Abstract
Environmentally benign and biodegradable lactic acid is identified as alternative solvent and catalyst for the tandem one-pot synthesis of Hantzsch 2-aminothiazole derivatives (4) from readily available aralkyl ketones (1) through in situ regioselective α-bromination using N-bromosuccinimide (2) followed by heterocyclization using thiourea (3) at 90–100°C. The major advantages of the present method include short reaction times (10–15 min), practical, simple to perform, easy work-up, good yield of products (up to 96%), productive for large-scale applications, free from apply of α-bromoketones (lachrymator) as substrates, avoids column purification. Hence, the present method meets with the concepts of both Wender’s “ideal synthesis” and sustainable chemical process.
Public Interest Statement
The reported method provides wide scope and quick access to Hantzsch 2-aminothiazole derivatives in good to excellent isolated yields within a short period of time from readily available aralkyl ketones through in situ regioselective α-bromination using NBS and subsequent heterocyclization using thiourea in presence of environmentally benign and biodegradable lactic acid at 90–100°C in a single step operation. Major advantages of the present method include scale-up process, non-explosive, easy to perform, simple work-up, easy isolation of products, improved worker safety and use of environmentally benign, non-volatile and biodegradable lactic acid as an alternative solvent and catalyst. Further, the present protocol is free from (i) column purification, (ii) the use of hazardous solvents and (iii) the isolation of lachrymatory α-bromoketones. Hence, the present method meets the concept of Wender’s “ideal synthesis”. Finally, it is concluded that the present method is an attractive addition to the sustainable chemical processes.
1. Introduction
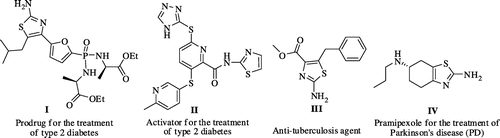
The growing interest in developing simple, more convenient methods for the synthesis of medicinally important thiazole moiety has great demand both in academic, chemical, and pharmaceutical domains (Dondoni, Citation1985). Consequently, extensive use of conventional solvents and hazardous catalysts became mandatory for their preparation which is leading to environmental pollution. Besides, these are mostly prepared from α-bromoketones that are difficult to store and not easily accessible. Further, these α-bromoketones are lachrymatory and cause other severe health hazards to the operating chemists. As a result, the growing interest in developing simple, safe, more convenient, and sustainable scale-up methods for the synthesis of these thiazole synthetic precursors from readily available ketones has great demand in academic, chemical, and pharmaceutical domains. The Hantzsch thiazole synthesis (Hantzsch & Weber, Citation1887) is a powerful synthetic tool for the construction of the five-membered 2-aminothiazole derivatives. Even though it was introduced more than one century ago, still the development of new Hantzsch-based methods is warranted. As a result, numerous methods have been reported with the improvement of many aspects of the original synthetic protocol for their syntheses from either ketones or α-brominated ketones or other key starting materials (Jacques & Vernin, Citation2008). 2-aminothiazole derivatives possess broad range of pharmacological activities (Biagetti et al., Citation2010; González Cabrera et al., Citation2011; Jaen et al., Citation1990; Parekh, Juddhawala, & Rawal, Citation2013; Patt et al., Citation1992; Shah, Shah, Patel, & Patel, Citation2012; Shao et al., Citation2013; Singh et al., Citation2014; Spector, Liang, Giordano, Sivaraja, & Peterson, Citation1998; Yang et al., Citation2010) and plant growth activities (An Tran, Anil Kumar, Jung-Ae, Lee, & Park, Citation2015). Recently, 2-aminothiazole derivatives are identified as a prodrug for the treatment of type 2 diabetes (I and II) (Erion et al., Citation2005), anti-tuberculosis agent (III) (Makam & Kannan, Citation2014), and anti-Parkinsonian agent-pramipexole (IV) (Chau, Cooper, & Schapira, Citation2013) as shown in Figure . Consequently, thiazole moiety is considered as “Important Structural motif” and the desirable properties of 2-amino thiazole derivatives render them attractive targets for new drug discovery. There are many reports on the synthesis of thiazole derivatives from ketones or their derivatives (Kumar, Minh An, Lee, Park, & Lee, Citation2015; Heravi, Poormohammad, Beheshtiha, & Baghernejad, Citation2011; Huang, Zhu, & Zhang, Citation2002; Janardhan, Krishnaiah, Rajitha, & Crooks, Citation2014; Kidwai, Chauhan, & Bhatnagar, Citation2011; Meshram, Thakur, Madhu Babu, & Bangade, Citation2012; Nitta et al., Citation2012; Potewar, Ingale, & Srinivasan, Citation2008; Zhuravel, Kovalenko, Vlasov, & Chernykh, Citation2005; Zhu et al., Citation2012) and thiourea or substituted thiourea. However, most of the reported methods suffer from one or more disadvantages including use of hazardous and toxic reagents, long reaction times, low selectivity of the products, use of toxic and volatile solvents as well as catalysts, environmentally hazardous processes, low yields and difficulties in work-up and isolation of products and oppressive operational procedures.
