?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
To develop the quantitative structure–activity relationship (QSAR) for predicting the anticonvulsant activity of α_substituted acetamido-N-benzylacetamide derivatives. Density Functional Theory (B3LYP/6-31G*) quantum chemical calculation method was used to find the optimized geometry of the studied molecules. Nine types of molecular descriptors were used to derive a quantitative relation between anticonvulsant activity and structural properties. The relevant molecular descriptors were selected by genetic algorithm approximation. The high value of the correlation coefficient, (R2) of 0.98, indicates that the model was satisfactory. The proposed model has good stability, robustness, and predictability on verifying with internal and external validation.
Keywords:
Public Interest Statement
The principal investigator and other authors have been actively engaged in the research of the area of Quantitative Structural Activity Relationship (QSAR) of α_substituted acetamido-N-benzylacetamide derivatives as an anticonvulsant activity. QSAR as a major factor in drug design, are mathematical equations relating chemical structure to their biological activity and it is also used to understand the structural features controlling the activities of neurotransmitters. The modeled α_substituted acetamido-N-benzylacetamide derivatives as an anticonvulsant activity will be used to inhibit/ deactivate the activities of Gamma Amino Butyrate Acid Transferases (GABAAT). An enzyme that causes epilepsy.
1. Introduction
Epilepsy is defined as a brain disorder characterized by an enduring predisposition to generate epileptic seizures (Fisher, Boas, & Blume, Citation2005). John Hughlings Jackson recommended that seizures were caused by “occasional, sudden, severe, extreme, rapid and local discharge of gray matter”, and that a generalized convulsion resulted when normal brain tissue invaded by the seizure activity initiated in the abnormal focus (Brunton, Lazo, & Parker, Citation2006). Ant-epilepsy campaign conducted by World Health Organization (WHO) in conjunction with International Bureau for Epilepsy (IBE) and International League Against Epilepsy (ILAE) suggests that 1% of world population at any time is afflicted with this neurological disorder. Every year about 2.4 million new cases are added to these figures (WHO/IBE/ILAEGlobal Campaign Against Epilepsy, Citation2009; Shadows & Hoofddorp, Citation2003). Several new compounds such as zonisamide, vigabatrin, gabapentin have emerged following the wide use of the classical antiepileptic drugs such as phenytoin, Phenobarbital, carbamazepine, valproic acid and various benzodiazepines. The need to synthesize newer compounds persists to treat those cases that are resistant to the available drugs and to reduce the side effects to the lowest possible level (Kathuria & Pathak, Citation2012). Ant-epileptic drugs exert their action by different mechanisms. They include an enhancement of the GABA-ergic neurotransmission effects on neuronal voltage-gated sodium and/or calcium channels (Anger, Madge, Mulla, & Riddall, Citation2001).
Quantitative structure–activity relationships (QSAR) are mathematical equations relating chemical structure to their biological activity (Krogsgaard-Larsen, Liljefors, & Ulf, Citation2002). The far reaching utilization of QSAR models was from the improvement of new basic descriptors and measurable mathematical statements relating different physical and organic properties of the substance structure. The main hypothesis in the QSAR approach is that all properties of a chemical substance are statistically related to its molecular structure. The success of the QSAR approach can be explained by the insight offered into the structural determination of chemical properties and biological activities, and the possibility to estimate the properties of new chemical compounds without the need to synthesize and test them. These molecular design techniques, which significantly reduce the cost and time involved in obtaining compounds with desired properties, were applied to a wide range of properties, such as melting and boiling temperature, molar heat capacity, standard Gibbs energy of formation, vaporization enthalpy refractive index, density, aqueous solubility, 1-octanol/water partition coefficient, solvation free energy, receptor binding affinities, pharmacological activities, and enzyme inhibition constants (Tarkoa & Ivanciuc, Citation2001; Palluotto et al., Citation1996).
The purpose of this research is to perform a quantum chemical QSAR study of a series of α_substituted acetamido-N-benzylacetamide derivatives to investigate the experimental activities of the molecules as anticonvulsant activity agents and obtain a linear model using the Genetic function Approximation (GFA) method.
