Abstract
Oebalus pugnax (F.), is a late-season pest of rice during the reproductive stage. A two-year field study was conducted in Stoneville, Mississippi, to characterize and evaluate various O. pugnax infestation durations on rice yield and quality at the milk stage (R5.5-R6.5) of panicle development. In 2011, twenty adult O. pugnax were caged individually on ˈWellsˈ panicles for 3, 5, or 7 days regardless of sex. The experiment was repeated in 2012 to include a 1-day infestation duration and a sex-controlled infestation of 10 males and 10 females caged individually on ‘Wells’ and ‘Cocodrie’ rice identification of panicles. The weight and percentages of the filled, undamaged, damaged, and blank kernels per panicle were evaluated. Filled kernel weight did not differ significantly amongst the various O. pugnax infestation durations. The undamaged kernels decreased with increasing infestation duration between 11% and 21% relative to the control treatment. The damaged and blank kernels increased with increasing infestation duration between 113–262% and 27–192% for the damaged and blank kernels, respectively. The average single kernel weight for undamaged, damaged, and blank kernels was 24, 13, and 4 mg, respectively. The damaged kernel resulted in a 46% weight reduction relative to the undamaged kernel. Female O. pugnax infestations resulted in a greater yield and quality reduction than male infestations. This study provides Mississippi rice producers, with first-hand information of O. pugnax feeding duration on kernel yield and quality. Future studies on the milling quality and identification of fungi pathogens associated with Mississippi rice may be desirable.
REVIEWING EDITOR:
1. Introduction
The rice stink bug, Oebalus pugnax, is a major late-season insect pest of rice (Oryza sativa L.) during the reproductive stage. Severe O. pugnax infestations can cause significant yield reduction in commercial rice production in the southern United States (Wilson & Stout, Citation2020). This phytophagous hemipteran pest causes feeding injury through a stylet (rostrum) penetration in a piercing-sucking process that extracts liquefied grain contents (Esquivel, Citation2015, Citation2019). This feeding process oftentimes results in stylet sheath deposition (Bowling, Citation1979). The feeding injury caused by this pest in rice can result in empty (blank) or damaged kernels. This primarily results in yield and quality reduction depending on the stage of panicle development (Awuni et al., Citation2015; Brorsen et al., Citation1988; Krinski & Foerster, Citation2017; Lorenz et al., Citation2021; Patel et al., Citation2006). The damaged kernels are often discolored and are commonly referred to as “pecky” rice.
Several factors have been reported to cause reduced grain yield and quality. For example, increase in temperature (1 °C) at nighttime (Peng et al., Citation2004), excessive nitrogen application (Pizzatti & Cortesi, Citation2008), planting date (Pizzatti & Cortesi, Citation2008), fungal infection (Marchetti & Peterson, Citation1984), and cultivar susceptibility (Bernhardt et al., Citation2004; Nilakhe, Citation1976). However, O. pugnax is generally accredited as the most important insect pest of rice capable of inflicting significant grain yield and quality reduction in rice production regions of the southern United States (Douglas & Tullis, Citation1950).
Grain yield from the field consists of undamaged and damaged kernels. The damaged kernels are often partially filled, shriveled, and shrunken with or without discolorations. Hence, such kernels consist of broken or whole grains distinctively discolored or chalky, atrophied or shriveled, and characterized by shrunken depression causing structural weakness at the point of injury. Marchetti and Peterson (Citation1984) described damaged kernels as black or dark brown circular discolorations around the stylet insertion point and under light intensity can be differentiated from undamaged rice kernels (Wang et al., 2002).
Except for the first instar, O. pugnax adults and nymphs feed similarly (Lorenz et al., Citation2021) and cause similar injury to rice from panicle exertion (R3-R4) (Moldenhauer et al., Citation2021) through pollination, caryopsis expansion (R4-R5) to grain filling (R6-R7) stages (Krinski & Foerster, Citation2017; Moldenhauer et al., Citation2021). Oebalus pugnax infestation at panicle exertion through pollination to caryopsis expansion causes blank kernels, while infestation at grain filling stage typically results in damaged kernels (Awuni et al., Citation2015; Krinski & Foerster, Citation2017). The grain-filling stage is divided into milk, soft, and hard dough stages (Counce et al., Citation2000; Moldenhauer et al., Citation2021), with each stage responding differently to O. pugnax injury (Awuni et al., Citation2015; Krinski & Foerster, Citation2017). Previous research suggests that the milk stage (R5.5–6.5) suffers greatly in both blank and damaged kernels due to O. pugnax feeding injury (Awuni et al., Citation2015; Krinski & Foerster, Citation2017; Patel et al., Citation2006).
The damaged kernels are of significant economic importance due to ‘pecky’ kernels that impact negatively on the milling quality, downgrading premium price, and consumer preference. In the U.S., milled rice containing more than 0.1 percent pecky grain by weight may not be designated No. 1 grade rice with consequences in discounted price premiums to rice producers (Brorsen et al., Citation1988; USDA-FGIS, Citation2002). The discounted price premiums result in considerable economic losses to rice producers and sometimes possible rejection by rice consumers. To reduce the impact of O. pugnax on grain quality, economic thresholds have been developed based on the week of panicle development (Harper et al., Citation1993).
Feeding duration studies have been performed on some stink bug species to determine the economic impact on various crops (Depieri & Panizzi, Citation2011; Simmons & Yeargan, Citation1988). However, there are limited studies on the impact of O. pugnax infestation duration in rice, especially during the grain-filling period considered the most vulnerable to this pest damage. Additionally, there is significant asynchronicity within and within panicle development, and within and between rice plants. This asynchronicity provides a complex sink-source balance in kernel development (Chen et al., Citation2019; Okamura et al., Citation2018). Besides, the basic determinants of grain yield in rice are the panicle number per plant, kernel number per panicle, and kernel filling capacity.
This study was designed to elucidate the impact of O. pugnax infestation duration during the milk stage (R5.5-R6.5) of panicle development on grain yield and quality and to compare the feeding injury of adult O. pugnax males and females.
2. Materials and methods
2.1. Site description
A two-year field study was conducted at the Delta Research and Extension Center (DREC) in Stoneville, Mississippi, United States. Stoneville is within Latitude 33.4240° N and Longitude 90.9151° W at 39 meters above sea level (U.S. Climate Data, Citation2024). The experimental site had soils described as fine-textured alluvial Sharkey Series (Snipes et al., Citation2005).
