?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Advancing understanding of the diverse properties of essential oils has spurred a significant increase in both their production and application. The current research aimed to study the chemical, physical, and antifungal characteristics of Vinca rosea leaves’ essential oil compared to its extract. The leaf’s essential oil and extract composition were examined using GC-MS and further tested for antifungal activity against Fusarium graminearum and Bipolaris sorokiniana by poisoned food technique. 34 compounds were identified in leaves essential oil predominantly composed of butylated hydroxytoluene (32.74%). The methanolic extract showed the presence of alkaloids, mainly vindoline, and the non-polar extract showed the presence of cholesterol as the primary compound. The methanolic extract demonstrated stronger antifungal activity against F. graminearum, with ED50 and ED90 of 80 and 240 µg/mL, compared to the essential oil, which had higher ED50 and ED90 of 98 and 865 µg/mL, respectively. In the case of B. sorokiniana, ED50 values of methanol extract, essential oil, and hexane extract were 76, 100, and >1000 µg/ml, respectively, and ED90 were observed to be 290, 875, and >1000 µg/ml. Vinca rosea essential oil can potentially be used to develop natural antifungal agents. For the first time, the chemical composition of the essential oil of V. rosea cultivated in the Northern Indian region is being reported.
REVIEWING EDITOR:
1. Introduction
The use of synthetic fungicides is restricted in agriculture due to the increasing resistance of fungi against them and awareness about their harmful effects, which leads to the inclination to use organic fungicides. Various natural substances derived from plants, including extracts and essential oils, serve crucial roles in medicine and agriculture due to their versatility. Different plant parts offer a wealth of phytochemicals with a wide range of biological activities, which the agricultural and pharmaceutical sectors have utilized to develop numerous drugs and chemicals.
Vinca rosea (also known as Catharanthus roseus) belongs to the Apocynaceae family, is native to Madagascar, and is cultivated worldwide for its medicinal properties. In India, it is commonly known as Sadabahar and Nityakalyani, mainly in Punjab, Kerala, Gujarat, Uttar Pradesh, and Assam. Vinca rosea has been traditionally used to treat high blood pressure, diabetes, wasp stings, and various other treatments (Das et al., Citation2020). This plant’s terpenoid indole alkaloid pathway synthesizes over one hundred thirty alkaloids (Almagro et al., Citation2015). Various alkaloids found in this plant are of commercial importance. Vincristine and vinblastine are used as anticancer drugs, and ajmalicine is used as an antihypertensive drug approved by the World Health Organization for their clinical usage (Almagro et al., Citation2015). In previous studies, dry and fresh leaves were used to extract the essential oil of V. rosea. Leaves showed the presence of esters, fatty acids, alkanes, terpenoids, aldehydes, alcohols, ketones, and various unidentified volatile components in essential oil, some of which were isolated for commercial purposes (Pandey et al., Citation2006; Cavaleiro et al., Citation2006). Various categories of compounds, like phenols, flavonoids, anthocyanins, volatile compounds, alkaloids, etc., were also reported in the extracts of V. rosea plants. It has also been gaining acceptance for its diverse application in various agricultural and pharmacological properties such as anthelmintic (Agarwal et al., Citation2011), antioxidant (Barkat & Mujeeb, Citation2013), antimicrobial (Prajakta & Ghosh, Citation2010), antibacterial (Ibrahim et al., Citation2011), antifungal (Balaabirami & Patharajan, Citation2012), antidiarrheal (Rajput et al., Citation2011), to name a few. Vindoline, vincristine, vinblastine, catharanthine, leurosine, syringic acid, petunidin, quercetin, para-coumaric acid, heneicosane, tricosane, and palmitic acid are some of the known crucial constituents of this plant responsible for its applications in agronomic and therapeutic practices (Pandey et al., Citation2006; Renault et al., Citation1999; Hisiger & Jolicoeur, Citation2007).
Vinca rosea has been established as an essential plant for pharmaceutical purposes, but its usage has yet to be explored much when it is categorized in agriculture. According to the literature, these plants could vary in their constituents based on their location, resulting from their geographical and climatic conditions. Little work has been reported on the V. rosea leaves essential oil obtained from the Northern Indian region and their antifungal activity against phytopathogenic fungi. Fusarium graminearum is a fungal pathogen causing billions of dollars of economic losses worldwide (Bai & Shaner, Citation2004; De Wolf et al., Citation2003). This infection alters the amino acid composition in wheat, leading to shrivelled kernels and contaminating the remaining grains with mycotoxins, primarily deoxynivalenol, which also hinders protein synthesis (Beyer & Aumann, Citation2008; Cavaleiro et al., Citation2006). Bipolaris sorokiniana is the fungal agent that causes a wide variety of cereal diseases, which cause millions of tons of wheat loss each year (Kumar & Rai, Citation2018).To study the relationship between the active ingredients and biological activity, the present study evaluated the antifungal activity of leaves essential oil and extracts against pathogenic wheat fungi, i.e. F. graminearum, which causes Fusarium head blight and B. sorokiniana, causing spot blotch and root rot in wheat.
2. Material and methods
2.1. Chemicals and equipment
All solvents, reagents, and absorbents employed in the experiments were of high analytical grade. The analysis of V. rosea essential oil was performed using Gas Chromatography-Mass Spectrometry (GC-MS) at the Advanced Instrumentation Research Facility (AIRF) located at Jawaharlal Nehru University, New Delhi. The specific GC-MS equipment used was a Thermo Focus-DSQ model.