2. Material and methodology
2.1. Data collection
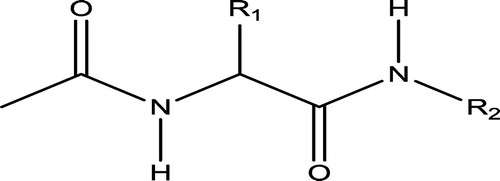
Thirty-five molecules belonging to α_substituted acetamido-N-benzylacetamide derivatives were used as anticonvulsant activities were taken from the literature and used for the present study (Kohn, Conley, & Leander, Citation1998). The observed structures, the biological activities of the training, outliers and test sets of these compounds are presented in Figure and Table , respectively.
Table 1. Biological activities of training and test set derivatives
2.2. Biological activity
The logarithm of measured ED50 (μM) against anticonvulsant activity as pED50 (pED50 = log 1/ED50) was used as dependent variable, consequently correlating the data linearly to the independent variable/descriptors.
2.3. Molecular optimization
The process of finding the equilibrium or lowest energy geometry of molecules is called Molecular Optimization. All molecular modeling studies were carried out using Spartan’14 version 1.1.2 (Wavefunction. Inc, Citation2013) and PaDEL-Descriptor version 2.18 (Yap, Citation2011) running on Toshiba Satellite, Dual-core processor window eight (8) operating system. The molecular structures of the compounds in the selected series were drawn in the graphic user interface of the software. Structures were built using 2D application tool and exported in 3D format. All 3D structures were geometrically optimized by minimizing energy. Calculation of the structural electronic and other descriptors of α_substituted acetamido-N-benzylacetamide derivatives was conducted by means of Density functional theory (DFT) using the B3LYP version and 6-31G* basis set. The lowest energy structure was used for each molecule to calculate their physicochemical properties (molecular descriptor).
2.4. Descriptor calculations
Molecular descriptors are mathematical values that describe properties of molecules. The quantum chemical descriptors were calculated using the Spartan’14 version 1.1.2 (Wavefunction. Inc, Citation2013) quantum chemistry package while the 1D, 2D, and 3D descriptors were calculated using PaDEL-Descriptor version 2.18 tool kit (Yap, Citation2011).
2.5. Training and test set
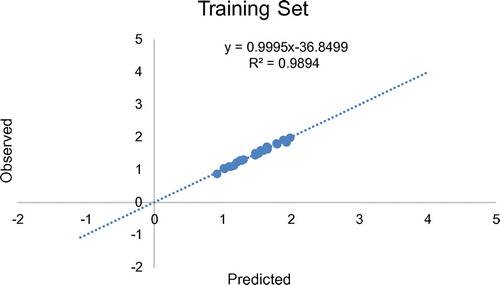
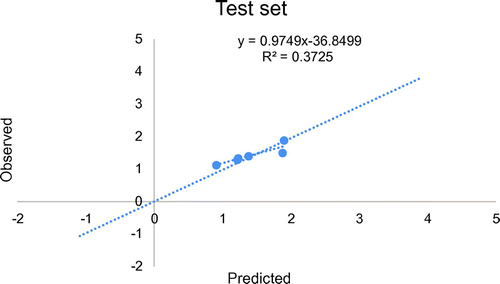
The training set comprises of molecules used in model development while the test set is made up of molecules not used in building the model, they were used in the external validation of the model generated by training set. The data-set for the biological activity was split into training set and test set. At least 70% of the data-set was used as training set while the remaining 30% was used as test set in line with the optimum splitting pattern of data-set in QSAR study (Patil, Citation2011). Consequently, the data-set of 35 derivatives was split into 25 training sets and 10 test sets. Four outliers were removed as shown in Table . The training set was used to generate the model while the test set was used to evaluate its predictive abilities (Figure ).