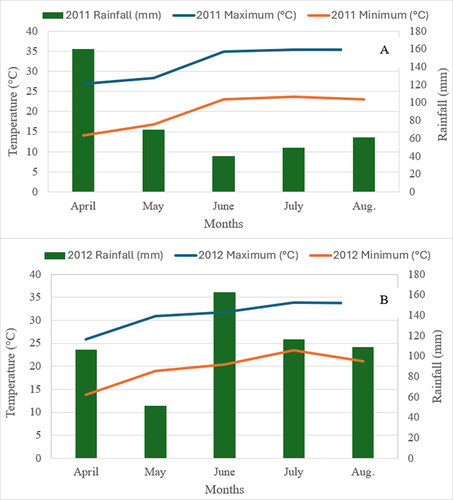
The average total annual rainfall was 1333 mm with average annual maximum and minimum temperatures of 23.3 °C and 11.7 °C, respectively (U.S. Climate Data, Citation2024). The total monthly rainfall during the study period was highest (160 mm) in April and lowest (40 mm) in June for 2011 (), while the highest (162 mm) and lowest (52 mm) rainfall occurred in June and May, respectively, for 2012 (). In both years, temperatures were relatively higher from June to August compared to April and May ().
Figure 1. Rainfall (mm) and temperature (°C) during the study period at Stoneville, Mississippi, United States in 2011 (A) and 2012 (B).
The research was conducted following guidelines provided by the Delta Research and Extension Center (DREC) and the Mississippi Agricultural and Forestry Experiment Station (MAFES).
2.2. Rice cultivars
Rice is cultivated in three key regions in the United States: the Mississippi Delta (Arkansas, Mississippi, northeast Louisiana, Southeast Missouri), the Gulf Coast (Southwest Louisiana, Southeast Texas), and the Central Valleys of California. The availability of rice seeds is made through the foundation seed programs in each rice-producing state. The rice cultivars ‘Cocodrie’ (Reg. no. CV-111, PI 606331) and ‘Wells’ (U.S. Patent 6,281,416 B1) (Linscombe et al., Citation2000; Moldenhauer et al., Citation2007) were made available for this experiment through the foundation seed program. The cultivars are high-yielding, semi-dwarf, short-season, and long-grain commercial rice cultivars popular among rice producers in the mid-southern rice-producing states (Linscombe et al., Citation2000; Moldenhauer et al., Citation2007). ‘Cocodrie’ was developed and maintained by the Louisiana State University Agricultural Center (Linscombe et al., Citation2000); while ‘Wells’ was developed and maintained by the University of Arkansas System Division of Agriculture’s Rice Research and Extension Center in Stuttgart, Arkansas (Moldenhauer et al., Citation2007).
2.3. Insect collection
Adult O. pugnax were collected from heading grasses in and around Stoneville, MS, with a 38 cm diameter sweep net (BioQuip Products, Rancho Dominguez, CA, USA). Samples consisting of ten sweeps of adult O. pugnax were collected, removed, and placed in Bugdorm (29 × 29 × 29 cm) rearing cages (BioQuip Products, Rancho Dominguez, CA, USA) made of white polypropylene screen. The sampled insects were transported to the laboratory and placed individually in 37.0 ml plastic cups and maintained on a 10% (w/v) sugar solution. The insects were kept under controlled conditions (25 ± 2 °C, 60 ± 5 RH, and photoperiod of 14:10 L:D) for at least 12 h to stabilize before field-level infestation. The test insects were transported to the field in a cooler to minimize heat stress during transportation for field-level infestation.
2.4. Experimental design
The experiment was a completely randomized design (CRD) with a one-factorial split plot arrangement on O. pugnax infestation durations at four infestation levels on ˈWellsˈ rice panicles in 2011. Twenty (20) panicles were individually caged with an adult O. pugnax for 3, 5, or 7 infestation durations (days) regardless of sex. In 2012, the experiment utilized a two-factorial split plot arrangement of O. pugnax sex and infestation duration at five infestation levels on ‘Wells’ and ‘Cocodrie’ rice panicles. The O. pugnax sex factor was controlled per cultivar with ten (10) panicles caged individually with adult males and another set of ten (10) panicles caged individually with adult females within an infestation duration for 1, 3, 5, or 7 days. Un-infested (0 days infested duration) panicles were included in each level of infestation duration as untreated controls to account for the cage effect on yield and quality for the entire infestation duration.
2.5. Treatment procedure
The milk stage (R5.5–6.5) was determined, and O. pugnax infestation duration was imposed approx. 115 days after planting (DAP) with removal timings (RTs) of 114, 116, 118, and 120 DAP corresponding to 1, 3, 5, and 7 days RTs. The two cultivars appeared to differ in maturity resulting in asynchronous treatments between cultivars in 2012 (Awuni et al., Citation2015). Nylon sleeve cages (25.4 × 50.8 cm) constructed from white polyester/nylon nettings with 20 mesh size (approx.) with drawstrings and toggles to open and close after infestation were used to cage test insects on the panicles. Twenty (20) rice panicles served as replicates within an infestation duration with individual O. pugnax adult caged per panicle during the milk stage of panicle development. The twenty panicles were randomly selected from a 6.6 m2 block of rice plants per cultivar at each level of infestation duration. Each treatment level (panicles) was identified with different colored tags (Snap-on, A. M. Leonard Inc., Piqua, OH, USA) placed around the stem of the infested panicles just above the flag leaf to facilitate identification at harvest. The panicles of ‘Wells’ were infested on August 10, 2011, and August 15, 2012, whereas ‘Cocodrie’ panicles were infested on August 3, 2012.
2.6. Crop management
‘Wells’ was tested for the two years, whereas ˈCocodrieˈ was included during the second year. The two rice cultivars were drill-seeded at 90 kg ha−1 into separate blocks in plots of eight rows (18 cm) wide and 4.57 m long on April 13, 2011, and April 26, 2012. The crops were managed under field conditions using standard agronomic practices for Mississippi drill-seeded rice (Buehring et al., Citation2008). Urea (46%) granular (90% 2–4 mm) nitrogen (Cargill LTD, No. 26, Saint-Petersburg, FL) was applied at 202 kg ha−1 on June 1, 2011, and June 19, 2012, at the 5–6 leaf stage. The rice plots were permanently flooded on June 2, 2011, and June 20, 2012, and was maintained until two weeks before harvest. The panicles were individually hand-harvested with scissors at maturity on September 8, 2011, and September 11, 2012. Individual panicles were placed in brown paper bags (W 12.5 ×L 27 cm) and allowed to air dry to approximately 12% moisture.
2.7. Data collection
The kernels per panicle were manually threshed in the laboratory and placed per panicle in 37.0 ml plastic cups for yield and quality analysis. The blank kernels were first separated from the filled kernels by the “finger feel” texture method as described (Moorberg & Crouse, Citation2021). This separation procedure was by placing each test kernel between the thumb and forefinger to determine unfilled regions. The filled kernels were separated into undamaged and damaged categories with a Gagne Porta-Trace Brand LED Lightbox (Gagne, Inc., Johnson City, NY, USA) (Wang et al., Citation2002). Green grain or immature rice kernels were included in the damaged category. Random samples from the filled kernel category were characterized through an imaging process using a Leica DFC 495 digital camera (Leica Microsystem Inc., Buffalo Grove, IL, USA) and a Leica Z16 microscope with motorized Z-stepping. The image stacks were merged using the Leica Application Suite v. 4.1.0 with Montage Module.
