Abstract
An efficient condition for the microwave synthesis of nitroalkanes from α,β-unsaturated nitroalkenes using tri-n-butyltin hydride as a mild reducing reagent under aqueous conditions is described. A variety of conjugated nitroalkenes have undergone reduction chemoselectively to yield corresponding nitroalkanes up to 95% yield in 8–30 min. Also, a possible ionic mechanism involving a six-membered cyclic transition state has been proposed.
Public Interest Statement
The paper reports significant new work pertaining to the reduction of conjugated nitroalkenes, and is of high contemporary interest because of the aqueous and environmentally compatible conditions employed. There is also a renaissance of interest in aliphatic nitro compounds, in view of their rich functional group transformation chemistry. The essential novelty of this work lies in a fact that microwave conditions and water as solvent were used to develop greener method for this tri-n-butyltin hydride reduction. The unavoidable importance of nitroalkane chemistry in the pharmaceutical and fine chemicals industry makes the present method reasonable for current significance. Therefore, this article can be general chemistry and materials chemistry readers.
1. Introduction
Nitroalkane chemistry is of inevitable importance in the pharmaceutical and fine chemicals industry (Bogusz, Citation1995; Ballini, Bosica, Fiorini, & Palmieri, Citation2005; Ballini, Palmieri, & Righi, Citation2007; Litzinger et al., Citation2011; Markofsky, Citation2005). Nitroalkanes are very important intermediates in organic synthesis as they can be converted into other functionalities, such as amines, carbonyl compounds, denitrated compounds, complex heterocyclic structures, and more (Ono, Citation2002; Ballini & Petrini, Citation2004; Li & Corey, Citation2009). Several methods have been developed for the synthesis of nitroalkanes by reduction of conjugated nitroalkenes from last five decades (Shechter, Ley, & Roberson, Citation1956; Meyers & Sircar, Citation1967; Barrett & Graboski, Citation1986; Kabalka, Laila, & Varma, Citation1990; Ranu & Chakraborty, Citation1991; Denmark & Marcin, Citation1993; Anbazhagan, Kumaran, & Sasidharan, Citation1997; Lucet, Sabelle, Kostelitz, Le Gall, & Mioskowski, Citation1999; Duursma, Minnaard, & Feringa, Citation2002; Xiang et al., Citation2012). These ionic metal hydride reduction methods generally employ toxic organic solvents as reaction medium under inert gas atmosphere.
Palomo and co-workers have reported the reduction of nitroalkenes using tri-n-butyltin hydride (1) as reducing agent in dichloromethane followed by treatment with acid in the absence of any radical initiators (Aizpurua, Oiarbide, & Palomo, Citation1987). The drawback of this methodology is that it reduces only those α-alkyl-substituted nitroalkenes when the β-position of nitroalkenes was substituted by a β-lactum moiety (Palomo et al., Citation1990; Shirode, Likhite, Gumaste, & Deshmukh, Citation2008). Moreover, the method involves two steps, takes longer reaction time, and uses organic solvents and corrosive acids in the reaction. Thus, a methodology for the selective reduction of nitroalkenes to nitroalkanes using 1 as reducing agent which can minimize the above drawbacks seems important.
In recent years, developing “green” conditions is increasing prerequisite in industrial research (Anastas, Citation2010; Dunn, Wells, & Williams, Citation2010). Use of water as a solvent is a powerful strategy for developing “green” chemical synthesis (Dallinger & Kappe, Citation2007). However, the use of ionic metal hydrides under aqueous conditions is challenging and often requires more equivalents because they can easily undergo decomposition (Lo, Karan, & Davis, Citation2007). An alternative reducing agent can be 1, which is relatively stable towards water as compared to ionic metal hydrides. Even though 1 is toxic, its use in organic synthesis is often unavoidable because it is a fairly weak and selective reducing agent (1 has nonionic bond between tin and hydrogen) (Chatgilialoglu, Griller, & Lesage, Citation1988). Furthermore, the aqueous organotin chemistry is still at its infancy (Rai, Aubrecht, & Collum, Citation1995; Sarma & Maitra, Citation1998, Citation2001) and more study in this field will be worth developing by considering environmental concerns. Herein, chemoselective reduction of conjugated nitroalkenes to nitroalkanes using 1 under mild aqueous microwave conditions has been reported.
2. Results and discussion
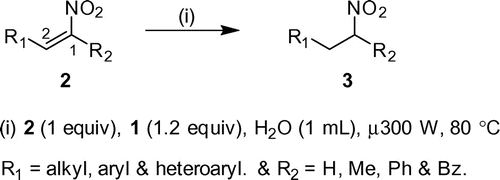
Initial exploratory studies were begun with (E)-2-nitrostyrene as a model substrate. When a heterogene-ous mixture of (E)-2-nitrostyrene (2a, 1.3 mmol), 1 (1.5 mmol), and water was subjected to microwave heating at 80°C (300 W) in CEM Discover-focused microwave™ synthesis system, (2-nitroethyl)benzene (3a) was formed (95% yield) in 10 min (Scheme , Table ).
