Abstract
Groundnuts (Arachis hypogaea L.) are produced and consumed globally due to their nutritious and hardy nature. However, they are vulnerable to Aspergillus spp. infection, which is active in the field or the store and produces aflatoxins. Aflatoxins are quite important as they impact food safety. Both humans and animals are at high risk of aflatoxin toxicity when exposed to aflatoxin contaminated food and feed, respectively. Aflatoxin is a major problem in the world particularly in sub-Saharan Africa because of climatic circumstances such as drought, floods, and extreme temperatures. Biological control, cultural practices, good agronomic practices, proper post-harvest handling, chemical control, and breeding of tolerant groundnut varieties are found to be effective for the prevention and management of aflatoxins. Therefore, there is a high need for increased awareness of mitigation practices for aflatoxins contamination to groundnut-producing farmers to ensure the production and availability of aflatoxins free groundnuts.
REVIEWING EDITOR:
1. Introduction
1.1. Groundnuts
Groundnut is one of the most important leguminous crops, cultivated primarily as a food and feed source around the world (Bediako et al., Citation2019). It is an important legume crop in tropical and semi-arid areas, where it serves as a source of protein and edible oil. Groundnut kernels contain between 16% and 36% protein, between 36% and 54% oil and between 10% and 20% carbohydrates (Singh et al., Citation2021). Groundnut shells are the byproducts when the groundnut seed is removed from the pod. There is a sizable amount of shell residue left over after processing groundnuts because groundnut shells comprise about 20% of the weight of dried peanut pods (Duc et al., Citation2019).
The crop is grown between latitudes 40° N and 40° S of the equator and up to an altitude of 1,050 meters (Cervini et al., Citation2022). Globally, Groundnut covers 32.7 million hectares with the production of 53.9 million tonnes with the productivity of 1.648 tonnes per hectare (FAOSTAT, Citation2022). According to FAOSTAT (Citation2021), Africa accounted for about 32% of the global groundnut production in 2021. Farmers in Tanzania grow groundnuts on flat seedbeds or ridges. Yields of groundnut in Tanzania are reported to be 0.5 t/ha to 1 t/ha compared with 1.5 t/ha to 2.5 t/ha reported in other African countries such as Nigeria and Guinea-Bissau (FAOSTAT, Citation2021).
According to FAOSTAT (Citation2021), the potential average yield of groundnuts is 2.5 t/ha to 2.7 t/ha but the low production is caused by diverse abiotic and biotic factors. Extreme temperatures, drought stress, soil characteristics including alkalinity, poor soil fertility, and nutrient deficits are among the abiotic factors affecting groundnut production (Daudi et al., Citation2018). The most important biotic factors affecting groundnut production and productivity in the country include groundnut rosette disease (groundnut rosette assistor virus, groundnut rosette virus, and a satellite RNA), rust (Puccinia arachidis), early leaf spot (Cercospora arachidicola) late leaf spot (Phaseoisariopsis personata) and aflatoxins contamination (Aspergillus species) (Giordano et al., Citation2021; Rago et al., Citation2017).
Groundnut grows well in the neutral soil pH of sandy loam with a light texture. The crop needs annual rainfall ranging between 500 mm and 600 mm of evenly distributed, and an ideal temperature between 28 °C and 30 °C (Halder et al., Citation2020). Drought and high heat stress are the main yield limiting abiotic factors in the semiarid areas (Fahad et al., Citation2017). Before blooming, during flowering, and during early pod formation, the periods of reproductive development are particularly sensitive to these restrictions (Ajeigbe et al., Citation2015; Hoffmann et al., Citation2018).
Shortages of nutrients such as Nitrogen (N), Phosphorus (P), Potassium (K), Calcium (Ca), Iron (Fe), and Boron (B) also generate significant yield losses (Singh & Basu, Citation2005). However, pests, diseases, and weeds are the main biotic factors limiting groundnut production. Leaf spots, rusts, and the toxin-producing fungus Aspergillus are the three most significant infections in groundnuts (Kumar & Thirumalaisamy, Citation2016).
Aflatoxins produced by Aspergillus spp. contaminate common foods, raise consumer health risks and result in large yearly economic losses (Guchi, Citation2015; Massomo, Citation2020).
1.2. Fungal infections in groundnuts
Several fungal species can penetrate and invade plant tissue given their ubiquitous nature especially under favorable conditions (Boni et al., Citation2021). According to Munthali et al. (Citation2016), many of these fungi are free-living organisms that can endure in the environment even in the absence of commonly cultivated crops. Fungal development is favored by high moisture (90–95%) and moderate temperature (25 and 30 °C), it is more likely to occur in the field and when groundnuts are not adequately dried (more than 5% humidity) (Bediako et al., Citation2019). The quality and viability of groundnut seeds are significantly reduced as a result of fungal pathogen invasion (Tiwari & Dubey, Citation2023). Different fungal species may develop, although Aspergillus species (A. parasiticus, A. flavus, A. niger, A. terreus) is frequently the most prevalent (Qi et al., Citation2023). A. flavus and A. parasiticus are the two species, which produce aflatoxins, and the infection begins in the field when groundnuts overstay in the field after reaching maturity (Mohammed, Citation2011).
1.3. Aflatoxins in groundnuts
Mycotoxins are secondary metabolites produced by a variety of fungi (Reverberi et al., Citation2010). They contaminate a lot of food products and local crops during pre- and post-harvesting under favorable conditions like high temperature and dampness (Bishnu et al., Citation2022). Due to its carcinogenic properties, aflatoxin is the principal mycotoxin that harms both animal and human health (). Aspergillus flavus and Aspergillus parasiticus are the principal sources of aflatoxins (Kumar et al., Citation2021).
Figure 1. The structure of aflatoxins (Martínez et al., Citation2023).
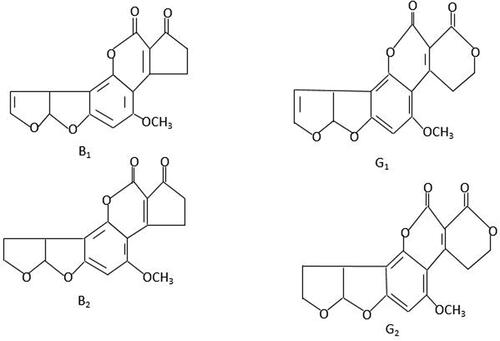
The high nutritional content of groundnuts makes them the ideal substrate for fungus growth and possible aflatoxin contamination (Mupunga et al., Citation2017). Aspergillus contamination of groundnuts with aflatoxin is a significant pre- and post-harvest issue that reduces kernel quality (Waliyar et al., Citation2015). Aflatoxigenic infestation at the pre-harvest stage is the root cause of post-harvest aflatoxin contamination, which leads to decreased kernel quality (Agbetiameh et al., Citation2020). Furthermore, aflatoxin contamination is directly caused by improper handling of pods and storage techniques following harvest, which results in high moisture and ambient temperatures (Waliyar et al., Citation2015).
