ABSTRACT
Enset, a perennial diploid plant from the Musaceae family, is a key crop in Ethiopia, supporting food, feed, and employment for over 25 million people. Despite the economic, social, and environmental importance of the crop, diseases like enset bacterial wilt pose a bottleneck for crop production and productivity. On the other hand, enset cultivation is started expanding to low-moisture area without basic information. Currently, experiments on enset diseases screening and stress conditions are being widely conducted using different pot size and enset varieties to overcome these issues. However, the absence of recommended pot sizes leads to use inappropriate pot sizes, affecting final results. Therefore, this experiment was conducted to determine the ideal pot size and stress-tolerant varieties by using three improved enset varieties and varying pot sizes. Results revealed significant effects of pot size on all traits and variety on most root, shoot, and corm traits tested. The interaction effect was significant only for some of the traits. Enset allocates more resources to root establishment in the first six months and then to vegetative growth. The crop can undergo normal growth for up to 10 months in small (7 L) and medium (23 L) pots and up to 14 months in large (34 L) pots without experiencing any stress. The variety Endale’s higher yield of dry corm in small pots and overall performance in pseudostem circumference may suggest resilience to stress conditions, as these are key yield determinant components. Further research is needed to validate these findings under field conditions.
REVIEWING EDITOR:
Introduction
Enset (ensete ventricosum (Welw.) Cheesman) is a hapaxanth perennial diploid (2n = 18) plant that belongs to the Musaceae family. The domestication of the enset can be traced back to the Ethiopian highlands 10,000 years ago (S. Stanley, Citation1966; Steven et al., Citation1997). Ethiopia is both the center of origin and diversity of the enset (N. I. Vavilov, Citation1951). This diversity is maintained on farms through the exchange, sharing, and purchasing of seedlings for cultivation among farmers. Enset is a large perennial crop with an average height of 4 to 11 m, a corm length of 0.7–1.8 m, and a diameter of 1.5–2.5 m at maturity (Tsegaye & Westphal, Citation1992). It is a very traditional crop in most growing areas and largely contributes to the food security and rural livelihoods of more than 20 million people in the country (Birmeta et al., Citation2004). In addition, it significantly contributes to animal feed, especially in the dry season, starch, and fiber as raw materials to industries and for medicinal use (G. Yemata, Citation2020). The economic contribution from enset plants in the cultivation areas accounts for 16.28%, which is approaching the benefit gained from grain crops, which accounts for 22.53% (CSA Ethiopia, Citation2019).
Pots are used to grow plants for experiments and production purposes. Years of research on the effects of pots has shown that variations in pot size have an effect on the morphological and physiological traits of plants (Al-Menaie et al., Citation2012; N. S. Robbins & D. M. Pharr, Citation1988). These effects are more pronounced in smaller containers (NeSmith & Duval, Citation1998). Plants require various resources, such as nutrients, an available growth medium, and space for roots to spread and undergo different morphological and physiological processes during growth. However, these resources can be affected by different pot sizes, particularly small pots, leading to root restriction, which disrupts resource use, causes stress to the crop, and reduces the overall growth of the plant (H. S. Al-Menaie et al., Citation2012; O. A. Geply et al., Citation2011; Oagile et al., Citation2016; N. S. Robbins & D. M. Pharr, 1988; M. A. Salisu et al., Citation2018). Many studies (Al-Menaie et al., Citation2012; NeSmith & Duval, Citation1998; Schrader, Citation2000; Townend & Dickinson, Citation1995; Whitfield et al., Citation1996) have shown that small pot sizes generally resulted in less growth, and it has been shown that both solid media and hydroponic nutrient solutions can cause these growth reductions.
There is ample evidence with respect to pot size that the physical restriction of the available volume for root growth limits plant growth. The effect is not only restricted to the roots, but also to the amount of water and nutrients available for plant growth (Hess & de Kroon, Citation2007; W. J. Arp, Citation1991). The majority of plants grown in pots experience root restriction because the roots of most species grow longer than the space provided by the container, and the effect of container size increases with the length of time the plant remains in the pot, which increases root density (Poorter et al., Citation2012). Increased root density creates a competitive environment within the root zone and leads to an oxygen shortage (hypoxia), which reduces root respiration (Kharkina et al., Citation1999; van Iersel, Citation1997), changes in the communication process from the roots to the aerial parts of the plant (Ternesi et al., Citation1994), nutrient deficiencies (Rieger & Marra, Citation1994), hormonal imbalance, especially for cytokinin and gibberellins (D. Benedetto et al., Citation2020), abscisic acid (Ternesi et al., Citation1994), leaf conductance, and intercellular CO2 concentration (Shi et al., Citation2007). The factors that affect root growth can also affect the growth of shoots, as the extent of root and shoot growth is interdependent. The shoots rely on the roots for water, nutrients, and cytokinins, whereas the roots rely on the shoots for carbohydrates and hormones such as auxins and gibberellins. Given the symbiotic relationship between roots and shoots, any stress placed on the roots also affects the ability of shoots to grow (Tonutti & Giulivo, Citation1990).
