 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We report on the synchrotron hard X-ray-induced decomposition of strontium oxalate (SrC2O4) pressurized to 7 GPa inside a diamond anvil cell (DAC). After some 4 h of irradiation in a white X-ray synchrotron beam, a dark reddish/brown region formed in the area of irradiation which was surrounded by a yellowish brown remainder in the rest of the sample. Upon depressurization of the sample to ambient conditions, the reacted/decomposed sample was recoverable as a dark brown/red and yellow waxy solid. Synchrotron infrared spectroscopy confirmed the strong presence of CO2 even under ambient conditions with the sample exposed to air and other strongly absorbing regions, suggesting that the sample may likely be polymerized CO (in part) with dispersed CO2 and SrO trapped within the polymer. These results will have significant implications in the ability to readily produce and trap CO2 in situ via irradiation of a simple powder for useful hard X-ray photochemistry and in the ability to easily manufacture polymeric CO (via loading of powders in a DAC or high volume press) without the need for the dangerous and complex loading of toxic CO. A novel means of X-ray-induced polymerization under extreme conditions has also been demonstrated.
Public Interest Statement
We have discovered an alternative and novel route of synthesis of polymeric carbon monoxide (CO) using strontium oxalate as the starting material via a combination of X-ray irradiation and high pressure without the need to use toxic CO as the reactant. This is the first reported polymerization of any compound using a combination of high-pressure and hard X-ray irradiation to the best of our knowledge. It is hoped that these techniques will enable the synthesis of novel polymers and other novel materials.
1. Introduction
Our recent efforts to develop useful hard X-ray photochemistry (Pravica, Bai, & Bhattacharya, Citation2012; Pravica et al., Citation2011, 2012; Pravica, Sneed, Bai, & Park, Citation2013; Pravica, Hulsey, et al., Citation2014; Pravica, Liu, & Bai, Citation2013; Pravica, Popov, et al., Citation2013; Pravica, Sneed, Smith, & Bai, Citation2013; Pravica, Sneed, White, & Wang, Citation2014a, Citation2014b; Pravica, Yulga, Tkachev, & Liu, Citation2009; Pravica, White, & Wang, Citation2015) have thus far enabled us to produce simple diatomic molecules (O2 (Pravica et al., Citation2011; Pravica, Bai, & Bhattacharya, Citation2012; Pravica et al., Citation2012; Pravica, Bai, et al., Citation2013; Pravica, Hulsey, et al., Citation2014; Pravica, Popov, et al., Citation2013; Pravica, Sneed, White, et al., Citation2014b), Cl2 (Pravica, Sneed, et al., Citation2013), N2 (Pravica, Liu, et al., Citation2013), and F2 (Pravica, Sneed, White, et al., Citation2014a; Pravica et al., Citation2015)) in situ inside a sealed and pressurized diamond anvil cell (DAC) by harnessing the highly penetrating, highly energetic, and highly focused properties of hard X-rays (>7 keV) to initiate decomposition reactions (e.g. KClO4 + hν → KCl + 2O2 (Pravica, Hulsey, et al., Citation2014). We have also initiated synthetic reactions of some of these newly generated species to form simple polyatomic products (H2O and OF2) via 2H2 + O2 → 2H2O (Pravica, Sneed, White, et al., Citation2014b) and 2F2 + O2 → 2OF2 (Pravica et al., Citation2015), respectively. We thus have an interest of developing novel routes of chemical synthesis of more complex and challenging-to-synthesize molecules via our available techniques. We also seek to produce detonation products (CO2, H2O, N2, N2O, etc.) in a highly controllable way utilizing useful hard X-ray photochemistry as a means to examine the effect of mixing (Pravica, Sneed, White, et al., Citation2014b; Pravica et al., Citation2015) on intermolecular potentials via Raman and infrared (IR) spectroscopy to aid in developing better codes to predict detonation behavior of explosives (https://www-pls.llnl.gov/?url=science_and_technology-chemistry-cheetah).