Since the 1960s, groundnut contamination with aflatoxin has caused concern on a global scale (Gichohi-Wainaina et al., Citation2023; Mohammed et al., Citation2016). According to Bediako et al. (Citation2019), pre- and post-harvest environmental conditions, inadequate management practices including delayed harvesting, mechanical damage at the time of harvest, and minimal curing and drying before storage are the main causes of aflatoxin contamination of groundnuts.
Aflatoxins are linked to serious toxicological consequences such as liver cancer, immunosuppression, irritation, and respiratory problems in both humans and animals (Massomo, Citation2020). Intake of high doses of aflatoxins can lead to acute aflatoxicosis and deaths all around the world with some examples as indicated in below where it caused multiple human deaths after consumption of aflatoxin contaminated maize.
Table 1. Aflatoxin contamination cases and deaths caused by the contamination.
Moreover, aflatoxin contamination can result in significant financial loss for groundnut growers, handlers, processors, and marketers (Bediako et al., Citation2019). Due to the numerous variables in the pre-harvest, harvesting, post-harvest stages of production, and transportation, effective and efficient fungal growth and aflatoxins control are imperative along the groundnut value chain (Bediako et al., Citation2019; Diao et al., Citation2015).
This review therefore details the management techniques to control the aflatoxins contamination in groundnuts.
2. Management practices to mitigate aflatoxins contamination in groundnuts
Aflatoxin contamination of groundnuts by Aspergillus is a significant pre- and post-harvest issue; hence management techniques to reduce aflatoxin contamination in groundnuts are required in both pre-harvest and post-harvest phases. To reduce the entry of aflatoxin into the food and feed systems, various pre-harvest and post-harvest tactics need to be used. However, no one approach will suffice to address this issue. An integrated management from the field until food or feed processing is necessary to reduce the impact of aflatoxins (Torres et al., Citation2015).
2.1. Biological control
One of the suggested mitigating techniques for aflatoxin is biological control (Dorner, Citation2004). Before being widely used, biocontrol products must be developed and their effectiveness must be demonstrated (Agbetiameh et al., Citation2020). Numerous species of microorganisms including bacteria, yeasts, and nontoxigenic fungal strains have been studied for biological control activity against toxigenic mold and aflatoxin contamination (Veras et al., Citation2023). Applying specific atoxigenic strains of A. flavus and A. parasiticus to the soil of crops like groundnuts, cotton, and corn has resulted in the majority of field successes to date because when crops are vulnerable to infection, the applied strains compete with the naturally occurring toxigenic strains for the same niche and successfully drive them out (Dorner, Citation2009).
The non-toxic strains are applied to the soil using different formulations, however, the most productive techniques involve combining the target strain with a carrier or substrate, like a tiny grain (Agbetiameh et al., Citation2019). This is done by growing either a small amount of the desired strain on sterile grain or the strain’s conidia applied to the grain’s surface (Agbetiameh et al., Citation2019). The fungus completely colonizes the grain after being applied to the field and absorbs moisture, and profuse sporulation produces inoculum levels high enough to give the nontoxigenic strain a competitive edge (Agbetiameh et al., Citation2020).
In some parts of Africa, they apply biocontrol products containing atoxigenic Aspergillus flavus strains to lower the aflatoxin level in crops (Senghor et al., Citation2020). For instance, the biocontrol product Aflasafe SN01 was created in Gambia and Senegal-West Africa. The four components which make up Aflasafe SN01 are atoxigenic strains of A. flavus that are local to Gambia and Senegal and are different from the components that make up other biocontrol products. The majority of the crops grown in treated fields usually have aflatoxin levels that do not exceed the threshold levels for both domestic and international markets (Senghor et al., Citation2020; Citation2021). This suggests that Aflasafe SN01 is a useful tool for peanut and maize aflatoxin reduction. Application of Aflasafe SN01 may improve Senegal and other countries’ health, trade, and economy regarding aflatoxin control if is made available and used widely (Senghor et al., Citation2020). Reductions of aflatoxin contamination in the range of 70–90% have been regularly attained in several years of field tests, particularly with cotton and groundnuts (Mahuku et al., Citation2023).
Biological control can also be done using lactic acid bacteria (LAB) and Bacillus spp. which is an environment friendly strategy for phytopathogenic fungi management (Hirozawa et al., Citation2023). Several molecules produced by these bacteria affect fungal growth and viability in different crops including groundnuts (Raman et al., Citation2022). The antifungal effect and compounds produced by LAB (e.g. Organic acids, peptides, cyclic dipeptides, fatty acids, and volatile compounds) and Bacillus spp. (e.g. Peptides, enzymes, and volatile compounds) highly affect fungal growth and viability (Hirozawa et al., Citation2023; Raman et al., Citation2022). Results indicate that the efficacy of the biocontrol product in limiting aflatoxin contamination is stable regardless of the environment and cropping system (Bandyopadhyay et al., Citation2019).
2.2. Cultural practices
The use of cultural practices, such as habitat management, pre- and post-harvest management, physical control measures, and use of resistant groundnut cultivars, is crucial for the management of aflatoxin contamination in groundnuts (Guchi, Citation2015). However, integration of practices such as summer plowing, seed treatment with carbendazim 50 WP (2 g/kg), furrow application of castor cake enriched with Trichoderma (2.5 kg in 50 kg) at sowing, application of gypsum (500 kg/ha) at flowering, spray applications of neem oil (2%) at 45 days after sowing (DAS), application of carbendazim (0.05%) plus mancozeb (0.2%) at 60–70 DAS, harvesting at 75% pod maturity and removal of damaged pods can be effective in reducing the soil population of A. flavus, seed infection and colonization hence greatly contributing to mitigation of aflatoxin contamination (Kumar et al., Citation2009).
