Abstract
The present study unveils major phenolic antioxidant compounds from Camellia sinensis fruits, followed by their investigation, purification and characterization using HPLC, ESI-MS and NMR studies. The spectrophotometric estimation results have clearly demonstrated that C. sinensis (tea) fruits contain up to 14% of total polyphenols (as gallic acid equivalent) and 7% of flavonoids (as quercetin equivalent) on dry weight basis. Differential solvent-mediated extractions have been performed for quantitative assessment of major phytoconstituents by RP-HPLC analysis. And the results have revealed that these fruits contain adequate amount of tea catechins (4%) along with caffeine (1%) and theanine (0.4%) on dry weight basis. Moreover, purification and characterization of major phytoconstituents such as epigallocatechin, epicatechin, epigallocatechin gallate and epicatechin gallate along with caffeine have been accomplished. Thus, it is clearly demonstrated that tea fruits could act as a possible and reliable source for obtaining major phenolic antioxidants.
Public Interest Statement
Tea is one of the major plantation crop cultivated in an area of 3.6 million hectares. During the manufacture of various types of tea (green, black and oolong), only fresh tender tea shoots comprising apical bud along with two to three leaves are used, while other parts of tea plant have remained unutilized, despite their abundance. In this report, we have explored the major bioactive constituents of green tea fruits. Hence, this study reveals a new sustainable and economical source for obtaining green tea bioactives for their utilization in nutraceuticals and cosmetic formulations.
1. Introduction
Tea (Camellia sinensis) is a well-known plant, whose fresh leaves are processed for manufacturing various types of tea: green, black, oolong and white. Tea is a rich source of flavonoids called as catechins. Major catechins present in tea are epigallocatechin (EGC), epicatechin (EC), epigallocatechin gallate (EGCG) and epicatechin gallate (ECG). Apart from these four major catechins, non-epimeric catechins such as gallocatechin (GC), catechin, gallocatechin gallate (GCG) and catechin gallate (CG) have also been reported in tea. Tea catechins have gained extensive recognition as natural antioxidant compounds, with evident support of being beneficial in prevention of diverse range of diseases. The use of catechins as natural antioxidants in oils, fats, animal feeds, animal products (Yilmaz, Citation2006) and as dietary supplements and food preservatives have gained huge popularity. Due to the high demand for tea catechins in pharmaceutical, drug and cosmetic industries across the world, there is a vast hunt for sustainable bioresources to obtain these bioactive compounds. In the recent past, numerous studies have been carried out, reporting occurrence of catechins in underutilized tea leaves (Vuong, Golding, Nguyen, & Roach, Citation2012) and tea flowers along with their extraction, isolation (Lin, Wu, & Lin, Citation2003; Way et al., Citation2009) and usage. Few studies have also been documented on tea fruits and their chemical composition (Bhardwaj, Kaur, Nagar, & Arora, Citation2007; Bhattacharya, Nagar, & Ahuja, Citation2002; Xu, Bao, Gao, Zhau, & Wang, Citation2012). But, despite these study reports, the investigations on phenolic antioxidants from whole tea fruit have remained unresolved till now. Therefore, the main endeavour of the present study was to investigate the phenolic antioxidant compounds from underutilized tea fruits along with their purification and characterization using HPLC, LC-MS and NMR techniques.
2. Experimental
2.1. Chemical and reagents
All the chemicals and reagents were obtained from Merck Specialities India, Mumbai, India. Various standards viz. GC, EGC, EC, catechin, EGCG, GCG, ECG and caffeine were purchased from Sigma–Aldrich Corporation, Bangalore, India.
2.2. Plant material
Fresh green tea fruits were collected from the experimental tea garden of CSIR-Institute of Himalayan Bioresource Technology, Palampur, HP, India (36° N and 78.18° E and 1,290 m above mean sea level) during the month of May. A sample voucher PLP-16160 has been deposited in the herbarium section of the CSIR-IHBT, Palampur. Freshly harvested tea fruits were allowed to dry. For drying, about 100 g of tea fruits were sliced in to 2–3 small pieces so as to expose the internal surface. These slices were immediately placed in a conventional microwave oven (IFB Appliances Microwave—Convection Domestic Oven model 30SC3) for 2 min for 30 s in order to inactivate the endogenous enzymes. After microwave treatment, these fruits were dried in a hot air oven at 50°C to constant weight. The dried tea fruits were ground to powder with mixer grinder (Anjani Goldline, India) and the powder was stored in airtight containers at ambient temperature until further use.