2.2. Materials
2.2.1. Plant collection and authentication
Vinca rosea leaves were harvested in the spring from the botanical gardens of Punjab Agricultural University (PAU) in Ludhiana, Punjab. The precise location of the collection is at coordinates 30.900965°N latitude 75.857277°E longitude. The plant species was formally identified and authenticated by the Principal Scientist and Head of the Department of Floriculture and Landscaping at PAU, with an authentication certificate provided for reference (refer to SI).
2.2.2. Pathogen cultures
Cultures of two phytopathogenic fungi, Fusarium graminearum and Bipolaris sorokiniana, were obtained from Dr. Jaspal Kaur, a Senior Plant Pathologist at PAU. These fungal strains were maintained on slants of potato dextrose agar (PDA) and stored at 4 °C to preserve viability.
2.3. Isolation of essential oil
Freshly harvested Vinca rosea leaves were washed thoroughly under running water and rinsed twice with sterilized water to remove soil and debris. A batch of 250 g of leaves was soaked overnight in 2.5 L of water in a 5-liter round-bottom flask. The essential oil was extracted using the steam distillation method with a Clevenger apparatus for a duration of 4 h (Pandey et al., Citation2006). This process was repeated 20 times to gather a sufficient quantity of essential oil, which could be further used to study bioactive compounds as well as for antifungal study. The collected essential oil was then dried over anhydrous sodium sulfate and stored at 4 °C to maintain its integrity.
2.4. Physical properties of essential oil
The optical rotation of the essential oil was measured using a polarimeter, and the refractive index was determined with a refractometer (Perfit India). All measurements were conducted at a controlled room temperature of 25 °C (Kaur et al., Citation2021).
2.5. Gas Chromatography-mass spectrometry (GC-MS) analysis of Vinca rosea leaf essential oil
The essential oil from the leaves of V. rosea was analyzed to determine its chemical composition using a Shimadzu QP2010 Plus gas chromatography-mass spectrometry (GC-MS) system. During the analysis, a single injection was made while keeping the split valve closed for the initial minute to enhance the introduction of the sample. The injector was maintained at a temperature of 280 °C. Helium served as the carrier gas at a steady pressure of 69 kPa. The oil’s components were separated using an Rtx-5 MS capillary column, measuring 30 m in length, 20 mm in internal diameter, and with a film thickness of 0.25 μm. The temperature of the column was programmed to start at 50 °C, held steady for 2 minutes, then increased at a rate of 3 °C per minute to 180 °C, and finally raised to 280 °C at a rate of 10 °C per minute. In the mass spectrometry stage, conditions were set to electron ionization at 70 eV, with an interface temperature also at 280 °C. The scan range was from 40 to 600 amu, providing a detailed examination of the sample’s components. Retention indices of the detected peaks were calculated using a series of n-alkanes (C9-C33), and reference values were calculated under the same conditions using the ADAM RI system (Adams, Citation2007). The identification of compounds was further supported by comparing mass spectra with those in databases like NIST08, WILEY8, and specialized libraries focused on fragrance and flavor compounds. The calculated retention indices were cross-verified with data from the NIST Chemistry WebBook, ensuring accurate identification of the oil’s components. This meticulous, analytical approach was essential for assessing the antifungal properties of the essential oil.
The chemical composition of Vinca rosea leaf extracts was examined using a Shimadzu QP2010 Plus GC-MS system. The analysis entailed injecting the sample in single-injection mode while keeping the split valve closed for the first minute to ensure optimal sample introduction, with the injector temperature set to 280 °C. Helium gas was utilized as the carrier, maintaining a steady pressure of 69 kPa. The components of the extracts were separated using an Rtx-5 MS capillary column, which measured 30 meters in length, had an internal diameter of 20 mm, and a film thickness of 0.25 μm. The oven’s temperature program started at 50 °C for two minutes, then gradually increased to 180 °C at a rate of 3 °C per minute, and finally rose to 200 °C at 10 °C per minute. During mass spectrometry, electron ionization was performed at 70 eV, with an interface temperature of 200 °C, and scanning was done over a mass range from 40 to 600 amu. The samples were injected in split mode (120:1 ratio) and diluted in methanol (1/100 v/v). The chemical constituents were quantified by normalizing the peak areas observed in the chromatogram (Hashmi et al., Citation2013).
2.6. Preparation of extract
Extract of the leaves was prepared using the maceration technique (Kapoor & Rani, Citation2019). Dried leaf powder of V. rosea (50 g) was soaked with 100 ml of polar solvent (methanol) and was kept at room temperature for 72 h with occasional stirring. The extracted solution was filtered using Whatman filter paper no. 2 and the excess solvent was removed under rotary evaporation. The extract was then stored at 4 °C. The same procedure was used to prepare the non-polar extract, i.e. hexane.
2.7. Yield calculation
The yield of essential oil and various extracts was calculated using the following formula:
2.8. Phytochemical analysis
Extracts of leaves of V. rosea were subjected to phytochemical analysis to detect bioactive compounds. These phytochemical tests were carried out by taking into consideration the standard procedure as described by Kapoor and Rani (Kapoor & Rani, Citation2019).
2.8.1. Alkaloids
To test for alkaloids, combine 1.0 ml of the extract with 2.0 ml of concentrated hydrochloric acid, followed by a few drops of Mayer’s reagent. The formation of yellow precipitates indicates the presence of alkaloids.
