 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Both Re2C and Re2N are ultra incompressible and have a bulk modulus of about 400 GPa. These materials are synthesized under high pressure and high temperature. The synthesis pressures are about 10 GPa or below for Re2C and 20–30 GPa for Re2N. If the synthesis pressure of Re2N was about 10 GPa or below, a large volume high-pressure cell like a multi-anvil apparatus can be used to synthesize Re2N. To realize this, a proper solid nitrogen source is needed instead of liquid or gas nitrogen. We used a precursor of a mixture of rhenium and home-made nanoscale amorphous two-dimensional carbon nitride as a solid nitrogen source. Consequently, the synthesis reaction produced Re2C but not Re2N. We characterized the synthesized Re2C by various techniques including high-pressure x-ray diffraction (XRD). The bulk modulus B0 of the synthesized Re2C under hydrostatic conditions was estimated to be 385.7 ± 18.0 GPa. This value is a little smaller than the previous data. When the pressure medium became non-hydrostatic, the peculiar compression behaviour occurred; the rate of broadening of XRD lines increased and the compression became negligible in the range of a few GPa. The reason for this peculiar behaviour is not known.
Public Interest Statement
Pursuing to synthesize a material harder than a diamond is interesting for both scientific and industrial aspects. The reported material is not harder than diamond but it may convince us that we will go further.
1. Introduction
If a proper solid nitrogen source could be used for synthesizing target nitrogen compounds under high pressure conditions, the synthesis experiment would be easier and faster than when using liquid or gas nitrogen to explore nitride compounds with novel physical properties.
High pressure and high temperature synthesis of rhenium carbide was reported 43 years ago by Popova and Boiko, and rhenium carbide and other transition metal carbides were expected to be superhard and have high melting points (Popova & Boiko, Citation1971). The study of rhenium carbides has continued over four decades. The scientific motivation for this long-term study is exploring novel materials. Rhenium itself is known to be ductile but has high bulk and shear moduli, high strength and a high melting point of 3,453 K (the second highest of all metals). Its bulk modulus has been repeatedly investigated by powder X-ray diffraction (XRD) up to 144 GPa, and the bulk modulus has been determined to be 352.6 GPa (Anzellini, Dewaele, Occelli, Loubeyre, & Mezouar, Citation2014). To make a stronger material than rhenium, the strategy is to introduce a new chemical bond with another element. Zhao et al. proposed in a recent paper (Zhao et al., Citation2014) the idea that a combination of electron-rich 4d transition metals (TMs) with light covalent-bond forming elements (LEs), like boron, carbon, nitrogen and oxygen, has been proved to be an effective way to design novel superhard materials over the past decade. The idea is that the combination of the TM’s high electron concentration (EC) and the directional bonding formed by strong hybridizations between TM d electrons and LE s and p electrons can effectively withstand both elastic and plastic deformation. This is a good place to start to understand experimentalists searching for novel materials to be used with TM metals like rhenium (Re). This paper proposes that ReN4, which has a Vickers hardness of 38.72 GPa, is the hardest rhenium nitride. The same idea of the combination of TM and LE was confirmed theoretically in previous papers (Miao, Sa, Zhou, Sun, & Ahuja, Citation2011; Zang, Sun, Tse, & Chen, Citation2012). Zhang et al. investigated indentation strength, but it is rather difficult to obtain a correct numerical value for it (Zang et al., Citation2012). Table shows the compilation of experimental bulk moduli of ReX reported so far.
Table 1. Compilation of experimental bulk moduli reported so far
Friedrich et al. solved the remaining problem of the carbon site in Re2C by Raman study in conjunction with a theoretical and comparative study for Re2N (Friedrich, Winkler, Refson, & Milman, Citation2012). This study confirms the first proposed site of C, which was suggested by Zhao et al. (Citation2010). The site of C and N in those crystals is the same Wyckoff position 2(d) at 1/3, 2/3 and 1/4. The rhenium atom is at Wyckoff position 4(f) at 1/3, 2/3 and z. Accordingly, Re2C and Re2N are isomorphic with the hexagonal space group P63/mmc, and the lattice constants of both are also almost identical, a = 2.83–2.84 Å and c = 9.85–9.88 Å (Friedrich et al., Citation2012).