2.8. Data analysis
The weight of filled (yield), undamaged, damaged, and blank kernels per panicle were evaluated. The filled kernels (g) per panicle consisted of undamaged and damaged kernels. The analysis was expressed as weight and percentage number per panicle. Cultivar within a year was considered a dataset and analyzed as three independent datasets, namely ‘Wells’ 2011, ‘Wells’ 2012, and ‘Cocodrie’ 2012. In the first analysis, all three datasets were combined and analyzed without considering stink bug sex for a one-factor analysis of infestation duration (days). The second analysis considered the 2012 dataset for a two-factorial analysis of O. pugnax sex by infestation duration. The infestation duration, sex, and interaction were fixed effects, while the year, replication, and replications within the year were considered random effects. All data were analyzed using PROC-MIXED in SAS version 9.4 (Littell et al., Citation1996; SAS Institute, Citation2023). Means and standard errors were calculated using an LSMEANS statement and separated according to Fisher’s protected least significant difference test (LSD) at α = 0.05.
3. Results
3.1. Characterization of grain quality
The characterization of grain quality is illustrated in . The categories of kernels included undamaged (), which appear translucent, versus damaged kernels (), which appear chalky, discolored, shriveled, and sunken. The damaged kernels included stylet sheath () deposition, and discolored, shrunken kernels with or without sunken circular lesions (). The discolorations ranged from brown to black (dark), with a blend of yellow or red in various positions and shapes on part or whole kernel (). In the advanced stage, the damaged kernels may be severed by dark brown fruiting bodies of saprophytic fungi developed under the kernel coats (). Other forms of kernel damage were with or without discolored shrunken and sunken lesions at the proximal (embryo) portion of the kernel and extending half of the kernel length () or a similar damage from the distal (flower) section of the kernel and extending half the kernel length (). Green grain or immature rice kernels with reduced grain size were identified and included in the damaged category i ().
Figure 2. Impact of O. pugnax on kernel. Undamaged kernel (A), kernel with volcanic-like stylet sheath (B), circular lesions with or without sunken portions (C), enlargement of lesions to whole kernel (D), advanced stage of damage with sclerotial bodies (E), kernels damaged from the embryo (F), kernel damaged from the flower end (G), and immature kernels (H).

3.2. Impact of O. pugnax infestation duration
3.2.1. Mean kernel weight per panicle
The filled kernel weight per panicle was not significantly different on O. pugnax various infestation durations during the milk stage of panicle development (). The filled kernel weight per panicle averaged 2.9 and 2.5 g in 2011 and 2012, respectively, across O. pugnax infestation durations (; Supplementary Table S1). The undamaged kernel weight per panicle differed significantly in 2012 (). The undamaged kernel weight per panicle was decreased with increased O. pugnax infestation duration by 15%, 19%, 19%, and 23% relative to the un-infested control ().
Figure 3. O. pugnax infestation duration on the weight of filled (g) (A), undamaged (g) (B), damaged (mg) (C), and blank (mg) (D) kernels per panicle in 2011 and 2012.
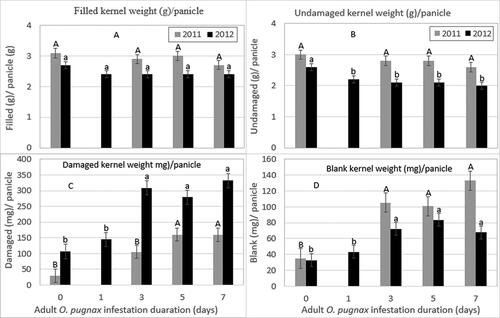
Table 1. Probability (F-values) for various infestation durations of adult O. pugnax to cause observed differences in yield (filled), undamaged, damaged, and blank kernels per panicle during the milk stage of panicle development.
Table 2. Probability (F-values) for adult O. pugnax sex by infestation durations on weight and percent (filled), undamaged, damaged, and blank kernels per panicle during the milk stage of panicle development.
In contrast, the weight of the damaged and blank kernels per panicle significantly increased with increased infestation durations in both years ().
The weight of damaged kernels was increased by 93%, 452%, and 498% for 3, 5, and 7-day infestation durations, respectively, relative to the un-infested control panicles in 2011. Similarly, the weight of damaged kernels was increased by 36%, 189%, 161%, and 210% for 1, 3, 5, and 7-day infestation durations, respectively, relative to the un-infested control panicles in 2012 (; Supplementary Table S1).
The blank kernel weight per panicle was increased by 200%, 189%, and 280% for 3, 5, and 7-day infestation durations, respectively, relative to the un-infested control panicles in 2011, while an increase of 30%, 118%, 154%, and 106% was recorded for 1, 3, 5, and 7-day infestation durations, respectively, when compared with the un-infested control panicles in 2012 (; Supplementary Table S1).
3.2.2. Percent kernel number per panicle
The percentage of filled, undamaged, damaged, and blank kernel numbers per panicle differed significantly on the various O. pugnax infestation durations in both years (). The percentage of filled and undamaged kernel numbers per panicle decreased with increased infestation durations. In 2011, the percentage of filled kernel number per panicle was reduced by 11%, 11%, and 17% for 3, 5, and 7-day infestation durations, respectively, compared to the un-infested control treatment; while in 2012, the percentage of filled kernel number per panicle was reduced by 3%, 8%, 13%, and 7% for 1, 3, 5, and 7-day infestation durations, respectively (; Supplementary Table S2). Similarly in 2011, the percentage of undamaged kernel number per panicle decreased by 12%, 16%, and 24% for 3, 5, and 7-day infestation durations, respectively, compared to the un-infested control panicles; while in 2012, the percentage of undamaged kernel number per panicle was reduced by 8%, 23%, 26%, and 26% for 1, 3, 5, and 7-day infestation durations relative to the un-infested control panicles (; Supplementary Table S2).
Figure 4. O. pugnax infestation duration on percent (%) filled (A), undamaged (B), damaged (C), and blank (D) kernels per panicle in 2011 and 2012.
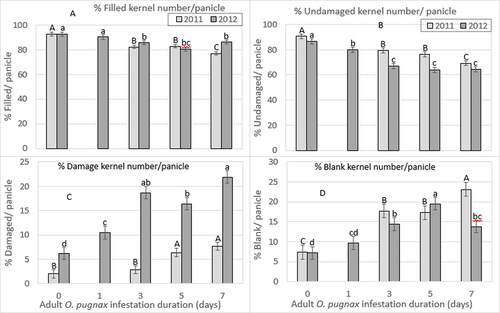
In contrast, the percentage of damaged and blank kernel numbers per panicle increased significantly with increased O. pugnax infestation durations. In 2011, the percentage of damaged kernel number per panicle increased by 45%, 220%, and 285% for 3, 5, and 7-day infestation durations, respectively, compared to the un-infested control; while in 2012, there was an increase in the percentage of damaged kernel number per panicle by 69%, 202%, 165%, and 253% for 1, 3, 5, and 7-day infestation durations, respectively, compared to the un-infested control panicles (; Supplementary Table S2). Similarly in 2011, the blank kernel weight per panicle was increased by 139%, 134%, and 212% for 3, 5, and 7-day infestation durations, respectively, compared to the un-infested control treatment, while in 2012, the percentage of blank kernel number per panicle was increased by 35%, 100%, 1711%, and 92% for 1, 3, 5, and 7-day infestation duration, respectively, compared to the un-infested control panicles (; Supplementary Table S2).