2.3. Estimation of total phenolics
Total phenolic content in tea fruits was determined by the Folin–Ciocalteu’s method following Rana, Bhangalia, & Singh, Citation2012. Tea fruit extract (100 µL) was taken into 25-mL volumetric flasks in triplicate. 0.5-mL 1 N Folin–Ciocalteu’s phenol reagent and 1.0-mL saturated solution of Na2CO3 were added to the flasks. The final volume was made up to 25 mL with distilled water. The flasks were vortexed and then incubated for 30 min. The final absorbance was recorded at 730 nm against a blank, using Hitachi 150-20 spectrophotometer. Aliquots (20, 40, 60, 80 and 100 μL) of 0.1% aqueous gallic acid were used for the preparation of standard curve.
2.4. Estimation of total flavonoids
For the determination of total flavonoids content in tea fruits, standard colorimetric method (Kosalec, Bakmaz, & Pepeljnjak, Citation2004) was used. Aliquots (500 μL) of tea fruit extract (triplicate) were taken in 5-mL volumetric flasks. Aluminium chloride (10% w/v, 0.1 mL) and potassium acetate (1 M and 0.1 mL) were added to the flasks. The volume was made up to 5 mL with distilled water and vortexed. After the incubation period of 25–30 min, the absorbance was measured at 415 nm against blank. Aliquots (20, 40, 60, 80 and 100 μL) of quercetin (mg/mL) were used for the preparation of standard curve.
2.5. DPPH free radical scavenging assay
The standard DPPH free radical scavenging assay (Brand-Williams, Cuvelier, & Berset, Citation1995) was used for evaluating radical scavenging potential of tea fruits. For this, five aliquots of varying concentrations of tea fruit extracts were mixed with DPPH solution (100 μM, 2.9 mL) in 80% aqueous ethanol. The reaction mixture was vortexed and then incubated at room temperature in dark. The colour change of the solution after 30 min was recorded at 517 nm against a blank. The minimum inhibitory concentration (IC50) of DPPH by tea fruit was calculated.
2.6. Differential solvent extraction for qualitative analysis
The powdered tea fruits were extracted using two solvents, i.e. 70% aqueous acetone and hot water (80–90° C). For extraction with both solvents, triplicate sets of accurately weighed 500-mg powdered sample were taken in centrifuge tubes and 10 mL of solvent was added followed by 30 s vortex mixing. The samples were then centrifuged at 6,000 rpm (3743×g) for 10 min. The supernatant was collected and the process was repeated two more times, with residue using 10 mL of solvent every time. The supernatants were pooled and the solvent was removed on a rotavapour (Buchi R-210, Buchi-India, Mumbai). The volume was constituted up to 50 mL with distilled water. All the extracts were filtered with 0.45-μm nylon filter (Millipore) prior to injection into HPLC.
2.7. RP-HPLC analysis
The HPLC analyses were performed on a Waters analytical HPLC system equipped with 2998 PDA detector, 2707 auto sampler, temperature control module and a column oven following a published HPLC method (Rana, Singh, & Gulati, Citation2015). The separation of the compounds was achieved with Synergi Max RP 80A column (4.6 × 250 mm, 4 µ). Acetonitrile (A) and water with 0.01% TFA (B) were used as solvents at a constant flow rate of 1 mL/min. For the separation of compounds, a gradient elution programme was used, i.e. starting from 10% A at 0 min to 15% at 3 min, which becomes 25% at 5 min, increased to 30% at 9 min. It is brought to 25% at 12 min and then 20% at 15 min, to 15% at 18 min and finally reaches 10% A at 20 min. The injection volume was 10 μL and column temperature was maintained at 32°C. Data were monitored continuously at 200–600 nm using photodiode array detector and the final data were recorded at 205 nm.