The first synthesis of Re2N was reported by Friedrich et al. in 2010, which is 39 years after the first rhenium carbide synthesis. The synthesis was conducted at about 20–30 GPa using a laser-heated diamond anvil cell with the chemical reaction between rhenium and loaded nitrogen occurring in a gasket. The bulk modulus of Re2N was reported to be greater than 400 GPa (Friedrich, Winkler, Bayarjargal, Morgenroth, & Chen, Citation2010). In 2012, Kawamura et al. reported the synthesis of ReN2, not Re2N, with a metathesis reaction of all solid precursors of ReCl5 and Li3 N using a belt-type high-pressure apparatus under a moderate pressure of 7.7 GPa (Kawamura, Yusa, & Taniguchi, Citation2012).
We have been synthesizing nanoscale amorphous two-dimensional carbon nitride, na-2D-C3N4HxOy (x, y = 0.5−2), using nitrogen atmospheric pressure plasma (Tabuchi et al., Citation2007). If this particle with rhenium was subjected to the chemical reaction under pressure, either of the following chemical reactions would take place,(1)
(1)
(2)
(2)
For simplicity, the HxOy of na-2D-C3N4HxOy on the left is omitted. At ambient pressure and high temperature, na-2D-C3N4HxOy decomposes as nitrogen goes out into the air, but under pressure the release of nitrogen into the air is suppressed. We report on the synthesis experiment using the starting material of rhenium and na-2D-C3N4HxOy under high pressure and high temperature.
2. Experimental section
Rhenium (99.99%, 100–200 mesh) bought from the Nilaco Corporation in Japan and home-made na-2D-C3N4HxOy were mixed in the mole ratio for Re2N in accordance with Equation 2. The mixture was put into a Pt capsule, the Pt capsule was sealed and then it was subjected to a pressure of 10 GPa and a temperature of 1,500 K for four hours using a multi-anvil apparatus of the Institute for Study of the Earth’s Interior, Okayama University. The synthesized material was investigated with JEOL JSM-7001F and JSM-7000F scanning electron microscopes (SEMs) equipped with energy dispersive spectroscopy (EDS) and Raman spectroscopy of Thermo-Electron Nicolet Almega XR with an Nd:YAG laser (532 nm excitation line) at 4 mW with a micrometer spot size of about 1. Micro-Vickers hardness was measured with the Akashi HM-114. The XRD measurement was conducted with Rigaku RINT Rapid II with a CuKα radiation source and synchrotron radiation source (λ = 0.418041 Å) at the Photon Factory (PF), Tsukuba. High-pressure XRD was performed at up to 30 GPa using a diamond anvil cell (DAC) with a pressure transmitting medium with a 4:1 methanol–ethanol mixture. A ruby scale was used to estimate the pressure value.
3. Results and discussion
Figure (a) is a cross-sectional photograph of a synthesized sample in the Pt capsule. The white-grey part that looks like a cloud is the synthesized sample, and the smooth shining dark part is epoxy resin. The whole Pt capsule was covered with epoxy resin and polished with a diamond powder to prepare a cross-sectional surface for microscope observation. At a certain stage of polishing, the epoxy resin was further introduced into the Pt capsule to fill the void. After the introduction of epoxy resin, the existence of epoxy resin in the synthesized sample means that the synthesized material includes a gaseous component, and the void was left after the gaseous component went out into the air while grinding. On the other hand, this means that the Pt seal was perfect and indicates that chemical reaction (1) or (2) likely took place during the high-pressure and high-temperature (HPHT) synthesis. The magnified SEM photograph is shown in Figure (b). The red line indicates each grain with a scale of about 1 μm. Accordingly, the synthesized material consists of 1-μm-scale grains.
Figure 1. (a) Cross-sectional view of synthesized sample that looks like cloud in Pt capsule (white). Dark smooth part in white-grey sample is epoxy resin. Scale bar at bottom right is 100 μm. Initial outer diameter of Pt capsule is 2 mm, and it is about 3 mm in height. (b) Magnified SEM photograph.