3.3. Effect of O. pugnax sex by infestation duration
3.3.1. Mean weight per panicle
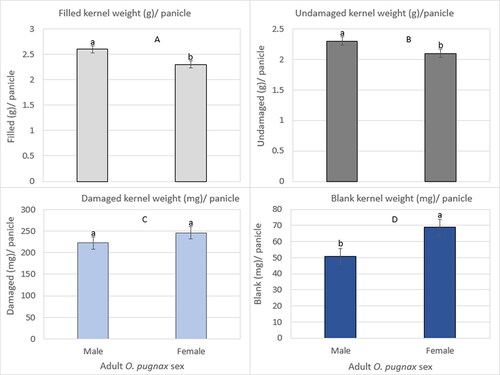
There was not significant O. pugnax sex × infestation duration on filled, undamaged, damaged, and blank kernel weight per panicle across cultivars ().
The main effect of O. pugnax sex significantly influenced the filled, undamaged, and blank kernel weight per panicle (). The filled () and undamaged () kernel weight per panicle was increased by 13% and 19%, respectively, in male infestations compared to female infestations (Supplementary Table S3). O pugnax sex was not significantly different on damaged kernel weight per panicle (), but did differ significantly on blank kernel weight per panicle with an increase of 36% in female infestations compared to male infestations (; Supplementary Table S3).
Figure 5. O. pugnax sex on weight of filled kernel (g) (A), undamaged (g) (B), damaged (mg) (C), and blank (mg) (D) per panicle across infestation durations and cultivars in 2012.
The O. pugnax infestation durations did not differ significantly on filled kernel weight per panicle (; Supplementary Table S3). However, O. pugnax infestation duration significantly influenced the undamaged, damaged, and blank kernel weight per panicle (). The undamaged kernel weight per panicle decreased with increasing O. pugnax infestation duration of 1, 3, 5, and 7 days by 15%, 19%, 19%, and 23% compared to the caged uninfested panicles at, respectively (; Supplementary Table S3).
Figure 6. O. pugnax infestation duration on the weight of filled kernel (g) (A), undamaged (g) (B), damaged (mg) (C), and blank (mg) (D) per panicle infestation duration across sex and cultivars in 2012.
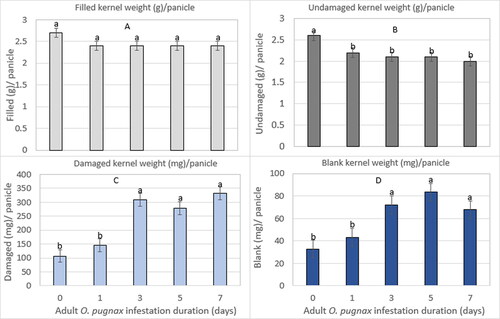
In contrast, the damaged kernel weight per panicle was increased with increasing O. pugnax infestation duartions of 1, 3, 5, and 7-day by 35%, 119%, 161%, and 210% for relative to the caged un-infested panicles (; Supplementary Table S3). Similarly, the blank kernel weight per panicle was increased by 32%, 120%, 155%, and 107% for 1, 3, 5, and 7-day infestation durations relative to the un-infested control panicles (; Supplementary Table S3).
3.3.2. Mean category percentages per panicle
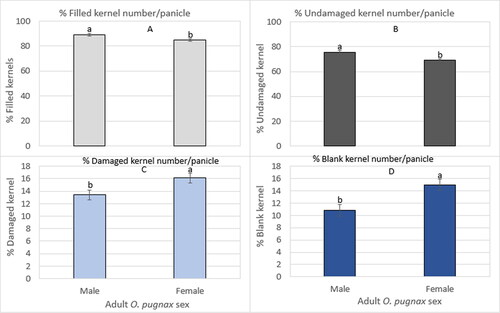
There was a significant O. pugnax sex × infestation duration interaction on the percentage of undamaged kernels per panicle (). The percentage of undamaged kernels number per panicle was highest in the un-infested control and 1-day infestation durations for both sexes with a significant decrease from the 3-day infestation duration for both the male infestation and female infestation (Supplementary Table S4). The O. pugnax sex × infestation duration did not differ significantly on the percentage of filled, damaged, and blank kernel number per panicle (). The main effect of O. pugnax sex differed significantly in the percentage of filled, undamaged, damaged, and blank kernel numbers per panicle across the categories (). The percentage of filled kernels per panicle () was higher by 5% and that of the undamaged () kernels per panicle was higher by 10% in the male infestations compared to the female infestations (Supplementary Table S4). In contrast, the percentage of damaged kernels () and blank kernels () number per panicle were higher in the female infestations by 20% and 39%, respectively, compared to the male infestations (Supplementary Table S4).
Figure 7. O. pugnax sex on percent (%) number of filled kernel (A), undamaged (B), damaged (C), and blank (D) per panicle across infestation durations and cultivars in 2012.
Similarly, the main effect of O. pugnax infestation duration on the percentage of the filled, undamaged, damaged, and blank kernel numbers per panicle differed significantly across the categories (). The percentage of filled kernel () and undamaged kernel () numbers per panicle decreased with increased O. pugnax infestation durations. Compared to the un-infested control treatment, the percentage of filled kernel number per panicle was decreased by 3%, 8%, 15%, and 8% in 1, 3, 5, and 7-day infestation durations (Supplementary Table S4). Similarly, the percentage of undamaged kernel number per panicle when compared with the un-infested control was decreased by 13%, 29%, 35%, and 35% in 1, 3, 5, and 7-day infestation durations, respectively.
Figure 8. O. pugnax infestation durations on percent (%) number of filled kernel (A), undamaged (B), damaged (C), and blank (D) per panicle across sex and cultivars in 2012.