In that spirit, we sought to produce polyatomic CO2 inside a DAC in situ using hard X-rays. Prior efforts to produce this important greenhouse atmospheric constituent molecule via X-ray irradiation of BaCO3 failed (Pravica, Bai, et al., Citation2013). We thus sought to examine the oxalate ion () as a potential source of CO2 and/or CO. As there is one study of SrC2O4 under ambient conditions which evolved CO and CO2 upon γ- irradiation at elevated temperatures (Bose, Bhatta, & Bhatta, Citation1998), we chose this compound as the candidate for hard X-ray irradiation.
2. Experimental
We used a symmetric-type DAC to perform the irradiation experiment. A 250-μm-thick stainless steel gasket was preindented to ~20 μm thickness, and a sample-containing hole of diameter ~130 μm was drilled via electric discharge machining. The diamonds each had a culet diameter of ~500 μm and were low fluorescence, type Ia quality. Fresh powdered SrC2O4 (Alfa Aesar > 95% purity) was manually loaded into the gasket hole using a needle along with one ruby for pressure measurement. The assembly was then sealed and pressurized to 7 GPa. No pressure-transmitting medium was used in this experiment which was conducted at room temperature.
The pressurized sample was irradiated at the 16 BM-B beamline in the Advanced Photon Source (APS) with white X-rays for approximately 4 h. Raman spectroscopy was attempted on the irradiated sample unsuccessfully due to very high sample fluorescence.
The sample was then transported (still at high pressure) to the Canadian Light Source (CLS) for post-irradiation IR studies some days later. We note in passing that in prior IR studies, the exciting IR beam does not excite fluorescence in samples and is a far-superior method to acquiring vibrational mode behavior in organic and stressed/damaged samples at high pressure (Pravica, Yulga, Tkachev, & Liu, Citation2009).
Mid-IR spectra were acquired at the 01B1–1 beamline using a Bruker Vertex 77v/S, Hyperion 3000 IR microscope. A liquid nitrogen-cooled MCT detector was used. The mid-IR microscope system typically focuses the IR beam which is then spatially filtered using a 100-μm-diameter circular aperture. The investigated spectral range was from 700 to 4,000 wavenumbers with a resolution of 1 cm−1. An improvised jacket that surrounded the DAC and fit snugly between the objective and sample stage of the microscope with constantly flowing argon gas was used to reduce water vapor contamination.
Far-IR studies were also performed on the irradiated samples at the 02B1-1 beamline at the CLS. The collection optics and DAC were housed in front of the FT-IR system with a plexiglass enclosure. The system was continuously purged from water vapor (as measured with a humidity sensor) using positive pressure nitrogen blowoff gas from a nearby liquid N2 dewar. Far-IR spectra were collected using a Horizontal Microscope system on the Far-Infrared Beamline at the CLS. Far-IR synchrotron radiation was redirected from the sample compartment of a Bruker IFS 125 HR spectrometer ® to a long working distance Schwarzchild objective focusing the light on the sample. A similar objective behind the sample collected the transmitted light and directed it to an off-axis parabolic mirror which refocused the light into an Infrared Laboratories® Ge:Cu detector. The spectrometer was equipped with a 6-micron mylar beamsplitter, and the data were collected using a scanner velocity of 40 kHz, 12.5-mm entrance aperture, and a resolution of 1 cm−1. The Ge:Cu detector was set for 16× gain. The interferograms were transformed using a zero filling factor of 8 and a 3-term Blackman-Harris apodization function.
To obtain a background mid-IR reference spectrum, an IR-transmitting CaF slide was prepared with virgin, unirradiated SrC2O4 powder (~3-μm thick) for comparison purposes.