2.6. Culture process
The correlation between activity values of the molecules against neurotransmitter and the calculated descriptors was obtained through correlation analysis using the Material studio software version 8. Pearson’s correlation matrix was used as a qualitative model, in order to select the suitable descriptors for regression analysis. The generated descriptors were subjected to regression analysis with the experimentally determined activities as the dependent variable and the selected descriptors as the independent variables using the Genetic Function Approximation (GFA) method in material studio software version 8. 25 samples in the training set were included in the training set. The number of descriptors in the regression equation was 9, and Population and Generation were set to 2,000 and 2,000, respectively. The number of top equations returned was 5. Mutation probability was 0.1, and the smoothing parameter was 0.5. The models were scored based on Friedman’s LOF. In GFA algorithm, an individual or model was represented as one-dimensional string of bits. It was a distinctive characteristic of GFA that it could create a population of models rather than a single model. GFA algorithm, selecting the basic functions genetically, developed better models than those made using stepwise regression methods. And then, the models were estimated using the “lack of fit” (LOF), which was measured using a slight variation of the original Friedman formula, so that best model received the best fitness score (Wu et al., Citation2015). In materials studio version 8, LOF is measured using a slight variation of the original Friedman formula (Friedman, Citation1990). The revised formula is:(1)
(1)
where SSE is the sum of squares of errors, c is the number of terms in the model, other than the constant term, d is a user-defined smoothing parameter, p is the total number of descriptors contained in all model terms (ignoring the constant term), and M is the number of samples in the training set. Unlike the commonly used least squares measure, the LOF measure cannot always be reduced by adding more terms to the regression model. While the new term may reduce the SSE, it also increases the values of c and p, which tends to increase the LOF score. Thus, adding a new term may reduce the SSE, but actually increases the LOF score. By limiting the tendency to simply add more terms, the LOF measure resists over fitting better than the SSE measure (Materials Studio 8.0 Manual). The significant regression is given by F-test, and the higher the value, the better the model (Khaled & Abdel-Shafi, Citation2011).
2.7. Model validation
The fitting ability, stability, reliability, and predictive ability of the developed models were examined by internal and external validation parameters. The validation parameters were compared with the minimum recommended value for a generally acceptable QSAR model (Ravinchandran et al., Citation2011) shown in Table .
Table 2. Minimum recommended value of validated parameters for generally acceptable QSAR
2.7.1. Internal validation parameters
The square of the correlation coefficient (R2) describes the fraction of the total variation attributed to the model. The closer the value of R2 is to 1.0, the better the regression equation explains the Y variable. R2 is the most commonly used internal validation indicator and is expressed as follows:(2)
(2)
where, Yobs; Ypred;Ytraining are the experimental property, the predicted property, and the mean experimental property of the samples in the training set, respectively (Brandon-Vaughn, Citation2015).
Adjusted value varies directly with the increase in number of regressors i.e. descriptors, thus, R2 cannot be a useful measure for the goodness of model fit. Therefore, R2 is adjusted for the number of explanatory variables in the model. The adjusted R2 is defined as:
(3)
(3)
where p = number of independent variables in the model (Jalali-Heravi & Kyani, Citation2004).
The leave one out cross validation coefficient (Q2) is given by;(4)
(4)
where Yp and Y represent the predicted and observed activities respectively of the training set and Ym the mean activity value of the training set (Jalali-Heravi & Kyani, Citation2004).
2.7.2. External validation parameters
Internal validation is an essential step in QSPR model development. The desired internal validation results show that the model exhibits higher stability and prediction ability. However, no real prediction ability is shown for external samples. Therefore, the external predictive ability and extrapolation of the models should be evaluated (Jalali-Heravi & Kyani, Citation2004).
is the predictive R2 of a development model and is an important parameter that is used to test the external predictive ability of a QSAR model. The predicted R2 value is calculated as follows;
(5)
(5)
Ypred(test) and Y(test) indicate predicted and observed activity values respectively of the test set compounds and Ym(tr) indicates mean activity value of the training set (Patil, Citation2011).
3. Results and discussion
Five QSAR models were built using GFA algorithm, but only the best model (model 1) was selected and reported due to the statistical significance, and its statistical parameters were calculated as well. The name and symbol of the descriptors used in the QSAR optimization model are shown in Table .
Table 3. List of some descriptors used in this study
The Pearson’s correlation matrix for descriptors used in the model are shown in Table . The result from this Correlation matrix shows clearly that the correlation coefficients between each pair of descriptors is very low, thus, it can be inferred that there exist no significant inter-correlation among the descriptors used in building the model.
Table 4. Pearson’s correlation matrix for descriptors used in QSAR model for the activities of anticonvulsant molecules
Table gives the result of Validation of the Genetic function Approximation (GFA) of model 1 that was generated from material studio.
Table 5. Validation of the genetic function approximation from material studio
Model 1 gives the best QSAR model among the five models generated based on statistical significance as it has the highest R2, R2adj. Q2, F, and lower LOF value. Also, it has the lowest LOF value and error. Based on this analysis, Model 1 was selected and reported as the best optimization model.