2.8.2. Amino acids
For amino acid identification, 2.0 ml of extract is treated with 2% copper (II) sulfate drop and 1.0 ml of 95% ethanol. Adding excess potassium hydroxide pellets results in a pink layer in the ethanolic solution, confirming the presence of amino acids.
2.8.3. Carbohydrates
To detect carbohydrates, boil 1.0 ml of the extract with 1.0 ml of each of Fehling’s solutions A and B. The presence of carbohydrates is confirmed by the formation of a red precipitate.
2.8.4. Flavonoids
Add 2.0 ml of dilute sodium hydroxide to 1.0 ml of extract to test for flavonoids. Adding 3.0 ml of dilute hydrochloric acid turns the yellow solution colourless, indicating flavonoids.
2.8.5. Glycosides
Glycosides were detected by adding 1.0 ml of extract to 2.0 ml of chloroform and 2.0 ml of concentrated sulfuric acid. A reddish-brown ring indicates glycosides.
2.8.6. Phenols
Add 3–4 little drops of ferric chloride to 2.0 ml of the extract for phenols. A dark green colour signifies the presence of phenols.
2.8.7. Saponins
Mix 1.0 ml of extract with 5.0 ml of distilled water to test for saponins and shake vigorously. Stable foam formation confirms the presence of saponins.
2.8.8. Tannins
Detect tannins by treating 2.0 ml of extract with 10% alcoholic ferric chloride solution. The appearance of a dark blue or greenish-grey colour indicates tannins.
2.8.9. Terpenoids
Mix 1.0 ml of extract with 1.0 ml of chloroform and layer with concentrated sulfuric acid for terpenoids. A reddish-brown interface indicates terpenoids.
2.8.10. Cardiac glycosides
Mix 5.0 ml of the extract with 2.0 ml sodium picrate reagent to test for cardiac glycosides. A yellow-orange colour indicates their presence.
2.9. Antifungal activity
The poisoned food technique was employed to test the antifungal efficacy of V. rosea essential oil and extracts against Fusarium graminearum and Bipolaris sorokiniana. Potato dextrose agar (PDA) was prepared by combining 250 g of potato, 20 g of agar, and 20 g of dextrose in 1 L of distilled water. The mixture was sterilized in an autoclave at 15 psi for 30 minutes at 121 °C (Sharma et al., Citation2019). In this technique, the concentration was 20 times higher than the required concentration, which caused the solution to be diluted to the required concentration. The stock solution was prepared in water along with Tween 80 and then added to molten PDA to obtain concentrations of 1000, 500, 250, and 125 μg/ml. The solutions of varying concentrations were poured into sterilized 90 mm Petri dishes and cooled. A 5 mm mycelial disk, taken from a seven-day-old culture, was placed in the center of each PDA plate, sealed with paraffin tape, and incubated in darkness at 25 ± 1 °C for seven days in triplicate. A Petri plate containing an unamended PDA served as a positive control. The percentage inhibition was recorded using formula (Gupta & Tripathi, Citation2011).
AC = Average increase in mycelial growth in control
AT = Average increase in mycelial growth in treatment
The result obtained from F. graminearum and B. sorokiniana was compared with standard fungicide propiconazole (Tilt 25 EC), which served as a negative control and represented in terms of ED50 and ED90 values (effective doses that inhibit 50 per cent and 90 per cent mycelial growth) (Gupta & Tripathi, Citation2011).
2.10. Statistical analysis
All experiments for the antifungal analysis on both fungi were conducted in triplicates. The data obtained was expressed as mean ± standard deviation. Statistical analysis was performed using the Tukey multiple range test with SPSS 16.0 and one-way ANOVA via CPCS 1 software (Software package for social studies, Version 16.0, Armonk, NY). A P-value of less than 0.05 was considered statistically significant.
3. Results and discussion
The gas chromatography-mass spectrometry (GC-MS) analysis identified and quantified thirty-four compounds in the essential oil extracted from V. rosea leaves. provides detailed information on these compounds, and the GC-MS chromatogram of the essential oil is provided in the Supporting Information (SI). The essential oil extracted from the leaves of V. rosea was light yellow in colour and had an average yield of 0.055% (w/v) based on the dry weight of the leaves. The oil exhibited a strong and unpleasant odor, consistent with previous literature reports (Pandey et al., Citation2006; Lawal et al., Citation2015; Aziz et al., Citation2015). Physicochemical properties of the oil include a density of 0.84 g/cm³ at 20 °C and a refractive index of 1.38. Its optical rotation was measured to be 84°6’. Solubility tests showed that the essential oil is soluble in methanol and ethanol, sparingly in hexane, and insoluble in water (Aziz et al., Citation2015).
Table 1. Chemical composition of essential oil of Vinca rosea leaves.
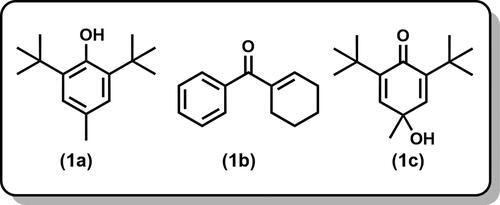
Among the compounds identified, none exceeded a concentration of 32.74% of the total essential oil. The three major constituents were: butylated hydroxytoluene () (32.74%), 1-cyclohexenyl phenyl ketone () (10.36%), and 2,5-cyclohexadien-1-one, 2,6-bis(1,1-dimethylethyl)-4-hydroxy-4-methyl- () (9.9%); together, these three compounds accounted for 53.0% of the essential oil.