On the other hand, now-a-days, recycle and reduce of solvent usage or change to other solvents with better environmental profiles (Jessop, Citation2011; Kerton, Citation2009) become prominent. Accordingly, we found that environmentally benign lactic acid (Yang, Tan, & Gu, Citation2012) can act as catalyst cum solvent for in situ regioselective α-bromination and subsequent heterocyclization in the synthesis of pharmacologically active 2-aminothiazole derivatives. The present method is scale-up process and also meets the concept of “ideal synthesis” as described by Wender, Handy, and Wright (Citation1997).
Herein, we report a simple, more convenient, practical method in which lactic acid acts as green catalyst and solvent for the one-pot synthesis of Hantzsch 2-aminothiazole derivatives (4) within 10–15 min from readily available aralkyl ketones (1) through in situ regioselective α-bromination using N-bromosuccinimide (2) followed by heterocyclization using thiourea (3) at 90–100°C (Scheme ).
2. Results and discussion
It is planned to develop a simple, more convinient method for the preparation of Hantzsch 2-amino thiazole derivatives (4). For this purpose, initially we have choosen to synthesize the 4-(4-bromophenyl)thiazol-2-amine (4a) using 4′-bromoacetophenone (1a). It is used as model substrate for the optimization of reaction conditions as discusssed below.
2.1. Selection of suitable catalyst and solvent
For the success of the present hypothesis, an acidic organic liquid which can function as a catalyst and solvent is needed to identify for the accomplishment of both in situ regioselective α-bromination and heterocyclization in a single step operation, as the solubility of key reactants and their α-bromination and subsequent heterocyclization become easy in the presence of acidic organic liquid compared to solid catalysts alone. At the same time, it is evident that both the α-bromination and hetrocyclization processes proceed in the presence of acidic catalysts.
Toward this direction, a reaction is carried out using 4′-bromoacetophenone (1a) and N-bromosuccinimide (2) followed by addition of thiourea (3) in the presence of acetic acid (entry 1, Table ) and the isolated yield of desired product (4a) is 30% at RT (entry 1, Table ) and 58% at 90–100°C. To increase the yield of 4a, the same reaction is carried out in lactic acid and the obtained yield of product (4a) is increased to 45% at RT (entry 3). The same reaction at 90–100°C provided 96% yield (entry 4, Table ). It may be due to more acidic nature of lactic acid compared to acetic acid. The evaluation of suitable acidic organic liquid which acts both as catalyst and solvent disclosed that lactic acid is the best option to obtain maximum yield (96%) of the desired product (4a) (entry 4, Table ) compared to acetic acid (entry 2, Table ).
Table 1. Screening of suitable acidic organic liquid (act as catalyst and solvent) for the synthesis of 4-(4-bromophenyl)thiazol-2-amine (4a)Table Footnotea
Later, other reaction conditions such as effect of brominating agent and mode of addition of brominating agent on the course of in situ regioselective α-bromination and also the effect of temperature both on in situ regioselective α-bromination and subsequent heterocyclization is investigated and the results obtained are discussed below.
In the present method, the in situ α-bromination reaction plays a vital role as the yield of target 2-aminothiazoles (4) is directly proportional to the yield of in situ generated α-brominated ketone (5). For this reason, various brominating agents and their mode of addition are studied. In particular, greater attention has been paid to improve the efficiency of α-bromination of ketones in terms of regioselectivity to generate α-monobrominated ketones exclusively compared to α-dibrominated ketones and also to achieve maximum conversion. The results obtained are presented in Tables and .
Table 2. Effect of brominating agent on the course of in situ α-bromination reactionTable Footnotea
Table 3. Effect of mode of addition of N-bromosuccinimide (NBS) on course of in situ α-bromination reactionTable Footnotea
2.2. Screening of suitable brominating agent
The effect of brominating agent is studied on the course of in situ regioselective α-bromination and the results obtained are presented in Table . Accordingly, a reaction is carried out using molecular bromine and obtained lower yield (55%) of product 5a (entry 1, Table ). The toxicity, difficulties in handling and low selectivity of molecular bromine encouraged us to use other user-friendly and readily available brominating agents such as HBr-H2O2, dioxane dibromide, N-bromosuccinimide (NBS) and CuBr2 which provided 60, 76, 97, and 81% yields of product 5a, respectively (entries 2–5, Table ).
The study revealed that NBS is the best brominating agent as it gave maximum yield (97%) of product 5a when added in 4 portions (entry 3, Table ).
2.3. Effect of temperature
In general, the solubility of reactants and products, rate of reaction, efficiency and selectivity of catalyst are highly dependent on operating temperature of the reaction. Hence, we desired to study the effect of temperature on the course of both in situ α-bromination (A) and subsequent heterocyclization (B) process. Accordingly, reactions are conducted at various ranges of temperature and the results obtained are presented in Table .