Model 1
pED50 = 0.1172*D.M + 0.6148*Polariza−0.7632*SP-1−0.8680*SP-5 + 0.4990*nssNH + 0.1769*SHBint2 + 1.6086*MAXDP−8.3221*Wnu2.unity + 0.8251*Wnu2.polar−36.8499
N = 25, R = 0.9947, R2 = 0.9895, Ra = 0.9832, = 0.9627, S.E = 0.0428, LOF = 0.0103, Min expt. error for non-significant LOF (95%) = 0.0331, F = 156.9263.
A univariate analysis is performed on the inhibition efficiency data in Table as a tool to assess the quality of the data available and its suitability for next statistical analysis. Data in Table show acceptable normal distribution. Statistical parameters presented in Table have been discussed in detail in our previous study (Khaled, Citation2011).
But Pred-R2 =1 −
Thus, pred- R2=1− (0.214556/0.8092) = 0.7349
Comparison of the validation parameters of model 1 with the optimum standard proposed by Ravinchandran et al. (Citation2011), in Table shows that the parameters were in agreement with the standard as R = 0.9947, R2 0.9895, Ra = 0.9832, 0.9627, S.E = 0.0428, LOF = 0.0103,
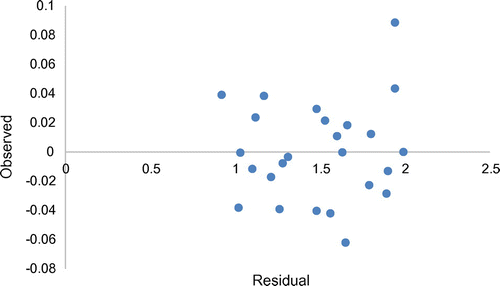
= 0.734854. This confirms the robustness of the model. Likewise, on comparing observed and predicted anticonvulsant activities (as presented in Table ) high predictability of model 1 was evidenced by the low residual values observed in the Table. Also, Figure gives the plot of predicted activities against observed activities on Microsoft excel package, the R2 value of 0.9895 was in agreement with GFA-derived R2 value, this further confirms the reliability of the model. Furthermore, the plot of observed activities versus residual activities (Figure ) indicated that there was no systemic error in model development as the propagation of residuals was observed on both sides of zero (Jalali-Heravi & Kyani, Citation2004).
Table 6. Experimental and predicted values of activity data
The inhibitory concentration of a molecule is inversely proportional to its biological activity. As shown in model 1 above, the PED50 of the α_substituted acetamido-N-benzylacetamide derivatives increases with increase in the values of the descriptors; Polariza (Polarizability), D.M (Dipole moment), and nssNH (Count of atom-type E-State: -NH-), SHBint2 (Sum of E-State descriptors of strength for potential Hydrogen Bonds of path length 2), MAXDP (Maximum positive intrinsic state difference in the molecule), Wnu2 Polar (Directional WHIM, weighted by unit weights) this is evidenced by their positive correlation with the dependent variable (Tables and ). It implies that the inhibitory concentration of the molecules against the neurotransmitter was directly proportional to these descriptors in the molecules. Also, it can be inferred that the inhibitory concentration of the molecules increases with the decrease in SP-1 (Simple Path, order 1), SP-5 (Simple Path, Order 5), Wnu2 Unity (Directional WHIM, Weighted by atomic Polarizabilities) descriptor in the molecules due to its negative correlation with pED50 as shown in model 1 (Table ).
Table 7a. External validation of Model-1
Table 7b. External validation of Model 1
Table 8. Univariate analysis of the Inhibition data
4. Conclusion
This research addresses the QSAR between a set of α_substituted acetamido-N-benzylacetamide derivatives and their inhibitory concentration against neurotransmitters. Our study developed five GFA-derived models out of which the optimal model was selected on the basis of its superior statistical significance. The robustness and applicability of QSAR equation has been established by internal and external validation techniques. This study provides an effective approach for the design and synthesis of new anticonvulsant drug that will be more effective in inhibiting neurotransmitter.
Acknowledgments
We acknowledge the authors of various literature consulted in the course of this research who anonymously helped at improving the quality of the manuscript. We also greatly acknowledge the contribution of Emmanuel Israel Edache and David Ebuka Arthur for their valuable comments and suggestions.
Additional information
Funding
Notes on contributors
Usman Abdulfatai
Usman Abdulfatai is a research scholar in the Department of Chemistry, Ahmadu Bello University, Zaria-Nigeria. He is working in the field of Quantitative structural activity relationship study of anticonvulsant activity under supervision of Adamu Uzairu and Sani Uba.