2.8. Catechins extraction and purification
For extraction of catechins, 1 kg of green tea fruits was collected and washed with distilled water. The fruits were ground in a mixer grinder. The fruit slurry obtained by grinding was extracted at least thrice with adequate amount of hot water (70–80°C) to obtain a final pooled extract of 10 L. This extract was filtered and concentrated in a rotavapour to 2.5 L or ¼ of its original volume. A portion of the concentrated extract (500 mL) was lyophilized to obtain powdered fruit extract.
For isolation and purification of catechins from tea fruits, about 25 g of powdered fruit extract were dissolved in lukewarm distilled water (37–40°C) to make a slurry. The catechins were purified with the help of column chromatography. The RP-C18 silica column saturated with water was used as a stationary phase to separate catechins. The catechins were eluted from the column by altering the polarity in reverse order using 10–60% of aqueous ethanol. The eluted fractions were collected (70–100 mL each) and analysed continuously using HPLC analysis to obtain individual catechins.
2.9. Purification of caffeine
For isolation and purification of caffeine from tea fruits, solvent partitioning technique was used. The concentrate (100 mL) obtained above was partitioned with hexane (3 × 100 mL) and chloroform (3 × 100 mL). The chloroform fraction was collected and allowed to dry to obtain caffeine (110 mg) as a yellowish powder.
2.10. ESI- MS analysis
All the fractions containing pure compound as confirmed by HPLC analysis were taken for mass determination by ESI-Q-TOF- MS analysis. This was performed on a Waters Aquity System in positive ion mode. All the samples were dissolved in 50% aqueous acetonitrile prior to injection in LC-MS system. The positive ion mode mass determination was carried out for all samples.
2.11. NMR studies
All isolated compounds obtained from tea fruits were analysed by 13C NMR. The 13C NMR analyses were performed on Bruker Avance 300 NMR unit. The samples were dissolved in deuterated methanol.
3. Result and discussion
Tea is an excellent source of phenolic antioxidant compounds which mainly comprise a group of monomeric flavonoids called catechins. The occurrence of catechins in adequate amount in green tea as an essential natural antioxidant has made tea a potential source of functional bioactive molecules (Yilmaz, Citation2006). As tea plants produce plenty of fruits every year and despite the abundance of fruits on the tea bush, the chemical composition of green tea fruits has not been properly investigated. Therefore, in this study, we have scrutinized the phytochemical (phenolic antioxidants) analysis of tea fruits for their utilization as novel source of tea bioactives. Earlier, Bhardwaj et al. (Citation2007) have isolated five brassinosteroids from immature seeds of tea. For the quantitative analysis of total phenolics and flavonoids, we have used standard assay methods. The total phenolics in tea fruit extract were recorded as 14.2% gallic acid equivalent on dry weight basis (Figure (a)). The phenolic antioxidants of tea fruits were also analysed earlier by Forrest and Bendall (Citation1969). The adequate quantity of phenolics in tea fruits have clearly underscored their importance as an economical and reliable source to obtain these valuable phytoconstituents. The flavonoids assay results have revealed total flavonoid content of 9% (dry weight basis) as quercetin equivalent (Figure (a)). The flavonoids are considered as functional ingredients of various foods, beverages, herbal remedies, extracts and dietary supplements, especially in tea (Punyasiri et al., Citation2004). The major class of flavonoids present in tea are flavan-3-ols. These flavonoids are also known for their free radical scavenging potential. Thus, it has become evident that antioxidants (phenolics and flavonoids) help in prevention of various metabolic diseases by acting as anti-inflammatory, anticarcinogenic and antiatherosclerotic. Therefore, we have evaluated the free radical scavenging potential of tea fruits by DPPH free radical scavenging assay. The IC50 value of dried tea fruits extract was calculated and compared with trolox as the standard antioxidant. The results have showed that 60.97 µg of fruit extracts inhibit 50% of 100 μM DPPH free radical solution in comparison to IC50 value of trolox (13.8 µg). These results suggest that sufficient amount of antioxidant compounds are present in tea fruits.