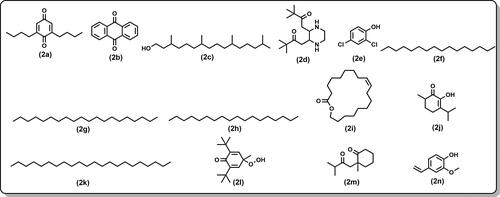
The essential oil also contained several minor compounds, contributing to 39% of the characterized components. These included: 2,6-di-butyl-2,5-cyclohexadiene-1,4-dione () (6.43%), anthraquinone () (5.64%), phytol () (4.75%), 1-[3-(3,3-dimethyl-2-oxo-butylidene)piperazin-2-ylidene]-3,3-dimethyl-butan-2-one () (4.58%), 2,4-dichlorophenol () (2.64%), hexadecane () (2.47%) eicosane () (2.06%), octadecane () (1.79%), oxacyclononadec-10-en-2-one () (1.64%), pseudodiosphenol () (1.54%), docosane () (1.34%), 2,6-di-tert-butyl-4-hydroperoxy-4-methyl-2,5-cyclohexadien-1-one ()(1.23%), 2-methyl-2-(3-methyl-2-oxobutyl)cyclohexanone () (1.22%) and 4-vinylguaiacol () (1.13%). The other identified components’ relative percentage was lower than 1%. Alkanes and aromatic hydrocarbons predominate the chemical composition of the essential oil.
In addition to the primary constituents identified in the V. rosea leaf essential oil, its composition has several noteworthy features. One significant finding is the presence of butylated hydroxytoluene (BHT), a compound known for its antioxidant properties (Huang et al., Citation2020). This underscores the potential of V. rosea leaf oil as a natural source of antioxidants and piques interest in its potential applications. Another notable compound in considerable concentration is phytol, which is involved in the biosynthesis of vitamins and is known for its biological activity (Islam et al., Citation2018). The essential oil composition suggests possible interference with the plant’s genetic regulation pathways, especially given the role of leaves in photosynthesis. Leaves, with their photosynthetically active chloroplasts, might influence the expression of terpenoid biosynthetic genes, leading to organ-specific production of these compounds (Islam et al., Citation2018; Li et al., Citation2020; Verdeguer et al., Citation2020).
Moreover, the volatile profile of the leaf oil shows considerable variation depending on its geographical origin. Comparative analysis of essential oils from V. rosea leaves collected from different regions such as France, India (Delhi), Portugal, Bangladesh, and Africa reveal significant differences in their chemical compositions (Lawal et al., Citation2015; Brun et al., Citation2001; Aziz et al., Citation2015). These variations underscore the impact of agro-climatic conditions and genetic differences on the essential oil profiles and emphasize these factors’ significance in our research (Islam et al., Citation2018; Li et al., Citation2020).
For instance, Lawal et al. reported that the major components of the essential oil from V. rosea leaves were linolenic acid ethyl ester (43.9%), phytol (7.3%), stearic acid (10.6%), and hexadecanoic acid (6.8%). Minor components in their study included oleic acid ethyl ester (5.8%), 2,6-bis(1,1-dimethylethyl)-2,5-cyclohexadiene-1,4-dione (6.2%), and linoleic acid (5.6%). These specific compounds were not detected in our analysis (Lawal et al., Citation2015). In contrast, essential oil from V. rosea leaves in France was found to contain high levels of palmitic acid (64.9%), methyl hexahydrofarnesyl acetone (4.0%), and palmitate (7.2%). Portuguese leaf oil was characterized by compounds such as 2,3-epoxy-α-ionone, β-ionone, benzaldehyde dihydroactinidiolide, trans-2-decen-1-ol, phenylacetaldehyde, ethyl hexanoate, and palmitic acid ethyl ester among others (Brun et al., Citation2001). Data from Bangladesh showed a predominance of phytol (57.47%) and geraniol (7.9%) as major components, with neophytadiene (3.37%), (E)-Octenal (2.30%), 3,4,4-trimethylcyclohexa-2-en-1-ol (2.28%), and menthol (2.07%) as minor compounds. Meanwhile, the essential oil from India (Delhi) had high concentrations of citronellol (7.9%), phytol (6.4%), pentadecanal (6.6%), (E, E)-2,4-hexadienal (7.7%), and geraniol (7.9%). It also contained a variety of other minor compounds, such as di-tert-butyl-p-cresol, hexadecenoic acid, and palmitic acid (Aziz et al., Citation2015).
Comparatively, our analysis identified phytol (4.75%) and hexadecane (2.47%) as minor components, and we did not detect butylated hydroxytoluene (BHT) in the previously mentioned studies. These discrepancies could be attributed to growth conditions or plant genetic variations. The sample preparation method (fresh vs dried leaves) could also affect the oil’s composition. For example, dried leaves were used for essential oil extraction in France and Africa, while fresh leaves were used in the study from India (Delhi) (Huang et al., Citation2020; Islam et al., Citation2018; Li et al., Citation2020). Overall, the variation in our findings compared to previously reported literature suggests that the composition of the essential oil of Vinca rosea leaves is highly influenced by environmental and genetic factors (Li et al., Citation2020). This variability allows the discovery of new compounds within the leaf oil that have not been previously identified (Li et al., Citation2020).
