 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Rice False Smut (RFS) disease (Ustilaginoidea virens) has become a severe problem in many parts of the world. Managing this disease is a major priority as the disease causes a significant yield loss. In the present study, we have investigated the effectiveness of bioformulations, biocontrol agents, and fungicides for managing RFS disease at the lab and field levels. The study evaluated bioformulations LBD 1 and 6, biocontrol agents Trichoderma viride, Bacillus velezensis (P-42 and A-6), and Pseudomonas fluorescens strain Pf-1, and various fungicides such as trifloxystrobin 25% + tebuconazole 50% WG, propiconazole 25% EC, difenconazole 25% EC, flusilazole 12.5% + carbendazim 25% EC, and azoxystrobin 23% SC against RFS. All fungicides tested in the in vitro assay exhibited complete mycelial inhibition against U. virens. On the other hand, biocontrol and bioformulations showed 41.03%–93.53% mycelial inhibition. Pooled data from two seasons (Kharif 2019 & 2020) indicated that all the tested biocontrol agents and fungicides effectively reduced the severity of the RFS disease in field conditions. The fungicide trifloxystrobin 25% + tebuconazole 50% WG at 0.4 gL-1 was found to be the most effective, with the lowest disease severity of 18.23%, followed by 20.24% severity by flusilazole 12.5% + carbendazim 25% EC at 1.0 mLL-1. Fungicides were found to be the best for the management of the disease among different treatments. This is the first comprehensive record of successfully managing the RFS disease using seaweed extracts, fungicides, and microbial bioagents and to standardize the spray schedule for better disease control.
REVIEWING EDITOR:
1. Introduction
Rice, scientifically known as Oryza sativa L., is the most widely cultivated food crop in Asia and a significant source of nourishment for half of the world’s population (Birla et al., Citation2017). It is the primary source of energy and protein for 4.5 billion people living in the most populous nations of Asia. More than 90% of the world’s rice is grown and consumed in Asia, where 60% of the world’s population resides (Bin Rahman & Zhang, Citation2023). Rice accounts for 35%–60% of the caloric intake of three billion Asians (Guyer et al., Citation1998). In the Karnataka state of India, rice is grown on an area of 1.2 million hectares, producing approximately 3.5 million tonnes and productivity of 3038 kg per hectare (Anonymous, Citation2020).
Biotic and abiotic stress are major constraints in rice production, resulting in significant crop losses every year (Kazan, Citation2018). These losses are expected to increase with the predicted effects of climate change. Therefore, researching sustainable approaches to alleviate these stresses faced by rice crops is a high priority (Bird et al., Citation2016). Among the biotic stress, rice is vulnerable to many diseases caused by fungal, bacterial, viral, and nematodes (Amoghavarsha et al., Citation2022; Raghunandana et al., Citation2023). One of the emerging fungal diseases that cause significant damage to rice yield and quality worldwide is rice false smut (Abbas et al., Citation2014; Hosagoudar, Citation2018; Huded et al., Citation2022).
Rice false smut (RFS) is caused by an ascomycete fungus Ustilaginoidea virens (Cooke, Citation1878) Takahashi (teleomorph: Villosiclava virens). It was first reported from the Tirunelveli district of Tamil Nadu State of Southern India in 1878 (Cook, 1878). For many years, the RFS was considered a minor disease with sporadic occurrence in a few rice belts of southeast Asia (Sun et al., Citation2020). However, in recent times, RFS has become one of the most devastating diseases of rice worldwide. The annual average incidence area of RFS in China is 3.06 Mha, with 158.6 million kilograms of loss per year (Lu et al., Citation2018).
Previous reports have indicated that the percentage of RFS-infected tillers ranged from 5% to 85%, leading to yield losses of 0.2%–49% depending on rice varieties (Dodan & Singh, Citation1996; Ladhalakshmi et al., Citation2012; Kumari & Kumar, Citation2015). Yield loss estimation due to RFS disease on different rice varieties grown in diverse ecosystems revealed up to 4.25% yield loss (Muniraju et al., Citation2017a). This disease is favored by certain climatic factors such as cloudy weather, high relative humidity (>95%), temperature (25 to 30 °C), water stress, and rainy days during the flowering stage (Sanghera et al., Citation2012; Raji et al., Citation2016a; Alase et al., Citation2021).
Breeding and deploying resistant varieties is the most economical and effective way to manage the disease (Rimbaud et al., Citation2021). However, little is known about the molecular mechanisms and genes underlying rice resistance against U. virens, and hence, no effective resistant cultivars are available for cultivation (Han et al., Citation2015) due to which farmers are left with the option of the use fungicides for the management of the disease. Several attempts were made in India to identify effective fungicides for field management of rice disease. A mixture of different fungicides often provides high control efficiency for different rice ecosystems (Muniraju et al., Citation2017b; Hosagoudar, Citation2018; Pandey et al., Citation2018; Savitha et al., Citation2019; Sharanabasav et al., Citation2020). Apart from the chemicals, biological control methods are eco-friendly and effective in managing plant diseases. Unlike fungicides, they do not leave any harmful residues. Therefore, several biocontrol agents, including Bacillus subtilis, Trichoderma sp., and Antennariella placitae, that have demonstrated high levels of inhibition against U. virens can be used to manage the disease (El-Naggar et al., Citation2015; Kannahi et al., Citation2016; Andargie et al., Citation2017).
Given these facts and the critical importance of the spray schedule, the present study aimed to evaluate the baseline sensitivity of fungicidal and biological treatments under laboratory setup followed by on-field-scale testing at different spraying schedules. This integrated approach will help the farmers to overcome the losses caused by the RFS pathogen at the field level.
2. Material and methods
2.1. Description of the study area
The experiment was conducted at the Rice Pathology Laboratory and experimental fields of Agricultural Research Station, Gangavathi, Karnataka, India (5.4319° N, 76.5315° E).
2.2. Pathogen, bioformulations, and biocontrol agents
The RFS disease sample was collected from the rice fields of the Agricultural Research Station, Gangavathi. The RFS pathogen was isolated and cultured as described by Pramesh et al. (Citation2020). Well-characterized seaweed-derived bioformulations such as LBD 1 and LBD 6 (Sahana et al., Citation2022) were used in the study. The LBD-1 and LBS-6 (20% solid extract) used in the study were provided by Sea6 Energy Pvt Ltd, Bengaluru, India. The biocontrol agents viz. Trichoderma viride, B. velezensis (P-42 and A-6), and Pseudomonas fluorescens (Pf-1) were obtained from the Pathogenomics Lab, Department of Plant Pathology, UAS, Bengaluru, Karnataka, India. Pure cultures of bioagents were used for further in-vitro and in-vivo studies.