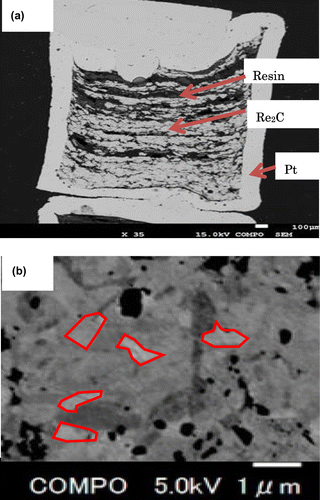
Figure shows the energy dispersive spectrum measured by the EDS on the JEOL JSM-7000 SEM. To avoid surface charging, the Os was coated on the synthesized sample. The signals of Re, C and a small amount of N and C were recorded. The composition was determined by measuring the energy dispersive spectrum by EDS on the Carl Zeiss Ultra Plus ULV-SEM with an accelerating electron voltage 15 kV. The composition is calculated by the ZAF correction method implemented in the SEM. The results are shown in Table . The ratio of atomic composition of Re to C is almost 2.
Figure 2. Measured EDS signals of synthesized sample. Re and C, and small amount of N and O were detected.
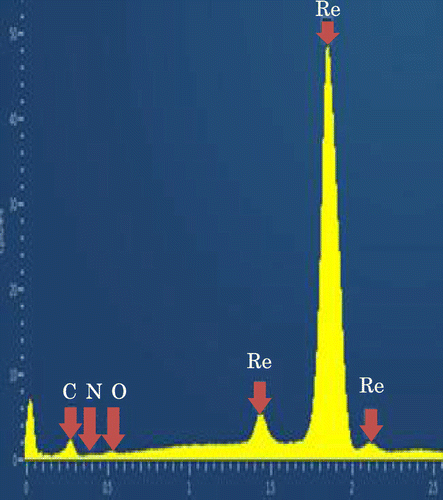
Table 2. Composition of synthesized Re compound
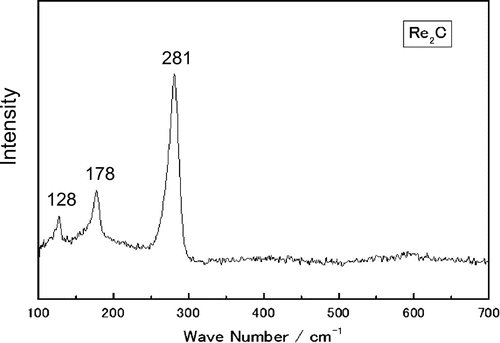
Figure is the Raman spectrum of the synthesized sample. The prominent Raman line is at 281 cm−1. The Raman spectra of Re2N and Re2C (Friedrich et al., Citation2012) show a strong peak at 271 and 278 cm−1 for Re2N and Re2C, respectively. The line at 281 cm−1 is close to that of Re2C.
Figure 3. Raman spectra of synthesized sample.
All the data mentioned above confirm that the synthesized material in this experiment is Re2C. From now on, in this paper the synthesized material is called Re2C.
Micro-Vickers hardness was measured by applying a load of 245.2 mN to the polished surface. The average hardness of five measurement points is 15.85 ± 0.67 GPa. This value is smaller than the theoretical value of 22.5 GPa (Zang et al., Citation2012). The plausible explanation for this is that the void is present in the sample. Figure (a) is the Rietveld refinement from the XRD pattern of Re2C recorded with RINT Rapid II with CuKα radiation source with RIETAN-FP (Izumi & Momma, Citation2007). All the measured XRD lines coincide with the calculated lines with the single hexagonal cell (a = 2.8437 (3) Å and c = 9.8467 (8) Å) of the 63/mmc symmetry. These cell parameters coincide well with the previous data (Friedrich et al., Citation2012; Klotz, Chervin, Munsch, & Le Marchand, Citation2009; Yamaoka et al., Citation2012; Zhao et al., Citation2010). In the refinement, the weak diffractions of Pt contaminated by the Pt capsule and residual graphite were taken into account. The residual errors of the Rietveld refinement are Rwp = 22.770% and Re = 28.879%. The Rwp number is reduced by more than half compared to the refinement for the previous Re2C single phase. The space group P63/mmc fixes the fractional coordinate, and only the rhenium z-coordinate is adjustable: the present z value of 0.6085 and the previously reported coordinate of 0.6085 (Zhao et al., Citation2010). These values completely coincide with each other. Figure (b) shows the crystal structure model used in the Rietveld refinement.