Regardless of the crop size potential, the arbitrary use of larger and smaller pots is not economically feasible. Pots larger than the sucker’s growth potential in the given time incur unnecessary costs for users and pots of small size, while putting stress on the root zone, leading to the recording or harvest of inappropriate data from this pot trial. Pots of appropriate size based on the genetic size potential of each crop species are important to produce exact data from the trial as well as to grow various crops in a convenient pot size. The experiment was conducted using various pot sizes at various times to screen for diseases in the greenhouse. These pots were not used based on the genetic potential size of the varieties or the length of time needed to hold the plant, which could mislead the final results. One of the problems that appeared in an experiment creating stress conditions for early floral development using various pot sizes was that the pots were unable to hold until flowering and started to be disrupted. This could not be achieved even in larger pots because enset was a large-sized plant, and the experiment was halted in the third season (after 18 months). Therefore, an experiment was conducted to determine the appropriate pot sizes for the early pot-based enset experiments, as well as the responses of enset varieties to the stress conditions imposed from the pot.
Materials and methods
Description of the study sites
A pot experiment was conducted at the Areka Agricultural Research Center (on-station), located approximately 390 km southwest of Addis Ababa in Southern Ethiopia, from January 2021 to June 2022. The site is geographically located at 7°3’25" to 7°4’24" northern latitude and from 37°40’52" to 37°41’30" eastern longitude, with an altitude ranging from 1700 to 1800 m above sea level. The maximum and minimum daily average temperatures were 25 °C and 13 °C, respectively, and the average annual rainfall was 1500 mm. The area experiences bimodal rainfall with an extended rainy season from March to September; April, July, and August are the peak rainy seasons. The soils in Areka are deep and highly weathered Haplic Alisols, with a pH of 5.2 and a tropical climate.
Treatments and experimental design
The Areka Agricultural Research Center as a national enset research program coordinating center has collected, characterization, and maintained over 700 enset land race in the enset botanical garden. In the first phase characterization and evaluation three early-maturing (Yambule, Gewada, and Endale) and three late maturing types (Kelisa, Zereta and Mesena) enset varieties have been released (Y. Mikias et al., Citation2011). Of which the three early maturing enset varieties in combinations with three pots of varying sizes (7 liters, 23 liters, and 34 liters) in volume were used for evaluation ( and ). The experiment was conducted in a factorial, completely randomized design with three replicates. Pots first filled with a mixture of sand, manure, and soil in proportions of 1:2:3, respectively. One-year-old enset suckers were transplanted into these various-sized cone-shaped pots and watered immediately after planting since the season was dry. Each plot consisted of four pots, and each pot contained a single enset plant so that the single plot consisted of four plants.
Table 1. Descriptions of treatments.
Data collection and analysis
Data were collected on four shoot traits, including plant height, pseudo-stem circumference, leaf number, and leaf length, in three rounds with six-month intervals, on five root growth traits, such as root number, root length, root diameter, root fresh weight, and root dry weight at the harvesting stage, and corm attributes, corm fresh weight, and corm dry weight at harvesting. A normality test was conducted to determine the distribution of the normality of the data. All analyses were performed using the GLM procedure of SAS software package 9.2. The treatment means were compared using LSD at p < 0.05. Excel growth trend graphs were used to conduct the growth trend analysis.
Results and discussion
Shoot and root growth
Analysis of variance (ANOVA) showed that the main effect of varieties had a substantial effect on traits, including plant height, pseudo-stem circumference, root number, root length, root fresh weight, root dry weight, and corm dry weight; however, this effect was not significant for leaf number, leaf length, root diameter, and fresh corm weight. The main effect pot size was highly significant for all traits tested in the experiment (). The interaction effect was significant for plant height, leaf length, root fresh weight, root dry weight, and corm dry weight ().
Table 2. Mean square analysis of variance for shoot and root growth as influenced by the variety and pot size.
Shoot growth as influenced by the main and interaction effects of enset variety and pot size
Pseudostem circumference
The main effect of varieties was highly significant (p < 0.05) () for pseudostem circumference, indicating the existence of adequate genetic variability among the varieties for this traits. As the trait has significant effects on yield, varieties that express the trait more effectively could yield better than those that express it less effectively. Endale had the thickest pseudostem with an average value of 0.55 m, and Yambule had the thinnest pseudostem, recording 0.40 m (). The observed differences among the varieties in similar growing media could imply variations in the genetic potential of varieties adapting to the tested media. However, these varieties responded differently to the pseudostem circumference in the open field experiment Endale (1.5 m); a higher pseudostem circumference was found in the Yambule variety (1.6 m) in mature plants, which produced a higher yield than the varieties with the highest pseudostem circumference recorded in this experiment. The difference in the responses of the varieties observed in this experiment might be due to their genetic ability to adapt to different growing media or conditions.
Table 3. Pseudo stem circumference and leaf number of enset as affected by variety and pot size.