2.3. In-vitro testing
An in-vitro evaluation was conducted to test the antagonistic effect of selected bio-control agents against U. virens by dual culture assay on the Potato Dextrose Agar (PDA) medium using a completely randomized design (CRD) with three replications. The method described by Bunbury-Blanchette and Walker et al. (2019) was used for the T. viride, whereas the antifungal activity of B. velezensis (P-42 and A-6), P. fluorescens (Pf-1) was tested following the method described by Fokkema (Citation1978). The radial growth of the pathogen was measured in control and treatments after 21 days, and the average was recorded. The percent inhibition of U. virens was calculated using the formula given by Vincent (Citation1947).
The efficacy of selected fungicides viz., trifloxystrobin 25% + tebuconazole 50% WG, propiconazole 25% EC, difenconazole 25% EC, flusilazole 12.5% + carbendazim 25% EC, and azoxystrobin 23% SC were tested using the poisoned food technique (Singh & Milne, Citation1974) and incubated at 27 ± 2 °C. The mycelial growth of U. virens was recorded after 21 days (as the growth of the pathogen in the control plate reached 90 mm). Growth inhibition was calculated using the formula suggested by Vincent (Citation1947). The efficacy of fungicides was expressed as percent inhibition of mycelial growth over control. The experiment was repeated two times.
2.4. Field-scale experiment
2.4.1. Experimental design
A field experiment was conducted at the experimental plots of the Agricultural Research Station, Gangavathi, during Kharif 2019 and 2020. The experiment was laid out in a Factorial Randomized Block Design (RBD) with three main factors, 12 treatments, and three replications. The bio-efficacy of each treatment was evaluated under natural epiphytotic conditions.
2.4.2. Experimental materials and procedure
The seedbed was prepared 30 days before transplanting. Seeds of GNV-10–89 were soaked overnight by incubating under warm conditions for 36–48 hours until sprouting and were broadcasted on the seed bed. From the seedbed, 30-day-old seedlings were transplanted in the puddled main field at 20 cm × 10 cm spacing, and the crop was maintained by following the standard package of practices of the University of Agricultural Sciences, Raichur, Karnataka, India.
2.4.3. Treatment imposition
The field experiment consists of 12 sub-treatments viz., LBD (1 and 6) (seaweed extracts), T. viride, B. velezensis (P-42 and A-6), P. fluorescens (Pf-1), trifloxystrobin 25% + tebuconazole 50% WG, propiconazole 25% EC, difenconazole 25% EC, flusilazole 12.5% + carbendazim 25% EC, azoxystrobin 23% SC, and control. The treatment was imposed at predefined stages of the crop, i.e. booting, 50% flowering, and ten days after flowering (which serves as the main factor). A single spray was given for each treatment at the predefined stage, as mentioned in .
Table 2. Effect of bio formulations, bio-control agents, and fungicides on rice false smut during Kharif 2019.
2.4.4. Assessment of disease variables and yield
The observations were taken on the number of infected tillers per m2 and smut balls per panicle for each treatment. The disease severity was calculated by multiplying the percentage of infected tillers by the percentage of infected grains. Harvesting was done manually in each treatment, and the yield was recorded. Disease incidence was recorded at the time of harvesting as percent infected tillers per square meter by using the formulae given by Mandhare et al. (Citation2008) and Singh and Dube (Citation1978).
2.5. Statistical analysis
The replicated data obtained from in-vitro tests conducted by following CRD and one-way analysis of variance (ANOVA) was applied at 1% of the level of significance. Subsequently, the disease variable data collected from treated and untreated control plots were compared and analyzed by following the factorial two-way ANOVA at a 5% level of significance. The percent values from the in-vitro and field data were transformed, and transformed values were considered for the statistical analysis by the SPSS package (SPSS version 2.0 software, IBM New York).
3. Results
3.1. Symptoms of rice false smut
The pathogen produces the typical symptoms characterized by the conversion of healthy florets into yellowish-orange to greenish-black smut balls. The smut balls were covered with powdery chlamydospores, often producing sclerotia ().
3.2. Identification of the pathogen
The pathogen was isolated on a PDA medium, observed for the morphological characters, and confirmed as U. virens (). The pathogen was also characterized by sequencing the ITS region (data not shown).
3.3. In-vitro evaluation of different bioagents, bioformulations, and fungicides
In-vitro study was conducted to evaluate bio agents, bio formulations, and chemical fungicides; it was found that the highest percent inhibition of U. virens was observed in the fungicide treatments, followed by bioagents and bio formulations (). In the different fungicides treated, trifloxystrobin 25% + tebuconazole 50% WG, flusilazole 12.5% + carbendazim 25% EC, propiconazole 25% EC, azoxystrobin 23% SC, and difenconazole 25% EC showed 100% inhibition of U. virens. In the case of bioagents, T. viride (93.53%) showed the highest percent inhibition, followed by B. velezensis P-42 (88.69%), B. velezensis A6 (87.86%), and P. fluorescens Pf-1 (83.81%). Whereas bioformulations viz., LBD 6 (38.69%) and LBD 1 (41.03%) showed less percent inhibition of U. virens, respectively ().
Figure 2. Box and Whisker plot represents the potency of fungicides, bioagents, and bioformulations against U. virens under in-vitro conditions. Details of T1 to T11 were furnished in .
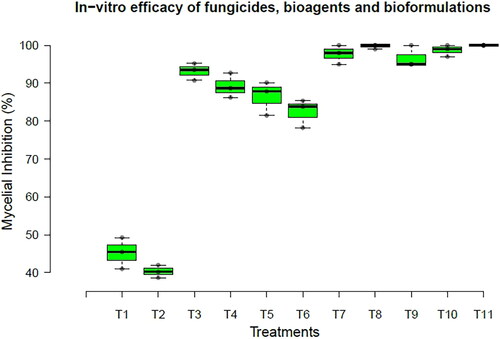
Table 1. In-vitro evaluation of bio formulations, bio-control agents, and fungicides on Ustilaginoidea virens.