Figure 4. Rietveld refinements from XRD data with RIETAN-FP (Izumi & Momma, Citation2007). (a) Crystalline phases in simulation are Re2C, Pt, and graphite. (b) Crystal structure of Re2C parallel (left) or perpendicular (right) to c-axis.
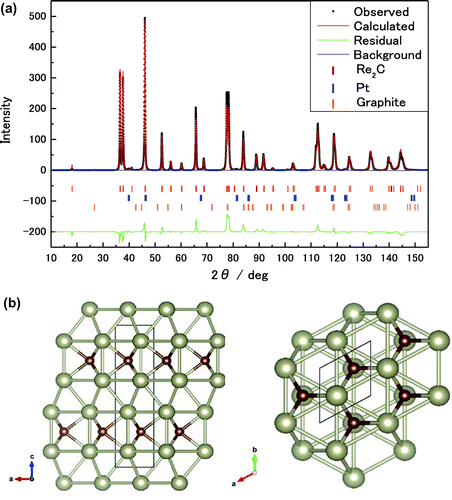
Figure shows the measured high-pressure XRD pattern, pressure dependence of lattice constants and volume of hexagonal Re2C synthesized in this experiment for increasing and decreasing pressure. No phase change was observed in the high-pressure XRD patterns (a) and (b). The 4:1 methanol–ethanol pressure medium is hydrostatic below 10 GPa (Klotz et al., Citation2009; Yamaoka et al., Citation2012). The estimated bulk modulus of this Re2C under hydrostatic conditions is B0 = 385.7 ± 18.0 GPa, which is determined by the non-linear least squares curve fitting with the third-order Birch–Murnaghan equation of state (EOS) with both increasing and decreasing pressure volume data to 10 GPa as shown in Figure (c). The initial volume at ambient pressure is V0 = 206.54 ± 0.05 Å3 and B0′ = 5.25 ± 3.55. The previously reported value of the bulk modulus of Re2C is 405–423 GPa (Juarez-Arellano et al., Citation2009), which is 5–10% larger than the present result. In the previous experiment, the pressure-transmitting medium was NaCl, the first pressure interval was about 10 GPa from the ambient pressure, and then there was an interval of a few to several GPa’s up to 70 GPa (Juarez-Arellano et al., Citation2009). The kink observed at 10 GPa shown in Figure (c) was not observed. This is probably due to the larger first pressure interval or the non-hydrostatic conditions from the initial pressure in the NaCl pressure transmitting medium. If the volume data beyond 10 GPa in Figure (c) are fitted with the EOS, the larger bulk modulus is estimated. Not only the hydrostatic conditions but also the composition of synthesized material should be taken into account to estimate the bulk modulus. The correct composition of the present synthesized Re2C has not been determined. Considering the experimental data including the previous (Juarez-Arellano et al., Citation2009) and present reports, it is fair to say that the correct bulk modulus of the hexagonal stoichiometric Re2C has not been determined experimentally yet. At 10 GPa at which the pressure medium starts becoming non-hydrostatic, the peculiar compression occurs, this material becomes extremely hard because almost no compression occurs in volume, and then after a few GPa’s more this material again becomes compressive. And its volume is larger than the extrapolated volume with the EOS. We need to conduct a further study to understand this peculiar behaviour.
Figure 5. High-pressure XRD patterns of increasing (a) and decreasing (b) pressure were recorded at PF, Tsukuba using DAC. Dependences of volume, and a- and c-axes on pressure are shown in panel (c).