In addition to analyzing the essential oil from V. rosea leaves, extracts were prepared using methanol and hexane as solvents. The methanolic extract had the highest yield at 9.3%, followed by the hexane extract at 4.4%, presented in . This aligns with previous studies, which found that polar solvents like methanol generally extract more compounds from plant materials than non-polar solvents like hexane (Agarwal et al., Citation2011; Renault et al., Citation1999; Kapoor & Rani, Citation2019). For instance, earlier reports noted higher extraction yields from Vinca rosea leaves and Taxus wallichiana stem, bark, and needles when using polar solvents (Adhikari et al., Citation2018). The observed yield of 9.3% for the methanolic extract in this study closely matches the 8.8% yield reported in the literature (Agarwal et al., Citation2011; Renault et al., Citation1999; Kapoor & Rani, Citation2019).
Table 2. Percentage yield of extracts of leaves.
Phytochemical analysis is crucial for identifying the bioactive profiles of plants. The Vinca rosea leaf extracts were examined for various phytochemicals, including alkaloids, terpenoids, tannins, saponins, phenols, glycosides, flavonoids, carbohydrates, amino acids, and cardiac glycosides (). Each class of compounds has distinct biological activities: Phenols exhibit antioxidant, antibacterial, and antiviral properties. Tannins contribute to the plant’s flavor, astringency, and colour. Alkaloids have antimicrobial properties by interacting with microbial DNA. Flavonoids are crucial for the plant’s colour and aroma, attracting pollinators. Saponins are known for their cholesterol-lowering, anti-inflammatory, and anticancer effects (Renault et al., Citation1999, Kapoor & Rani, Citation2019, Islam et al., Citation2018). The extracts were prepared in methanol and hexane to maximize the diversity of bioactive compounds extracted. The methanolic extract contained many bioactive constituents, including alkaloids, amino acids, flavonoids, phenols, saponins, tannins, carbohydrates, glycosides, cardiac glycosides, and terpenoids. In contrast, the hexane extract contained saponins, tannins, and terpenoids. Both extracts were found to have phenols and flavonoids (Pandey et al., Citation2006; Renault et al., Citation1999). The findings of this study are consistent with previously reported results, demonstrating similar profiles of bioactive compounds in V. rosea leaf extracts from various sources (Renault et al., Citation1999; Kapoor & Rani, Citation2019).
Table 3. Phytochemical identification test for extracts of leaves.
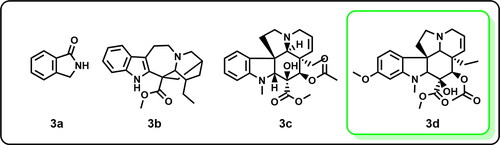
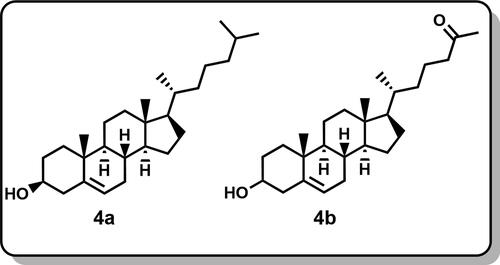
The GC-MS analysis of V. rosea leaves’ methanolic extract revealed several bioactive alkaloids and flavonoids (). Key alkaloids identified include isoindolinine (), coronaridine (), Desmethoxyvindoline (), and vindoline (). These compounds are noteworthy because vindoline is a commercially significant anti-cancer drug precursor synthesizing various pharmaceutical agents. In contrast, the GC-MS analysis of hexane extract () showed the presence of lipid compounds, specifically cholesterol (), 26-Nor-5-cholesten-3á-ol-25-one (). This distinct lipid profile highlights the hexane extract’s potential effectiveness in antifungal applications and utility in isolating specific lipid compounds (Rajput et al., Citation2011; Renault et al., Citation1999; Daniel, Citation2006).The GC-MS chromatograms for both extracts are available in the Supporting Information (SI), providing detailed insights into their chemical compositions. The results show the different extraction efficiencies and compound profiles obtained with polar (methanol) versus non-polar (hexane) solvents, supporting the targeted extraction of specific bioactive molecules from V. rosea leaves (Rajput et al., Citation2011; Renault et al., Citation1999; Daniel, Citation2006).
Table 4. The results of GC-MS of methanolic extract of leaves of Vinca rosea.
Table 5. The results of GC-MS of hexane extract of leaves of Vinca rosea.
In recent years, the medicinal and agricultural sectors have increasingly turned to natural products as alternatives to synthetic compounds. This shift is driven by environmental and health concerns and a growing consumer demand for natural ingredients. Despite the broad exploration of natural antifungal agents, the antifungal activity of V. rosea essential oil against wheat fungi such as F. graminearum and B. sorokiniana has yet to be extensively studied. These fungi are significant agricultural problems in India (De Wolf et al., Citation2003).
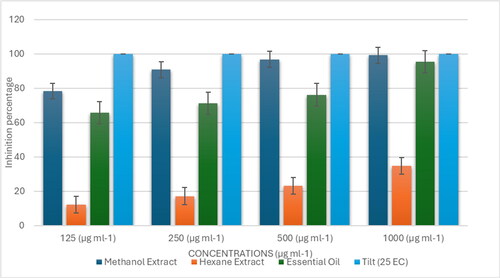
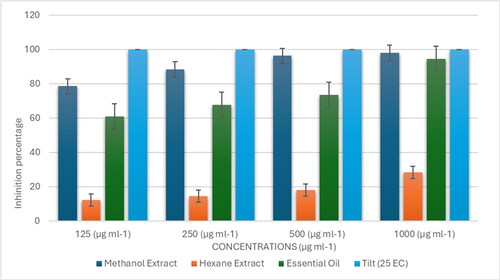
Given this context, we screened the antifungal potential of V. rosea leaf extracts and essential oil against these wheat pathogens using the poisoned food technique. Many plant species have demonstrated natural fungicidal properties, and we investigated whether V. rosea could serve a similar role. The percent mycelial inhibition for F. graminearum and B. sorokiniana under different treatments is presented in and , respectively. Additionally, the ED50 (effective dose to inhibit 50% of mycelial growth) and ED90 (effective dose to inhibit 90% of mycelial growth) values for the essential oil and extracts are detailed in .