3.4. Field evaluation of different bioagents, bioformulations, and chemical fungicides
3.4.1. Control efficiency of treatments
The experiment was sprayed with different bioagents, bioformulations, and fungicides at different crop growth stages and compared with the grain yield. During Kharif 2019, among the bio-control agents and bio formulations sprayed at the booting stage, the block treated with T. viride recorded a PDI of 38.72 and reduced the disease severity significantly by 29.10% over control (54.61%), followed by bio-formulation LBD-1 with PDI 41.91, reduced disease severity by 23.25%. Other treatments viz., LBD-6 (42.72%), B. velezensis (P-42) (43.34%), and B. velezensis (A6) (43.35%) were statistically on par with each other with percent reduction over control of 21.77, 20.64, and 20.62, respectively. P. fluorescens (Pf-1) with a PDI of 46.23 reduced the disease severity by 15.35% over control.
Among the fungicides sprayed at the booting stage, trifloxystrobin 25% + tebuconazole 50% WG at 0.4 gL−1 with PDI 9.54, followed by flusilazole 12.5% + carbendazim 25% EC at 1.0 mLL−1 with PDI of 12.48 reduced the disease severity by 82.53% and 77.14%, respectively, over control. Propiconazole 25% EC (14.01%), azoxystrobin 23% SC (17.32%), and difenconazole 25% EC (19.20%) reduced the disease severity (74.35%, 68.29%, and 64.84%) significantly over the control, respectively. A similar trend was observed at 50% flowering and ten days after flowering. Among the sprayings at different stages of the crop, i.e. booting, 50% flowering, and ten days after flowering, spraying at the booting stage was statistically significant in reducing the disease severity, followed by 50% flowering and ten days after flowering. Spraying at ten days post-flowering, though, recorded higher disease severity but was significantly less than the control ().
During Kharif 2020, among the bio-control agents and bio formulations sprayed at booting stage, T. viride (21.69%) reduced disease severity significantly (27.99%) over control (30.12%), followed by B. velezensis (P-42) (23.23%) where 22.86% of disease severity reduction over control was recorded. Whereas treatments viz., LBD-1 (23.63%), LBD-6 (23.86%), and B. velezensis (A-6) (24.60%) showed higher disease severity compared to T. viride (21.69%) and they are statistically on par with each other. P. fluorescens (Pf-1) (26.33%) and B. velezensis (A-6) (24.60%) are statistically on par with each other. Among the fungicides, trifloxystrobin 25% + tebuconazole 50% WG at 0.4 gL−1 (5.97%) recorded the lowest disease severity and highest percent reduction (80.19%) over control (30.12%) followed by flusilazole 12.5% + carbendazim 25% EC at 1.0 mLL−1 (8.69%) with 71.14% reduction. Rest of the chemical fungicides, i.e. propiconazole 25% EC (10.06%), azoxystrobin 23% SC (11.14%), and difenconazole 25% EC (13.03%), also reduced disease severity significantly with percent reduction of 66.58%, 63.01%, and 56.75%, respectively, over control. A similar trend was recorded on spraying at 50% flowering. Maximum disease severity was observed when spraying was carried out ten days after flowering ().
Table 3. Effect of bio formulations, bio-control agents, and fungicides on rice false smut during Kharif 2020.
3.4.2. Influence of treatments on yield
Data on grain yield during 2019 revealed that the highest grain yield was recorded in treatment sprayed at the booting stage with trifloxystrobin 25% + tebuconazole 50% WG at 0.4 gL−1 (65.40 qha−1) followed by flusilazole 12.5% + carbendazim 25% EC at 1.0 mLL−1 (63.27 qha−1). Fungicides viz., propiconazole 25% EC (62.13 qha−1), azoxystrobin 23% SC (60.00 qha−1), and difenconazole 25% EC (59.06 qha−1) recorded significantly higher yield than bio-control agents, bio formulations, and control (46.96 qha−1). Among the bio-control agents and bio formulations sprayed at booting stage, T. viride (54.45 qha−1) recorded higher yield followed by LBD-1 (53.40 qha−1) than LBD-6 (52.49 qha−1), B. velezensis (P-42) (52.25 qha−1), B. velezensis (A-6) (51.91 qha−1), and P. fluorescens (Pf-1) (51.42 qha−1). A similar trend was observed at 50% flowering, where grain yield was less than that recorded at the booting stage and higher than spraying ten days after flowering. The lowest yield was recorded when spraying was done ten days after flowering, but it was significantly higher than the control (54.29 qha−1) ().
During Kharif 2020, when sprayed at the booting stage, the highest yield was recorded in the plot treated with trifloxystrobin 25% + tebuconazole 50% WG at 0.4 gL−1 (66.08 qha−1) followed by flusilazole 12.5% + carbendazim 25% EC at 1.0 mLL−1 (65.00 qha−1) over control (52.15 qha−1). Remaining fungicides viz., propiconazole 25% EC (62.75 qha−1), azoxystrobin 23% SC (62.25 qha−1), and difenconazole 25% EC (61.65 qha−1) recorded significantly higher yield than bio-control agents, bio formulations, and control (52.15 qha−1). Among the bio-control agents and bio formulations, T. viride (57.58 qha−1) recorded a higher yield followed by LBD-1 (57.48 qha−1) than B. velezensis (P-42) (56.83 qha−1), B. velezensis (A6) (56.63 qha−1), LBD-6 (56.58 qha−1), and P. fluorescens (Pf-1) (55.83 qha−1). A similar trend was observed at 50% flowering, where grain yield was less than that recorded at the booting stage and higher than spraying at ten days after flowering. The lowest yield was recorded when spraying was done ten days after flowering, which is significantly higher than the control (51.03 qha−1) ().
In the pooled data of 2019 and 2020, it was observed that at the booting stage, the highest yield was recorded when treated with trifloxystrobin 25% + tebuconazole 50% WG at 0.4 gL−1 (65.74 qha−1) with disease severity of 7.75% followed by flusilazole 12.5% + carbendazim 25% EC at 1.0 mLL−1 (64.14 qha−1) with disease severity of 10.59% compared to control (49.56 qha−1) with disease severity of 42.37%. Among the bio-control agents and bio formulations, T. viride (56.01 qha−1) recorded a higher yield with a disease severity of 30.20% followed by LBD-1 (55.44 qha−1) with a disease severity of 32.77%. Overall, fungicides reduced the disease severity and increased the yield significantly compared to bio-control agents and formulations ().