Table 1. Microwave reduction of nitroalkenes
Different α-unsubstituted and β-monosubstituted-conjugated nitroalkenes (2a-g) subjected to the above reduction conditions, corresponding nitroalkanes furnished in 73–92% yield in 8–15 min. α,β-Monosubstituted nitroalkenes (2h-s) were also reduced to the corresponding nitroalkanes, but over a longer period of time (10–25 min) as compared to conjugated nitroalkenes 2a-g (Table ). In the case of heteroaryl and aliphatic–substituted nitroalkenes, yields were moderate may be because of side reactions occurring in parallel during microwave heating as evidenced by TLC.
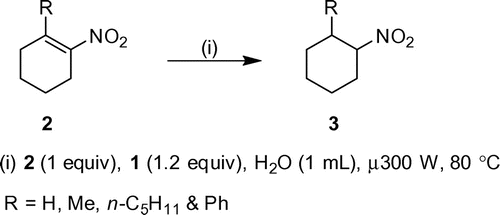
Moreover, α-disubstituted-β-monosubstituted conjuga-ted nitroalkenes (2t-w) have undergone reduction under conditions in excellent yield (78–94%, Scheme , Table ). This extends the versatility of method to reduction of hindered endocyclic double bonds.
Table 2. Microwave reduction of 1-substituted-2-nitro-1-cyclohexenes to 1-substituted -2-nitrocyclohexanes
All products were purified by flash column chromatography to remove nonpolar tin impurities and characterized by common spectroscopic techniques.
Even though the effect of substituents on reaction was less, the reaction was highly dependent on the nature of solvent. The reaction furnished best yields of nitroalkanes when water was used as solvent, rather than water–organic solvent mixtures and the reaction has not occurred in pure organic solvents at all. Purity of reactants and maintenance of microwave conditions (power, temperature, and time) during the reaction are also important; the change in any of parameters resulted in the deviation of yield. This method is highly chemoselective as the reagent has reduced only double bonds not the nitro groups, even though 1 is well-known to reduce nitro groups (Ono, Miyake, Tamura, & Kaji, Citation1981; Tanner, Blackburn, & Diaz, Citation1981).
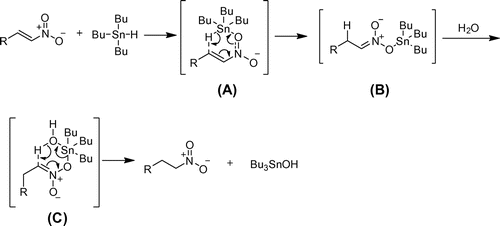
Interestingly, the use of radical initiators (AIBN or DBP) and radical inhibitors (TEMPO or galvinoxyl) in the reaction (of case 2a) have shown very little effect on the outcome. This directed us to propose an ionic mechanism where the formation of tin nitronate (B) was believed to be a key intermediate for the transformation. Moreover, no nonpolar solvents, no polar solvents and even no organic–water solvent mixtures were successful in giving better yield of 3a as compared to pure water. Water is known to stabilize the transition state by enforced hydrophobic effect and hydrogen bonding effect (Chandrasekhar, Shariffskul, & Jorgensen, Citation2002). This might have stemmed the enhancement of forward reaction when water was used as solvent in the present case.
Furthermore, we have observed that the reaction utilizes only one equivalent (or slight excess) of 1 for the completion of reaction which shows that the reaction occurs via 1,4-addition of 1 to the nitroalkene followed by hydrolysis of the tin nitronate product by water (see Supplementary material).
Based on the above experimental studies, mechanism of the reaction can be written as shown in Scheme . Therefore, the nitro group of nitroalkene interacts with tin atom of 1 because of hydrophobic effect of water to form the six-membered transition state (A). The transition state A rearranges to another tin nitronate intermediate B followed by the attack of a water molecule furnishing the nitroalkane. The alternative mechanism, involving hydration of nitroalkene to give the alcohol, which is then reduced by 1, would require excess 1 as understood in the dehydroxylation of 2-nitroalcohols (Chandrasekhar & Shrinidhi, Citation2014).
In conclusion, the method described above for the chemoselective reduction of a wide variety of nitroalkenes with tri-n-butyltin hydride–water was achieved under microwave heating. Products were formed within few min in good yields. Also, the use of neutral nonionic hydride reducing agent (1) was the better choice as compared to other ionic hydride reducing agents (aluminium or sodium borohydrides) under microwave conditions in water. In the case of ionic hydrides as reducing agents, there is a chance of accumulation of lot of pressure in the reaction flask and even there is a chance of explosion in extreme cases. The use of water as green solvent and microwave conditions adds to the benignness of this tri-n-butyltin hydride reduction strategy.