An IR transmitting diamond was used to hold a thin film (~3-μm thick) of virgin SrC2O4 for a far-IR standard measurement, also for comparison purposes. This fresh sample had been prepared by manually compressing fresh SrC2O4 between two diamonds in a symmetric-style DAC (without a gasket) and removing one of the diamonds. All measurements were performed in transmission and at room temperature. All presented spectra constituted the average of 512 scans with 2 s/scan.
3. Results
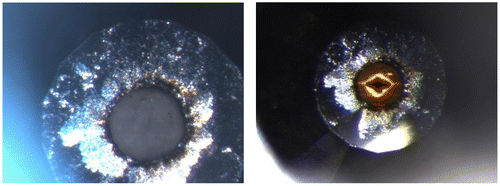
We present images of the virgin, unirradiated sample loaded at the APS in Figure (left) and the same sample after a 4-h irradiation (right). As in prior irradiation studies (e.g. Pravica et al., Citation2011, Citation2012), there is no evidence that the sample temperature altered beyond ambient temperature. It is immediately evident that a very dark cross pattern has been formed after irradiation which was in the shadow of the incident X-ray beam (including a small amount of damage caused during the alignment of the sample into the beam). Outside of the irradiated region, the sample yellowed and darkened significantly. Raman spectroscopy was attempted to compare with the unirradiated spectrum taken before irradiation, but the irradiated sample was far too fluorescent in the presence of 532-nm laser light to collect any useful spectra. The Raman spectrum of the sample collected just before it was irradiated agreed well with http://webbook.nist.gov/cgi/cbook.cgi?ID=B6000066&Mask=80, suggesting that our sample commenced as highly pure SrC2O4.
Figure 1. (Left) Strontium oxalate sample loaded at 7 GPa before irradiation looking through one diamond. (Right): The same pressurized sample after ~ 4 h of irradiation examined one day later.
Unfortunately, as the diamonds were not of sufficient IR-transmitting quality, no useful IR signal could be obtained in either the far- or mid-IR range with the sample squeezed in between the diamonds. The DAC was then depressurized and the gasket was removed. It was immediately noted that the colored sample remained in the gasket so that IR spectra of the depressurized and reacted sample were successfully recorded without diamond interference. The far-IR patterns of the irradiated sample (still inside the depressurized gasket but with one diamond removed) and an unirradiated virgin sample (placed on the same IR-transmitting diamond) are displayed in Figure . Both spectra are background subtracted.
Figure 2. Background-subtracted far-IR transmission spectra of the irradiated/recovered (red trace) and virgin (black trace) SrC2O4 samples.
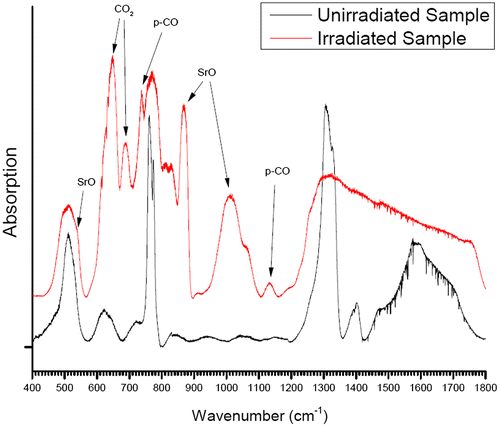
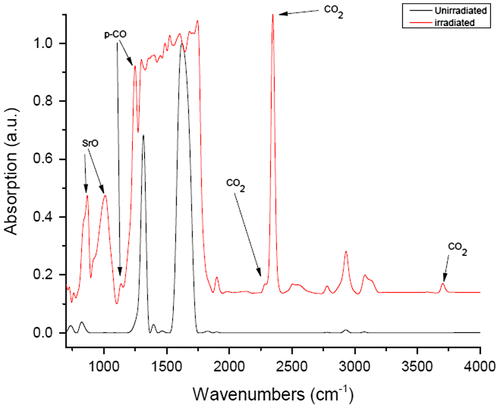
Figure displays the background-subtracted mid-IR spectra of the ambient, unirradiated SrC2O4 sample along with that of the irradiated/decompressed sample. The virgin, unirradiated mid- and far-IR spectra agree generally well with the spectrum in Ito and Bernstein (Citation1956). The far-IR unirradiated spectrum possesses two main modes near 515 and 775 cm−1 which are assigned to COO out-of-plane rocking [δ(CO2)] and anion deformation modes, respectively (Ito & Bernstein, Citation1956; D’Antonio, Torres, Palacios, González-Baró, & Baran, Citation2015). Progressing toward higher frequencies (Figures and ), two main modes are observed near 1,300 and 1,660 cm−1 which are associated with νas (CO2) and νs (CO) stretching modes, respectively (D’Antonio et al., Citation2015).