Pot size significantly affected the pseudostem circumference of the enset plant (p < 0.05). The largest pseudostem circumference was found in the widest pot size (34 L), with an average value of 0.84 m, while the shortest pseudostem circumference (0.23 cm) was found in the smallest pot size of 7 L (). A similar study was conducted on other crops such as water lilies (Nymphaea spp.) (Al-Menaie et al., Citation2012) and rubber (Hevea Brasiliensis Mull. Arg.) (M. A. Salisu et al., Citation2018), showed that larger pot sizes produce larger plants than smaller pot sizes. A decrease in shoot growth due to small pot sizes has been reported for various crops like tomatoes (Peterson et al., Citation1991), watermelon (Liu & Latimer, Citation1995), and muskmelon (Maynard et al., Citation1996). Reducing pot size limits resources and leads to decreased plant growth (Oagile et al., Citation2016). Varieties showed varied responses to different pot sizes and amounts of growth media for various traits. Endale exhibited superior pseudostem circumference (0.98 m) compared to other varieties in the larger pot size (34 L), while Yambule displayed inferior pseudostem circumference (0.65 m) in both the large and small pot sizes (7 L). This shows the ability of varieties to adapt to the prevailing environmental conditions, whether resources are abundant or limited. The varying responses of the varieties in different growth mediums indicate potential differences in gene expression, influencing their growth rates under specific conditions.
Leaf number
The main effect of pot size was highly significant for enset leaf number (p < 0.05); however, this effect was not significant in variety (), indicating that varieties were genetically not responsive to leaf number. The highest leaf number (6.15) was found in the widest pot size (34 L), whereas the lowest leaf number (4.13) was found in the smallest pot size (7 L) (), indicating a significant impact of the growing medium on leaf numbers. Larger pots provide more space for root growth, minimizing resource competition and stress levels. This can lead to better shoot development and an increased leaf number. On the other hand, plants in small pots may experience stress from confinement, affecting their overall health and leaf production. This could be comparable to soil management at the field level, where planting in loose and fertile soil with sufficient moisture could improve shoot growth and result in a higher leaf number compared to poorly managed soils. Chhetri et al. (Citation2022) discovered that various types of growing media and varieties have a significant impact on the number of leaves in leafy vegetables.
Plant height
Plant height was significantly affected by the interaction effect of variety and pot size at p < 0.05 (). The interaction effect suggests that larger pot sizes generally support greater plant heights across all varieties, with Gewada showing the most significant growth advantage in larger pots. The results imply that pot size has a critical role in enabling the plants to reach their genetic potential in height. The tallest plant height (4.5 m) was observed in the variety Gewada and in the large pot size 34 L followed by Endale (4.25 m) and Yambule (3.92 m) (). Conversely, the shortest plant height (1.22 m) was observed in the variety Gewada and in the small pot size of 7 L, suggesting that the variety exhibited linear growth in plant height as the pot size increased. Large pots exhibited a 58% plant height advantage over small pots, with varieties showing more robust growth in the larger pots than in the smaller ones (). This indicates that large pots provide optimal conditions for plant growth, consisting of adequate nutrients, moisture, and space that reduce physical limitations. The shortest plant height of the varieties in the small pot size was the result of a competitive environment within the root zone, which affected the root volume physically and physiologically and affected the plant height as the shoot and root growth are interdependent. Tonutti and Giulivo (Citation1990) noted that any stress on roots affects shoot growth due to their symbiotic relationship.
Table 4. Plant height and leaf length of enset as affected by the interaction effects of variety and pot size.
The growth trend of plant height in Yambule was slightly different in small pots compared to other varieties tested. Yambule showed superior plant height when grown in small pots, which was not significantly different from the height of other varieties grown in medium-sized pots. This suggests that Yambule could be more resilient to stressful growing conditions, as evidenced by the results obtained from the small pot experiment, which had limited nutrients, moisture, and space that could hinder plant growth.
Leaf length
The interaction effect of variety and pot size significantly affected (p < 0.01) the leaf length of enset, as indicated in . The longest leaf length (2.86 m) was observed in the variety Gewada grown in a large pot size of 34 L, followed by Endale. On the other hand, the shortest leaf length (0.8 m) was observed in the same variety grown in a small pot size of 7 L (). The differences in response of the varieties to the trait depend on the pot size, indicating that adequate resources and sufficient space are important for optimal trait expression. The varieties Gewada and Endale exhibited better leaf length when grown in larger pots, with an increase in leaf length as pot size was increased. Whereas, Yambule was categorized as medium and showed a similar response in both large and medium pot sizes. The low response of variety Yambule for the trait in the variable growing media may indicate resilience to limited resources, useful for growing in different environments. Similarly, pot size increases linearly in leaf area, shoot biomass, and root biomass linearly (Cantliffe, Citation1993).
Root growth as influenced by the main and interaction effect of variety and pot size
Root number
The differences among the main effect of varieties were highly significant (p < 0.05) for the average number of roots per plant (). Differences among tested enset varieties indicate genetic variation. The variety Endale had the highest average number of roots per plant (171), while Yambule had the lowest (115.54) (). Root architectural characteristics, particularly root numbers have been shown to relate to genotypic tolerance to low soil fertility in various crops. A study conducted on common beans showed that a higher number of basal roots in the top soil improved phosphorus uptake and plant vigor in low-phosphorus soils (Miguel et al., Citation2013). However, in Enset the increased number of roots in the Endale variety improved the growth and development of pseudostem circumference and dry corm weight which are the most yield determinant components. This indicates that the increased number of roots in enset could improve both the efficient nutrient uptake and water utilization by intercepting larger plot area.