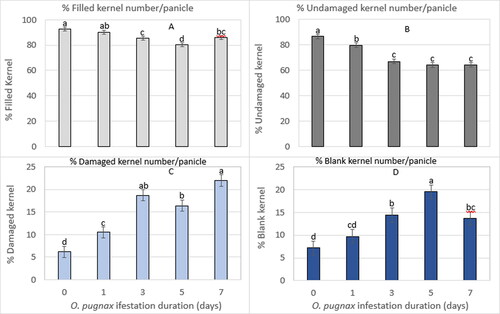
In contrast, the percentage of damaged kernel () and blank kernel () numbers per panicle increased with increased infestation durations. The highest percentage of damaged kernels per panicle was from the 7-day infestation duration with a decrease of 72%, 52%, 15%, and 26% in the control, 1, 3, and 5-day infestation durations, respectively (Supplementary Table S4). Similarly, the highest percentage of blank kernels per panicle was from the 5-day infestation duration with a decrease of 63%, 50%, 26%, and 30% in the control, 1, 3, and 7-day infestation durations, respectively (Supplementary Table S4).
3.4. Oebalus pugnax sex × infestation duration on kernel weight (mg) per category
3.4.1. Mean weight per kernel category
The interaction effect of O. pugnax sex × infestation duration, the main effect of sex, and infestation duration on single kernel weight of undamaged, damaged, and blank kernels were not statistically different within the kernel categories (). Therefore, regardless of O. pugnax sex () or infestation duration (), the weight per kernel of undamaged, damaged, and blank kernels averaged 24, 13, and 4 mg, respectively (Supplementary Table S5). This represented a 52% and 84% weight reduction in damaged and blank kernels relative to undamaged kernels, respectively.
Figure 9. O. pugnax sex (A) and infestation duration (B) on the weight of undamaged, damaged, and blank per kernel.
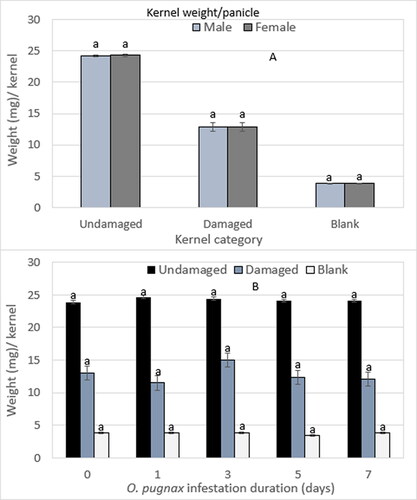
Table 3. Probability (F-values) for adult O. pugnax sex by infestation durations on the weight of undamaged, damaged, and blank per kernel during the milk stage of panicle development.
4. Discussion
Numerous studies have investigated the impact of O. pugnax on grain yield and quality (Awuni et al., Citation2015; Patel et al., Citation2006; Swanson & Newson, Citation1962; Weber et al., Citation2020; Wilson & Stout, Citation2020). Common among these studies suggest that rice is most susceptible to the injurious effects of O. pugnax from the flowering (R4/R5) stage with damaging effects during the milk (R5/R6) and soft dough (R6/R7) stages of panicle development. This further reaffirms that the damaging effect of this pest on rice is critical during the first two weeks of panicle development from flowering to the milk stage (Awuni et al., Citation2015; Patel et al., Citation2006).
Although most authors report significant yield and quality loss in all panicle development stages (Awuni et al., Citation2015; Krinski & Foerster, Citation2017; Patel et al., Citation2006), various authors have reported differently to the damaging effect of this pest on the grain-filling phase of panicle development. Espino et al. (Citation2007) observed a greater percentage of damaged kernels from infestations during the milk and soft dough stages compared to the flowering stage. Awuni et al. (Citation2015) and Krinski and Foerster (Citation2017) reported significant yield losses from infestations during flowering due to a greater percentage of blank kernels and the greatest number of damaged kernels from milk-stage infestations. These studies suggest that flowering and milk stages are the most susceptible to Oebalus spp. infestations. In our current study, O. pugnax infestation duration was controlled during the milk stage (R5.5-R6) to examine differences in grain yield and quality reductions in rice when allowed to feed at various duration timings.
The results indicated that 1-day was often enough to cause measurable impacts, with significant increases in the blank and damaged kernels from the 3-day infestation. Therefore, increases in the number of damaged and blank kernels will lead to a reduction in yield. This is with the assumption that no within-kernel and between-panicle compensation during the milk stage of panicle development. This study also indicated that O. pugnax damage was significant even at 1 day of infestation compared with the un-infested control. This suggests that a 1-day infestation duration can significantly impact rice yield and quality. The study indicated a 50% and 80% weight reduction per kernel of damaged and blank kernels, respectively, when compared to undamaged kernels. The summary from this study is that significant yield and quality reduction can occur within 1 to 3-day infestation duration during the milk stage of panicle development.
Yield loss per insect is one of the major variables in determining economic injury level (Poston et al., Citation1983). Tammes (Citation1961) observed three responses from the insect injury-yield loss relationship. The tolerant response, where some injury can occur before yield declines with increasing injury; the susceptive response indicates little compensation yield declines linearly with increasing injury; and the hyper susceptive response, where the yield loss is greatest at the low levels of injury with smaller yield loss as injury increases (Poston et al., Citation1983). This study indicated no compensation in the form of increased kernel weight and seems to demonstrate a tolerant response to infestation duration.
The damaged rice kernels (pecky, chalky, shriveled, or sunken) potentially impact milling quality and grade yield. The U.S. standard for milled rice is set at maximum limits of 1.0% damaged kernels for U.S. No. 1% to 6.0% damaged kernels for U.S. No. 6 (USDA-FGIS, Citation2002). Hence, damaged rice kernels pose a considerable challenge to the rice industry and require an integrated approach to ensure consistency in high-quality rice standards (Childs, Citation2012; Fitchette, Citation2024). Therefore, the damage to rice kernels due to O. pugnax infestation has serious economic implications for the rice industry.
Douglas and Tullis (Citation1950) observed that damaged rice kernels at the grain-filling stage were often discolored and associated the phenomena with the vectoring of O. pugnax. The characterization of the damaged kernels in this study supported that reported by Douglas and Tullis (Citation1950). However, the inclusion of immature rice grains in this study was arguably from the implication of that the stink bug species, Nezara viridula (southern green stink bug) caused delayed soybean maturity and reduced grain size (Boethel et al., Citation2000; Tood & Turnipseed, Citation1974). The immature rice kernels observed in this study were typically greenish tint, reduced in grain size, and structurally weak compared to matured kernels previously described by Boethel et al. (Citation2000) and Tood and Turnipseed (Citation1974)., and may be prone to breakage during milling (Siebenmorgen et al., Citation2023).
Espino and Way (Citation2007) previously infested rice panicles for 48 h with one adult O. pugnax male or female at the heading and grain-filling stages of panicle development and observed not a significant difference in grain yield during all the stage of panicle development. In this study, there was not a significant yield reduction in grain yield, however, there was a significant reduction of undamaged, and a significant increase in the damaged and blank kernel weight across infestation durations and sex. Thus, supporting the findings of Espino and Way (Citation2007), Naresh and Smith (Citation1984), and Rashid et al. (Citation2005). Bowling (Citation1979) used stylet sheath deposition rates of O. pugnax to determine feeding behavior and observed that females deposited more sheaths than males. The aggressive feeding by females may be an adaptation to provide nutrition for their large reproductive system. Cherry et al. (Citation2018) observed that adult O. pugnax females are generally larger in size and that their reproductive activities may require more nutrition than males. Weber et al. (Citation2020) reported similar findings, suggesting that female O. poecilus, an important pest of rice in South America, fed more on reproductive-stage rice and caused greater kernel damage.