Regarding the effective control of the infection, the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) and its partners have developed an integrated approach to mitigate A. flavus infestation and aflatoxin contamination by combining: (i) host plant resistance, (iii) organic supplements to enhance water holding capacity, plant vigor and seed health, (iii) timely harvest and postharvest drying methods, and (iv) awareness campaigns and training courses to disseminate technology to the end-users. This approach successfully reduces aflatoxin contamination in groundnuts and has been effective in West and Central Africa (Waliyar et al., Citation2013). This strategy is easy, affordable, appropriate for conditions of subsistence farming and, can be scaled up for use in commercial farming in developing countries (Kaale et al., Citation2021).
2.3. Good agronomic practices
Pre-harvest aflatoxin formation can be decreased by practicing good agronomic techniques such as crop rotation, resistant varieties, pest control, timely planting and harvesting, weed management, appropriate fertilization, and late-season irrigation (Engels, Citation2011).
Other good agricultural practices (GAPs) such as farmyard manure application, gypsum application, protective irrigation, drying of pods on tarpaulins after harvest in farmers’ fields have also proven to be of importance in effective control of aflatoxins (Parimi et al., Citation2018).
2.4. Proper post-harvest handling
Aflatoxin contamination in groundnuts at post-harvest stage is reduced through sorting, proper post-harvest drying, safe transportation and packaging, and post-harvest insect control (Bediako et al., Citation2019). Removal of high-risk components from shelled lots and severely contaminated nuts is the main post-harvest management strategy for reducing mycotoxin contamination in groundnuts (Lavkor & Var, Citation2017). Farmers cover their crops when it rains, after harvest and after the first drying of the pods. However, drying on tarpaulin has also been demonstrated to prevent aflatoxin contamination by reducing spores on groundnut (Yahaya et al., Citation2022).
Moreover, storing groundnuts in hermetically-sealed bags reduce the favorable conditions for fungal growth compared with storing in traditional poly bags hence this practice of post-harvest handling also plays a role in the mitigation of aflatoxin contamination (Jordan et al., Citation2020). Farmers who adopt post-harvest management procedures reduce the chances of aflatoxin contamination in their dried kernels and grains (Seetha et al., Citation2017).
However, Waliyar et al. (Citation2015) reported that other management tools can be used for reducing post-harvest aflatoxin contamination such as post-harvest machinery sanitation, disinfestation, detoxification, inactivation of the fungal pathogens, addition of binding agents which hinder fungal growth to some extent, and addition of antifungal compounds. These post-harvest management options and enhanced use of good agricultural practices such as dehulling, steeping, wet milling, dry milling, heat treatment, and irradiation play a major role in mitigating the problem of aflatoxin contamination in groundnuts (Pickova et al., Citation2021; Waliyar et al., Citation2015).
2.5. Breeding for aflatoxin tolerance in groundnut
To significantly reduce groundnut contamination in farmers’ fields, varietal resistance is one of the methods to address aflatoxin contamination in groundnuts (Nigam et al., Citation2009). Genotype-Environment (G x E) interaction limits the selection of resistant breeding materials based on seed characteristics as it reduces the association between phenotypic and genotypic values, and may cause selections from one environment to perform poorly in another (Jahanzaib et al., Citation2019). Aflatoxin management using crop genetics could benefit from pre-breeding, the creation of technological tools, knowledge of the diversity of the pathogen population, and an understanding of gene networks to help with selection (Ncube & Maphosa, Citation2020).
To effectively reduce infection in farmers’ fields, common crop varieties should be genetically resistant to aflatoxin. Sustainable breeding for new groundnut varieties that are resistant to aflatoxin contamination might be done using resistant donor parents with precise selection (Pandey et al., Citation2019).
2.6. Chemical control
Fungi, insects and aflatoxins may be serious problems in storage systems for grains when conditions are predisposing (Nesci et al., Citation2016). The use of synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) against Aspergillus and insect vectors has shown fungicidal and insecticidal effects when used in groundnuts (Jordan et al., Citation2020). Fungicides such as azoxystrobin, cyprodinil (EC10), and fludioxonil (EC10) are also found to be effective in chemical control of aflatoxins as they reduce fungal growth, sporulation, aflatoxin production, and completely inhibit conidia germination (Lagogianni & Tsitsigiannis, Citation2018).
The impact of synthetic and natural compounds on the decrease of aflatoxins in food items has been assessed by some researches (Tahir et al., Citation2018). Citric acid’s impact on the degradation of aflatoxin-B1 and aflatoxin-B2 in extruded sorghum is a good illustration of this action (Yang, Citation2019). According to Fatah (Citation2019), aflatoxin reduction in crops was studied using pressure and sodium hydrosulfite (Na2S2O4) and according to the study, applying 2% Na2S2O4 under high pressure led to a greater percentage reduction of aflatoxin B1, aflatoxinB2, aflatoxinG, and aflatoxinG without causing crop damage. However, it is typically discovered that Aflatoxin-B2 is the most resistant to the administered therapy.
In addition, insect respiration raises the temperature and moisture content of grains, creating ideal circumstances for the growth of fungi (Mohapatra et al., Citation2017). Because of this, Barra et al. (Citation2015) examined the effectiveness of 2, 6-di (t-butyl)-p-cresol (BHT) and the entomopathogenic fungus Purpureocillium lilacinum in preventing the buildup of aflatoxin B1 in stored crops like maize and groundnuts. According to the findings, BHT and Purpureocillium lilacinum together significantly reduced aflatoxin-B1 levels in stored produce. Aflatoxin-B1 was reduced most effectively by tartaric acid, followed by citric acid, lactic acid, succinic acid, and tartaric acid, in that order (Lee et al., Citation2015). Aflatoxin-B1 is changed by these acid treatments into -keto acid, which then changes into aflatoxin-D1, which is less harmful than aflatoxin-B1 (Lee et al., Citation2015). According to Zhang et al. (Citation2012), acidic electrolyzed oxidizing water, an electrolyte solution made with an electrolysis apparatus with an ion-exchange membrane, has been used to decontaminate samples of naturally contaminated groundnuts to prevent the contamination of groundnuts with aflatoxin-B1. After soaking in the solution, the amount of aflatoxin-B1 in groundnuts drops by roughly 85%. Moreover, after treatment, neither the nutritional value nor the color of the groundnuts alters considerably (Xiong et al., Citation2012; Zhang et al., Citation2012)
2.7. Provision of education to farmers about aflatoxins and their management
Aflatoxin is a technical term to most farmers and most of them fail to comprehend the issue or the significance of managing aflatoxin contamination, unlike the extension staff and agro-dealers (Ngotho, Citation2017). On the other hand, marketing strategies also demonstrate a failure to address the issue of aflatoxin contamination during both the production and marketing phases (Ola et al., Citation2022).