Table 5. Effect of different varieties and pot sizes on enset root growth parameters and corm fresh weight.
The differences between pot sizes were highly significant (p < 0.05) for the average number of roots per plant (). The largest root number was found in the pot size of 34 L, with an average number of 269.89, whereas the smallest number of roots (52.28) was found in the narrowest pot size of 7 L (). The variation attributed to the number of roots in various pot sizes demonstrates how the variable size of the pot and growing medium could affect the development of enset roots. The biggest pot had more soil, nutrients, and moisture than the smallest pot, resulting in more roots growing with less physical obstruction. The extra space enabled the roots to spread out and intercept large area, ultimately leading to healthier and more vigorous plant growth. It has been noted in other related studies that the size of pots could have an effect on the amount of water and nutrients available for plant growth (W. J. Arp, Citation1991; H. Hadizadeh et al., Citation2010).
Root length
Root length was significantly different (p < 0.05) for all varieties tested (). The presence of variation among the tested varieties indicates significant genetic potential for selecting enset genotypes based on root length. The longest root length (1.04 m) was found in the variety Gewada, whereas the shortest root length (0.74 m) was found in the variety Yambule. Endale had showed medium root length (0.79 m) (). Enset varieties with the longest root length did not show better tolerance to stress in smaller pots. This is in contrary with the study of M. Araya et al. (Citation1998), who found that the deep rooting system in banana plants enables better tolerance to drought conditions. Similarly, this has been demonstrated in other crops (Henry et al., Citation2011; Lynch, Citation2013; Wasson et al., Citation2012).
There was a significant effect of pot size on average root length (p < 0.05 ()). The longest root length (1.16 m) was observed in the largest pot size of 34 L, whereas the shortest root length (0.61 m) was observed in the smallest pot size of 7 L (). The largest pot size produced about 32% more root length compared to the smaller pot size in this experiment. This is consistent with the study by A. Salisu et al. (Citation2018), which revealed that plants grown in larger pots showed a significant increase in root length compared to those in smaller pots. The variable response in root length in different pot sizes indicates the effect of pot size and growing medium on root growth, either suppressing or promoting it, similar to the impact of compact and loose soil in field conditions.
Root diameter
The main effect of pot size was highly significant for root diameter (p < 0.05), whereas this effect was not significant for the varieties (). Pot size and growing media significantly influenced root diameter growth; nevertheless, the minimal variation among varieties in trait attributes indicates the limited genetic potential of the varieties for root diameter. The largest pot size of 34 L had the thickest root diameter (2.45 mm), whereas the smallest pot size of 7 L had the thinnest root diameter (1.55 mm) (). The variation in root diameter for various pot sizes revealed differences in growing media, which consist of variable amounts of nutrients, water, and space to grow the root, and matter for the root diameter. The constraints of space, coupled with limited nutrients and water in a small pot, affect the growth of the root diameter of the enset, potentially reducing its penetration into the soil. Fan et al. (Citation2003), Saengwilai et al. (Citation2014), and Lynch et al. (Citation2014) have suggested that nutritional and hydraulic constraints could impact root diameter and the relative arrangement of anatomical characteristics. In such instances, the root diameter would have become thin, and root penetration into the hard and dry soil would have been reduced (Materechera et al., Citation1992). Besides, regardless of water, nutrients, or other factors, pot size influences plant root volume, limiting root growth depending on the genetic size potential of the plant (Hess & de Kroon, Citation2007; McConnaughay & Bazzaz, Citation1991; Poorter et al., Citation2012).
Fresh corm weight
The main effect of pot size was highly significant (p < 0.05) for fresh corm weight, whereas this effect was not significant in variety (). The biggest fresh corm weight (3.58 kg) was scored in the largest pot size 34 L, whereas the smallest fresh corm weight (0.23 kg) was scored in the narrowest pot size 7 L (). The corm weight increased proportionally as the pot size increased. According to M. A. Salisu et al. (Citation2018), larger pots appear to benefit from the balance between water usage and plant growth increases, which are controlled by the container’s capacity. H. Hadizadeh et al. (Citation2010) also found that the growth of ornamental plants was limited by pot size, which restricted nutrient availability and root length. The effect of large and small pot sizes on the corm fresh weight attributed to the increase or decrease in corm weight would be comparable to the field condition of compact and loose soils.
Fresh and dry root weight
The interaction effect of variety and pot size was highly significant (p < 0.05) for fresh and dry root weight (). The highest fresh root weight (5.74 kg) was recorded in the variety Gewada and the large pot size 34 L, followed by the variety Endale (5.44 kg), while the lowest fresh root weight was found in the variety Endale and small pot size 7 L (0.24 kg) (). The root biomass of the varieties Gewada and Endale increased as the pot size increased, indicating the varieties are more responsive to the growing environment than other varieties in terms of root fresh weight. On the other hand, the highest dry root weight (700 g) was recorded in the variety Gewada and large pot size 34 L, followed by Endale (628 g), while the lowest dry root weight (57 g) was found in the variety Endale and small pot size 7 L (). The increased production of root biomass of the varieties could be an indicator of tolerating drought conditions. Genotypes with the highest root volume, total plant biomass production, and root dry weight are drought-tolerant (Nansamba et al., Citation2020). The small pot size was significantly reduced the amount of fresh and dry root weight in all varieties, suggesting the effect of small pot in the growth and development of roots.