Insect crop damage is two-dimensional, the injury per insect and damage per unit of injury (Pedigo et al., Citation1986). Arguably, caging one adult O. pugnax on one panicle for the infestation durations may appear high density relative to the natural field infestation condition. A previous study by Awuni et al. (Citation2015) using large cage (1.8 × 1.8 × 1.8 m3) infestation in Mississippi rice did not appear to have a significant impact on yields or grain quality across the stages of panicle development compared to the sleeve cage infestation. However, an examination of the infestations across cages appear to have higher quantitative yield losses during the flowering stage and higher qualitative yield loses more common for infestations during the milk and soft dough stages of panicle development. In this study, qualitative damage increased with increased infestation duration. A limitation of most cage studies is the non-inclusion of uncaged rice panicles as a natural control treatment to account for natural infestation. Buntin (Citation2001) noted that both caged and uncaged controls should be provided as treatments where data from the caged control is used for yield loss relationship and the comparison of the caged and uncaged controls accounts for cage effects. Cage effects may impose such limitations as altering the microclimate under the cage by reducing wind and light levels, thereby increasing the relative humidity, and ambient temperature. Arguably, it is generally observed that the cage effect becomes minimal when the pest is infested for a duration of 1–2 weeks (Buntin, Citation2001; Litsinger (Citation2009). Admittedly, quantifying the relationship between damage and insect numbers requires expensive detailed studies which the authors acknowledge and consider for future experiments.
Nonetheless, this study is important as it generates a gradient of artificial injury on various levels of O. pugnax infestation duration (days) mimicking the effect in field-level damage. While this experiment like many does provide some estimate of field-level infestation damage of O. pugnax, it does not account for milling quality. The damaged kernels (pecky, chalky, atrophied, shriveled, or sunken kernels) have a potential effect on both milling and grade yield. The determination of milling quality is a critical step in the assessment of O. pugnax damage in rice that needs consideration for future experiments.
5. Conclusion
This publication provides valuable data and information to practitioners as long as O. pugnax remains a concern to rice producers, his study is still valuable to rice producers in Mississippi.
In summary, longer infestation durations of O. pugnax during the milk stage resulted in a significant reduction in grain quality. The 1-day infestation was significant in reducing grain yield and quality. However, an infestation lasting 3 days consistently caused a significant yield and quality reduction. Based on this study, it is recommended that rice during the reproductive phase be vigorously monitored at least twice weekly from flowering through the milk stage to minimize economic yield reduction in rice. Management decisions should be based on the effects of O. pugnax infestation duration rather than the sex. Further study on milling quality and identifying fungi-associated pathogens in Mississippi rice is recommended.
Authors’ contributions
George Awuni, Jeffrey Gore, Donald Cook, Fred Musser, Tom Allen, and Maria Tomaso-Peterson: Conceptualization, data curation, formal analysis, methodology, writing review, and editing. George Awuni, Jeffrey Gore, Donald Cook, and Fred Musser: Data curation, funding acquisition, investigation, project administration, and writing the original draft.
Supplemental Material
Download MS Word (43 KB)Supplemental Material
Download MS Word (51.8 KB)Supplemental Material
Download MS Word (52.6 KB)Supplemental Material
Download MS Word (41.1 KB)Supplemental Material
Download MS Word (39.2 KB)Acknowledgment
The author(s) are grateful to Mr. Joseph McGown, Mr. Boise Stokes, Mr. David Cross, and Ms. Katherine Knighten for their invaluable technical contribution to this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting this article will be made available by the authors upon reasonable request.
Additional information
Funding
Notes on contributors
George A. Awuni
George A. Awuni (PhD) is an Assistant Research/Extension Professor in the Department of Agricultural Science and Plant Protection in the College of Agriculture and Life Sciences, at Mississippi State University. George obtained a PhD in Agricultural Entomology in row crops and is passionate about international agriculture research in promoting global food security with a focus on smallholder agriculture. He has over 10 years of international collaborative research with the USAID Soybean Innovation Laboratory (SIL) and the Savanna Agricultural Research Institute (SARI) managing the Soybean Management with Appropriate Research and Technology (SMART) Farm project in northern Ghana.
Jeffrey Gore
Jeffrey Gore (PhD) is an Extension/Research Professor in the Department of Agricultural Science and Plant Protection in the College of Agriculture and Life Sciences, at Mississippi State University. Jeff currently Heads the Delta Research and Extension Center (DREC) in Stoneville, Mississippi. Before assuming this administrative role as Head of DREC, he previously led a variety of field and laboratory Research/Extension activities in the management of insect pests of row crops in Mississippi. Previously, Jeff worked in several Research/Extension roles at Louisiana State University where he obtained a PhD. He has over 30 years of Research and Extension experience in agricultural entomology in row crops.
Maria Tomaso-Peterson
Maria Tomaso-Peterson (PhD) is an Extension/Research Professor Emerita in the Department of Agricultural Science and Plant Protection in the College of Agriculture and Life Sciences, at Mississippi State University. Her discipline is in Plant Pathology with a specialty in turf grass pathogens. Maria’s research interests are focused on turfgrass diseases, disease management strategies, and biological control tactics. Maria has over 15 years of research/extension experience at Mississippi State University.
Tom W. Allen
Tom W. Allen (PhD) is an Extension/Research Professor in the Department of Agricultural Science and Plant Protection in the College of Agriculture and Life Sciences, at Mississippi State University’s Delta Research and Extension (DREC) in Stoneville, Mississippi. Tom’s Extension/Research activities focus on plant disease management strategies with a specialty in row crop plant pathology. Tom has over 15 years of Research/Extension experience at Mississippi State University.
Donald R. Cook
Donald R. Cook (PhD) is an Associate Extension/Research Professor in the Department of Agricultural Science and Plant Protection in the College of Agriculture and Life Sciences, at Mississippi State University’s Delta Research and Extension (DREC) in Stoneville, Mississippi. He currently leads a variety of field and laboratory research/extension activities on the management of insect pests in row crops in Mississippi. Previously, Don worked in several research/extension roles at Louisiana State University where he obtained a PhD. Don has over 30 years of research and extension experience in agricultural entomology in various roles.
Fred R. Musser
Fred R. Musser (PhD) is Professor and Interim Head of the Department of Agricultural Science and Plant Protection in the College of Agriculture and Life Sciences, at Mississippi State University. Fred has over 25 years of research/teaching experience in agricultural entomology. His research includes Integrated Pest Management (IPM) tactics for insect pests of row crops, particularly cotton and soybeans, insect movement and modeling, feeding preferences, and sampling methods, especially tarnished plant bugs and stink bugs, insect resistance monitoring and management of tarnished plant bugs, cotton bollworms, and bean leaf beetle.