Table 4. Effect of bio formulations, bio-control agents, and fungicides on rice false smut (pooled 2019 and 2020).
3.4.3. Impact of application strategy on efficacy of treatments
Results from the ANOVA () revealed that the application of treatments at particular crop stages has significantly (P < 0.05) influenced the control of RFS disease under field conditions. Similarly, treatments and their interaction with crop stages had significant differences in the disease severity of RFS. Hence, spraying of fungicides, bioagents, or bioformulations at specific and defined crop stages has a substantial role in deciding the potency of treatments in controlling RFS under natural epiphytotic conditions.
Table 5. Analysis of Variance (ANOVA) for the influence of crop stages and treatments on disease severity in rice under field conditions.
4. Discussion
As RFS has attained a major disease status, there is an urgent need to develop strategies for the efficient and economical management of the disease. Efforts have been made worldwide to manage the RFS menace by finding resistance sources, employing biological agents, modifying cultural practices, and applying fungicidal molecules (Sun et al., Citation2020). However, RFS management relies largely on the application of fungicides, as they provide immediate control against RFS pathogens (Hu et al., Citation2018). The present study was carried out in vitro and at the field level to find effective biocontrol, bio formulation, and chemical fungicide for the management of the RFS. In vitro results implicated that all the tested fungicides significantly inhibited the mycelial growth of U. virens over control. The findings of our work were also supported by previous studies (Bhargava et al., Citation2018; Banasode & Hosagoudar, Citation2020; Singh et al., Citation2021). Biocontrol agents such as B. subtilis (Liang et al., Citation2014), and Trichoderma sp. (Kannahi et al., Citation2016) were found to reduce the disease in controlled and in-vitro conditions against U. virens. Among Trichoderma sp., T. viride, T. virens, T. harzianum, and T. reesei, have been reported previously to exhibit antagonistic activity against U. virens (Kannahi et al., Citation2016).
Subsequently, the antagonistic effects of Trichoderma sp. have been described and demonstrated under in-vitro conditions (Baite et al., Citation2022). The study reported maximum inhibition by T. harzianum (66.88%) followed by T. atroviride (51.16%), Dendryphiella sp. (41.50%), B. amyloliquefaciens (36.56%), and B. subtilis (36.40%) (Baite et al., Citation2022). T. harzianum and T. atroviride control the pathogen by parasitism and the production of volatile metabolites. B. subtilis is also known to produce volatile metabolites that control U. virens (Baite et al., Citation2022).
Previous studies on bioformulations reported the efficacy of LBD on blast disease (Sahana et al. Citation2022). The present study reported the antagonist activity of LBD in the management of RFS disease. Studies on bio-control agents such as B. subtilis (Liang et al., Citation2014) and Trichoderma sp. (Kannahi et al., Citation2016) against U. virens reduce the disease in controlled and in-vitro conditions. However, the present study found the inefficient field performance of bioformulations and bio-control agents under field conditions compared to fungicides. In this study, T. viride was found to be more efficient than the other formulations and bio-control agents but less efficient than fungicides. Compared to all the inputs, the management involving the fungicides and their combination found best against RFS disease, such as propiconazole (Chen et al., Citation2013; Kumari & Kumar, Citation2015; Singh & Mahendra, Citation2020), propiconazole and trifloxystrobin 25% + tebuconazole 50% (Ladhalakshmi et al., Citation2014; Singh & Sunder, Citation2015; Raji et al., Citation2016b), trifloxystrobin 25% + tebuconazole 50% 75 WG (Hosagoudar, Citation2018; Savitha et al., Citation2019; Sharanabasav et al., Citation2020), and azoxystrobin 18.2% + difenoconazole 11.4% SC (Pandey et al., Citation2018; Kumar & Shailbala, Citation2019).
Combination fungicides are more efficient in managing RFS disease compared to solo fungicides due to their lower dose, broad range of action, and also lower risk of fungicide resistance in target pathogens (Bag & Saha, Citation2009; Bhuvaneswari & Raju, Citation2012; Kumar & Veerabhadraswamy, Citation2014; Pramesh et al., Citation2017; Pramesh et al., Citation2017; Savitha et al., Citation2019). In the present study, both combination and solo fungicides such as trifloxystrobin 25% + tebuconazole 50% 75 WG, flusilazole 12.5% + carbendazim 25% EC, propiconazole 25% EC, azoxystrobin 23% SC, and difenconazole 25% EC showed higher efficacy in reducing the disease severity and hence can be recommended for the successful management of the disease.
The trifloxystrobin 25% + tebuconazole 50% 75 WG fungicide found best in the present study consists of two different molecules, trifloxystrobin and tebuconazole. Chemically, trifloxystrobin is methyl (2E)–2–(methoxyimino)–2–[2–({[(E)–{1–[3–(trifluoromethyl) phenyl] ethylidene} amino] oxy} methyl) phenyl] acetate, is a broad-spectrum site-specific fungicide that is active against both higher and lower fungi (Kegley et al., Citation2016). This molecule acts by inhibiting the mitochondrial respiration of fungal cells by targeting the cytochrome bc1 in mitochondria. Since it’s a site-specific fungicide, the sub-populations of the fungal pathogens can be resistant against the strobilurin; under such circumstances, the compound tebuconazole (RS–1–(4–chlorophenyl)–4,4–dimethyl–3– (1H, 1,2,4–triazol–1–yl methyl) pentan–3–ol) helps to manage the disease. Tebuconazole is a systemic triazole fungicide that acts by inhibiting the ergosterol biosynthesis in fungi, thereby providing protective, curative, and eradicative action (Kegley et al., Citation2016; Klix et al., Citation2007).
In conclusion, the present study found that the application of trifloxystrobin 25% + tebuconazole 50% WG at 0.4 gL−1 is highly effective during the booting stage of the crop in reducing the RFS under field conditions. Hence, this fungicide should be included in the RFS disease management practice. Although different methods are available for disease management, the use of fungicides stands best over others due to their fast and site-specific action. The action of bio-control agents and bioformulations in field conditions is not as efficient as chemicals. Therefore, chemical control is inevitable and reliable for farmers to manage false smut disease. Following proper cultural practices and a foliar spray of combi fungicide at the booting stage are most important to manage the disease in integrated pest management (IPM) systems where the application of need-based fungicide has been recommended.