Table 4. Effect of temperature on in situ regioselective α-bromination and subsequent heterocyclizationTable Footnotea
For instance, when the reaction is carried out at room temperature, the rate of α-bromination (A) process is slow (3 h) and provided lower yield (53%) of product 5a (entry 1, Table ) and subsequent heterocyclization (B) using thiourea (3) provided only 40% yield of final product 4a (entry 1). This may be due to partial solubility of the reactants. To improve the solubility of reactants and yield of α-bromoketone (5a) further, the reaction is conducted at different temperatures, for example at 40–50, 50–60, 70–80, 80–90, and 90–100°C provided 65, 69, 75, 84, and 97% isolated yield of α-bromoketone (5a) (entries 2–6).
While we consider heterocyclization (B) step alone, this stage is also slightly influenced by the operating temperature. For example, at room temperature, the yield of product 4a (40%) is decreased (entry 1, Table ) significantly due to the partial solubility of thiourea (3) in lactic acid. When the same reaction is conducted at 40–50°C, improved yield (58%) of product 4a is obtained compared to RT (entry 2). But, when the reaction is operated at 50–60, 70–80, 80–90, and 90–100°C provided yields of 62, 71, 82, and 96% of product 4a, respectively (entries 3–6, Table ).
These results indicate that the isolated yield of product 4a is directly proportional to the percentage of formation of in situ regioselective α-bromoketone 5a (entries 2–6, Table ).
When we consider the overall reaction i.e. both in situ regioselective α-bromination (A) and heterocyclization (B), the optimum operating temperature is 90–100°C for maximum conversion and isolated yield (96%) of target product 4a (entry 7, Table ). Based on our present study, we found that in case of laboratory scale, the optimum temperature is 90–100°C for the synthesis of 2-aminothiazoles (4).
2.4. Scope of the method
The substrate scope of the present method is studied with the help of above optimized reaction conditions using different types of aralkyl ketones (1a-o) as key starting substrates and the results obtained are presented in Table . The study disclosed that the substrate with fluoro, chloro, and bromo groups at para-position provided excellent yields (90–96%) of the products 4a, 4c, 4d (entries 1, 3, and 4, Table ), but meta monohalogenated substrate, for example 1-(3-bromophenyl)ethanone (1b) provided good yield (85%)of the product 4b (entry 2, Table ). Whereas the dihalogenated substrates, 1-(2,4-dichlorophenyl)ethanone (1e) and 1-(3,4-dichlorophenyl) ethanone (1f) provided moderate yields of products 4e and 4f in 74% and 78%, respectively (entries 5 and 6, Table ). It is found that the isolated yield of the respective products depends on the position of halogen(s) on aromatic ring of aralkyl ketone (entries 1–6, Table ).
Table 5. Tandem one-pot synthesis of 2-amino thiazole derivatives (4a-o) from readily available aralkyl ketones (1a-o)Table Footnotea
Interestingly, substrates, 1-(4-nitrophenyl)ethanone (1g) and 1-(3-nitrophenyl)ethanone (1h) with high deactivating groups (-NO2) provided lower yields of product 4g (53%) and 4h (46%) (entries 7 and 8, Table ) compared to all other substrates used in the present study. This may be due to the presence of strong electron withdrawing groups on the aromatic ring cause to reduce the percentage of formation of in situ α-brominated ketones which play vital role on yield of final product. Simple aralkyl ketones, for instance acetophenone (1i) and propiophenone (1l) provided good yields of the products 4i (92%) and 4l (84%) (entries 9 and 12, Table ) and the substrates with moderate activating groups, for example 1-(p-tolyl)ethanone (1j), 1-(4-ethylphenyl)ethanone (1k) provided acceptable yields (79–84%) of respective products (4j and 4k) (entries 10–11, Table ). But, the substrate with high activating group, for example 1-(4-methoxyphenyl)ethanone (1m) provided good yield (88%) of the product 4m (entry 13, Table ). Interestingly, in case of acenaphthanones (1n and 1o), the 1-acetyl naphthalene (1n) gave lower yield (81%) of product 4n when compared to 2-acetyl naphthalene (1o) which afforded higher yield (90%) of product 4o (entries 14 and 15, Table ). It may be due to steric effects.
3. Conclusions
In summary, the developed method provides wide scope and quick access to Hantzsch 2-aminothiazole derivatives (4) in good yields within 10–15 min from readily available aralkyl ketones (1) through in situ regioselective α-bromination using NBS (2) and subsequent heterocyclization using thiourea (3) in the presence of lactic acid at 90–100°C in a single step operation when compared to an alternative two-step procedures or other reported one-step methods. Major advantages of the present method include scale-up process, non-explosive, easy to perform, simple work-up, easy isolation of products, improved worker safety and use of environmentally benign, non-volatile and biodegradable lactic acid as alternative solvent and catalyst. Further, the current protocol is free from (i) column purification, (ii) the use of hazardous solvents, and (iii) the isolation of lachrymatory α-bromoketones. Finally, it is concluded that the present method is an attractive addition to the sustainable chemical processes.
