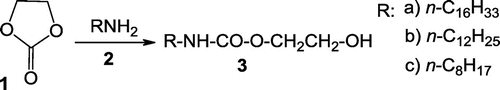?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A method for the synthesis of nonionic surfactants – N-alkyl-O-(2-hydroxyethyl) carbamates is proposed by acylation of fatty amines with ethylene carbonate without any solvent or catalyst. The surface tension of the prepared surfactants was measured, toxicity and biodegradability were determined for the surfactant with n-dodecyl as a hydrophobic group and N-monosubstituted amide and hydroxyl groups for their hydrophilic part.
Public Interest Statement
This paper describes a simple method for the preparation of nonionic surfactants that are useful in different exploitation areas. These surfactants form by heating fatty amines with ethylene carbonate at 150°C without any other solvent or catalyst. Obtained surfactants have surface activity comparable to other nonionic surfactants, and they are highly biodegradable materials. Therefore, they may be used when the simplicity of their preparation is important.
1. Introduction
Surfactants can be found among the most widely spread man-manufactured substances in the world, and they are produced in millions of tons per year. Very strict regulations are in place for their utilization/exploitation and production. They should not only be highly efficient in washing and cleaning processes but also practically nontoxic, sufficiently biodegradable, and reasonably cheap to make their application favorable to people. Various methods are elaborated for production of modern surfactants, both chemical and enzymatic (Friedli, Citation2001; Holmberg, Citation2003; Rosen & Kunjappu, Citation2013; Rosenholm, Citation2014; Somasundaran, Citation2006) allowing the preparation of anionic, cationic, zwitterionic, and nonionic surfactants, useful for different applications. Syntheses of these surfactants usually include several stages and demand time. Prices of reagents and the degree of complexity of their preparations together with the biological properties of these surfactants quite frequently limit exploitation of such surfactants. Therefore, the elaboration of a straightforward method for the synthesis of nonionic surfactants is discussed in this communication.
2. Results and discussion
Recently we have found that a simple acylation reaction of primary alkyl amines (2) with ethylene carbonate (1) at reasonably high temperatures (~150°C) resulted in the formation of promising nonionic surfactants—N-alkyl carbamates with hydrophobic alkyl group at nitrogen atom and N–H and O–H bonds in the hydrophilic parts of the surfactants (3) (Scheme ).
Preparation of these surfactants is extremely simple—just heating both reagents 1 and 2 in equimolar ratio without any solvent, any catalyst and any inert gas protection. The simplicity of the proposed method differs it from approaches described in literature for similar purposes (Fujita, Bhanage, Kanamaru, & Arai, Citation2005; Hazard, Cheymol, Charbrier, Sekera, & Eche-Fialaire, Citation1961; Sekera, Citation1962). Acylation reactions of alcohols with freshly prepared carbamoyl chlorides (Hazard et al., Citation1961; Sekera, Citation1962) or transesterification reactions of corresponding carbamates with alcohols proposed by various authors (Fujita et al., Citation2005; Hazard et al., Citation1961) are very much more complicated and time-consuming methods for fabrication of carbamates. Even the recently shown acylation of octylamine with ethylene carbonate has required the use of a poisonous catalyst—Bu2Sn(OCH3)2, and the necessity to remove the excess of amine under vacuum at the end of the process (Hellawell et al., Citation2014). Traces of poisonous and dangerous catalyst have been acceptable in compositions of greases—the subject of a patent (Hellawell et al., Citation2014) but their presence is certainly not imaginable in materials foreseen for washing and cleaning.
Structures of obtained carbamates (3) were confirmed by their 1H-NMR and IR spectra; acceptable data of elemental analyses were also obtained. Good correspondence of spectral and analytical data was achieved (Table ).
Table 1. Yields and analyses of surfactants–carbamates
Sufficiently long linear carbon atom chains (C8 – C16) form the lipophilic part of the proposed surfactants, and N–H bond of monosubstituted amide group together with O–H bond provide acceptable surface tension (32–43 mN/cm) and critical micelle concentration (cmc, 8.4–11.9 mg/L) for these novel surfactants (3) (Table ). The surface tension of obtained N-alkyl carbamates (3) measured by the du Noüy ring method at 25°C differs a little depending on the length of alkyl chain but remains in range typical for nonionic surfactants (Friedli, Citation2001; Holmberg, Citation2003; Rosen & Kunjappu, Citation2013; Somasundaran, Citation2006).