References
- Anger, T., Madge, D. J., Mulla, M., & Riddall, D. (2001). Medicinal chemistry of neuronal voltage-gated sodium channel blockers. Journal of Medicinal Chemistry, 44, 115–137.10.1021/jm000155h
- Brandon-Vaughn, O. K. A. (2015). Comprehensive R archive network (CRAN). Retrieved from http://CRAN.R-project.org
- Brunton, L. L., Lazo, J. S., & Parker, K. L. (2006). Goodman and Gilman’s The pharmacological basis of therapeutics (11th ed., pp. 610–611). New York, NY: McGraw-Hill Medical Publishing Division.
- Fisher, R. S., Boas, W., & Blume, W. (2005). Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia, 46, 470–472.10.1111/epi.2005.46.issue-4
- Friedman, J. F. (1990). Multivariate adaptive regression splines Laboratory for Computational Statistics (Stanford Technical Report No. 102). Stanford, CA: Department of Statistics, Stanford University.
- Jalali-Heravi, M. J., & Kyani, A. (2004). Use of computer-assisted methods for the modeling of the retention time of a variety of volatile organic compounds: A PCA-MLR-ANN approach. Journal of Chemical Information and Modeling, 44, 1328–1335.10.1021/ci0342270
- Kathuria, V., & Pathak, D. P. (2012). Synthesis and anticonvulsant activity of some N-substituted-phthalimide analogs. The Pharma Innovation Journal, 1, 55–59.
- Khaled, K. F. (2011). Modeling corrosion inhibition of iron in acid medium by genetic function approximation method: A QSAR model. Elsevier Corrosion Science, 53, 3457–3465.
- Khaled, K. F., & Abdel-Shafi, N. S. (2011). Quantitative structure and activity relationship modeling study of corrosion inhibitors: Genetic function approximation and molecular dynamics simulation methods. International Journal of Electrochemical Science, 6, 4077–4094.
- Kohn, H., Conley, J. D., & Leander, J. D. (1998). Marked stereospecificity in a new class of anticonvulsants. Brain Research, 457, 371–375.
- Krogsgaard-Larsen, P., Liljefors, T., & Ulf, Madsen (2002). Textbook of drug design and discovery (554 p.). London: Taylor and Francis.
- Palluotto, F., Carotti, A., Casini, G., Campagna, F., Genchi, G., Rizzo, M., & De Sarro, G. B. (1996). Structure-activity relationships of 2-aryl-2,5-dihydropyridazino [4,3-b]indol-3(3H)-ones at the benzodiazepine receptor. Bioorganic & Medicinal Chemistry, 4, 2091–2104.10.1016/S0968-0896(96)00220-9
- Patil, S. S. (2011). A least square approach to analyze usage data for effective web personalization. International Journal of Computer Engineering Research, 2, 68–74.
- Ravinchandran V., Rajak, H., Jain, A., Sivadasan, S., Varghese, C. P., & Kishore-Agrawal, R. (2011). Validation of QSAR models-strategies and importance. International Journal of Drug Design and Discovery, 2, 511–519.
- Shadow, P., & Hoofddorp, G. (2003). Annual report of the WHO/IBE/ILAE global campaign against epilepsy. Zwanenburg.
- Tarkoa, L., & Ivanciuc, O. (2001). QSAR modeling of the anticonvulsant activity of phenylacetanilides with Preclav. Communications in mathematics and in computer chemistry, 44, 201–214.
- Wavefunction. Inc. (2013). Spartan’14 (Version 1.1.2). Irvine, CA.
- WHO/IBE/ILAEGlobal Campaign Against Epilepsy. (2009). Atlas epilepsy care in the world, programme for neurological diseases and neuroscience department of mental health and substance abuse. Geneva: World Health Organization. Retreived from http://www.who.int/entity/mediacentre/factsheets/fs999/en
- Wu, W., Zhang, C., Lin, W., Chen, Q., Guo, X., & Qian, Y. (2015). Quantitative structure-property relationship (QSPR) modeling of drug-loaded polymeric micelles via genetic function approximation. PLoS ONE, 10(3), 0119575. doi:10.1371/journal.pone.0119575
- Yap, C. W. (2011). Inc. PaDEL-Descriptor, version 2.18: An open source software to calculate molecular descriptors and fingerprints. Journal of Computational Chemistry, 32, 1466–1474.