4. Materials and method
4.1. Materials
Melting points of various obtained products are determined and uncorrected. 1H NMR spectra are recorded on a Varian 400 MHz and 13C NMR spectra on a Jeol/AL-100 MHz. Chemical shifts were expressed in parts per million (ppm), coupling constants are expressed in Hertz (Hz). Splitting patterns describe apparent multiplicities and were designated as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet). High-resolution mass spectra (HRMS) and compound purity data are acquired on a Exactive Orbitrap Mass Spectrometer (ThermoScientific, Waltham, MA, USA) equipped with electro spray ionization (ESI) source. Thin-layer chromatography is performed on 0.25 mm Merck silica gel plates and visualized with UV light. Column chromatography is performed on silica gel. Chemicals and solvents are purchased from Sigma Aldrich and Merck. Isolated compounds are identified on the basis of spectroscopic data (1H & 13C NMR, Mass and HRMS).
4.2. Method for the synthesis of 2-aminothiazole derivatives (4a-o)
In a 100-mL 3 necked round bottom flask, aralkyl ketone (1) (25.0 mmole) and lactic acid (20 mL) are taken. The substrate (1) is partially soluble in lactic acid at room temperature. The temperature of the reaction mass is raised to 90–100°C. At this temperature, the reaction mass became homogeneous and N-bromosuccinimide (2) (30.0 mmol) is added in 4 portions (7.5 × 4 = 30 mmol). On addition of each portion of NBS (3.0 mmol), the color of the reaction mass is changed from colorless to orange red. This indicates that the bromonium ion is released from NBS (2). Later, within few minutes, the disappearance of the orange red color is observed. The same situation is observed when another 3 portions of NBS (2) is added. After the completion of α-bromination as per TLC, thiourea (3) (30.0 mmol) is added and stirred at the same temperature for 1 min. Then, the reaction mass is slowly cooled down to RT. As the temperature of the reaction mass is below 80°C, the final product (4) is slowly thrown out from the lactic acid. At room temperature, 20 mL of water is added for the precipitation of product (4). Then, it is filtered-off and washed with 10 mL of water. The crude solid product (4) is collected and to this 100 mL of cold water is added and quenched with NaHCO3.Footnote1 Again, it is filtered-off and washed twice with water (2 × 25 mL) for the removal of by-product and inorganic salts.Footnote2 The pure solid product (4) is collected and dried in vacuum oven at ambient temperature and the isolated yields of respective products are presented in Table . All the prepared products (4a-o) are characterized by physical and spectroscopic data (1H &13C NMR, Mass, and HRMS).
4.3. Characterization data of the corresponding compounds are as follows
4.3.1. 4-(4-bromophenyl) thiazol-2-amine (4a)
Off-white solid, yield: 96%; m.p. 180–181°C; 1H NMR (400 MHz, DMSO-d6, δ/ppm): 7.73 (2H, dd, J = 6.8 Hz, J = 2.0 Hz, arom H), 7.69 (2H, br s, -NH2), 7.61 (2H, dd, J = 6.8 Hz, J = 2.0 Hz, arom H), 7.16 (1H, s, thiazole H); 13C NMR (100 MHz, DMSO-d6, δ/ppm): 169.12, 144.39, 131.59 (3C), 127.61 (2C), 121.09, 102.91; HRMS (ESI): calcd for C9H8BrN2S [M + H]+254.9586, found 254.9596;[M + H + 2]+256.9572.
4.3.2. 4-(3-bromophenyl) thiazol-2-amine (4b)
Yellow solid, yield: 85%; m.p. 162–163°C; 1H NMR (400 MHz, DMSO-d6, δ/ppm): 7.98 (1H, s, arom H), 7.79 (1H, d, J = 7.6 Hz, arom H), 7.44 (1H, d, J = 6.8 Hz, arom H), 7.32 (1H, t, J = 8.0 Hz, arom H), 7.16 (1H, s, thiazole H), 7.15 (2H, br s, -NH2); 13C NMR (100 MHz, DMSO-d6, δ/ppm): 168.35, 148.06, 137.11, 130.62, 129.73, 128.15, 124.32, 121.99, 103.10; HRMS (ESI): calcd for C9H8BrN2S [M + H]+ 254.9507, found 254.9596; [M + H + 2]+ 256.9550.
4.3.3. 4-(4-chlorophenyl) thiazol-2-amine (4c)
Off-white solid, yield: 95%; m.p. 166–167°C; 1H NMR (400 MHz, DMSO-d6, δ/ppm): 7.80 (2H, d, J = 8.4 Hz, arom H), 7.41 (2H, d, J = 8.8 Hz, arom H), 7.09 (2H, s, -NH2), 7.07(1H, s, thiazole H); 13C NMR (100 MHz, DMSO-d6, δ/ppm): 168.39, 148.56, 133.71, 131.57, 128.45 (2C), 127.20(2C), 102.28; HRMS (ESI): calcd for C9H8N2ClS [M + H]+ 211.0091, found 211.0082.