Figure 3. Mid IR absorption (1—transmission) spectra of the irradiated (raised red trace) and virgin (lower black trace) SrC2O4 samples.
Examining the irradiated/decompressed far- and mid-IR spectra, dramatic changes are apparent. In the case of the far-IR irradiated/decompressed spectrum, moving from left to right in wavenumber, a strong peak near 654 cm−1 and a lesser one near 680 cm−1 are likely associated with the O=C=O bending mode (ν2) (Isokoski, Poteet, & Linnartz, Citation2013). Progressing toward higher vibrational energies, there is a very sharp peak near 725 cm−1 which was also observed in Evans et al. (Citation2006), Lipp, Evans, Baer, and Yoo (Citation2005), except at a lower frequency of 704 cm−1. We suspect that this peak may be associated with CO2 (ν3 (Gunzler & Gremlich, Citation2002)).Footnote1 The relatively narrow peak near 880 cm−1 and the broad line near 1,020 cm−1 are likely SrO multiphonon bands (Jacobson & Nixon, Citation1968). Finally, the small peak near 1,130 cm−1 was also observed in the recovered poly-CO samples from Evans et al. (Citation2006) and Lipp et al. (Citation2005). Beyond this, there is strong absorption and mixing of bands complicating interpretation of the spectra above 1,130 cm−1.
In the case of the irradiated mid-IR spectrum, the two primary peaks near 1,275 and 1,650 cm−1 appear severely diminished (1,275 cm−1 line) or largely disappear (1,650 cm−1 line) again suggesting a significant reaction/transformation/polymerization of the sample. In their place, a number of new modes appear. At least three of these new lines are associated with CO2: The vibration near 2,349 cm−1 is the ν3 antisymmetric 12C=O stretch (Isokoski et al., Citation2013). The peak near 1,850 cm−1 may possibly be associated with the CO2 bending mode with an overtone observed near 3,700 cm−1 (Evans et al., Citation2006) or the ν3 + ν1 mode (Hansen, Citation1997; Isokoski et al., Citation2013).
Two other new peaks near 880 and 1,030 cm−1 are from SrO (Jacobson & Nixon, Citation1968). Finally, other newly appearing peaks may be attributed to polymeric-CO near 1,120 and 1,270 cm−1 (Evans et al., Citation2006; Bernard, Chiarotti, Scandolo, & Tosatti, Citation1998; Santoro et al., Citation2015).
4. Discussion
When pressure was released, the recovered sample appeared to be quite intact and stable—even sticking completely to the gasket when removed from the DAC with no residue left behind on the diamonds. We attempted Raman spectroscopy but found, as did other researchers (Evans et al., Citation2006; Lipp et al., Citation2005) that the sample appeared to rapidly decompose/burn where the laser light was incident upon the material and the region where the laser was incident transformed into a shiny/metallic-looking material which we suspect to be graphitic carbon.