Figure 1. (a) A column chart showing percentage of total phenolics, flavanoids, catechins, caffeine and theanine in tea fruits on dry weight basis. (b) RP-HPLC chromatograms showing quantitative analysis of major phytoconstituents.
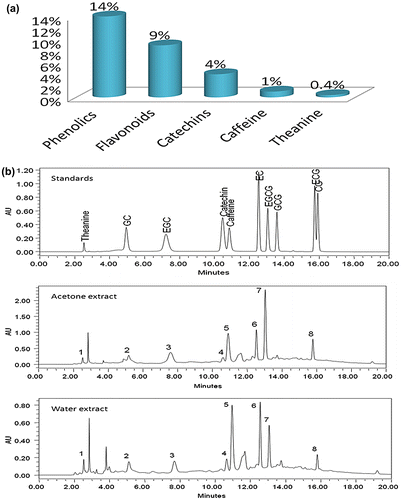
The promising results of phenolics, flavonoids and free radical scavenging antioxidants in tea fruits have clearly highlighted the ample content of extractable bioactive constituents in tea fruits. The results of qualitative analysis of phytoconstituents have led us to perform HPLC analysis of the major tea constituents (theanine, caffeine and catechins). The optimization of solvent extraction was carried out using hot water (80–90° C) and 70% aqueous acetone. The triplicate sets of extracts of both solvents were evaluated using our HPLC method (Rana et al., Citation2015) for determining theanine, caffeine and catechins contents (Figure (b)). From the HPLC results (Table S1), we have concluded that tea fruit contains up to 6% of these major constituents with catechins recording up to 4%, caffeine up to 1% and theanine 0.4% on dry weight basis (Figure (a)). Earlier, Suzuki and Waller (Citation1987) have reported the presence of caffeine in tea seeds but did not observe any catechins. The HPLC analysis of tea fruit extracts have confirmed that adequate amount of bioactive constituents are present in it. Subsequently, the next footstep was the purification and characterization of major bioactive molecules. For the extraction of major phytoconstituents from tea fruits, hot water (80–90°C) was taken as the extraction solvent. Major constituents isolated from the aqueous extracts of tea fruits include all the four major catechins: (1) EGC;, (2) EC; (3) EGCG; and (4) ECG; along with (5) caffeine (Figure ). The purification of catechins was performed using reverse phase column chromatography (RP-CC), while for the purification of caffeine, partition chromatography technique was employed. The eluted fractions obtained during column chromatography were monitored continuously on HPLC. The fractions containing individual compounds were dried on a rotavapour to remove the solvent. All purified compounds were further characterized with the help ESI-Q-TOF mass and 13C NMR studies.
The ESI-Q-TOF mass analyses of purified catechins were performed on Waters Acquity system. The expected quasi-molecular ions of m/z 307.5, [M + H+] for EGC; 291.4, [M + H+] for EC, 459.7, [M + H+] for EGCG; 443.1, [M + H+] for ECG and 195.1, [M + H+] for caffeine were observed in positive ion mode. The results of LC-MS for catechins and caffeine were compared with that of the literature data (Wu, Xu, Héritier, & Andlauer, Citation2012). The 13C NMR data of purified catechins and caffeine were recorded on 300 MHz NMR. All the samples were dissolved in CD3OD. The NMR data are given in Table S2, and also compared with the reported data of Kumar and Rajapaksha (Citation2005). From these results, it has become evident that C. sinensis (tea) fruits are a rich source of polyphenolic antioxidant compounds. The availability of caffeine, theanine and catechins in adequate quantity in tea fruits has emphasized their significance as a valuable part of the tea plant, which could be harvested for obtaining bioactive natural constituents.