Table 6. Root growth and dry corm weight of enset as affected by the interaction effect of variety and pot size.
Dry corm weight
Variety significantly interacted (p < 0.05) with pot size for dry corm weight (). The highest dry corm weight was from Endale variety and 34 L pot size, yielding a mean of 1267 g, while the lowest was from Gewada variety, yielding a mean of 37 g (). The variety that performed well in pseudostem, root number and leaf length could also excel in corm dry matter yield. The dry corm weight increased significantly in varieties as the pot size increased, except for Yambule, which attributed non-significant difference in the large and medium pot size. The limited response of varieties to different growing media may be due to their wide adaptation. This trial also indicated that varieties with a high interaction with pot size produced better dry corn weight than those with a low interaction.
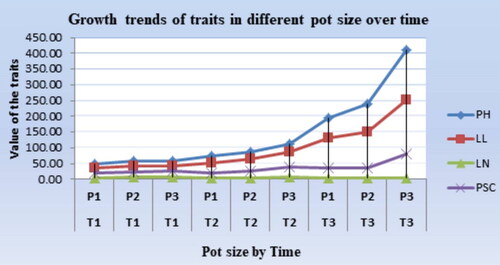
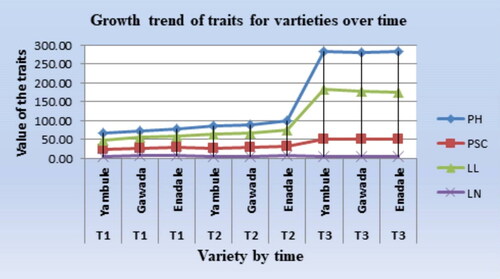
Growth trends of shoot
The plant growth trend indicated that enset showed a slow and similar growth rate in all varieties and pot sizes for the traits in the early growth stages of the two consecutive seasons (12 months), indicating that the enset plant mobilized the sink to the root growth system in these early growth stages for better field establishment ( and ). However, this growth trend was rapidly changed after two growth seasons in the large pot and showed a clear difference between the large and small pots in the third season. The reduced growth rate observed in the third season in the small pot size suggests a scarcity of moisture, nutrients and creation of a competitive environment in the root zone. However, this growth trend rapidly changed after two growth seasons in the large pot and showed a clear difference between the large and small pots in the third season. The reduced growth rate observed in the third season due to the small pot size suggests a scarcity of moisture, and nutrients and the creation of a competitive environment in the root zone. This is agrees with the study of Oagile et al. (Citation2016); A. Monsuru et al. (2018), who found that pots with small size limits resources such as nutrients, available growth medium, and space for roots to spread, resulting in reduced plant growth. Under such conditions, stunted plant growth occurs, even for the best-performing plants (Ursine, Citation2008).
Figure 3. Comparison of the growth trends of each variety over a six-month interval for the tested traits.
T = Time interval for data collection (6 months interval).
T1 = 0-6months, T2 = 7-12 months, T3 = 13-18 months.
SH = pseudo stem height, LL = Leaf length, LN = Leaf Number, PSC = Pseudo stem circumference.
Correlation of shoot and root traits
The correlation between shoot and root growth traits, and corm fresh and dry weights was determined using Pearson’s correlation coefficient, and the results are presented in . Shoot growth traits, such as plant height, pseudo stem circumference, leaf length, and leaf number, had strong to very strong, significantly positive correlations with each other, ranging r from 0.68 to 0.96 for leaf number with plant height and leaf length with plant height, respectively. The root traits were positively and significantly correlated with each other, ranging r from 0.55 to 0.97, except root numbers with root length (r = 0.38) and root diameter with root length (r = 0.20), which were not significantly correlated with each other.
Table 7. Correlation among shoot and root growth traits of enset varieties.
Root traits had a strong and significantly positive correlation with shoot characteristics, ranging from root length with leaf number of r = 0.32 to fresh root weight with pseudo circumference of r = 0.95, except root length with leaf number, which was not significantly correlated (), suggesting how the root architecture could support the shoot growth system by providing adequate nutrients and water. Shoots rely on the roots for water, nutrients, and cytokinins, and roots rely on shoots for carbohydrates and hormones such as auxins and gibberellins, as reported by Tonutti and Giulivo (Citation1990). An imbalance in these factors can affect plant growth and development. Corm fresh and dry weights were strongly and positively correlated with all shoot and root traits except root dry weight and root length. All traits, except root length, significantly contributed to the yield of corm fresh and dry weights, which implies that the longer the root length, the greater the demand for food from the corm. Corm yield increased as the number of roots increased, indicating the significant role of root numbers in providing the necessary nutrients and water from the top soil for the growth and development of the plant.