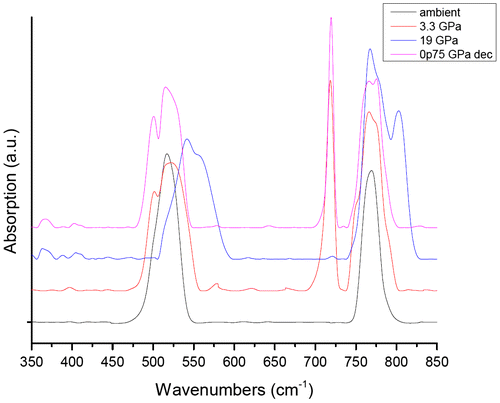
We sought to prove that the observed polymeric synthesis was largely X-ray induced by conducting a separate far-IR high-pressure study of SrC2O4 up to 19 GPa using petroleum jelly as a pressure-transmitting medium which will be reported in more detail in a subsequent paper. We present the preliminary results in Figure . The far-IR pattern found no evidence of pressure-induced polymerization or decomposition as the acquired IR spectrum after pressure release returned to that obtained under ambient conditions. Examining Figure (left), there is also no visual evidence of chemical reaction when pressurized to 7 GPa until irradiation (right photo) at the same pressure.
Figure 4. Far-IR spectra of virgin, unirradiated strontium oxalate.
By irradiating the pressurized sample, we produced molecular CO2 and an energetic polymer which largely appears to be polymeric carbon monoxide that is recoverable—similar to experiments that studied pure CO under high pressure (Evans et al., Citation2006; Katz, Schiferl, & Mills, Citation1984; Lipp et al., Citation2005). We also produced SrO which, considering just the stoichiometry of C and O, would support the idea that we formed polymerized carbon suboxide (p-C3O2) (Katz et al., Citation1984; Snow, Haubenstock, & Yang, Citation1978). However, given the observation that CO2 remained in the reacted sample/polymer (even after over one month after the sample was opened to ambient conditions) and the similar varying colored appearance of the sample observed in earlier studies of CO (Evans et al., Citation2006; Lipp et al., Citation2005), we suspect that the synthesized polymer is poly-CO. We also did not observe a maximum in transmission near 2,200 cm−1 (Katz et al., Citation1984; Snow et al., Citation1978)—rather, a broad window of transmission in that region which suggests that p-C3O2 was not present. Therefore, we propose the following reaction sequence:
The CO would be highly reactive especially under the highly ionizing conditions wrought by highly penetrating and highly energetic hard X-rays and would then polymerize into poly-CO with further irradiation and within the matrix of inert CO2 and SrO, explaining the absence of CO in the mid-IR spectra (Ewing, Citation1962). At a pressure of 7 GPa and 300 K, the newly produced CO would react to form poly-CO based on the phase diagram in Ceppatelli, Serdyukov, Bini, and Jodl (Citation2009).
5. Conclusion
We have observed that hard X-ray irradiation of strontium oxalate (pressurized inside a diamond anvil cell to 7 GPa) has initiated a powerful chemical reaction(s) which polymerized the sample and produced CO2 and SrO in situ within the poly-CO matrix. The CO2 appears to be trapped within the polymer even when the gasket was depressurized to ambient pressure and removed from the DAC. The Sr atoms appear to have bonded with oxygen to form SrO. Future experiments will ascertain if the SrO segregates or is merely dispersed throughout the sample. The polymer is a highly colored substance with waxlike, even “gooey (Evans et al., Citation2006)” consistency after exposure to air that appears to be very similar to recovered polymeric CO that has been produced by pressurizing CO above 5 GPa in earlier studies (Evans et al., Citation2006; Lipp et al., Citation2005). We found no evidence that pressure by itself has any significant role in the polymerization of SrC2O4 as it does in the case of pure CO. However, pressure in combination with X-ray irradiation is likely vital to this process. This enables far more control in the synthesis.
We have thus discovered a novel and easy route of chemical synthesis for a form of polymeric CO. We have also, for the first time, produced CO2 via useful hard X-ray photochemistry which expands our repertoire for future chemical decomposition reactions (and synthesis) and which will aid us in our efforts to study the effects of diffusion, mixing, and segregation on detonation products (and other chemical species) under extreme conditions. It also enables the synthesis of an interesting polymer interspersed with an inert oxide which may have future electronic and/or optical applications.