4.3.4. 4-(4-fluorophenyl) thiazol-2-amine (4d)
Off-white solid, yield: 94%; m.p. 101–102°C; 1H NMR (400 MHz, DMSO-d6, δ/ppm): 8.8 (2H, br s, -NH2), 7.82 (2H, m, arom H), 7.34 (2H, t, J = 6.8 Hz, arom H), 7.21 (1H, s, thiazole H); 13C NMR (100 MHz, DMSO-d6, δ/ppm): 170.33, 162.51(1C, d, 1JC-F=246.2 Hz), 137.66, 128.17, (2C, d, 3JC-F = 8.3 Hz), 125.20 (1C, d, 4JC-F = 3.3 Hz), 116.08 (2C, d,2JC-F = 21.4 Hz), 102.82; HRMS (ESI): calcd for C9H8N2FS [M + H]+ 195. 0387, found 195. 0377.
4.3.5. 4-(2, 4-dichlorophenyl) thiazol-2-amine (4e)
Off-white solid, yield: 74%; m.p. 189–191°C; 1H NMR (400 MHz, DMSO-d6, δ/ppm): 8.6 (2H, Br s, -NH2), 7.79 (1H, d, J = 2.4 Hz, arom H), 7.71 (1H, d, J = 8.4 Hz, arom H), 7.57 (1H, dd, J = 8.4 Hz, J = 2.0 Hz, arom H), 7.14 (1H, s, thiazole H); 13C NMR (100 MHz, DMSO-d6, δ/ppm): 169.21, 135.40, 134.92, 132.87 (2C), 129.65, 127.81, 127.58, 108.13; MS (ESI) m/z: [M + H]+ 245.00, [M + H + 2]+ 247.00.
4.3.6. 4-(3, 4-dichlorophenyl) thiazol-2-amine (4f)
Off-white solid, yield: 78%; m.p. 193–194°C; 1H NMR (400 MHz, DMSO-d6, δ/ppm): 8.016 (1H, d, J = 2.0 Hz, arom H), 7.78 (1H, dd, J = 8.8 Hz, J = 1.6 Hz, arom H), 7.61 (1H, d, J = 8.4 Hz, arom H), 7.23 (1H, s, arom H), 7.16 (2H, br s, -NH2); 13C NMR (100 MHz, DMSO-d6, δ/ppm): 168.58, 146.95, 135.18, 131.38, 130.59, 129.44, 127.19, 125.44, 103.76; HRMS (ESI): calcd for C9H7N2Cl2S [M + H]+ 244.9702, found 244. 9691;[M + H + 2]+246.9658.
4.3.7. 4-(4-nitrophenyl) thiazol-2-amine (4g)
Orange solid, yield: 53%; m.p. 285–286°C; 1H NMR (400 MHz, DMSO-d6, δ/ppm): 8.23 (2H, d, J = 8.8 Hz, arom H), 8.04 (2H, d, J = 8.8 Hz, arom H), 7.42 (1H, s, thiazole H), 7.25 (2H, br s, -NH2); 13C NMR (100 MHz, DMSO-d6, δ/ppm): 168.79, 146.85, 146.02, 140.28, 126.32 (2C), 124.00 (2C), 106.67; HRMS (ESI): calcd for C9H8O2N3S [M + H] + 222.0332, found 222.0341.
4.3.8. 4-(3-nitrophenyl) thiazol-2-amine (4h)
Yellow solid, yield: 46%; m.p. 282–283°C; 1H NMR (400 MHz, DMSO-d6, δ/ppm): 8.60 (1H, s, arom H), 8.23 (1H, d, J = 7.6 Hz, arom H), 8.14 (1H, d, J = 8.0 Hz, arom H), 7.69 (1H, t, J = 8.0 Hz, arom H), 7.38 (1H, s, thiazole H);13C NMR (100 MHz, DMSO-d6, δ/ppm): 169.03, 148.19, 144.93, 134.93, 131.69, 130.19, 122.19, 120.05, 104.65; MS (ESI) m/z:[M + H]+ 222.00.
4.3.9. 4-phenylthiazol-2-amine (4i)
Off-white solid, yield: 92%, m.p. 149–151°C; 1H NMR (400 MHz, DMSO-d6, δ/ppm): 8.6 (2H, br s, -NH2), 7.73 (2H, d, J = 7.6 Hz, arom H), 7.48 (2H, t, J = 8.0 Hz, arom H), 7.41 (1H, d, J = 7.6 Hz, arom H), 7.21 (1H, s, thiazole H); 13C NMR (100 MHz, DMSO-d6, δ/ppm): 170.30, 138.53, 129.54, 129.10 (2C), 128.46, 125.80 (2C), 103.06; HRMS (ESI): calcd for C9H9N2S [M + H]+ 177.0481, found 177.0486.