5. Conclusion
Rice False Smut is an important emerging disease of rice in the world. Managing this disease is of major concern as the disease causes a significant yield loss and threatens human and animal health by producing mycotoxins. Currently, the information on on-field disease management is limited, and several previous studies focused only on fungicides or bio-agents without standardizing the spray schedule. In the present study, we have investigated the effectiveness of bio-formulations (seaweed derivatives), biocontrol agents, and fungicides for managing RFS at the field level. The study evaluated two seaweed-derived bioformulations (LBD 1 and 6), biocontrol agents T. viride, B. velezensis (P-42 and A-6), and P. fluorescens strain Pf-1, and various fungicides. Our study indicated that the timing of the application of fungicides, bio-agents, and bio-formulations plays a crucial role in managing the disease.
To the best of our knowledge, this is the first comprehensive record of managing the RFS disease using seaweed extracts, fungicides, and microbial bioagents and to standardize the spray schedule for better disease control. We hope this work will be more interesting to the researchers working on rice diseases in general and RFA in particular.
Authors’ contributions statement
Saddamhusen Alase: Data curation, investigation, writing original draft. D. Pramesh: Conceptualization, supervision, data curation, investigation. M. K. Prasanna Kumar: Investigation, supervision, and data curation. Shankarappa Sridhara: Reviewing and fund acquisition. Hosam O. Elansary: draft writing and fund acquisition. Mohamed A. Rashwan: Reviewing and fund acquisition. Ihab Mohamed Moussa: Reviewing and fund acquisition. B. S. Chethana: Data curation, Investigation. Balanagouda Patil: Formal analysis, Writing–review, and editing. Amoghavarsha Chittaragi: Formal analysis, Writing – review & editing. A. Ragunandana: Writing– review & editing. Sharanabasav Huded: Writing– review & editing and A. Nagaraja: Conceptualization, supervision, data curation, investigation.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgments
The authors would like to thank the Researchers Supporting Project (No. RSPD2024R741), King Saud University, Riyadh, Saudi Arabia. The work was also supported by the University of Agricultural Sciences, Raichur. The authors are grateful for the funding from the testing chemical project of UAS Raichur (Ab.Ac. 8096).
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
Additional information
Funding
Notes on contributors
Saddamhusen Alase
Saddamhusen Alase obtained his Ph.D. from the Department of Plant Pathology, University of Agricultural Sciences, GKVK, Bangalore. He has worked on False smut of rice for his Ph.D. work. He earned his M.Sc. in Plant Pathology from the Department of Plant Pathology, University of Agricultural Sciences Raichur. His research interests include host plant resistance and disease management. He has published around 15 research papers in peer-reviewed journals.
D. Pramesh
D. Pramesh obtained his Master’s and Doctoral degrees from ICAR-IARI, New Delhi, with a specialization in plant virus genomics and diagnostics. He was also a visiting scientist at the Department of Plant Pathology and Entomology, North Carolina State University, Raleigh, NC, USA. After joining UAS Raichur, with the support of national and international research grants, his research was mainly focused on pathogenomics, virulence profiling, diagnostics, host plant resistance, microbiome, and disease management. He has published more than 100 papers in peer-reviewed journals. He served as an academic editor of PLOS ONE journal and a peer-reviewer for more than 60 international journals.
M. K. Prasanna Kumar
M. K. Prasanna Kumar, Professor of Plant Pathology at the University of Agricultural Sciences, Bangalore, obtained his doctoral degree from UAS, Bangalore. Before joining the university, he worked as a consultant for FAO-TCDC projects in Middle and Far Eastern countries and later as a biologist at Dow AgroSciences. In his current position, he has characterized several fungal and bacterial pathogens infecting rice, pomegranate, and tomato using next-generation sequencing technologies. He has identified many endophytes and phylloplane bacteria along with their antimicrobial compounds and has characterized them. He continues to advance research in pathogenomics while imparting this knowledge to his master’s and doctoral students.
Shankarappa Sridhara
Shankarappa Sridhara, a Professor at the Department of Agronomy, KSNUAHS, Shivamogga, obtained his Ph. D. from UAS-GKVK, Bengaluru in 1997. In addition to holding five patents, he has over 140 articles published in reputable journals. He had overseen the execution of initiatives linked to climate change and mentored several M. Sc. and Ph. D. students. Crop modeling, meteorology, climate change, weed biology, and forecasting are among his areas of interest in research.
Hosam O. Elansary
Hosam O. Elansary, Professor at the Department of Plant Production, College of Food and Agricultural Sciences, King Saud University. Previous visiting faculty at the Biodiversity Institute of Ontario, University of Guelph, Canada. He was a Ph.D. graduate of the faculty of Science at Charles University in Prague. During his studies in Europe, he worked on a project between the USA & the Czech Academy of Sciences. He has more than 100 ISI publications in prestigious plant physiology and molecular Biology journals. He was selected as an editorial board of several international journals. He participated either as a PI, Co-PI, or consultant in several local and international projects like EIFAD & TreeBOL.
Mohamed A. Rashwan
Mohamed A. Rashwan, Assistant Professor, Department of Agricultural Engineering, College of Food and Agricultural Sciences, King Saud University, Saudi Arabia. Professor, Department of Agricultural and Biosystems Engineering, Faculty of Agriculture, Alexandria University, Alexandria, Egypt. He did post-doctoral studies at the University of Alexandria and obtained his doctorate from Kagoshima University, Japan, in 2005 in the field of agricultural engineering. He is interested in working in environmental engineering fields such as compost production and greenhouse cooling. He also has an interest in solar energy and its uses in agriculture. He supervised a number of Master’s theses. He published and peer-reviewed many papers and wrote many books in the field of agricultural engineering.
Ihab Mohamed Moussa
Ihab Mohamed Moussa is working as a Professor of Plant Microbiology and Biotechnology in the Department of Botany & Microbiology at King Saud University, Saudi Arabia. His current research focuses on Plant microbiology, plant physiology, diversity, Plant succession, Phytoremediation, and Conservation of the microbiology of the Egyptian Mediterranean coast, the Nile Delta, and Saudi Arabia. He is actively engaged in several scientific projects that have been successfully completed. He has published over 40 papers in peer-reviewed journals of international repute.