To develop strategies for addressing the issue of aflatoxin contamination, partnerships between public, private and international research institutions, local governments, marketing organizations, non-governmental organizations, farmers’ groups, consumer groups, agrochemical manufacturers, and other stakeholders should be enhanced whereby farmers who adopt the education and practice good crop husbandry manage to highly mitigate aflatoxin contamination. (Anitha et al., Citation2019; Migwi et al., Citation2020).
3. Current advancement in mitigation of aflatoxin contamination
Aflatoxin contamination and A. flavus infection are favored by dry conditions and hot temperatures and the contamination affects humanity on a variety of levels, including social, economic, and political, in addition to food insecurity and human health (Pickova et al., Citation2021). To control aflatoxin contamination in groundnuts, molecular and genetic engineering tools are currently being applied to the study of host resistance to biotic and abiotic factors affecting pre-harvest aflatoxin contamination groundnuts (Bhatnagar-Mathur et al., Citation2015).
Proteomics, differential display reverse transcription-polymerase chain reaction (DD-RTPCR), expressed sequence tag (EST), and gene chip technology (macro/microarray) are some of the molecular tools that are being applied to study gene expression in response to drought stress and genetic transformation (Song et al., Citation2023). One element that gives a clear picture of the effectiveness of control approach through genetic improvement is the genetic enhancement of crop resilience to drought stress (Berni & Brutti, Citation2023; Pandey et al., Citation2019).
4. Conclusions and recommendations
Aflatoxin contamination is an important problem all over the world, especially in developing countries, particularly in the African continent (countries) where the level of awareness of the contamination is low. Aflatoxin contamination has led to economic losses to groundnut producers, health concerns, and deaths to consumers when consumption of groundnuts exceeds the threshold level of contamination. Different strategies, both at pre-harvest and post-harvest stages have been applied to reduce the entry of aflatoxins to the food and feed chains.
Currently, no single strategy is enough to solve this problem. An integrated management strategy from the field until food or feed processing is necessary to reduce the impact of aflatoxins. However, mitigation measures to minimize aflatoxin contamination in groundnuts have been employed only by groundnut producers who are aware of the problem. There is a gap in the level of awareness between the farmers with the knowledge and without the knowledge of how to minimize aflatoxin contamination in groundnuts, majority of them are unaware of the situation. Therefore, efforts should be made to protect susceptible groundnut varieties especially groundnuts against aflatoxin contamination to sustain health, income, and trade benefits to the farmers.
Author contributions statement
The corresponding author (Zamu Mdindikasi) designed and drafted the paper while Andekelile Mwamahonje, Amarchius James, Felista Mpore and Athuman Mahinda critically revised the paper for intellectual content. The final version of the paper to be submitted for publication was approved by Andekelile Mwamahonje and all authors agree to be accountable for all aspects of the work.
Acknowledgments
The management of Tanzania Agriculture Research Institute (TARI) – Makutupora Centre and all members who had their inputs to this paper are highly acknowledged.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable because no new data were generated in this study.
Additional information
Notes on contributors
Zamu Mdindikasi
Zamu Mdindikasi (MSc.) is a Research Scientist at Tanzania Agricultural Research Institute (TARI)-Tanzania. Professionally, he is a Microbiologist. He holds a Master degree in Applied Microbiology from Sokoine University of Agriculture (Awarded in 2023) where his research focused on the PREVALENCE OF NON-TYPHOIDAL SALMONELLA INFECTION IN WILD AND DOMESTIC ANIMALS AROUND RUAHA NATIONAL PARK ECOSYSTEM, TANZANIA. He graduated BSc Agriculture General in 2017 at Sokoine University of Agriculture based in Morogoro region, Tanzania. His research interest is in Microbes particularly the pathogenic microbes in plants and animals.
Andekelile Mwamahonje
Andekelile Mwamahonje is a Senior Research Scientist at Tanzania Agricultural Research Institute (TARI) Makutupora Centre, Tanzania. He holds a PhD in Plant Breeding in 2021 from University of Ghana where his research focused on the Breeding for drought tolerance and high grain yield of sorghum varieties. He has developed three lines of sorghum which are climate resilience in semi-arid areas and participated in the release of five grape varieties at the centre. He graduated Masters of Science in Sustainable Agriculture in 2016 at the Nelson Mandela African Institution of Science and Technology Arusha Tanzania, and he graduated BSc Agriculture General in 2012 at the Sokoine University of Agriculture based in Morogoro region, Tanzania. His research of interest covers the application of marker assisted selection, genome wide association mapping and marker assisted backcrossing techniques for exploiting traits of interest for developing climate resilience crop varieties where his main focus has been on addressing challenges on abiotic and biotic stresses affecting crops in the tropics.
Felista Joseph Mpore
Felista Joseph Mpore (MSc.) is a Senior Research Scientist at Tanzania Agricultural Research Institute (TARI)-Tanzania. She is professionally a Food Scientist and she holds a Master degree in Food Science from Sokoine University of Agriculture. She graduated her master degree in 2013 where her research focused on the “Nutrient content, microbial stability and sensory acceptability of sun and solar drier raisin from Dodoma Tanzania”. She graduated BSc Food Science and Technology in 2008 at Sokoine University of Agriculture located in Morogoro region, Tanzania. Her research interest is in Post Harvest Management and Value Addition.
Armachius James
Armachius James (PhD) is a Research Scientist at Tanzania Agricultural Research Institute (TARI)-Tanzania. Professionally, he is a Food and Nutrition Scientist. His research interest is in Food systems, particularly food and nutrition security, and food safety. Currently, Armachius is a graduate research scholar at Beijing Technology and Business University, Beijing-China, working on cyclic nucleotide phosphodiesterases (PDEs) regulated pathways as the targets for the bioactive compounds in alleviating metabolic diseases.