References
- Awuni, G. A., Gore, J., Cook, D., Musser, F., Catchot, A., & Dobbins, C. (2015). Impact of Oebalus pugnax (Hemiptera: Pentatomidae) infestation timing on rice yields and Quality. Journal of Economic Entomology, 108(4), 1–17. https://doi.org/10.1093/jee/tov123
- Bernhardt, J. L., Moldenhauer, K. A. K., & Gibbons, J. W. (2004). Screening rice lines for susceptibility to rice stink bug: Results from the arkansas rice performance tests. In Pest Management: Insects. B. R. Wells Rice Research Studies. Arkansas Agricultural Experiment Station Research Series, 529, 159–249.
- Boethel, D. J., Russin, J. S., Wier, A. T., Layton, M. B., Mink, J. S., & Boyd, M. L. (2000). Delayed maturity associated with southern green stink bug (Heteroptera: Pentatomidae) injury at various soybean phonological states. Journal of Economic Entomology, 93(3), 707–712. https://doi.org/10.1603/0022-0493-93.3.707
- Bowling, C. C. (1979). The stylet sheath as an indicator of feeding activity of the rice stink bug. Journal of Economic Entomology, 72(2), 259–260. https://doi.org/10.1093/jee/72.2.259
- Brorsen, B. W., Grant, W. R., & Rister, M. E. (1988). Some effects of rice quality on rough rice prices. Southern Agricultural Economics Association, 20(1), 1–10, https://doi.org/10.22004/ag.econ.29707.
- Buehring, N., Walker, T., & Bond, J. (2008). Rice stand establishment. In Mississippi’s rice grower’s Guide (pp. 9–15). Mississippi State University Extension Service. Publication. 2255.
- Buntin, G. B. (2001). Techniques for evaluating yield loss from insects. In R. K. D. Peterson. & L. G. Higley (Eds.), Biotic stress and yield loss. CRC Press LLC.
- Chen, L., Deng, Y., Zhu, H., Hu, Y., Jiang, Z., Tang, S., Wang, S., & Ding, Y. (2019). The Initiation of Inferior Grain Filling is Affected by Sugar Translocation Efficiency in Large Panicle Rice. Rice (New York, N.Y.), 12(1), 75. https://doi.org/10.1186/s12284-019-0333-7
- Cherry, R., Odero, C., Karounos, M., & Fernandez, J. (2018). Insecticidal control for the rice stink bug (Hemiptera: Pentatomidae) complex found in Florida rice. Journal of Entomological Science, 53(3), 372–378. https://doi.org/10.18474/JES17-126.1
- Childs, N. (2012). U.S. 2012/2013 rice yearbook. A Report from the Economic Research Service (ERS). USDA. Electronic Outlook. https://www.ers.usda.gov/webdocs/outlooks/38623/32617_rcs-12j.pdf?v=3283
- Counce, P. A., Keisling, T. C., & Mitchell, A. J. (2000). A uniform, objective, and adaptive system for expressing rice development. Crop Science, 40(2), 436–443. https://doi.org/10.2135/cropsci2000.402436x
- Depieri, R. A., & Panizzi, A. R. (2011). Duration of feeding and superficial and in-depth damage to soybean seed by selected species of stink bugs (Heteroptera: Pentatomidae). Neotropical Entomology, 40(2), 197–203. https://doi.org/10.1590/S1519-566X2011000200007
- Douglas, W. A., & Tullis, E. L. (1950). Insects and fungi as causes of pecky rice. USDA. Technical Bulletin, 1015, 1–20.
- Espino, L., & Way, M. O. (2007). Relative susceptibility of stages of rice panicle development to male and female Oebalus pugnax. Southwestern Entomologist, 32(4), 203–211. https://doi.org/10.3958/0147-1724-32.4.203
- Espino, L., Way, M. O., & Olson, J. K. (2007). Most susceptible stage of rice panicle development to Oebalus pugnax (Hemiptera: Pentatomidae). Journal of Economic Entomology, 100(4), 1282–1290. https://doi.org/10.1603/0022-0493(2007)100[1282:mssorp[PMC]2.0.co;2]
- Esquivel, J. F. (2015). Stylet penetration estimates for a suite of phytophagous hemipteran pests of row crops. Environmental Entomology, 44(3), 619–626. https://doi.org/10.1093/ee/nvv027
- Esquivel, J. F. (2019). Stink bug rostrum length vs. stylet penetration potential. Entomologia Experimentalis et Applicata, 167(4), 323–329. https://doi.org/10.1111/eea.12782
- Fitchette, T. (2024). Rice quality at heart of U.S. market share woes in Latin America. Available online: https://www.farmprogress.com/rice/rice-quality-at-heart-of-u-s-market-share-woes-in-latin-america. Accessed: 01/18/2024
- Krinski, D., & Foerster, L. A. (2017). Quantitative and qualitative damage caused by Oebalus poecilus (Hemiptera, Pentatomidae) to upland rice cultivated in new agricultural frontier of the Amazon rainforest (Brazil). Ciência e Agrotecnologia, 41(3), 300–311. https://doi.org/10.1590/1413-70542017413036816
- Harper, J. K., Way, M. O., Drees, B. M., Rister, M. E., & Mjelde, J. W. (1993). Damage function analysis for the rice stink bug (Hemiptera: Pentatomidae). Journal of Economic Entomology, 86, 1250–1258.
- Linscombe, S. D., Jodari, F., Bollich, P. K., Groth, D. E., White, L. M., Chu, Q. R., Dunand, R. T., & Sanders, D. E. (2000). Registration of ‘Cocodrie’ rice. Crop Science, 40(1), 294–294. https://doi.org/10.2135/cropsci2000.0007rcv
- Litsinger, J. A. (2009). When is a rice insect a pest: Yield loss and the green revolution. In R. Peshin, A.K. Dhawan (Eds.), Integrated pest management: Innovation-development process (pp 1:391–498). https://doi.org/10.1007/978-1-4020-8992-316.
- Littell, R. C., Milliken, C. G., Stroup, W. W., & Wolfinger, R. D. (1996). SAS system for mixed models (pp. 633). SAS Institute Inc.
- Lorenz, G., Bateman, N., Hardke, J. T. &, Cato, A. (2021). Insect management in rice. In Hardke, J. T. (Ed.), Arkansas rice production handbook-MP192. University of Arkansas. Available online: https://www.uaex.uada.edu/publications/mp-192.aspx. Accessed 01/12/2024.