B. S. Chethana
B. S. Chethana, an Assistant Professor of Plant Pathology at the University of Agricultural Sciences Bangalore, Karnataka, excels in managing diseases of rice, tomato, and other crops. With an M.Sc. and Ph.D., her research focuses on molecular characterization of fungal pathogens and integrated disease management.
Balanagouda Patil
Balanagouda Patil is currently pursuing a Postdoctoral Degree from ITC, Life Sciences, India Ltd, Bengaluru, India. He graduated in Agriculture and obtained an M.Sc. and Ph.D. in Plant Pathology. He specializes in mycology, forest pathology, and disease management.
Amoghavarsha Chittaragi
Amoghavarsha Chittaragi is a Scientist at the Chaudhary Charan Haryana Agricultural University, Hisar, Haryana, India. He graduated in Agriculture and obtained an M.Sc. and Ph.D. in Plant Pathology. He specializes in Mycology and host-pathogen interaction.
A. Raghunandana
A. Raghunandana obtained his Ph. D. degree with a specialization in Plant Pathology from the University of Agricultural Sciences, Dharwad. During his doctoral program, he investigated molecular aspects of the rice sheath disease complex and the source of resistance in rice landraces against sheath blight disease. Currently, he is working as a senior research fellow in the Rice Pathology Lab, ARS, Gangavathi. His areas of expertise include pathogenomics, mycology, bacteriology, and disease management.
H. Sharanabasav
H. Sharanabasav is pursuing a Doctoral Research Scholarship (Ph.D.) in the Department of Plant Pathology, University of Agricultural Sciences, Raichur, Karnataka. He has published more than five Research papers in International and National journals.
A. Nagaraja
A. Nagaraja obtained a Ph.D. in Mycology and Plant Pathology from the ICAR-IARI, New Delhi, and started an academic career as an Assistant Professor (Plant Pathology) at UAS Dharwad and finally moved to UAS Bangalore. He has published over 100 research papers in journals of high repute.
References
- Abbas, H. K., Shier, W. T., Cartwright, R. D., & Sciumbato, G. L. (2014). Ustilaginoidea virens infection of rice in Arkansas: toxicity of false smut galls, their extracts and the ustiloxin fraction. American Journal of Plant Sciences, 05(21), 1–13. https://doi.org/10.4236/ajps.2014.521333
- Alase, S., Nagaraja, A., Pramesh, D., & Prasanna Kumar, M. K. (2021). Influence of weather parameters on false smut disease development in rice. The Mysore Journal of Agricultural Sciences, 55(4), 320–325.
- Amoghavarsha, C., Pramesh, D., Sridhara, S., Patil, B., Shil, S., Naik, G. R., Naik, M. K., Shokralla, S., El-Sabrout, A. M., Mahmoud, E. A., Elansary, H. O., Nayak, A., & Prasannakumar, M. K. (2022). Spatial distribution and identification of potential risk regions to rice blast disease in different rice ecosystems of Karnataka. Scientific Reports, 12(1), 7403. https://doi.org/10.1038/s41598-022-11453-9
- Andargie, M., Congyi, Z., Yun, Y., & Li, J. (2017). Identification and evaluation of potential bio-control fungal endophytes against Ustilagonoidea virens on rice plants. World Journal of Microbiology & Biotechnology, 33(6), 120. https://doi.org/10.1007/s11274-017-2273-y
- Anonymous. (2020). Final estimates of district wise area, production and yield of principal crops in Karnataka for the year 2018-19. Director of Economics and Statistics Bengaluru (pp. 3).
- Bag, M. K., & Saha, S. (2009). Fungitoxic effect of Nativo 75 WG (trifloxystrobin 25% + tebuconazole 50%) on grain discoloration (GD) disease of rice in West Bengal. Pestology, 33, 47–49.
- Baite, M. S., Prabhukarthikeyan, S. R., & Raghu, S. (2022). Biological control of a fungus Ustilaginoidea virens causing false smut of rice. BioControl, 67(3), 357–363. https://doi.org/10.1007/s10526-022-10148-4
- Banasode, M., & Hosagoudar, G. N. (2020). In vitro and in vivo evaluation of fungicides against false smut disease of rice in Hilly Zone of Karnataka, India. International Journal of Current Microbiology and Applied Sciences, 9(9), 3598–3609. https://doi.org/10.20546/ijcmas.2020.909.445
- Bhargava, P., Kumar, A., Kumar, S., & Azad, C. S. (2018). Impact of fungicides and nanoparticles on Ustilaginoidea virens causing false smut disease of rice. J. Pharmacogn. Phytochem, 7(1), 1541–1544.
- Bhuvaneswari, V., & Raju, K. S. (2012). Efficacy of new combination fungicide against rice sheath blight caused by Rhizoctonia solani (Kuhn). J. Rice Res, 5(1), &2): 212–215.
- Bin Rahman, A. R., & Zhang, J. (2023). Trends in rice research: 2030 and beyond. Food and Energy Secur, 12(2), e390.
- Bird, J., Roy, S., Shah, T., Aggarwal, P., Smakhtin, V., Amarnath, G., Amarasinghe, U. A., Pavelic, P., & Mccornick, P. G. (2016). Adapting to climate variability and change in India. In Biswas, A. K., Tortajada, C., (Eds.), Water security. Climate change and sustainable development (pp. 41–63). Springer.
- Birla, D. S., Malik, K., Sainger, M., Chaudhary, D., Jaiwal, R., & Jaiwal, P. K. (2017). Progress and challenges in improving the nutritional quality of rice (Oryza sativa L.). Critical Reviews in Food Science and Nutrition, 57(11), 2455–2481. https://doi.org/10.1080/10408398.2015.1084992
- Bunbury-Blanchette, A. L., & Walker, A. K. (2019). Trichoderma species show biocontrol potential in dual culture and greenhouse bioassays against Fusarium basal rot of onion. Biological Control, 130, 127–135. https://doi.org/10.1016/j.biocontrol.2018.11.007
- Chen, Y., Zhang, Y., Yao, J., Li, Y. F., Yang, X., Wang, W. X., Zhang, A. F., & Gao, T. C. (2013). Frequency distribution of sensitivity of Ustilaginoidea virens to four EBI fungicides, prochloraz, difenconazole, propiconazole and tebuconazole and their efficacy in controlling false smut in Anhui Province of China. Phytoparasitica, 41(3), 277–284. https://doi.org/10.1007/s12600-013-0288-y
- Cooke, M. C. (1878). Some extra-European fungi. Grevillea, 7, 13–15.