Athuman Mahinda
Athuman Mahinda (Ph.D) is an award-winning scholar in the field of Agriculture, Food security, Environment and Climate change. He graduated with the 1 st first class in BSc. Agronomy from Sokoine University of Agriculture (SUA). For the entire period of his studies at SUA, Dr. Mahinda received numerous academic awards within the University and Tanzania’s government. In 2014, he received a certificate of excellence in academics from the University of Nairobi for his outstanding performance in MSc. studies. Dr. Mahinda graduated his first doctoral program in Global Future of Life, Food and Environment from Kyoto University in 2018, followed by a second doctoral degree in Agricultural Sciences from the same University in 2020. With over twelve years of experience in the field of Agriculture and related sector, Dr. Mahinda has worked across various sectors including private sectors, research institutions, and higher learning intuitions. He served as a Research Scientist at the Tanzania Agricultural Research Institute (TARI) for five years before being seconded to the University of Dar es Salaam (UDSM) as an Assistant Lecturer. Currently, Dr. Mahinda is working at the same University, in the Department of Agricultural Engineering, College of Agricultural Science and Food Technology as a Lecturer, Researcher and Consultant, and holds various leadership roles representing his University, College and Department. At the UDSM he teaches Precision Agriculture, Principles of Agronomy, Fundamental of Soil Sciences, Environmental and Agricultural Waste Management. Dr. Mahinda’s research interest is on crop production and cropping systems, soil, water management, food security, climate smart agriculture, and natural resource management and economics.
References
- Agbetiameh, D., Ortega-Beltran, A., Awuah, R. T., Atehnkeng, J., Islam, M.-S., Callicott, K. A., Cotty, P. J., & Bandyopadhyay, R. (2019). Potential of atoxigenic Aspergillus flavus vegetative compatibility groups associated with maize and groundnut in Ghana as biocontrol agents for aflatoxin management. Frontiers in Microbiology, 10, 1. https://doi.org/10.3389/fmicb.2019.02069
- Agbetiameh, D., Ortega-Beltran, A., Awuah, R. T., Atehnkeng, J., Elzein, A., Cotty, P. J., & Bandyopadhyay, R. (2020). Field efficacy of two atoxigenic biocontrol products for mitigation of aflatoxin contamination in maize and groundnut in Ghana. Biological Control, 150, 104351. https://doi.org/10.1016/j.biocontrol.2020.104351
- Ajeigbe, H. A., Waliyar, F., Echekwu, C. A., Kunihya, A., Motagi, B. N., Eniaiyeju, D., & Inuwa, A. (2015). A farmer’s guide to profitable groundnut production in Nigeria. Technical Report. ICRISAT.
- Anitha, S., Tsusaka, T. W., Njoroge, S. M. C., Kumwenda, N., Kachulu, L., Maruwo, J., Machinjiri, N., Botha, R., Msere, H. W., Masumba, J., Tavares, A., Heinrich, G. M., Siambi, M., & Okori, P. (2019). Knowledge, attitude and practice of Malawian farmers on pre-and post-harvest crop management to mitigate aflatoxin contamination in groundnut, maize and sorghum—Implication for behavioral change. Toxins, 11(12), 716. https://doi.org/10.3390/toxins11120716
- Bandyopadhyay, R., Atehnkeng, J., Ortega-Beltran, A., Akande, A., Falade, T. D., & Cotty, P. J. (2019). “Ground-truthing” efficacy of biological control for aflatoxin mitigation in farmers’ fields in Nigeria: From field trials to commercial usage, a 10-year study. Frontiers in Microbiology, 10, 2528. https://doi.org/10.3389/fmicb.2019.02528
- Barra, P., Etcheverry, M., & Nesci, A. (2015). Efficacy of 2, 6-di (t-butyl)-p-cresol (BHT) and the entomopathogenic fungus Purpureocillium lilacinum, to control Tribolium confusum and to reduce aflatoxin B1 in stored maize. Journal of Stored Products Research, 64(2015), 72–10. https://doi.org/10.1016/j.jspr.2015.09.003
- Bediako, K. A., Ofori, K., Offei, S. K., Dzidzienyo, D., Asibuo, J. Y., & Amoah, R. A. (2019). Aflatoxin contamination of groundnut (Arachis hypogaea L.): Predisposing factors and management interventions. Food Control, 98, 61–67. https://doi.org/10.1016/j.foodcont.2018.11.020
- Benkerroum, N. (2020). Aflatoxins: Producing-molds, structure, health issues and incidence in Southeast Asian and Sub-Saharan African countries. International Journal of Environmental Research and Public Health, 17(4), 1215. https://doi.org/10.3390/ijerph17041215
- Berni, E., & Brutti, A. (2023). Electromagnetic radiations and their effect on filamentous fungi and mycotoxins: Recent advances and perspectives. Current Opinion in Food Science, 52, 101073. https://doi.org/10.1016/j.cofs.2023.101073
- Bhatnagar-Mathur, P., Sunkara, S., Bhatnagar-Panwar, M., Waliyar, F., & Sharma, K. K. (2015). Biotechnological advances for combating Aspergillus flavus and aflatoxin contamination in crops. Plant Science: An International Journal of Experimental Plant Biology, 234, 119–132. https://doi.org/10.1016/j.plantsci.2015.02.009
- Bishnu, P., De, P., Hayat, A., Roy, D., Das, S., & Mahato, M. K. (2022). Modern approaches in aflatoxin free groundnut production. In R. K. Naresh (Ed.), Advances in agriculture sciences (p. 24). AkiNik Publications.
- Boni, S. B., Beed, F., Kimanya, M. E., Koyano, E., Mponda, O., Mamiro, D., Kaoneka, B., Bandyopadhyay, R., Korie, S., & Mahuku, G. (2021). Aflatoxin contamination in Tanzania: Quantifying the problem in maize and groundnuts from rural households. World Mycotoxin Journal, 14(4), 553–564. https://doi.org/10.3920/WMJ2020.2646
- Cervini, C., Verheecke-Vaessen, C., He, T., Mohammed, A., Magan, N., & Medina, A. (2022). Improvements within the peanut production chain to minimize aflatoxins contamination: An Ethiopian case study. Food Control, 136, 108622. https://doi.org/10.1016/j.foodcont.2021.108622
- Daudi, H., Shimelis, H., Laing, M., Okori, P., & Mponda, O. (2018). Groundnut production constraints, farming systems, and farmer-preferred traits in Tanzania. Journal of Crop Improvement, 32(6), 812–828. https://doi.org/10.1080/15427528.2018.1531801
- Diao, E., Dong, H., Hou, H., Zhang, Z., Ji, N., & Ma, W. (2015). Factors influencing aflatoxin contamination in before and after harvest peanuts: A review. Journal of Food Research, 4(1), 148. https://doi.org/10.5539/jfr.v4n1p148
- Dorner, J. W. (2004). Biological control of aflatoxin contamination of crops. Journal of Toxicology: Toxin Reviews, 23(2–3), 425–450. https://doi.org/10.1081/TXR-200027877
- Dorner, J. W. (2009). Biological control of aflatoxin contamination in corn using a nontoxigenic strain of Aspergillus flavus. Journal of Food Protection, 72(4), 801–804. https://doi.org/10.4315/0362-028x-72.4.801
- Duc, P. A., Dharanipriya, P., Velmurugan, B. K., & Shanmugavadivu, M. (2019). Groundnut shell-a beneficial bio-waste. Biocatalysis and Agricultural Biotechnology, 20, 101206. https://doi.org/10.1016/j.bcab.2019.101206
- Engels, J. (2011). Groundnut grower’s guide for Mozambique: Production, harvesting and post-harvest handling. CNFA, 58 pp.