To the best of our knowledge, this study represents the first polymerization of any compound via a combination of hard X-ray irradiation and pressure.
Acknowledgments
We thank Scott Rosendahl and Xia Liu for beamline support. A portion of the research described in this paper was performed at the Mid-IR beamline of the Canadian Light Source, which is supported by the Natural Sciences and Engineering Research Council of Canada, the National Research Council Canada, the Canadian Institutes of Health Research, the Province of Saskatchewan, Western Economic Diversification Canada, and the University of Saskatchewan. Kamil Dziubek acknowledges the Polish Ministry of Science and Higher Education for financial support through the “Mobilność Plus” program.
Additional information
Funding
Notes on contributors
Michael Pravica
Michael Pravica’s research group studies matter subjected to extreme conditions of pressure, temperature, and ionizing radiation. They have also been developing the field of useful hard X-ray photochemistry by harnessing the highly ionizing, highly penetrating, and highly focused properties of synchrotron hard X-rays (>7 keV) to initiate novel in situ decomposition and synthetic chemistry under extreme and/or isolated conditions.
Notes
1. We note in passing that have routinely observed vibrational shifts from our X-ray photochemical products that differ slightly due to differing chemical environments (Pravica, Sneed, White, et al., Citation2014b) and our method of producing this polymer is very different from prior syntheses of poly-CO (Evans et al., Citation2006; Lipp et al., Citation2005).
References
- Bernard, S., Chiarotti, G., Scandolo, S., & Tosatti, E. (1998). Decomposition and polymerization of solid carbon monoxide under pressure. Physical Review Letters, 81, 2092–2095.10.1103/PhysRevLett.81.2092
- Bose, S., Bhatta, S., & Bhatta, D. (1998). Decomposition of γ-irradiated strontium oxialate. Radiation Effects and Defects in Solids, 145, 263–270.10.1080/10420159808225769
- Ceppatelli, M., Serdyukov, A., Bini, R., & Jodl, H. (2009). Pressure induced reactivity of solid CO by FTIR studies. The Journal of Physical Chemistry B, 113, 6652–6660.10.1021/jp900586a
- D’Antonio, M., Torres, M., & Palacios, D., González-Baró, A., & Baran, E. (2015). Vibrational spectra of the two hydrates of strontium oxalate. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 137, 486–489.10.1016/j.saa.2014.08.102
- Evans, W., Lipp, M., Yoo, C.-S., Cynn, H., Herberg, J., & Maxwell, R. (2006). Pressure-induced polymerization of carbon monoxide: Disproportionation and synthesis of an energetic lactonic polymer. Chemistry of Materials, 18, 2520–2531.10.1021/cm0524446
- Ewing, G. (1962). Infrared spectra of liquid and solid carbon monoxide. The Journal of Chemical Physics, 37, 2250–2256.10.1063/1.1732994
- Gunzler, H., & Gremlich, H-U. (2002). IR spectroscopy, Weinheim: Wiley-VCH.
- Hansen, G. (1997). The infrared absorption spectrum of carbon dioxide ice from 1.8 to 333 μm. Journal of Geophysical Research: Planets, 102, 21569–21587.10.1029/97JE01875
- Isokoski, K., Poteet, C. A., & Linnartz, H. (2013). Highly resolved infrared spectra of pure CO2 ice (15–75 K). Astronomy & Astrophysics, 555(A85), 1–6.