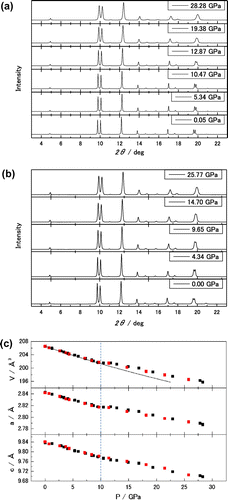
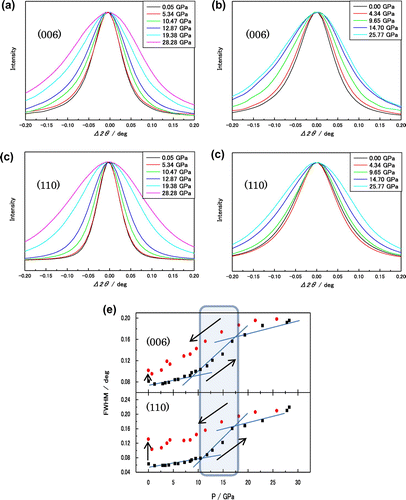
Figure is the full width at half maximum (FWHM) of the XRD (1 1 0) and (0 0 6) lines as a function of pressure. The FWHM increases as the applied pressure increases, and it increases significantly as the pressure becomes larger than about 10 GPa. The pressure medium of the methanol–ethanol mixture solidifies at about 10 GPa, and the pressure inhomogeneity increases. The increase in FWHM continues up to the largest pressure of the present study (30 GPa), but the rate of increase of FWHM decreases as the pressure increases beyond about 20 GPa. This increasing and decreasing behaviour of the slope of FWHM is not easy to understand, but we know that both the hydrostatic pressure and anisotropic stress are effective to increase this FWHM broadening. As the pressure decreases to ambient pressure, the FWHM decreases monotonically, and the slope of the FWHM is similar to the case of increasing pressure. But the FWHM of both (0 0 6) and (1 1 0) XRD lines is larger than those at ambient conditions. This means that the broadening of the a- and c-axes persists after the release of pressure at ambient conditions. The persistent broadening of lattice constants implies that the pressure induces a dislocation in Re2C. Zhang et al. considered the Vickers indentation strength theoretically and estimated the values of 22.5 GPa for Re2C and 64.3 GPa for c-BN (Zang et al., Citation2012). The Vickers hardness of Re2C is smaller by a factor of one-third, but the bulk moduli of both do not differ much (the bulk modulus of c-BN is 365–380 GPa (Aguado & Baonza, Citation2006)). Zhang et al. discusses the details of electronic charge distribution under stress to explain these differences in both materials (Zang et al., Citation2012).
Figure 6. Full width at half maximum (FWHM) of (0 0 6) and (1 1 0) diffraction lines of XRD spectra obtained from experiment at PF.
We summarize the broadening behaviour of Re2C under pressure as follows: (1) the broadening proceeds continuously from the beginning of applying of the pressure and (2) the rate of broadening depends on the non-hydrostaticity of the pressure medium. The broadening behaviour of the lattice in Re2C is a puzzle in the sense that we do not have an explanation for it. In this study, we have totally ignored the role of N and O in the synthesized, which is a theme for future study, and more investigation of N- and O-oriented spectroscopy, for example, neutron scattering, is necessary to determine the atomic position in the synthesized Re2C. Alternatively, nitrides like GaN and TaN are potential candidates for the precursor instead of C3N4HxOy.
The initial plan of this study was to synthesize Re2N using a mixture of Re and home-made nanoscale amorphous C3N4HxOy. If the synthesis pressure is set at a higher pressure, the chance of a chemical reaction (2) in the introduction increases because the enthalpy of nitrogen gas increases significantly and faster than that of solids. However, this is not our strategy. The alternative is to have a chemical reaction with a third highly reactive element with carbon like Ti; the Ti absorbs the carbon, and the rest is Re2N. We will pursue this possibility to find a “carbon absorber” that is better than Re.
4. Conclusion
The ultra incompressible Re2C was synthesized by subjecting the mixture of Re and home-made nanoscale amorphous two-dimensional C3N4HxOY to high pressure and high temperature conditions. We determined the bulk modulus of the synthesized Re2C to be B0 = 385.7 ± 18.0 GPa under hydrostatic conditions. Under non-hydrostatic conditions, the peculiar compression behaviour is found. For the future synthesis of Re2N using the above precursor, a strong carbon absorber like Ti is necessary.
Acknowledgements
Nozomu Yasui thanks Mr. N. Kusano and Prof. H. Nishido for their assistance with Raman spectral measurement.