- Dodan, D. S., & Singh, S. R. (1996). False smut of rice: Present status. Agricultural Reviews, 17(4), 227–240.
- El-Naggar, M. M., Elsharkawy, M. M., Almalla, R. A., El-Kot, G., Alwakil, A. M., Badr, M. M. (2015). Control of Ustilaginoidea virens, the causal agent of rice false smut disease in Egypt. Egypt. J. Pest Control, 25, 555–564.
- Fokkema, N. J. (1978). Fungal antagonism in the phyllosphere. Annals of Applied Biology, 89(1), 115–119. https://doi.org/10.1111/j.1744-7348.1978.tb02582.x
- Guyer, D., Tuttle, A., Rouse, S., Volrath, S., Johnson, M., Potter, S., Görlach, J., Goff, S., Crossland, L., & Ward, E. (1998). Activation of latent 171 transgenes in Arabidopsis using a hybrid transcription factor. Genetics, 149(2), 633–639. https://doi.org/10.1093/genetics/149.2.633
- Han, Y., Kang, Z., Jun, Y., Nan, Z., Anfei, F., Yong, Z., Yongfeng, L., Zhiyi, C., Tom, H., & Wenxian, S. (2015). Differential expression profiling of the early response to Ustilaginoidea virens between false smut resistant and susceptible rice varieties. BMC Genomics, 16(1), 955. https://doi.org/10.1186/s12864-015-2193-x
- Hana, S., Banakar, N., Rangaswami, K. T., & Kumar, M. K. P. (2018). Unravelling the effect of seaweed bio formulations in relieving biotic and abiotic stress in rice. International Journal of Current Microbiology and Applied Sciences, 7(10), 543–550. https://doi.org/10.20546/ijcmas.2018.710.060
- Hosagoudar, G. N. (2018). Evaluation of fungicides for the management of false smut of paddy. J. Pharmacogn. Phytochem, 7(6), 1870–1874.
- Hu, D. W., Liang, W. S., & Lai, C. H. (2018). Advances in the occurrence of rice false smut and its control. Plant Prot, 44, 1–5.
- Huded, S., Pramesh, D., Chittaragi, A., Sridhara, S., Chidanandappa, E., Prasannakumar, M. K., Manjunatha, C., Patil, B., Shil, S., Pushpa, H. D., Raghunandana, A., Usha, I., Balasundram, S. K., & Shamshiri, R. R. (2022). Spatial distribution patterns for identifying risk areas associated with false smut disease of rice in Southern India. Agronomy, 12(12), 2947. https://doi.org/10.3390/agronomy12122947
- Kannahi, M., Dhivya, S., & Senthilkumar, R, PG and Research Department of Microbiology, Sengamala Thayaar Educational Trust Women’s College, Mannargudi, Tamil Nadu, India. (2016). Biological control on rice false smut disease using Trichoderma species. International Journal of Pure & Applied Bioscience, 4(2), 311–316. https://doi.org/10.18782/2320-7051.2237
- Kazan, K. (2018). Plant-biotic interactions under elevated CO2: A molecular perspective. Environmental and Experimental Botany, 153, 249–261. https://doi.org/10.1016/j.envexpbot.2018.06.005
- Kegley, S. E., Hill, B. R., Orme, S., & Choi, A. H. (2016). PAN Pesticide Database, Pesticide Action Network. North America, Oakland, California, USA. http://www.pesticideinfo.org.
- Klix, M. B., Verreet, J. A., & Beyer, M. (2007). Comparison of the declining triazole sensitivity of Gibberella zeae and increased sensitivity achieved by advances in triazole fungicide development. Crop Protection, 26(4), 683–690. https://doi.org/10.1016/j.cropro.2006.06.006
- Kumari, S., & Kumar, J. (2015). Evaluation of yield losses and management practices of false smut in rice (Oryza sativa). Indian Phytopathol, 68(1), 45–49.
- Kumar, A., & Shailbala. (2019). Efficacy of different fungicides for the management of false smut disease of rice in Bihar. International Journal of Chemical Studies, 7(3), 4220–4222.
- Kumar, M. K. P., & Veerabhadraswamy, A. L. (2014). Appraise combination of fungicides against blast and sheath blight diseases of paddy (Oryza sativa L.). Journal of Experimental Biology and Agricultural Sciences, 2(1), 213–215.
- Ladhalakshmi, D., Laha, G. S., Krishnaveni, D., Prakasam, V., & Prasad, M. S. (2014). Evaluation of selected fungicides against rice false smut disease. 3rd Intl. Conference on Agri. Hort.
- Ladhalakshmi, D., Laha, G. S., Singh, R., Karthikeyan, A., Mangrauthia, S. K., Sundaram, R. M., Thukkaiyannan, P., & Viraktamath, B. C. (2012). Isolation and characterization of Ustilaginoidea virens and survey of false smut disease of rice in India. Phytoparasitica, 40(2), 171–176. https://doi.org/10.1007/s12600-011-0214-0
- Liang, Y., Zhang, X. M., Li, D. Q., Huang, F., Hu, P. S., & Peng, Y. L. (2014). Integrated approach to control false smut in hybrid rice in Sichuan Province, China. Rice Science, 21(6), 354–−360. https://doi.org/10.1016/S1672-6308(14)60269-9
- Lu, M. H., Liu, W. C., & Zhu, F. (2018). Epidemic law and control technique of rice false smut in recent years. China Plant Prot, 38, 44–47.
- Mandhare, V. K., Gawade, S. B., Game, B. C., & Padule, D. N. (2008). Prevalence and incidence of bunt and false smut in paddy (Oryza sativa L.) seeds in Maharashtra. Agricultural Science Digest, 28(4), 292–294.
- Muniraju, K. M., Pramesh, D., Mallesh, S. B., Mallikarjun, K., & Guruprasad, G. S. (2017a). Disease severity and yield losses caused by false smut disease of rice in different rice ecosystems of Karnataka. Int. J. Microbiol. Res, 9(10), 963–966.