Conclusion
The study confirmed that the variation attributed to enset varieties for various traits in the trails implies the genetic potential of these varieties to be selected for specific objectives, particularly stress tolerance. For instance, root characteristics, particularly root number in enset, could be used in selecting enset varieties with drought tolerance. Endale, a variety that excelled in dry corm weight and leaf length compared to others, even in limited pot sizes, could be classified as stress-tolerant variety. The growth trends of shoot traits for all enset varieties exhibited slow and comparable growth in the first six (6) months after planting. During this period, enset more invested on enhancing root system to optimize water and nutrient absorption for improved field establishment. Later, rapid and steady shoot growth was seen in all varieties in the subsequent season, particularly in the third season, suggesting a higher allocation of resources to shoot development, as active growth is usually linked to the presence of more sinks.
Pot size significantly affected shoot and root growth, as well as the corm traits, as the time of the plant in the pot increased. The enset suckers grew in all pot sizes without any effect on the shoot, root, and corm traits for ten months, as seen from the growth trend analysis. The difference, however, was observed clearly in shoot growth among the pots, particularly in small and medium pots, after ten months. Stunted shoot growth in small pots is caused by physical root restrictions, limited nutrients, and moisture in the root zone, as root and shoot growth is interdependent. Thus, it is impractical to cultivate enset plants in small and medium pots for over 10 months and in larger pots for 14 months to achieve optimal growth. Unlike many other plants that may bloom in such challenging circumstances, the enset plant tends to exhibit stunted growth over an extended period without flowering and eventually died if the conditions persist.
Variety and pot size were significantly correlated with plant height, leaf length, fresh root weight, dry root weight, and dry corm weight. These traits highly depend on pot size; as pot size increases, the trait growth also increased. A strong positive correlation was found with most shoot, root, and corm traits. The relationship between root and shoot traits highlights the interdependence of traits for overall growth and development.
Researchers, students, and others can typically use the identified pot size for optimal plant growth in any experiment. Farmers can benefit from the identified drought-tolerant variety with targeted field trial assistance.
Authors’ contributions
Henok Fikre: Conceptualization, Methodology, Validation, Formal analysis, Resources, Data curation: Writing (original draft), Visualization, Supervision, Project administration, Funding Acquisition. Nadew Boto: Formal analysis, Resources, Data curation: Writing (original draft), Visualization and Funding Acquisition.
Acknowledgment
I would like to thank the Southern Agriculture Research Institute and Areka Agricultural Research Center for all the support and facilities offered during the experiment.
Disclosure statement
The authors confirm that no commercial or financial relationship might be considered a potential conflict of interest formed during the execution of the research.
Data availability statement
Data will be provided promptly upon request.
Additional information
Funding
Notes on contributors
Henok Fikre
Mr Henok Fikre is an enset breeder and national enset program coordinator at former Southern Ethiopia Agricultural Research Institute, Areka Agricultural Research Center. He earned his MSc in plant breeding from Hawassa University in 2018. Mr. Henok has over 13 years of experience in crop improvement research and has published more than 4 peer-reviewed articles, 1 book and 1enset production manual. His research focuses on variety development for high yield, disease and moisture stress. In his spare time, Mr. Henok enjoys lessening spiritual songs, reading books and volunteering in community science outreach programs.
Nadew Boto
Nadew Boto, after his completion of high school, he joined Haramaya University, the leading agricultural University, and graduated with the Degree of Bachelor of Science in Plant Science in 2006G.C. Then after, he was employed and worked as an agronomist in Agricultural Development Office of Damot Gale District in southern Ethiopia. For fourteen years. And later on, he won the competition to join one of the research institutes in Ethiopia, South Agricultural Research Institute (SARI) and has been working as an agronomy associate researcher-I at Areka agricultural research center, South Ethiopia. After working for two years he requested SARI to have a chance of continuing his learning so as to quench his want to upgrade his profession. Fortunately, he was permitted to join School of Graduate Studies of Hawassa University in 2016 to pursue a study leading to the Degree of Master of Sciences in Agronomy and completed in 2018G.C. Till then he has been working as a horticultural crops agronomy researcher-I in the same center.
References
- Al-Menaie, H. S., Al-Ragam, O., Al-Dosery, N., Zalzaleh, M., Mathew, M., & Suresh, N. (2012). Effect of pot size on plant growth and multiplication of water lilies (Nymphaea spp). American-Eurasian Journal of Agricultural & Environmental, 12, 1–12.
- Araya, M., Vargas, A., & Cheves, A. (1998). Changes in distribution of roots of banana (Musa AAA cv. Valery) with plant height distance from the pseudostem, and soil depth. The Journal of Horticultural Science and Biotechnology, 73(4), 437–440. https://doi.org/10.1080/14620316.1998.11510996
- Arp, W. J. (1991). Effects of source‐sink relations on photosynthetic acclimation to elevated CO2. Plant, Cell & Environment, 14(8), 869–875. https://doi.org/10.1111/j.1365-3040.1991.tb01450.x
- Benedetto, D. A., Giardina, E., De Lojo, J., Gandolfo, E., & Hakim, G. (2020). Exogenous benzyl amino purine (BAP) applications for the ornamental pot industry. In Sonja Kortesmäki (Ed.), Cytokinins: Biosynthesis and uses (pp. 1–56). Nova Science Publishers, Inc.
- Birmeta, G., Nybom, H., & Bekele, E. (2004). Distinction between wild and cultivated enset (Ensete ventricosum) gene pools in Ethiopia using RAPD markers. Hereditas, 140(2), 139–148. https://doi.org/10.1111/j.1601-5223.2004.01792.x
- Cantliffe, D. J. 1993. Pre- and postharvest practices for improved vegetable transplant quality. hortTechnology 3, 415–417.
