (2018). Plant adaptations to the combination of drought and high temperatures. Physiologia plantarum, 162(1), 2–12. https://doi.org/10.1111/ppl.12540
- Zhang, C. X., Feng, B. H., Chen, T. T., Zhang, X. F., Tao, L. X., & Fu, G. F. (2017). Sugars, antioxidant enzymes and IAA mediate salicylic acid to prevent rice spikelet degeneration caused by heat stress. Plant Growth Regulation, 83(2), 313–323. https://doi.org/10.1007/s10725-017-0296-x
- Zhang, M., Gao, Y., Zhang, Y., Fischer, T., Zhao, Z., Zhou, X., Wang, Z., & Wang, E. (2020). The contribution of spike photosynthesis to wheat yield needs to be considered in process-based crop models. Field Crops Research, 257, 107931. https://doi.org/10.1016/j.fcr.2020.107931
- Zhang, Y., Xu, W., Wang, H., Dong, H., Qi, X., Zhao, M., Fang, Y., Gao, C., & Hu, L. (2016). Progress in genetic improvement of grain yield and related physiological traits of Chinese wheat in Henan Province. Field Crops Research, 199, 117–128. https://doi.org/10.1016/J.FCR.2016.09.022