Additional information
Funding
Notes on contributors
Kenichi Takarabe
I operate a very small research group pursuing a synthesis of carbon nitride. This emerging material would be a better catalyst and harder material.
Notes
1 This article is associated with 7th Asian Conference on High Pressure Research (ACHPR-7), Bangkok, Thailand.
References
- Aguado, F., & Baonza, V. G. (2006). Prediction of bulk modulus at high temperatures from longitudinal phonon frequencies: Application to diamond, c− BN, and 3 C− SiC. Physical Review B, 73, 024111.10.1103/PhysRevB.73.024111
- Anzellini, S., Dewaele, A., Occelli, F., Loubeyre, P., & Mezouar, M. (2014). Equation of state of rhenium and application for ultra high pressure calibration. Journal of Applied Physics, 115, 043511.
- Friedrich, A., Winkler, B., Bayarjargal, L., Morgenroth, W., Juarez-Arellano, E. A., Milman, V., … Chen, K. (2010). Novel rhenium nitrides. Physical Review Letters, 105, 085504.10.1103/PhysRevLett.105.085504
- Friedrich, A., Winkler, B., Refson, K., & Milman, V. (2012). Experimental evidence for the structural models of Re. Physical Review B, 86, 014114.10.1103/PhysRevB.86.014114
- Izumi, F., & Momma, K. (2007). Three-dimensional visualization in powder diffraction. Solid State Phenomena, 130, 15–20.10.4028/www.scientific.net/SSP.130
- Juarez-Arellano, E., Winkler, B., Friedrich, A., Bayarjargal, L., Milman, V., Yan, J., & Clark, S. M. (2009). Stability field of the high-(P, T) Re2C phase and properties of an analogous osmium carbide phase. Journal of Alloys and Compounds, 481, 577.10.1016/j.jallcom.2009.03.029
- Kawamura, F., Yusa, H., & Taniguchi, T. (2012). Synthesis of rhenium nitride crystals with MoS2 structure. Applied Physics Letters, 100, 251910.10.1063/1.4729586
- Klotz, S., Chervin, J.-C., Munsch, P., & Le Marchand, G. (2009). Hydrostatic limits of 11 pressure transmitting media. Journal of Physics D: Applied Physics, 42, 075413.10.1088/0022-3727/42/7/075413
- Miao, N., Sa, B., Zhou, J., Sun, Z., & Ahuja, R. (2011). Mechanical properties and electronic structure of the incompressible rhenium carbides and nitrides: A first-principles study. Solid State Communications, 151, 1842–1845.10.1016/j.ssc.2011.08.011
- Popova, S. V., & Boiko, L. G. (1971). A new rhenium carbide formed by high-pressure treatment. High Temperatures-High Pressures, 3, 237–238.
- Tabuchi, H., Sougawa, M., Takarabe, K., Sato, S., & Ariyada, O. (2007). Use of nitrogen atmospheric pressure plasma for sythesizing carbon nitride. Japanese Journal of Applied Physics, 46, 1596.
- Yamaoka, H., Zekko, Y., Jarrige, I., Lin, J.-F., Hiraoka, N., Ishii, H., … Mizuki, J. (2012). Ruby pressure scale in a low-temperature diamond anvil cell. Journal of Applied Physics, 112, 124503.10.1063/1.4769305
- Zang, C., Sun, H., Tse, J. S., & Chen, C. (2012). Indentation strength of ultraincompressible rhenium boride, carbide, and nitride from first-principles calculations. Physical Review B, 86, 014108.10.1103/PhysRevB.86.014108
- Zhao, Z., Cui, L., Wang, L.-M., Xu, B., Liu, Z., Yu, D., … Tian, Y. (2010). Bulk Re2C: Crystal structure, hardness, and ultra-incompressibility. Crystal Growth & Design, 10, 5024–5026.10.1021/cg100659g
- Zhao, Z., Bao, K., Li, D., Duon, D., Tian, F., Jin, X., … Cui, T. (2014). Nitrogen concentration driving the hardness of rhenium nitrides. Scientific Reports, 4, 4947. doi:10.1038/srep04797