- Chhetri, S., Dulal, S., Subba, S., & Gurung, K. (2022). Effect of different growing media on growth and yield of leafy vegetables in nutrient ilm technique hydroponics system. Archives of Agriculture and Environmental Science, 7(1), . 2–19. https://doi.org/10.26832/24566632.2022.070103
- CSA (Central Statistical Authority). (2019). The Federal Democratic Republic of Ethiopia Central Statistical agency Agricultural Sample Survey. Vol. 1. Report on area and production of major crops. Addis Ababa 23 pp.
- Fan, M., Zhu, J., Richards, C., Brown, K. M., & Lynch, J. P. (2003). Physiological roles for aerenchyma in phosphorus-stressed roots. Functional Plant Biology, 30, 493–506. https://doi.org/10.1071/FP03046
- Geply, O. A., Baiyewu, R. A., Adegoke, I. A., Ayodele, O. O., & Ademola, I. T. (2011). Effect of different pot sizes and growth media on the agronomic performance of Jatropha curcas. Pakistan Journal of Nutrition, 10(10), 952–954. https://doi.org/10.3923/pjn.2011.952.954
- Hadizadeh, H., Tehranifar, A., Shoor, M., & Nemati, H. (2010). Investing of the dwarfeness effect of paclobutrazol on tuberose (Polianthes tuberosa L.) and the possibility of pot tuberose production. Journal of Horticulture Science (Agriculture Science and Technology), 24, 7–13. https://doi.org/10.22067/JHORTS4.V1389I1.3637
- Henry, A., Gowda, V. R. P., Torres, R. O., McNally, K. L., & Serraj, R. (2011). Variation in root system architecture and drought response in rice (Oryza sativa): phenotyping of the Oryza SNP panel in rain fed lowland fields. Field Crops Research, 120(2), 205–214. https://doi.org/10.1016/j.fcr.2010.10.003
- Hess, L., & de Kroon, H. (2007). Effects of rooting volume and nutrient availability as an alternative explanation for root self/non-self-discrimination. Journal of Ecology, 95(2), 241–251. https://doi.org/10.1111/j.1365-2745.2006.01204.x
- Kharkina, T. G., Ottosen, C.-O., & Rosenqvist, E. (1999). Effects of root restriction on the growth and physiology of cucumber plants. Physiologia Plantarum, 105(3), 434–441. https://doi.org/10.1034/j.1399-3054.1999.105307.x
- Liu, A., & Latimer, J. G. (1995). Root cell volume in the planter flat affects watermelon seedling development and fruit yield. HortScience, 30(2), 242–246. https://doi.org/10.21273/HORTSCI.30.2.242
- Lynch, J. P. (2013). Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Annals of Botany, 112, 347–357. https://doi.org/10.1093/aob/mcs293
- Lynch, J. P., Chimungu, J. G., & Brown, K. M. (2014). Root anatomical phenes associated with water acquisition from drying soil: targets for crop improvement. Journal of Experimental Botany, 65, 6155–6166. https://doi.org/10.1093/jxb/eru162
- Materechera, S. A., Alston, A. M., Kirby, J. M. et al. 1992. Influence of root diameter on the penetration of seminal roots into a compacted subsoil. Plant Soil, 144, 297–303. https://doi.org/10.1007/BF00012888
- Maynard, E. T., Vavrina, C. S., & Scott, W. D. (1996). Containerized muskmelon transplants: Cell volume effects on pre-transplant development and subsequent yield. HortScience, 31(1), 58–61. https://doi.org/10.21273/HORTSCI.31.1.58
- McConnaughay, K. D. M., & Bazzaz, F. A. (1991). Is physical space a soil resource? Ecology, 72(1), 94–103. https://doi.org/10.2307/1938905
- Miguel, M. A., Widrig, A., Vieira, R. F., Brown, K. M., & Lynch, J. P. (2013). Basal root whorl number: a modulator of phosphorus acquisition in common bean (Phaseolus vulgaris). Annals of Botany, 112(6), 973–982. https://doi.org/10.1093/aob/mct164
- Mikias, Y., Zerihun, Y., Sadic, M., Abay, A., Fiseha, N., Kidus, M., Atnafua, B., Abebe, C., Fekadu, G. T., Dagmawi, M., & Getachew, W. M. (2011). Registration of enset (Enset ventricosum (Welw.) Cheesman) varieties Yanbule, Gewada, Endale, Kelisa, Zerita and Mesena. Ethiopian Journal of Agricultural Science.