Table 6. ED50 and ED90 value of different treatments against F. graminearum and B. sorokiniana.
Based on the ED50 and ED90 values, we established a classification for the effectiveness of the plant materials. For Fusarium graminearum, the methanolic extract of leaves was the most effective (ED50 = 80 µg/mL, ED90 = 240 µg/mL), followed by the essential oil (ED50 = 98 µg/mL, ED90 = 865 µg/mL). These results were compared to the standard fungicide propiconazole (Tilt 25 EC), which inhibited fungal growth at 125 µg/mL and had the lowest ED50 value of 62.5 µg/mL, making it the most effective treatment overall. The hexane extract was the least effective, with ED50 and ED90 values exceeding 1000 µg/mL.
A similar trend was observed for Bipolaris sorokiniana. The ED50 values for the methanolic extract, essential oil, hexane extract, and standard fungicide were 76, 100, >1000, and 62.5 µg/mL, respectively, while the ED90 values were 290, 875, >1000, and 110 µg/mL, respectively (). The order of effectiveness against both fungi, based on their ED50 and ED90 values, was as follows:
Our findings indicated that both the methanolic extract and the essential oil of V. rosea were highly effective in controlling the growth of both fungi. The strong antifungal activity of the methanolic extract is likely due to its high content of bioactive alkaloids, particularly vindoline, known for its anticancer properties, as well as phenols and flavonoids, which possess antimicrobial activities. The antifungal properties of the essential oil may be attributed to its components, such as butylated hydroxytoluene, known for its antioxidant effects, and other alkanes and aromatic hydrocarbons (Tyagi & Malik, Citation2010; Miron et al., Citation2014; Hashmi et al., Citation2013).
Alkaloids are nitrogen-containing compounds that disrupt fungal cell membranes, impairing cell function and inhibiting growth. Lipophilic terpenoids can similarly disrupt fungal cell membranes, inhibiting spore germination and mycelial growth (Tyagi & Malik, Citation2010; Miron et al., Citation2014; Hashmi et al., Citation2013). The antifungal activity of Vinca rosea extracts and essential oil may result from these compounds’ ability to alter cell wall synthesis, increase membrane permeability, and impair fungal enzymatic systems (Tyagi & Malik, Citation2010). This can cause leakage of intracellular contents, disrupt ion balance, and inhibit respiratory chain functions (Amber et al., Citation2010).
In summary, our antifungal data unequivocally demonstrate that the essential oil and methanolic extract of V. rosea hold significant promise as natural antifungal agents. These findings not only underscore the potential of V. rosea in the field of agriculture but also advocate for their use in formulating herbal solutions to combat fungal pathogens in agricultural settings.
4. Conclusion
This study provides the first detailed report on the chemical constituents of the essential oil derived from the fresh leaves of V. rosea cultivated in Ludhiana, Punjab. Through GC-MS analysis, thirty-four compounds were identified in the essential oil, with butylated hydroxytoluene being the most abundant. The essential oil and methanolic leaf extract demonstrated significant antifungal activity against the wheat pathogens F. graminearum and B. sorokiniana, outperforming the hexane extract. The strong antifungal properties of the methanolic extract are likely due to its high content of bioactive alkaloids, such as vindoline, and phenolic compounds, while the essential oil’s effectiveness can be attributed to its rich composition of hydrocarbons and aromatic compounds, including butylated hydroxytoluene. These findings highlight the potential of V. rosea extracts and essential oil as natural antifungal agents, offering a promising alternative to synthetic fungicides. Future research should focus on in vivo studies to justify and evaluate the commercial application of these natural products, paving the way for their potential integration into agricultural practices for sustainable disease management.
Authors’ contributions
Parul Sharma: Searched and worked on the project, extracted the data, wrote the original draft, given final approval of the version to be published. Ramandeep Kaur: Conceptualization, supervision, drafting and revising the manuscript, and giving the final approval of the version to be published. Urvashi Bhardwaj: Reviewed and final approval of the version to be published. Jaspal Kaur: Worked on getting the antifungal activity data, conceptualization, and final approval of the version to be published. All authors have read and approved the final version of the manuscript submitted for publication and are responsible for the final content.
Acknowledgments
The authors are thankful to Dr. Kiranjit Kaur Dhatt, Principal Scientist-cum-Head, Department of Floriculture and Landscaping, Punjab Agricultural University (Ludhiana), for the identification and authentication of the plant specimen and Head of the Department of Soil Science, Punjab Agricultural University, for providing the mineral analysis facility.
Disclosure statement
The authors confirm that they have no competing financial interests or personal relationships that could have influenced the work reported in this paper.
Data availability statement
We have carried out the research work and assure you that it can be provided whenever required from the corresponding author.
Additional information
Funding
Notes on contributors
Parul Sharma
Parul Sharma earned her Master’s in Natural Product Chemistry from Punjab Agricultural University, Ludhiana, India. She is pursuing a Ph.D. in Organic Chemistry at Oklahoma State University, Stillwater, USA, and is currently working as a Graduate Research Assistant. Her research focuses on discovering new methods for synthesizing pharmaceutically important compounds.