OACH_Supplementary_Material.docx
Download MS Word (17.8 KB)Additional information
Funding
Notes on contributors
Ajay Rana
Ajay Rana has completed his PhD from the Academy of Scientific and Innovative Research at CSIR-IHBT Palampur in 2014. Presently, he is working as a scientist fellow at CSIR-IHBT. He is well versed in the extraction, purification and characterization of phytochemicals (phenolics, flavonoids and iridoids) using various chromatographic techniques (column chromatography, RP-SPE, TLC, HPLC and UPLC), LC-MS/MS and NMR techniques. His current interest is in development of diversified value-added products and nutraceuticals using green, economical and sustainable process technologies. The process/technologies developed by Ajay Rana have been transferred to industry for scaled-up production of tea phytochemicals and development of value-added products. He has also published various research articles in high repute international journals and has filled patents of industrial relevance.
References
- Bhardwaj, R., Kaur, S., Nagar, P. K., & Arora, H. K. (2007). Isolation and characterization of brassinosteroids from immature seeds of Camellia sinensis (O) Kuntze. Plant Growth Regulation, 53, 1–5.
- Bhattacharya, A., Nagar, P. K., & Ahuja, P. S. (2002). Seed development in Camellia sinensis (L.) O. Kuntze. Seed Science Research, 12, 29–46.
- Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology, 28, 25–30.
- Forrest, G. I., & Bendall, D. S. (1969). The distribution of polyphenols in the tea plant (Camellia sinensis L.). Biochemistry Journal, 118, 741–755.
- Kosalec, I., Bakmaz, M., & Pepeljnjak, S. (2004). Quantitative analysis of the flavonoids in raw propolis from northern Croatia. Acta Pharmaceutica, 54, 65–72.
- Kumar, N. S., & Rajapaksha, M. (2005). Separation of catechin constituents from five tea cultivars using high-speed counter-current chromatography. Journal of Chromatography A, 1083, 223–228.
- Lin, Y. S., Wu, S. S., & Lin, J. K. (2003). Determination of tea polyphenols and caffeine in tea flowers (Camellia sinensis) and their hydroxyl radical scavenging and nitric oxide suppressing effects. Journal of Agricultural and Food Chemistry, 51, 975–980.
- Punyasiri, P. A. N., Abeysinghe, I. S. B., Kumar, V., Treutter, D., Duy, D., Gosch, C., … Fischer, T. C. (2004). Flavonoid biosynthesis in the tea plant Camellia sinensis: Properties of enzymes of the prominent epicatechin and catechin pathways. Archives of Biochemistry and Biophysics, 431, 22–30.
- Rana, A., Bhangalia, S., & Singh, H. P. (2012). A new phenylethanoid glucoside from Jacaranda mimosifolia. Natural Product Research, 27, 1167–1173.
- Rana, A., Singh, H. P., & Gulati, A. (2015). Concurrent analysis of theanine, caffeine, and catechins using hydrophobic selective C12 stationary phase. Journal of Liquid Chromatography & Related Technologies, 38, 709–715.
- Suzuki, T., & Waller, G. R. (1987). Allelopathy due to purine alkaloids in tea seeds during germination. Plant and Soil, 98, 131–136.
- Vuong, Q. V., Golding, J. B., Nguyen, M. H., & Roach, P. D. (2012). Production of caffeinated and decaffeinated green tea catechin powders from underutilised old tea leaves. Journal of Food Engineering, 110, 1–8.
- Way, T. D., Lin, H. Y., Hua, K. T., Lee, J. C., Li, W. H., Lee, M. R., … Lin, J. K. (2009). Beneficial effects of different tea flowers against human breast cancer MCF-7 cells. Food Chemistry, 114, 1231–1236.
- Wu, C., Xu, H., Héritier, J., & Andlauer, W. (2012). Determination of catechins and flavonol glycosides in Chinese tea varieties. Food Chemistry, 132, 144–149.
- Xu, P., Bao, J., Gao, J., Zhau, T., & Wang, Y. (2012). Optimization of phenolic antioxidants from tea (Camellia sinensis) fruit peel biomass using response surface methodology. Bioresources, 7, 2431–2443.
- Yilmaz, Y. (2006). Novel uses of catechins in foods. Trends in Food Science & Technology, 17, 64–71.