3. Experimental
All the reactions were carried out in CEM Discover-focused microwave™ synthesis system using 10 mL reaction vessel. The instrument possesses digital settings for microwave power, temperature, pressure, and time.
A heterogeneous mixture of nitroalkene (1.3 mmol), 1 (1.5 mmol), and H2O (2 mL) in 10 mL microwave vessel fitted with a septum, was irradiated in the CEM Discover-focused microwave™ synthesis system at 300 W and 80°C for 8–30 min. After cooling to room temperature, the residue was extracted with ether (2 × 20 mL). The combined organic extracts were dried (Na2SO4) and concentrated in vacuum to furnish corresponding nitroalkanes in crude form. It was purified by column chromatography on silica gel (100–200 mesh, eluent: 5:95 ethyl acetate–hexane) to obtain pure nitroalkane. The disappearance of alkene peak (IR: 1618–1655 cm−1 and 1H-NMR: δ 6.9–8.0) and the appearance of protons α and β to NO2 group in 1H-NMR (δ 4.3–4.7 for α protons and δ 1.9–3.8 for β protons) of products were the supporting information for the reduction. The melting point for the solid nitroalkanes were matching with the literature values and some of them were liquids whose boiling point was not measured as it was synthesized in very low amounts.
Suplementary_material.docx
Download MS Word (71.5 KB)Additional information
Funding
Notes on contributors
Annadka Shrinidhi
Annadka Shrinidhi is a post-doc in the Department of Pharmacy at University of Groningen, The Netherlands. He obtained his PhD in Organic Chemistry from Indian Institute of Science, India and his thesis was entitled as “Organic Transformations in Water: Synthetic and Mechanistic Studies towards Green Methodologies”. His research interests are focused in the areas of synthetic organic chemistry, physical organic chemistry and medicinal chemistry. His current research interest is in the development of novel gadolinium based MRI contrast agents via multi-component synthesis.
References
- Aizpurua, J. M., Oiarbide, M., & Palomo, C. (1987). Reduction of α,β-unsaturated nitrocompounds with tributyltin hydride. Tetrahedron Letters, 28, 5365–5366.
- Anastas, P. (2010). Handbook of green chemistry. Weinheim: Wiley-VCH.
- Anbazhagan, M., Kumaran, G., & Sasidharan, M. J. (1997). Selective conversion of nitroalcohols into nitroolefinsover zeolite under heterogeneous conditions. Journal of Chemical Research, 336–337.
- Ballini, R., Bosica, G., Fiorini, D., & Palmieri, A. (2005). Synthesis of fine chemicals by the conjugate addition of nitroalkanes to electrophilic alkenes. Journal of Fudan University Natural Science, 44, 629–630.
- Ballini, R., Palmieri, A., & Righi, P. (2007). Highly efficient one- or two-step sequences for the synthesis of fine chemicals from versatile nitroalkanes. Tetrahedron, 63, 12099–12121.
- Ballini, R., & Petrini, M. (2004). Recent synthetic developments in the nitro to carbonyl conversion (Nef reaction). Tetrahedron, 60, 1017–1047.
- Barrett, A. G. M., & Graboski, G. G. (1986). Conjugated nitroalkenes: Versatile intermediates in organic synthesis. Chemical Reviews, 86, 751–762.
- Bogusz, M. (1995). Retention and selectivity in liquid chromatography—Prediction, standardisation and phase comparisons. Journal of Chromatography Library, 57, 171–207.
- Chandrasekhar, J., Shariffskul, S., & Jorgensen, W. L. (2002). QM/MM simulations for Diels−Alder reactions in water: Contribution of enhanced hydrogen bonding at the transition state to the solvent effect. The Journal of Physical Chemistry B, 106, 8078–8085.
- Chandrasekhar, S., & Shrinidhi, A. (2014). Selective aldehyde reduction in ketoaldehydes with NaBH4 -Na2 CO3 -H2 O at room temperatures. Synthetic Communications, 44, 2051–2056.
- Chatgilialoglu, C., Griller, D., & Lesage, M. (1988). Tris(trimethylsilyl)silane. A new reducing agent. The Journal of Organic Chemistry, 53, 3641–3642.
- Dallinger, D., & Kappe, C. O. (2007). Microwave-assisted synthesis in water as solvent. Chemical Reviews, 107, 2563–2591.
- Denmark, S. E., & Marcin, L. R. (1993). A general method for the preparation of 2,2-disubstituted 1-nitroalkenes. The Journal of Organic Chemistry, 58, 3850–3856.