Ramandeep Kaur
Ramandeep Kaur has been a Chemist at Punjab Agricultural University, Ludhiana, since May 2018. She received the Maulana Azad National Fellowship (JRF and SRF) from the University Grants Commission (UGC), India. With over 40 research and review articles published in high-impact journals, her interests include Natural Product Chemistry, Agro-chemistry, Organic Chemistry, and Green Chemistry.
Urvashi Bhardwaj
Urvashi Bhardwaj has also been a Chemist at Punjab Agricultural University, Ludhiana, since May 2018. She has published over 38 research and review articles in prestigious journals. Her research interests encompass Natural Product Chemistry, Agrochemistry, and Green Chemistry.
Jaspal Kaur
Jaspal Kaur is a Principal Plant Pathologist in the Department of Plant Breeding and Genetics at Punjab Agricultural University. She specializes in wheat and barley pathology, focusing on identifying resistance sources for these diseases, monitoring and surveying them, identifying new rust pathotypes using differentials and trap nurseries, and studying their epidemiology and management.
References
- Adams, R. P. (2007). Identification of essential oil components by gas chromatography/mass spectrometry (4th ed.). Allured Publishing Corporation.
- Adhikari, P., Pandey, A., Agnihotri, V., & Pande, V. (2018). Selection of solvent and extraction method for determination of antimicrobial potential of Taxus wallichiana Zucc. Res. Pharm, 8, 1–9.
- Agarwal, S., Chettri, N., Bisoyi, S., Tazeen, A., Vedamurthy, A. B., Krishna, V., & Hoskeri, H. J. (2011). Evaluation of in-vitro anthelminthic activity of Catharanthus roseus extract. Int. J. Pharm. Sci. Drug Res, 3, 211–213.
- Almagro, L., Fernández-Pérez, F., & Pedreño, M. A. (2015). Indole alkaloids from Catharanthus roseus: Bioproduction and their effect on human health. Molecules (Basel, Switzerland), 20(2), 2973–3000. https://doi.org/10.3390/molecules20022973
- Amber, K., Aijaz, A., Immaculata, X., Luqman, K. A., & Nikhat, M. (2010). Anticandidal effect of Ocimum sanctum essential oil and its synergy with fluconazole and ketoconazole. Phytomedicine: international Journal of Phytotherapy and Phytopharmacology, 17(12), 921–925. https://doi.org/10.1016/j.phymed.2010.02.005
- Aziz, S., Saha, K., Sultana, N., Khan, M., Nada, K., & Afroze, M. (2015). Comparative studies of volatile components of the essential oil of leaves and flowers of Catharanthus roseus growing in Bangladesh by GC-MS analysis. Indian Journal of Pharmaceutical and Biological Research, 3(01), 06–10. https://doi.org/10.30750/ijpbr.3.1.2
- Bai, G., & Shaner, G. (2004). Management and resistance in wheat and barley to Fusarium head blight. Annual Review of Phytopathology, 42(1), 135–161. https://doi.org/10.1146/annurev.phyto.42.040803.140340
- Balaabirami, S. & Patharajan, S. (2012). In vitro antimicrobial and antifungal activity of Catharanthus roseus leaves extract against important pathogenic organisms. International Journal of Pharmaceutical Sciences, 4, 487–490.
- Barkat, A., & Mujeeb, M. (2013). The comparative study of Catharanthus roseus extract and extract loaded chitosan nanoparticles in alloxan induced diabetics rats. International Journal of Biomedical Research, 4(12), 670–678. https://doi.org/10.7439/ijbr.v4i12.412
- Beyer, M., & Aumann, J. (2008). Effects of Fusarium infection on the amino acid composition of winter wheat grains. Food Chemistry, 111(3), 750–754. https://doi.org/10.1016/j.foodchem.2008.04.033
- Brun, G., BessièRe, J.-M., Dijoux-Franca, M-G., David, B., & Mariotte, A.-M. (2001). Volatile components of Catharanthus roseus (L) G. Don (Apocyanaceae). Flavour and Fragrance Journal, 16(2), 116–119. https://doi.org/10.1002/ffj.956
- Cavaleiro, C., Pinto, E., Gonçalves, M. J., & Salgueiro, L. (2006). Antifungal activity of Juniperus essential oils against dermatophyte, Aspergillus, and Candida strains. Journal of Applied Microbiology, 100(6), 1333–1338. https://doi.org/10.1111/j.1365-2672.2006.02889.x
- Daniel, M. (2006). Medicinal plants: chemistry and properties (pp. 17–18). Science Publishers.