4.3.10. 2-Amino-4-(4-methylphenyl) thiazole (4j)
Off-white solid, yield: 84%; m.p. 136–137°C; 1H NMR (400 MHz, DMSO-d6, δ/ppm): 8.7 (2H, br s, -NH2), 7.62 (2H, d, J = 8.4 Hz, arom H), 7.29 (2H, d, J = 8.0 Hz, arom H), 7.15 (1H, s, thiazole H), 2.34 (3H, s, -CH3); 13C NMR (100 MHz, DMSO-d6, δ/ppm): 170.27, 139.28, 138.46, 129.61 (2C), 125.66 (3C), 102.05, 20.88; HRMS (ESI): calcd for C10H11N2S [M + H]+ 191.0637, found 191.0644.
4.3.11. 4-(4-ethylphenyl) thiazol-2-amine (4k)
Off-white solid, yield: 77%; m.p. 140–141°C; 1H NMR (400 MHz, DMSO-d6, δ/ppm): 8.75 (2H, br s, -NH2), 7.67 (2H, d, J = 8.4 Hz, arom H), 7.32 (2H, d, J = 8.6 Hz, arom H), 7.14 (1H, s, thiazole H), 2.64 (2H, q, J = 7.6 Hz, -CH2-), 1.19 (3H, t, J = 7.6 Hz, -CH3); 13C NMR (100 MHz, DMSO-d6, δ/ppm): 168.14, 149.97, 142.71, 132.57, 127.82 (2C), 125.56 (2C), 100.58, 27.92, 15.50; HRMS (ESI): calcd for C11H13N2S [M + H]+ 205.07940, found 205.07976.
4.3.12. 5-methyl-4-phenylthiazol-2-amine (4l)
Off-white solid, yield: 79%, m.p. 118–120°C; 1H NMR (400 MHz, DMSO-d6, δ/ppm): 8.96 (2H, br s, -NH2), 7.57–7.48 (5H, m, arom H), 2.28 (3H, s, -CH3); 13C NMR (100 MHz, DMSO-d6, δ/ppm): 179.46, 167.66, 133.12, 129.43, 128.95 (2C), 128.45 (2C), 114.83, 11.70; HRMS (ESI): calcd for C10H11N2S [M + H]+191.0637, found 191.0642.
4.3.13. 4-(4-methoxyphenyl) thiazol-2-amine (4m)
Off-white solid, yield: 88%; m.p. 205–206°C; 1H NMR (400 MHz, DMSO-d6, δ/ppm): 8.9 (2H, br s, -NH2) 7.71 (2H, d, J = 8.8 Hz, arom H), 7.085 (1H, s, thiazole H), 7.05 (H, d, J = 8.8 Hz, arom H), 3.81 (3H, s, -OCH3); 13C NMR (100 MHz, DMSO-d6, δ/ppm): 170.38, 160.12, 138.14, 127.26 (2C), 120.91, 114.49 (2C), 100.49, 55.42; HRMS (ESI): calcd for C10H11ON2S [M + H]+ 207.0587, found 207.0593.
4.3.14. 4-(naphthalen-1-yl) thiazol-2-amine (4n)
Brown solid, yield: 81%; m.p. 164–165°C; 1H NMR (400 MHz, DMSO-d6, δ/ppm): 9.06 (2H, br s, -NH2), 8.10–8.02 (4H, m, arom H), 7.69 -7.60 (3H, m, arom H), 7.07 (1H, s, arom H); 13C NMR (100 MHz, DMSO-d6, δ/ppm): 168.05, 149.86, 133.48, 133.39, 130.74, 128.13, 127.96, 126.61, 126.23, 125.89, 125.79, 125.41, 104.99; HRMS (ESI): calcd for C13H11N2S [M + H]+ 227.06375, found 227.06416.
4.3.15. 4-(naphthalen-2-yl) thiazol-2-amine (4o)
Brown solid, yield: 90%; m.p. 151–152°C; 1H NMR (400 MHz, DMSO-d6, δ/ppm): 8.32 (1H, s, arom H), 7.96–7.87 (4H, m, arom H), 7.52–7.47 (2H, m, arom H), 7.17 (1H, s, arom H), 7.13 (2H, br s, -NH2); 13C NMR (100 MHz, DMSO-d6, δ/ppm): 169.44, 153.37, 133.01, 132.74, 132.36, 128.36, 127.80, 127.49, 126.58, 126.42, 125.66, 123.53, 103.63; HRMS (ESI): calcd for C13H11N2S [M + H]+ 227.0637, found 227.0645.
4.3.16. 2-bromo-1-(4-bromophenyl)ethanone (5a)
Off-white solid, yield: 97%; m.p. 108–110°C; 1H NMR (400 MHz, CDCl3): δ = 7.53 (d, J = 8.8 Hz, 2H, arom H), 7.64 (d, J = 8.4 Hz, 2H, arom H), 4.39 (s, 2 H, -CH2-).
Supplementary_material_doc.doc
Download MS Word (6 MB)Additional information
Funding
Notes on contributors
N.C. Gangi Reddy
N.C. Gangi Reddy was born in 1981 in Naravakati Palle village, Kadapa, Andhra Pradesh, INDIA. He has been awarded the PhD degree from Sri Venkateswara University, Tirupati, INDIA in April 2007. Later, he joined as an assistant professor in the Department of Chemistry, Yogi Vemana University, Kadapa in June 2007. His research interests are design and synthesis of medicinally valuable organic compounds and development of catalyst-based synthetic methodologies. He published more than 25 research papers in journals of international repute. He completed two major research projects as principal investigator.