- Marchetti, M. A., & Peterson, H. D. (1984). The role of Bipolaris oryzae in floral abortion and kernel discoloration in rice. Plant Disease, 68(4), 288–291. https://doi.org/10.1094/PD-69-288
- Moldenhauer, K. A. K., Counce, P. & Hardke, J. T. (2021). Rice growth and development. In Hardke, J. T. (Ed.), Arkansas rice production handbook-MP192. University of Arkansas. Available online: https://www.uaex.uada.edu/publications/mp-192.aspx. Accessed 01/12/2024.
- Moldenhauer, K. A. K., Lee, F. N., Bernhardt, J. L., Norman, R. J., Slaton, N. A., Wilson, C. E., Anders, M. M., Cartwright, R. D., & Blocker, M. M. (2007). Registration of ‘Wells’ rice. Crop Science, 47(1), 442–443. https://doi.org/10.2135/cropsci2006.06.0419
- Moorberg, C. J., & Crouse, D. A. (2021). Soil laboratory manual: K-state edition, Version 2.0. New Prairie Press. Kansas State University Libraries. Electronic edition. Available at: https://newprairiepress.org/ebooks/39/. Accessed 01/25/2024.
- Naresh, J. S., & Smith, C. M. (1984). Feeding preference of the rice stink bug on annual and sedges. Entomologia Experimentalis et Applicata, 35(1), 89–92. https://doi.org/10.1111/j.1570-7458.1984.tb03365.x
- Nilakhe, S. S. (1976). Rice lines screened for resistance to the rice stink bug. Journal of Economic Entomology, 69(6), 703–705. https://doi.org/10.1093/jee/69.6.703
- Okamura, M., Arai-Sanoh, Y., Yoshida, H., Mukouyama, T., Adachi, S., Yabe, S., Nakagawa, H., Tsutsumi, K., Taniguchi, Y., Kobayashi, N., & Kondo, M. (2018). Characterization of high-yielding rice cultivars with different grain-filling properties to clarify limiting factors for improving grain yield. Field Crops Research, 219, 139–147. https://doi.org/10.1016/j.fcr.2018.01.035
- Patel, D. T., Stout, M. J., & Fuxa, J. R. (2006). Effects of rice panicle age on quantitative and qualitative injury by the rice stink bug (Hemiptera: Pentatomidae). Florida Entomologist, 89(3), 321–327. https://doi.org/10.1653/0015-4040(2006)89[321:EORPAO]2.0.CO;2
- Pedigo, L. P., Hutchins, S. H., & Higley, L. G. (1986). Economic injury levels in theory and practice. Annual Review of Entomology, 31(1), 341–368. https://doi.org/10.1146/annurev.en.31.010186.002013
- Peng, S., Huang, J., Sheehy, J. E., Laza, R. C., Visperas, R. M., Zhong, X., Centeno, G. S., Khush, G. S., & Cassman, K. G. (2004). Rice yields decline with higher night temperature from Global Warming. Proceedings of the National Academy of Science, 101, 9971–9975.
- Pizzatti, C., & Cortesi, P. (2008). Effects of chemicals, nitrogen, time of sowing and panicle brown spot epidemics on rice grain discoloration in Italy. Journal of Plant Pathology, 90, 197–209.
- Poston, F. L., Pedigo, L. P., & Welch, S. M. (1983). Economic injury levels: Reality and practicality. Bulletin of the Entomological Society of America, 29(1), 49–53. https://doi.org/10.1093/besa/29.1.49
- Rashid, T., Johnson, D. T., & Bernhard, J. L. (2005). Feeding preference, fecundity, and egg hatch of rice stink bug, Oebalus pugnax (F.) (Hemiptera: Pentatomidae) on artificial diet rice and alternate host grasses. Southwestern Entomologist, 30, 257–262.
- SAS Institute. (2023). SAS (r) Proprietary Software 9.4, Copyright (c) 2023, SAS Institute Inc., Cary, NC, USA.
- Siebenmorgen, T. J., Counce, P. A., & Hardke, J. T. (2023). Production factors affecting rice milling quality. pp Rice Growth and Development. In J. T. Hardke & L. Goforth (Eds.), Arkansas rice production handbook-MP192 (Modified 2023) (Chapter 14, pp 169–176). University of Arkansas. Available online: https://www.uaex.uada.edu/publications/pdf/mp192/mp192.pdf, accessed on 25 May 2024.
- Simmons, A. M., & Yeargan, K. V. (1988). Feeding frequency and feeding duration of the green stink bug (Hemiptera: Pentatomidae) on soybean. Journal of Economic Entomology, 81(3), 812–815. https://doi.org/10.1093/jee/81.3.812
- Snipes, C. E., Nichols, S. P., Poston, D. H., Walker, T. W., Evans, L. P., & Robinson, H. R. (2005). Current agricultural practices of the Mississippi Delta. Mississippi State University. MAFES Bulletin 1143.
- Swanson, M. C., & Newsom, L. D. (1962). Effects of infestation by the rice stink bug, Oebalus pugnax, on yield and quality in rice. Journal of Economic Entomology, 55(6), 877–879. https://doi.org/10.1093/jee/55.6.877
- Tammes, P. M. L. (1961). Studies of yield losses II. Injury as a limiting factor of yield. Tijdschrift Over Plantenziekten, 67(3), 257–263. https://doi.org/10.1007/BF01984045
- Tood, J. W., & Turnipseed, S. G. (1974). Effects of southern green stinkbug damage on yield and quality of soybeans. Journal of Economic Entomology, 67(3), 421–426. https://doi.org/10.1093/jee/67.3.421
- U.S. Climate Data. (2024). Climate Stoneville-Mississippi, ©U.S. Climate Data, version 3.0. https://www.usclimatedata.com/climate/stoneville/mississippi/united-states/usms0371, accessed on 29 May 2024
- USDA-FGIS. (2002). United States Standards for Rice. USDA Grain Inspection, Packers, and Stockyards Administration. Federal Grain Inspection Service (FGIS). 67 FR 61249, number: 02-24750. Washington, D. C. Available online: https://www.federalregister.gov/documents/2002/09/30/02-24750/united-states-standards-for-milled-rice Accessed: 12/28/2023
- Wang, D., Dowell, F. E., Lan, Y., Pasikatan, M., & Maghirang, E. (2002). Determining pecky rice kernels using visible and near-infrared spectroscopy. International Journal of Food Properties, 5(3), 629–639. https://doi.org/10.1081/JFP-120015497
- Weber, N. C., Redaelli, L. R., dos Santos, E. M., & Werner, F. M. (2020). Quantitative and qualitative damages of Oebalus poecilus on irrigated rice in southern Brazil. Revista Ceres, 67(2), 126–132. https://doi.org/10.1590/0034-737x20267020005
- Wilson, B. E., & Stout, M. J. (2020). Reexamination of the influence of Oebalus pugnax (Hemipter: Pentaomidae) infestations on rice yield and quality. Journal of Economic Entomology, 113(3), 1248–1253. https://doi.org/10.1093/jee/toaa063