- Muniraju, K. M., Pramesh, D., Mallesh, S. B., Mallikarjun, K., & Guruprasad, G. S. (2017b). Novel fungicides for the management of false smut disease of rice caused by Ustilaginoidea virens. International Journal of Current Microbiology and Applied Sciences, 6(11), 2664–2669. https://doi.org/10.20546/ijcmas.2017.611.313
- Pandey, S. K., Masurkar, P., & Singh, R. K. (2018). Status and incidence of false smut disease in rice and their chemical management. J. Pharmacogn. Phytochem, 7(3), 588–590.
- Pramesh, D, Muniraju, K, Mallikarjun, K, Guruprasad, G, Mahantashivayogayya, K, Reddy, B, Gowdar, S, Chethana, B, Maruti, 2017, Bio-efficacy of a combination fungicide against of blast and sheath blight disease of paddy.Journal of Experimental Agriculture International., 14(4): 1–8. https://doi.org/10.9734/JEAI/2016/28893
- Pramesh, D., Prasannakumar, M. K., Muniraju, K. M., Mahesh, H. B., Pushpa, H. D., Manjunatha, C., Saddamhusen, A., Chidanandappa, E., Yadav, M. K., Kumara, M. K., Sharanabasav, H., Rohith, B. S., Banerjee, G., & Das, A. J. (2020). Comparative genomics of rice false smut fungi Ustilaginoidea virens Uv-Gvt strain from India reveals genetic diversity and phylogenetic divergence. 3 Biotech, 10(8), 342. https://doi.org/10.1007/s13205-020-02336-9
- Pramesh, D., Saddamhusen Alase, K. M., Muniraju, K., & Kumara, M. (2017). A combination fungicide for the management of sheath blight, sheath rot and stem rot diseases of paddy. International Journal of Current Microbiology and Applied Science, 6(9), 3500–3509.
- Raghunandana, A., Pramesh, D., Sunkad, G., Amoghavarsha, C., Yadav, M. K., Ngangkham, U., Pushpa, H. D., Prasannakumar, M. K., Raghavendra, B. T., Naik, H. R., Manjunatha, S. E., & Yenjerappa, S. T. (2023). Genetic diversity and pathotype profiling of Xanthomonas oryzae pv. oryzae isolates from diverse rice growing ecosystems of Karnataka state of India. Plant Protection Science, 59(1), 31–47. https://doi.org/10.17221/76/2022-PPS
- Raji, P., Sumiya, K. V., Dhanya, S., Remya, K., & Narayanankutty, M. C. (2016a). Screening of rice varieties and in-vitro evaluation of botanicals against false smut pathogen, Ustilaginoidea virens. International Journal of Agricultural Science Research, 6(2), 79–86.
- Raji, P., Sumiya, K. V., Renjisha, K., Dhanya, S., & Narayanankutty, M. C. (2016b). Evaluation of fungicides against false smut of rice caused by Ustilaginoidea Virens. International Journal of Applied and Natural Sciences, 5(2), 77–82.
- Rimbaud, L., Fabre, F., Papaïx, J., Moury, B., Lannou, C., Barrett, L. G., & Thrall, P. H. (2021). Models of plant resistance deployment. Annual Review of Phytopathology, 59(1), 125–152. https://doi.org/10.1146/annurev-phyto-020620-122134
- Sahana, N. B., Prasanna Kumar, M. K., Mahesh, H. B., Parivallal, P. B., Puneeth, M. E., Gautam, C., Pramesh, D., Shiva Kumara, T. N., Girish, T. R., Nori, S., & Narayan, S. S. (2022). Red-seaweed biostimulants differentially alleviate the impact of fungicidal stress in rice (Oryza sativa L.). Scientific Reports, 12(1), 5993. https://doi.org/10.1038/s41598-022-10010-8
- Sanghera, G. S., Ahanger, M. A., Kashayp, S. C., Bhat, Z. A., Rather, A. G., & Parray, G. A. (2012). False smut of rice (Ustilaginoidea virens) under temperate agro-climatic condition of Kashmir. India. Elixir Bio. Tech, 49, 9827–9830.
- Savitha, A. S., Nagaraja, A., Pramesh, D., & Chethana, B. S. (2019). Bio efficacy of novel fungicide molecules in the management of false smut of rice caused by Ustilaginoidea virens. Int. J. Chem. Stud, 7(4), 3208–3212.
- Sharanabasav, H., Pramesh, D., Chidanandappa, E., Saddamhusen, A., Amoghvarsha, C., Raghunandana, A., Prasanna Kumar, M. K., Raghavendra, B. T., Harischandra, N., Mallesh, S. B., Mahantashivayogayya, K., Huruli, S., Reddy, B. G. M., & Gowdar, S. B. (2020). Field evaluation of fungicides against false smut disease of rice. Journal of Pharmacognosy and Phytochemistry, 9(3), 1453–1456. https://doi.org/10.22271/phyto.2020.v9.i3x.11515
- Singh, R. A., & Dube, S. (1978). International Rice Research Institute (pp.49–55).
- Singh, L., Kumar, P., Poonam, R., & Singh, B. (2021). In-vitro evaluation of different chemicals against Ustilaginoidea virens causing false smut of rice. International Journal of Chemical Studies, 9(1), 983–986. https://doi.org/10.22271/chemi.2021.v9.i1n.11353
- Singh, U. P., & Mahendra, A. (2020). Evaluation of fungicides for the false smut in Jaunpur of Eastern U.P. International Journal of Current Microbiology and Applied Sciences, 10, 101–105.
- Singh, G., & Milne, K. S. (1974). Laboratory evaluation of fungicides against fungi causing flower blight of chrysanthemums. New Zealand Journal of Agricultural Research, 2(2), 181–183. https://doi.org/10.1080/03015521.1974.10425758
- Singh, R., & Sunder, S. (2015). Identification of sources of resistance to blast and false smut of rice and their management with fungicides. Journal of Mycology and Plant Pathology, 45(1), 55–59.
- Sun, W., Fan, J., Fang, A., Li, Y., Tariqjaveed, M., Li, D., Hu, D., & Wang, W. M. (2020). Ustilaginoidea virens: Insights into an emerging rice pathogen. Annual Review of Phytopathology, 58(1), 363–385. https://doi.org/10.1146/annurev-phyto-010820-012908
- Vincent, J. M. (1947). Distortion of fungal hyphae in the presence of certain inhibitors. Nature, 159(4051), 850–850. https://doi.org/10.1038/159850b0