Notes
1. NaHCO3 is added slowly, because of rapid effervescences.
2. If the reaction mass contains unreacted substrate (1), after water wash, it is to be washed again with petroleum ether.
References
- An Tran, N. M., Anil Kumar, M., Jung-Ae, K., Lee, K. D., & Park, S. (2015). Synthesis, anticancer and antioxidant activity of novel carbazole-based thiazole derivatives. Phosphorus, Sulfur, and Silicon and the Related Elements, 190, 1–9.
- Biagetti, M., Leslie, C. P., Mazzali, A., Seri, C., Pizzi, D. A., Bentley, J., … Caberlotto, L. (2010). Synthesis and structure–activity relationship of N-(3-azabicyclo [3.1.0] hex-6-ylmethyl)-5-(2-pyridinyl)-1, 3-thiazol-2-amines derivatives as NPY Y5 antagonists. Bioorganic & Medicinal Chemistry Letters, 20, 4741–4744.
- Chau, K. Y., Cooper, J. M., & Schapira, A. H. (2013). Pramipexole reduces phosphorylation of α-synuclein at serine-129. Journal of Molecular Neuroscience, 51, 573–580.10.1007/s12031-013-0030-8
- Dondoni, A. (1985). New perspectives in thiazole chemistry. Phosphorus and Sulfur and the Related Elements, 24, 1–38.10.1080/03086648508073395
- Erion, M. D., van Poelje, P. D., Dang, Q., Kasibhatla, S. R., Potter, S. C., Reddy, M. R., … Lipscomb, W. N. (2005). MB06322 (CS-917): A potent and selective inhibitor of fructose 1, 6-bisphosphatase for controlling gluconeogenesis in type 2 diabetes. Proceedings of the National Academy of Sciences, 102, 7970–7975.10.1073/pnas.0502983102
- González Cabrera, D. G., Douelle, F., Feng, T.-S., Nchinda, A. T., Younis, Y., White, K. L., … Chibale, K. (2011). Novel orally active antimalarial thiazoles. Journal of Medicinal Chemistry, 54, 7713–7719.10.1021/jm201108k
- Hantzsch, A., & Weber, J. H. (1887). Ueber Verbindungen des Thiazols [Pyridins der Thiophenreihe]. Berichte der deutschen chemischen Gesellschaft, 20, 3118–3132.10.1002/(ISSN)1099-0682
- Heravi, M. M., Poormohammad, N., Beheshtiha, Y. S., & Baghernejad, B. (2011). Efficient synthesis of 2,4-disubstituted thiazoles under grinding. Synthetic Communications, 41, 579–582.10.1080/00397911003629440
- Huang, X., Zhu, Q., & Zhang, J.-Z. (2002). Synthesis of a new polymer-supported reagent-poly [4-hydroxy (tosyloxy) iodo] styrene and its application to the synthesis of 2-amino-4-arylthiazoles. Chinese Journal of Chemistry, 20, 1411–1414.
- Jacques, V. M., & Vernin, G. (2008). Chemistry of heterocyclic compounds: Thiazole and its derivatives (Part One, Vol. 34). Wiley-VCH. ISBN: 978-0-471-03993-8.
- Jaen, J. C., Wise, L. D., Caprathe, B. W., Tecle, H., Bergmeier, S., Humblet, C. C., … Pugsley, T. A. (1990). 4-(1,2,5,6-Tetrahydro-1-alkyl-3-pyridinyl)-2-thiazolamines: A novel class of compounds with central dopamine agonist properties. Journal of Medicinal Chemistry, 33, 311–317.10.1021/jm00163a051
- Janardhan, B., Krishnaiah, V., Rajitha, B., & Crooks, P. A. (2014). Sodium fluoride as an efficient catalyst for the synthesis of 2,4-disubstituted-1,3-thiazoles and selenazoles at ambient temperature. Chinese Chemical Letters, 25, 172–175.