- Fahad, S., Bajwa, A. A., Nazir, U., Anjum, S. A., Farooq, A., Zohaib, A., Sadia, S., Nasim, W., Adkins, S., Saud, S., Ihsan, M. Z., Alharby, H., Wu, C., Wang, D., & Huang, J. (2017). Crop production under drought and heat stress: Plant responses and management options. Frontiers in Plant Science, 8, 1147. https://doi.org/10.3389/fpls.2017.01147
- FAOSTAT. (2021). Statistical data on crops, groundnut, area, production quantity of Tanzania, Africa & World. http://faostat.fao.org.
- FAOSTAT. (2022). FAO Stat. Database, 2021.
- Fatah, A. A. (2019). Mycotoxins contamination of food in Somalia, 2019.
- Gichohi-Wainaina, W. N., Kimanya, M., Muzanila, Y. C., Kumwenda, N. C., Msere, H., Rashidi, M., Mponda, O., & Okori, P. (2023). Aflatoxin contamination, exposure among rural smallholder farming Tanzanian mothers and associations with growth among their children. Toxins, 15(4), 257. https://doi.org/10.3390/toxins15040257
- Giordano, D. F., Pastor, N., Palacios, S., Oddino, C. M., & Torres, A. M. (2021). Peanut leaf spot caused by Nothopassalora personata. Tropical Plant Pathology, 46(2), 139–151. https://doi.org/10.1007/s40858-020-00411-3
- Guchi, E. (2015). Aflatoxin contamination in groundnut (Arachis hypogaea L.) caused by Aspergillus species in Ethiopia. Journal of Applied & Environmental Microbiology, 3(1), 11–19.
- Halder, D., Kheroar, S., Srivastava, R. K., & Panda, R. K. (2020). Assessment of future climate variability and potential adaptation strategies on yield of peanut and Kharif rice in eastern India. Theoretical and Applied Climatology, 140(3–4), 823–838. https://doi.org/10.1007/s00704-020-03123-5
- Hirozawa, M. T., Ono, M. A., Suguiura, I. M. D. S., Bordini, J. G., & Ono, E. Y. S. (2023). Lactic acid bacteria and Bacillus spp. as fungal biological control agents. Journal of Applied Microbiology, 134(2), lxac083. https://doi.org/10.1093/jambio/lxac083
- Hoffmann, M. P., Odhiambo, J. J., Koch, M., Ayisi, K. K., Zhao, G., Soler, A. S., & Rötter, R. P. (2018). Exploring adaptations of groundnut cropping to prevailing climate variability and extremes in Limpopo Province, South Africa. Field Crops Research, 219, 1–13. https://doi.org/10.1016/j.fcr.2018.01.019
- Jahanzaib, M., Nawaz, N., Khurshid, H., Jan, S. A., Arshad, M., & Hassan, I. (2019). Estimating genotype × environment interaction for groundnut seed yield across different ecological zones. International Journal of Agriculture & Biology, 22, 139–145.
- Jordan, D., Appaw, W., Ellis, W. O., Akromah, R., Mochiah, M. B., Abudulai, M., Brandenburg, R. L., Jelliffe, J., Bravo-Ureta, B., & Boote, K. (2020). Evaluating improved management practices to minimize aflatoxin contamination in the field, during drying, and in storage in Ghana. Peanut Science, 3–15. https://doi.org/10.3146/PS20-3.1
- Kaale, L. D., Kimanya, M. E., Macha, I. J., & Mlalila, N. (2021). Aflatoxin contamination and recommendations to improve its control: A review. World Mycotoxin Journal, 14(1), 27–40. https://doi.org/10.3920/WMJ2020.2599
- Kamala, A., Shirima, C., Jani, B., Bakari, M., Sillo, H., Rusibamayila, N., De Saeger, S., Kimanya, M., Gong, Y. Y., Simba, A., & Investigation Team. (2018). Outbreak of an acute aflatoxicosis in Tanzania during 2016. World Mycotoxin Journal, 11(3), 311–320. https://doi.org/10.3920/WMJ2018.2344
- Kumar, A., Pathak, H., Bhadauria, S., & Sudan, J. (2021). Aflatoxin contamination in food crops: Causes, detection, and management: A review. Food Production, Processing and Nutrition, 3(1), 1–9. https://doi.org/10.1186/s43014-021-00064-y
- Kumar, V., & Thirumalaisamy, P. P. (2016). Diseases of groundnut. In Disease of field crops and their management (pp. 459–487). Indian Phytopathological Society, Today and Tomorrow’s Printers and Publishers.
- Kumar, V., Bagwan, N. B., & Singh, D. (2009). On-farm evaluation of cultural practices for management of aflatoxins in groundnut. Journal of Mycology and Plant Pathology, 39(2), 271.
- Lagogianni, C. S., & Tsitsigiannis, D. I. (2018). Effective chemical management for prevention of aflatoxins in maize. Phytopathologia Mediterranea, 57(1), 186–198.