- Ito, K., & Bernstein, H. (1956). The vibrational spectra of the formate, acetate, and oxalate ions. Canadian Journal of Chemistry, 34, 170–178.10.1139/v56-021
- Jacobson, J. L., & Nixon, E. R. (1968). Infrared dielectric response and lattice vibrations of calcium and strontium oxides. Journal of Physics and Chemistry of Solids, 29, 967–976.10.1016/0022-3697(68)90233-3
- Katz, A., Schiferl, D., & Mills, R. (1984). New phases and chemical reactions in solid carbon monoxide under pressure. The Journal of Physical Chemistry, 88, 3176–3179.10.1021/j150659a007
- Lipp, M., Evans, W., Baer, B., & Yoo, C.-S. (2005). High-energy-density extended CO solid. Nature Materials, 4, 211–215.10.1038/nmat1321
- Pravica, M., Bai, L., & Bhattacharya, N. (2012). High-pressure X-ray diffraction studies of potassium chlorate. Journal of Applied Crystallography, 45, 48–52.10.1107/S0021889811053957
- Pravica, M., Bai, L., Park, C., Liu, Y., Galley, M., Robinson, J., & Bhattacharya, N. (2011). Note: A novel method for in situ loading of gases via X-ray induced chemistry. Review of Scientific Instruments, 82, 106102.10.1063/1.3648062
- Pravica, M., Bai, L., Park, C., Liu, Y., Galley, M., Robinson, J., & Hatchett, D. (2012). Note: Experiments in hard X-ray chemistry: In situ production of molecular hydrogen and X-ray induced combustion. Review of Scientific Instruments, 83, 036102.10.1063/1.3682336
- Pravica, M., Bai, L., Sneed, D., & Park, C. (2013). Measurement of the energy dependence of X-ray-induced decomposition of potassium chlorate. The Journal of Physical Chemistry A, 117, 2302–2306.10.1021/jp4008812
- Pravica, M., Hulsey, B., Bai, L., Sneed, D., Smith, Q., & Guardala, G. (2014). 18th APS-SCCM and 24th AIRAPT. Journal of Physics: Conference Series, 500, 022009.
- Pravica, M., Liu, Y., & Bai, L. (2013). Hydrazine at high pressures. Chemical Physics Letters, 555, 113–115.
- Pravica, M., Popov, D., Sinogeikin, S., Sneed, D., Guardala, G., & Smith, Q. (2013). X-ray induced mobility of molecular oxygen at extreme conditions. Applied Physics Letters, 103, 224103.10.1063/1.4836475
- Pravica, M., Sneed, D., Smith, Q., & Bai, L. (2013). High pressure X-ray photochemical studies of carbon tetrachloride: Cl2 production and segregation. Chemical Physics Letters, 590, 74–76.10.1016/j.cplett.2013.10.056
- Pravica, M., Sneed, D., White, M., & Wang, Y. (2014a). Note: Loading method of molecular fluorine using x-ray induced chemistry. Review of Scientific Instruments, 85, 086110. doi:10.1063/1.4893384
- Pravica, M., Sneed, D., White, M., & Wang, Y. (2014b). Communication: A novel method for generating molecular mixtures at extreme conditions: The case of hydrogen and oxygen. The Journal of Chemical Physics, 141, 091101. doi:10.1063/1.4894402
- Pravica, M., White, M., & Wang, Y. (2015). A novel method for generating molecular mixtures at extreme conditions: The case of fluorine and oxygen. APS SCCM Conference Proceedings.
- Pravica, M., Yulga, B., Tkachev, S., & Liu, Z. (2009). High-pressure far- and mid-infrared study of 1,3,5-triamino-2,4,6-trinitrobenzene. The Journal of Physical Chemistry A, 113, 9133–9137.10.1021/jp903584x
- Santoro, M., Dziubek, K., Scelta, D., Ceppatelli, M., Gorelli, F. A., Bini, R., … Haines, J. (2015). High pressure synthesis of all-transoid polycarbonyl [–(C=O)–] n in a zeolite. Chemistry of Materials, 27, 6486–6489. doi:10.1021/acs.chemmater.5b02596
- Snow, A., Haubenstock, H., & Yang, N.-L. (1978). Poly(carbon suboxide). Characterization, polymerization, and radical structure. Macromolecules, 11, 77–86.10.1021/ma60061a015