- Jessop, P. G. (2011). Searching for green solvents. Green Chemistry, 13, 1391–1398.10.1039/c0gc00797h
- Kerton, F. M. (2009). Alternative solvents for green chemistry. In J. Clark (Ed.), RSC green chemistry series (pp. 1–218). Cambridge. ISBN 978-0-85404-163-3
- Kidwai, M., Chauhan, R., & Bhatnagar, D. (2011). Eco-friendly synthesis of 2-aminothiazoles using Nafion-H as a recyclable catalyst in PEG–water solvent system. Journal of Sulfur Chemistry, 32, 37–44.10.1080/17415993.2010.533773
- Kumar, M., Minh An, T. N., Lee, I. J., Park, S., & Lee, K. D. (2015). Synthesis and bioactivity of novel phenothiazine-based thiazole derivatives. Phosphorus, Sulfur, and Silicon and the Related Elements, 190, 1160–1168.10.1080/10426507.2014.978324
- Makam, P., & Kannan, T. (2014). 2-Aminothiazole derivatives as antimycobacterial agents: Synthesis, characterization, in vitro and in silico studies. European Journal of Medicinal Chemistry, 87, 643–656.10.1016/j.ejmech.2014.09.086
- Meshram, H. M., Thakur, P. B., Madhu Babu, B., & Bangade, V. M. (2012). Convenient and simple synthesis of 2-aminothiazoles by the reaction of α-halo ketone carbonyls with ammonium thiocyanate in the presence of N-methylimidazole. Tetrahedron Letters, 53, 5265–5269.10.1016/j.tetlet.2012.07.080
- Nitta, A., Fujii, H., Sakami, S., Satoh, M., Nakaki, J., Satoh, S., … Kawai, H. (2012). Novel series of 3-amino-N-(4-aryl-1, 1-dioxothian-4-yl) butanamides as potent and selective dipeptidyl peptidase IV inhibitors. Bioorganic & Medicinal Chemistry Letters, 22, 7036–7040.
- Parekh, N. M., Juddhawala, K. V., & Rawal, B. M. (2013). Antimicrobial activity of thiazolyl benzenesulfonamide-condensed 2, 4-thiazolidinediones derivatives. Medicinal Chemistry Research, 22, 2737–2745.10.1007/s00044-012-0273-x
- Patt, W. C., Hamilton, H. W., Taylor, M. D., Ryan, M. J., Taylor, Jr. D. G., Connolly, C. J. C., … Olson, S. C. J. (1992). Structure-activity relationships of a series of 2-amino-4-thiazole-containing renin inhibitors. Journal of Medicinal Chemistry, 35, 2562–2572.10.1021/jm00092a006
- Potewar, T. M., Ingale, S. A., & Srinivasan, K. V. (2008). Catalyst-free efficient synthesis of 2-aminothiazoles in water at ambient temperature. Tetrahedron, 64, 5019–5022.10.1016/j.tet.2008.03.082
- Shah, N. K., Shah, N. M., Patel, M. P., & Patel, R. G. (2012). Synthesis, characterization and antimicrobial activity of some new biquinoline derivatives containing a thiazole moiety. Chinese Chemical Letters, 23, 454–457.10.1016/j.cclet.2012.01.042
- Shao, H., Shi, S., Huang, S., Hole, A. J., Abbas, A. Y., Baumli, S., … Wang, S. (2013). Substituted 4-(Thiazol-5-yl)-2-(phenylamino)pyrimidines are highly active CDK9 inhibitors: Synthesis, X-ray crystal structures, structure–activity relationship, and anticancer activities. Journal of Medicinal Chemistry, 56, 640–659.10.1021/jm301475f
- Singh, S., Prasad, N. R., Chufan, E. E., Patel, B. A., Wang, Y.-J., Chen, Z.-S., … Talele, T. T. (2014). Design and synthesis of human ABCB1 (P-Glycoprotein) inhibitors by peptide coupling of diverse chemical scaffolds on carboxyl and amino termini of (S)-valine-derived thiazole amino acid. Journal of Medicinal Chemistry, 57, 4058–4072.10.1021/jm401966m
- Spector, F. C., Liang, L., Giordano, H., Sivaraja, M., & Peterson, M. G. (1998). Inhibition of herpes simplex virus replication by a 2-amino thiazole via interactions with the helicase component of the UL5-UL8-UL52 complex. Journal of Virology, 72, 6979–6987.
- Wender, P. A., Handy, S. L., & Wright, D. L. (1997). Towards the ideal synthesis. Chemistry and Industry, 1997, 765–769.
- Yang, B. V., Weinstein, D. S., Doweyko, L. M., Gong, H., Vaccaro, W., Huynh, T., … Barrish, J. C. (2010). Dimethyl-diphenyl-propanamide derivatives as nonsteroidal dissociated glucocorticoid receptor agonists. Journal of Medicinal Chemistry, 53, 8241–8251.10.1021/jm100957a
- Yang, J., Tan, J.-N., & Gu, Y. (2012). Lactic acid as an invaluable bio-based solvent for organic reactions. Green Chemistry, 14, 3304–3317.10.1039/c2gc36083g
- Zhu, Y.-P., Yuan, J.-J., Zhao, Q., Lian, M., Gao, Q.-H., Liu, M.-C., … Wu, A.-X. (2012). I2/CuO-catalyzed tandem cyclization strategy for one-pot synthesis of substituted 2-aminothiozole from easily available aromatic ketones/α, β-unsaturated ketones and thiourea. Tetrahedron, 68, 173–178.10.1016/j.tet.2011.10.074
- Zhuravel, I. O., Kovalenko, S. M., Vlasov, S. V., & Chernykh, V. P. (2005). Solution-phase synthesis of a combinatorial library of 3-[4- (coumarin-3-yl)-1,3-thiazol-2-ylcarbamoyl]propanoic acid Amides. Molecules, 10, 444–456.10.3390/10020444