- Lavkor, I., & Var, I. (2017). The control of aflatoxin contamination at harvest, drying, pre-storage and storage periods in peanut: The new approach. In Aflatoxin-control, analysis, detection and health risks (pp. 45–64). http://dx.doi.org/10.5772/intechopen.68675
- Lee, J., Her, J. Y., & Lee, K. G. (2015). Reduction of aflatoxins (B1, B2, G1, and G2) in soybean-based model systems. Food Chemistry, 189, 45–51. https://doi.org/10.1016/j.foodchem.2015.02.013
- Lewis, L., Onsongo, M., Njapau, H., Schurz-Rogers, H., Luber, G., Kieszak, S., Nyamongo, J., Backer, L., Dahiye, A. M., Misore, A., DeCock, K., & Rubin, C. (2005). Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environmental Health Perspectives, 113(12), 1763–1767. https://doi.org/10.1289/ehp.7998
- Mahuku, G., Mauro, A., Pallangyo, B., Nsami, E., Boni, S. B., Koyano, E., Mponda, O., Ortega-Beltran, A., Atehnkeng, J., Aquiline, F., Samuel, R., Njela, J., Cotty, P. J., & Bandyopadhyay, R. (2023). Atoxigenic-based technology for biocontrol of aflatoxin in maize and groundnuts for Tanzania. World Mycotoxin Journal, 16(1), 59–74. https://doi.org/10.3920/WMJ2021.2758
- Martínez, J., Hernández-Rodríguez, M., Méndez-Albores, A., Téllez-Isaías, G., Mera Jiménez, E., Nicolás-Vázquez, M. I., & Miranda Ruvalcaba, R. (2023). Computational studies of aflatoxin B1 (AFB1): A review. Toxins, 15(2), 135. https://doi.org/10.3390/toxins15020135
- Massomo, S. M. (2020). Aspergillus flavus and aflatoxin contamination in the maize value chain and what needs to be done in Tanzania. Scientific African, 10, e00606. https://doi.org/10.1016/j.sciaf.2020.e00606
- Migwi, B., Mutegi, C., Mburu, J., Wagacha, J., Cotty, P., Bandyopadhyay, R., & Manyong, V. M. (2020). Assessment of willingness-to-pay for Aflasafe KE01, a native biological control product for aflatoxin management in Kenya. Food Additives & Contaminants: Part A, 37(11), 1951–1962. https://doi.org/10.1080/19440049.2020.1817571
- Mohammed, A. (2011). Survey and identification of Aspergillus species and Aflatoxin contamination of groundnut (Arachis hypogaea. L.) in Eastern Ethiopia. Hawassa University.
- Mohammed, A., Chala, A., Dejene, M., Fininsa, C., Hoisington, D. A., Sobolev, V. S., & Arias, R. S. (2016). Aspergillus and aflatoxin in groundnut (Arachis hypogaea L.) and groundnut cake in Eastern Ethiopia. Food Additives & Contaminants: Part B, 9(4), 290–298. https://doi.org/10.1080/19393210.2016.1216468
- Mohapatra, D., Kumar, S., Kotwaliwale, N., & Singh, K. K. (2017). Critical factors responsible for fungi growth in stored food grains and non-Chemical approaches for their control. Industrial Crops and Products, 108, 162–182. https://doi.org/10.1016/j.indcrop.2017.06.039
- Munthali, W. M., Charlie, H. J., Kachulu, L., & Seetha, D. (2016). How to reduce aflatoxin contamination in groundnuts and maize a guide for extension workers. Monograph. ICRISAT.
- Mupunga, I., Mngqawa, P., & Katerere, D. R. (2017). Peanuts, aflatoxins and undernutrition in children in Sub-Saharan Africa. Nutrients, 9(12), 1287. https://doi.org/10.3390/nu9121287
- Ncube, J., & Maphosa, M. (2020). Current state of knowledge on groundnut aflatoxins and their management from a plant breeding perspective: Lessons for Africa. Scientific African, 7, e00264. https://doi.org/10.1016/j.sciaf.2020.e00264
- Nesci, A., Passone, M. A., Barra, P., Girardi, N., García, D., & Etcheverry, M. (2016). Prevention of aflatoxin contamination in stored grains using chemical strategies. Current Opinion in Food Science, 11, 56–60. https://doi.org/10.1016/j.cofs.2016.09.010
- Ngotho, E. N. (2017). Effects of social learning in adoption of aflatoxin reduction interventions [Doctoral dissertation, University of Nairobi].
- Nigam, S. N., Waliyar, F., Aruna, R., Reddy, S. V., Kumar, P. L., Craufurd, P. Q., Diallo, A. T., Ntare, B. R., & Upadhyaya, H. D. (2009). Breeding peanut for resistance to aflatoxin contamination at ICRISAT. Peanut Science, 36(1), 42–49. https://doi.org/10.3146/AT07-008.1
- Ola, O. T., Ogedengbe, O. O., Raji, T. M., Eze, B., Chama, M., Ilori, O. N., Awofisayo, M. A., Kaptoge, L., Bandyopadhyay, R., Ortega-Beltran, A., & Ndarubu, A. A. (2022). Aflatoxin biocontrol effectiveness in the real world—private sector-led efforts to manage aflatoxins in Nigeria through biocontrol-centered strategies. Frontiers in Microbiology, 13, 977789. https://doi.org/10.3389/fmicb.2022.977789
- Outbreak News Today. (2017). Aflatoxin kills 4 children in Tanzania, linked to consumption of maize. https://outbreaknewstoday.com/aflatoxin-kills-4-children-in-tanzania-linked-to-consumption-of-maize-54210/
- Pandey, M. K., Kumar, R., Pandey, A. K., Soni, P., Gangurde, S. S., Sudini, H. K., Fountain, J. C., Liao, B., Desmae, H., Okori, P., Chen, X., Jiang, H., Mendu, V., Falalou, H., Njoroge, S., Mwololo, J., Guo, B., Zhuang, W., Wang, X., Liang, X., & Varshney, R. K. (2019). Mitigating aflatoxin contamination in groundnut through a combination of genetic resistance and post-harvest management practices. Toxins, 11(6), 315. https://doi.org/10.3390/toxins11060315
- Parimi, V., Kotamraju, V. K. K., & Sudini, H. K. (2018). On-farm demonstrations with a set of good agricultural practices (GAPs) proved cost-effective in reducing pre-harvest aflatoxin contamination in groundnut. Agronomy, 8(2), 10. https://doi.org/10.3390/agronomy8020010