- Dunn, P. J., Wells, A., & Williams, M. T. (2010). Green chemistry in the pharmaceutical industry. Weinheim: Wiley-VCH.
- Duursma, A., Minnaard, A. J., & Feringa, B. L. (2002). One-pot multi-substrate enantioselective conjugate addition of diethylzinc to nitroalkenes. Tetrahedron, 58, 5773–5778.
- Kabalka, G. W., Laila, G. M. H., & Varma, R. S. (1990). Selected reductions of conjugated nitroalkenes. Tetrahedron, 46, 7443–7457.
- Li, J. J., & Corey, E. (2009). Name reactions for homologation. Part 1. Hoboken, NJ: John Wiley.
- Litzinger, T., Colket, M., Kahandawala, M., Lee, S. Y., Liscinsky, D., McNesby, K., … Stouffer, S. (2011). Fuel additive effects on soot across a suite of laboratory devices, Part 2: Nitroalkanes. Combustion Science and Technology, 183, 739–754.
- Lo, C.-T. F., Karan, K., & Davis, B. R. (2007). Kinetic studies of reaction between sodium borohydride and methanol, water, and their mixtures. Industrial & Engineering Chemistry Research, 46, 5478–5484.
- Lucet, D., Sabelle, S., Kostelitz, O., Le Gall, T., & Mioskowski, C. (1999). Enantioselective synthesis of α-amino acids and monosubstituted 1,2-diamines by conjugate addition of 4-phenyl-2-oxazolidinone to nitroalkenes. European Journal of Organic Chemistry, 1999, 2583–2591.
- Markofsky, S. B. (2005). Ullmann’s encyclopedia of industrial chemistry. Weinheim: Wiley-VCH.
- Meyers, A. I., & Sircar, J. C. (1967). Reduction of nitroalkenes to nitroalkanes with aqueous sodium borohydride. The Journal of Organic Chemistry, 32, 4134–4136.
- Ono, N. (2002). The nitro group in organic synthesis. New York, NY: Wiley-VCH.
- Ono, N., Miyake, H., Tamura, R., & Kaji, A. (1981). A new synthetic method: Direct replacement of the nitro group by hydrogen or deuterium. Tetrahedron Letters, 22, 1705–1708.
- Palomo, C., Aizpurua, J. M., Cossio, F. P., Garcia, J. M., Lopez, M. C., & Oiarbide, M. (1990). Tributyltin hydride addition to nitroalkenes: A convenient procedure for the conversion of nitroalkenes into nitroalkanes and carbonyl compounds. The Journal of Organic Chemistry, 55, 2070–2078.
- Rai, R., Aubrecht, K. B., & Collum, D. B. (1995). Palladium-catalyzed stille couplings of aryl-, vinyl-, and alkyltrichlorostannanes in aqueous solution. Tetrahedron Letters, 36, 3111–3114.
- Ranu, B. C., & Chakraborty, R. (1991). Highly selective reduction of conjugated nitroalkenes with zinc borohydride in DME. Tetrahedron Letters, 32, 3579–3582.
- Sarma, K. D., & Maitra, U. (1998). Aqueous organotin chemistry: Tin hydride mediated dehalogenation of organohalides and a novel organotin mediated nucleophilic substitution on 2-iodobenzoates in water. Tetrahedron, 54, 4965–4976.
- Sarma, K. D., & Maitra, U. (2001). Aqueous organotin chemistry: Part 3-triphenyltin hydride and di-n-butyltin dichloride mediated nucleophilic substitution of 2-iodobenzoates in water. Indian Journal of Chemistry Section B, 40, 1148–1150.
- Shechter, H., Ley, D. E., & Roberson, Jr., E. B. (1956). Nitroalkanes from conjugated nitroalkenes by reduction with complex hydrides 1. Journal of the American Chemical Society, 78, 4984–4991.
- Shirode, N. M., Likhite, A. P., Gumaste, V. K., & Deshmukh, A. R. A. S. (2008). Synthesis of (3S,4R)-4-benzylamino-3-methoxypiperidine, an important intermediate for (3S,4R)-cisapride. Tetrahedron, 64, 7191–7198.
- Tanner, D. D., Blackburn, E. V., & Diaz, G. E. (1981). A free-radical chain reaction involving electron transfer. The replacement of the nitro group by hydrogen using trialkyltin hydride, a variation of the Kornblum reaction. Journal of the American Chemical Society, 103, 1557–1559.
- Xiang, J., Sun, E.-X., Lian, C.-X., Yuan, W.-C., Zhu, J., Wang, Q., & Deng, J. (2012). The highly chemoselective transfer hydrogenation of the carbon–carbon double bond of conjugated nitroalkenes by a rhodium complex. Tetrahedron, 68, 4609–4620.