- Nansamba, M., Sibiya, J., Tumuhimbise, R., Karamura, D., Kubiriba, J., & Karamura, E. (2020). Breeding banana (Musa spp.) for drought tolerance: A review. Plant Breeding, 139(4), 685–696. https://doi.org/10.1111/pbr.12812
- NeSmith, D. S., & Duval, J. R. (1998). The effect of container size. HortTechnology, 8(4), 495–498. https://doi.org/10.21273/HORTTECH.8.4.495
- Oagile, O., Gabolemogwe, P., Matsuane, C., & Mathowa, T. (2016). Effect of container size on the growth and development of tomato seedlings. International Journal of Current Microbiology and Applied Sciences, 5(4), 890–896. https://doi.org/10.20546/ijcmas.2016.504.100
- Peterson, T. A., Reinsel, M. D., & Krizek, D. T. (1991). Tomato (Lycopersicon esculentum Mill. cv ‘Better Bush) plant response to root restriction. Alteration of plant morphology. Journal of Experimental Botany, 42(10), 1233–1240. https://doi.org/10.1093/jxb/42.10.1233
- Poorter, H., J., Buhler, D., van Dusschoten, Jose Climent., & J. A., Postma. (2012). Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Functional Plant Biology, 39, 839–850. https://doi.org/10.1071/FP12049
- Rieger, M., & Marra, F. (1994). Response of young peach trees to root confinement. Journal of the American Society for Horticultural Science, 119, 223–228.
- Robbins, N. S., & Pharr, D. M. (1988). Effect of restricted root growth on carbohydrate metabolism and whole plant growth of Cucumis sativus L. Plant Physiology, 87, 409–413. https://doi.org/10.1104/pp.87.2.409
- Saengwilai, P., Nord, E. A., Chimungu, J. G., Brown, K. M., & Lynch, J. P. (2014). Root cortical aerenchyma enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiology, 166, 726–735. https://doi.org/10.1104/pp.114.241711
- Salisu, M. A., Sulaiman, Z., Samad, M. Y. A., & Kolapo, O. K. (2018). Effect of various types and size of container on growth and root morphology of rubber (Hevea Brasiliensis Mull. Arg.). IJSTR: international journal of scientific & technology research, 7, 21–27.
- Schrader, W. L. (2000). Using transplants in vegetable production. Publication 8013 by Division of agriculture and natural resource. University of California. http://anrcatalog.ucdavis.edu/pdf/8013.pdf. Available online, retrieved 10-10-2014.
- Shi, K., Hu, W. H., Dong, D. K., Zhou, Y. H., & Yu, J. Q. (2007). Low O2 supply is involved in the poor growth in root-restricted plants of tomato (Lycopersicon esculentum Mill.). Environmental and Experimental Botany, 61(2), 181–189. https://doi.org/10.1016/j.envexpbot.2007.05.010
- Stanley, S. (1966). Enset in Ethiopian economy. Ethiopian Geographical Journal, 4(1), 30–37.
- Steven, A. B., Spring, A., Hiebsch, C., McCabe, J. T., Tabogie, E., Diro, M., Wolde-Michael, G., Yntiso, G., Shigeta, M., & Tesfaye, S. (1997). The Tree Against Hunger: Enset Cultivation in Ethiopia American Academy for the Advancement of Science. Available from: https://www.researchgate.net/publication/275969940. The_Tree Against_Hunger Enset Cultivation in_Ethiopia [accessed Sep 09 2023].
- Ternesi, A., Andrade, A. P., Jorrin, J., & Benlloch, M. (1994). Root-shoot ratios in sunflower with confined root systems. Plant and Soil, 166(1), 31–36. https://doi.org/10.1007/BF02185478
- Tonutti, P., & Giulivo, C. (1990). Effect of available soil volume on growth of young kiwi plants. Acta Horticulturae, 282(282), 283–290. https://doi.org/10.17660/ActaHortic.1990.282.36
- Townend, J., & Dickinson, A. L. (1995). A comparison of rooting environments in containers of different sizes. Plant and Soil, 175(1), 139–146. https://doi.org/10.1007/BF02413019
- Tsegaye, A., & Westphal, E. (1992). Ensete ventricosum (Welw.) Cheesman. Record from PROTA4U. L. P. A. Oyen, & R. H. M. J. Lemmens (Eds.), PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale). https://www.prota4u.org/protav8.asp?h=M4&t=ensete%2Cventricosum&p=Ensete%20ventricosum#Synonyms
- Ursine. (2008). Effects of varied soil composition (char, sand, potting mix) on the growth of radish starts. Available at terrapreta.bioenergylists.org/node/582.
- van Iersel, M. (1997). Root restriction effects on growth and development of Salvia (Salviasplendens). HortScience, 32(7), 1186–1190. https://doi.org/10.21273/HORTSCI.32.7.1186
- Vavilov, N. I. (1951). The origin, variation, immunity and breeding of cultivated plants. Chronica Botanica, 13, 1–366.
- Wasson, A. P., Richards, R. A., Chatrath, R., Misra, S. C., Prasad, S. V. S., Rebetzke, G. J., Kirkegaard, J. A., Christopher, J., & Watt, M. (2012). Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. Journal of Experimental Botany, 63, 3485–3498. https://doi.org/10.1093/jxb/ers111
- Whitfield, C. P., Davison, A. W., & Ashenden, T. W. (1996). Interactive effects of ozone and soil volume on Plantago major. New Phytologist, 134(2), 287–294. https://doi.org/10.1111/j.1469-8137.1996.tb04633.x
- Yemata, G. (2020). Ensete ventricosum: A Multipurpose Crop against Hunger in Ethiopia. The Scientific World Journal, 2020, 1–10. https://doi.org/10.1155/2020/6431849