- Pickova, D., Ostry, V., Toman, J., & Malir, F. (2021). Aflatoxins: History, significant milestones, recent data on their toxicity and ways to mitigation. Toxins, 13(6), 399. https://doi.org/10.3390/toxins13060399
- Qi, X., Chen, B., & Rao, J. (2023). Natural compounds of plant origin in the control of fungi and mycotoxins in foods. Current Opinion in Food Science, 52, 101054. https://doi.org/10.1016/j.cofs.2023.101054
- Rago, A. M., Cazón, L. I., Paredes, J. A., Molina, J. P. E., Conforto, E. C., Bisonard, E. M., & Oddino, C. (2017). Peanut smut: From an emerging disease to an actual threat to Argentine peanut production. Plant Disease, 101(3), 400–408. https://doi.org/10.1094/PDIS-09-16-1248-FE
- Raman, J., Kim, J. S., Choi, K. R., Eun, H., Yang, D., Ko, Y. J., & Kim, S. J. (2022). Application of lactic acid bacteria (LAB) in sustainable agriculture: Advantages and limitations. International Journal of Molecular Sciences, 23(14), 7784. https://doi.org/10.3390/ijms23147784
- Reverberi, M., Ricelli, A., Zjalic, S., Fabbri, A. A., & Fanelli, C. (2010). Natural functions of mycotoxins and control of their biosynthesis in fungi. Applied Microbiology and Biotechnology, 87(3), 899–911. https://doi.org/10.1007/s00253-010-2657-5
- Seetha, A., Munthali, W., Msere, H. W., Swai, E., Muzanila, Y., Sichone, E., Tsusaka, T. W., Rathore, A., & Okori, P. (2017). Occurrence of aflatoxins and its management in diverse cropping systems of central Tanzania. Mycotoxin Research, 33(4), 323–331. https://doi.org/10.1007/s12550-017-0286-x
- Senghor, A. L., Ortega-Beltran, A., Atehnkeng, J., Jarju, P., Cotty, P. J., & Bandyopadhyay, R. (2021). Aflasafe SN01 is the first biocontrol product approved for aflatoxin mitigation in two nations, Senegal and The Gambia. Plant Disease, 105(5), 1461–1473. https://doi.org/10.1094/PDIS-09-20-1899-RE
- Senghor, L. A., Ortega-Beltran, A., Atehnkeng, J., Callicott, K. A., Cotty, P. J., & Bandyopadhyay, R. (2020). The atoxigenic biocontrol product Aflasafe SN01 is a valuable tool to mitigate aflatoxin contamination of both maize and groundnut cultivated in Senegal. Plant Disease, 104(2), 510–520. https://doi.org/10.1094/PDIS-03-19-0575-RE
- Singh, A. L., & Basu, M. S. (2005). Integrated nutrient management in groundnut-A farmer’s manual. National Research Centre Forgroundnut.
- Singh, A., Raina, S. N., Sharma, M., Chaudhary, M., Sharma, S., & Rajpal, V. R. (2021). Functional uses of peanut (Arachis hypogaea L.) seed storage proteins. In Grain and seed proteins functionality (pp. 121–142). http://dx.doi.org/10.5772/intechopen.87503
- Song, T. K., Zamri, N. E. M., Ibrahim, R., Mohtar, J. A., Abbas, H., & Rahman, A. M. A. (2023). Differential display reverse transcription polymerase chain reaction (DDRT-PCR) for grey oyster mushroom samples grown with acoustic sound treatment. In IOP Conference Series: Earth and Environmental Science (Vol. 1216, p. 012018). IOP Publishing. https://doi.org/10.1088/1755-1315/1216/1/012018
- Tahir, N. I., Hussain, S., Javed, M., Rehman, H., Shahzady, T. G., Parveen, B., & Ali, K. G. (2018). Nature of aflatoxins: Their extraction, analysis, and control. Journal of Food Safety, 38(6), e12561. https://doi.org/10.1111/jfs.12561
- Tiwari, S., & Dubey, N. K. (2023). Nanoencapsulated essential oils as sustainable approach for control of fungal and mycotoxin contamination of food commodities. Current Opinion in Food Science, 52, 101053. https://doi.org/10.1016/j.cofs.2023.101053
- Torres, O., Matute, J., Gelineau-van Waes, J., Maddox, J. R., Gregory, S. G., Ashley-Koch, A. E., Showker, J. L., Voss, K. A., & Riley, R. T. (2015). Human health implications from co-exposure to aflatoxins and fumonisins in maize-based foods in Latin America: Guatemala as a case study. World Mycotoxin Journal, 8(2), 143–160. https://doi.org/10.3920/WMJ2014.1736
- Veras, F. F., Silveira, R. D., & Welke, J. E. (2023). Bacillus spp. as a strategy to control fungi and mycotoxins in food. Current Opinion in Food Science, 52, 101068. https://doi.org/10.1016/j.cofs.2023.101068
- Waliyar, F., Osiru, M., Ntare, B. R., Kumar, K. V. K., Sudini, H., Traore, A., & Diarra, B. (2015). Post-harvest management of aflatoxin contamination in groundnut. World Mycotoxin Journal, 8(2), 245–252. https://doi.org/10.3920/WMJ2014.1766
- Waliyar, F., Osiru, M., Sudini, H., & Njoroge, S. M. C. (2013). Reducing aflatoxins in groundnuts through integrated management and biocontrol. In Aflatoxins - finding solutions for improved food safety (pp. 1–2). International Food Policy Research Institute. http://oar.icrisat.org/id/eprint/7389
- Xiong, K., Liu, H. J., & Li, L. T. (2012). Product identification and safety evaluation of aflatoxin B1 decontaminated by electrolyzed oxidizing water. Journal of Agricultural and Food Chemistry, 60(38), 9770–9778. https://doi.org/10.1021/jf303478y
- Yahaya, I., Dankyi, A., Nboyine, J., Abudulai, M., Mahama, G., Mochia, B., Brandenburg, R., Ureta, B. B., & Jordan, D. (2022). Adoption of post-harvest strategies to minimize aflatoxin contamination in groundnut (Arachis Hypogaea) in Ghana. Archives of Agriculture Research and Technology, 3, 1042.
- Yang, Q. (2019). Decontamination of aflatoxin B1. In Aflatoxin B1 occurrence, detection and toxicological effects (177–180). http://dx.doi.org/10.5772/intechopen.77925
- Zhang, Q., Xiong, K., Tatsumi, E., Li, L.-T., & Liu, H.-J. (2012). Elimination of aflatoxin B1 in peanuts by acidic electrolyzed oxidizing water. Food Control, 27(1), 16–20. https://doi.org/10.1016/j.foodcont.2012.02.029