- Das, A., Sarkar, S., Bhattacharyya, S., & Gantait, S. (2020). Biotechnological advancements in Catharanthus roseus (L.) G. Don. Applied Microbiology and Biotechnology, 104(11), 4811–4835. https://doi.org/10.1007/s00253-020-10592-1
- De Wolf, E. D., Madden, L. V., & Lipps, P. E. (2003). Risk assessment models for wheat fusarium head blight epidemics based on within-season weather data. Phytopathology, 93(4), 428–435. https://doi.org/10.1094/PHYTO.2003.93.4.428
- Gupta, S. K., & Tripathi, S. C. (2011). Fungitoxic activity of Solanum torvum against Fusarium sacchari. Plant Protection Science, 47(3), 83–91. https://doi.org/10.17221/20/2010-PPS
- Hashmi, L. S., Hossain, M. A., Weli, A. M., Al-Riyami, Q., & Al-Sabahi, J. N. (2013). Gas chromatography-mass spectrometry analysis of different organic crude extracts from the local medicinal plant of Thymus vulgaris L. Asian Pacific Journal of Tropical Biomedicine, 3(1), 69–73. https://doi.org/10.1016/S2221-1691(13)60026-X
- Hisiger, S., & Jolicoeur, M. (2007). Analysis of Catharanthus roseus alkaloids by HPLC. Phytochemistry Reviews, 6(2-3), 207–234. https://doi.org/10.1007/s11101-006-9027-8
- Huang, Y., Sun, C., Guan, X., Lian, S., Li, B., & Wang, C. (2020). Butylated hydroxytoluene induced resistance against Botryosphaeria dothidea in apple fruit. Frontiers in Microbiology, 11, 599062. https://doi.org/10.3389/fmicb.2020.599062
- Ibrahim, M., Mehjabeen, S. S., & Narsu, M. L. (2011). Pharmacological evaluation of Catharanthus roseus. Int. J. Appl. Pharm, 2, 165–173.
- Islam, M. T., Ali, E. S., Uddin, S. J., Shaw, S., Islam, M. A., Ahmed, M. I., Chandra Shill, M., Karmakar, U. K., Yarla, N. S., Khan, I. N., Billah, M. M., Pieczynska, M. D., Zengin, G., Malainer, C., Nicoletti, F., Gulei, D., Berindan-Neagoe, I., Apostolov, A., Banach, M., … Atanasov, A. G. (2018). Phytol: A review of biomedical activities. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 121, 82–94. https://doi.org/10.1016/j.fct.2018.08.032
- Kapoor, M., & Rani, J. (2019). Qualitative and quantitative analysis of phytochemicals by GC-MS and antioxidant activity of Catharanthus roseus (L.) G. Don. Journal of Pharmacognosy and Phytochemistry, 8, 378–385.
- Kaur, H., Bhardwaj, U., Kaur, R., & Kaur, H. (2021). Chemical composition and antifungal potential of citronella (Cymbopogon nardus) leaves essential oil and its major compounds. Journal of Essential Oil Bearing Plants, 24, 1–11. https://doi.org/10.1080/0972060X.2021.1940111
- Kumar, P., & Rai, R. C. (2018). Estimation of yield loss at different disease levels of spot blotch of wheat in Bihar. International Journal of Chemical Studies, 6, 57–58.
- Lawal, O. A., Ogunwande, I. A., Ibirogba, A. E., Layode, O. M., & Opoku, A. R. (2015). Chemical constituents of essential oils from Catharanthus roseus (L.) G. Don grown in Nigeria. Journal of Essential Oil Bearing Plants, 18(1), 57–63. https://doi.org/10.1080/0972060X.2014.983435
- Li, Y., Kong, D., Fu, Y., Sussman, M. R., & Wu, H. (2020). The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiology and Biochemistry: PPB, 148, 80–89. https://doi.org/10.1016/j.plaphy.2020.01.006
- Miron, D., Battisti, F., Silva, F. K., Lana, A. D., Pippi, B., Casanova, B., Gnoatto, S., Fuentefria, A., Mayorga, P., & Schapoval, E. E. S. (2014). Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Rev. Bras. Farmacogn, 24(6), 660–667. https://doi.org/10.1016/j.bjp.2014.10.004
- Pandey, S. R., Mallavarapu, G. R., Naqvi, A. A., Yadav, A., Rai, S. K., Srivastava, S., Singh, D., Mishra, R., & Kumar, S. (2006). Volatile components of leaves and flowers of periwinkle Catharanthus roseus (L.) G. Don from New Delhi. Flavour and Fragrance Journal, 21(3), 427–430. https://doi.org/10.1002/ffj.1606
- Prajakta, J. P., & Ghosh, J. S. (2010). Antimicrobial activity of Catharanthus roseus: A detailed study. British Journal of Pharmacology and Toxicology, 1, 40–44.
- Rajput, M. S., Nair, V., Chauhan, A., Jawanjal, H., & Dange, V. (2011). Evaluation of antidiarrheal activity of aerial parts of Vinca major in experimental animals. Middle East Journal of Scientific Research, 7, 784–788.
- Renault, J. H., Nuzillard, J. M., Le Crouérour, G., Thépenier, P., Zèches-Hanrot, M., & Le Men-Olivier, L. (1999). Isolation of indole alkaloids from Catharanthus roseus by centrifugal partition chromatography in the pH-zone refining mode. Journal of Chromatography. A, 849(2), 421–431. https://doi.org/10.1016/s0021-9673(99)00495-1
- Sharma, M., Chahal, K. K., Kaur, R., Singh, R., & Kataria, D. (2019). Antifungal potential and structure activity relationship of carrot seed constituents. Journal of Food Biochemistry, 43, 1–9. https://doi.org/10.1111/jfbc.12613
- Tyagi, A. K., & Malik, A. (2010). Liquid and vapour-phase antifungal activities of selected essential oils against Candida albicans: microscopic observations and chemical characterization of Cymbopogon citratus. BMC Complementary and Alternative Medicine, 10(1), 65. https://doi.org/10.1186/1472-6882-10-65
- Verdeguer, M., Sánchez-Moreiras, A. M., & Araniti, F. (2020). Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plants (Basel, Switzerland), 9(11), 1571. https://doi.org/10.3390/plants9111571