Table 2. The behavior of surfactants–carbamates in water solutions
It should be mentioned that these surfactants are also comparatively nontoxic substances. Daphnia’s are the most commonly used crustacean test species for determination of the effects of xenobiotics on primary consumers in freshwater aquatic ecosystems (Daphtoxkit F Magna, Citation2000). EC50 (half maximum effective concentration) for the carbamate 3b after 24 and 48 h was found to be 2 and 0.5 mg L−1, respectively. The comparison of our results obtained for Daphnia magna with earlier literature in the context of ecotoxicity (EC50) of carbamates showed that surfactant 3b displayed similar or lower toxicity than other surfactants (Rosen & Kunjappu, Citation2013; Somasundaran, Citation2006). Germination tests with Lepidium sativum L., Raphanus sativus L. and Secale cereale L. in the concentration range of the surfactant 3b from 0.5 to 100 L−1 did not reveal any inhibition effect.
The biodegradability of the surfactant 3b was determined by CO2 evolution in sealed vessels (OECD Guidelines for the Testing of Chemicals, Citation2014). BOD3 test has revealed some difference between sets amended with surfactant 3b (10 mg L−1) and control without any chemical. After 72 h incubation period, oxygen consumption and without it was found to be 18.4 ± 2 mg L−1 and 8.4 ± 0.0 mg L−1, respectively. The results indicate potentially high biodegradability of the surfactant 3b by microorganisms (Holmberg, Citation2003; Rosen & Kunjappu, Citation2013; Somasundaran, Citation2006).
3. Experimental
All reagents were used as purchased from commercial suppliers without further purification. All melting points were determined in open capillaries, using Thomas Hoover melting point apparatus, expressed in °C, and are uncorrected. 1H-NMR spectra of the compounds were recorded on Varian 400 MR NMR spectrometer using TMS as an internal standard. Infrared spectra (IR) were recorded on Perkin Elmer Spectrum One Infrared Spectrometer, and samples were screened in potassium bromide (KBr) pellets. Elemental analyses were performed on a Flash EA1112 CHNS analyzer (Thermo Electron Corporation).
N-Dodecyl-O-(2-hydroxyethyl)-carbamate (3b) (a typical experiment). Dodecyl amine (5.55 g; 30 mmol) and ethylene carbonate (2.64 g; 30 mmol) were stirred in a round bottom flask at 150°C (±5°C) for 3 h. No inert gas protection was required. tert-butyl methyl ether (40 mL) was added to the cooled to ~90°C reaction mixture, and the obtained solution was carefully mixed. The precipitate was separated from the mother liquid by suction using a Büchner funnel after keeping the reaction mixture at room temperature during 16 h. The precipitate was dried in open air. N-Dodecyl-O-(2-hydroxyethyl)-carbamate (3b, 6.48 g, 79.1%) was obtained in the form of white crystals with m.p. 58–60°C. Found, %: C, 66.01; H, 11.60; N, 5.14. C15H31NO3. Calculated, %: C 65.89; H, 11.43; N, 5.12. 1H-NMR spectrum (CDCl3, δ-ppm): 4.78 (m, 1H); 4.20 (t, 2H); 3.80 (t, 2H); 3.18 (m, 2H); 2.42 (m, 1H); 1.49 (t, 2H); 1.27 (m, 18H); 0.88 (t, 3H). IR spectrum (KBr νmax cm−1): 3,298 (ν, N–H); 2,951–2,853 (ν, C–H); 1,685 (ν, C=O); 1,568 (δ, N–H).
Other carbamates (3a and 3c) were prepared and characterized in a similar way.
N-Hexadecyl-O-(2-hydroxyethyl)-carbamate (3a), yield 79.8%, white crystals, m.p. 78–80°C. Found, %: C, 69.46; H, 12.16; N, 4.28. C19H39NO3. Calculated, %: C 69.25; H, 11.93; N, 4.25. 1H NMR spectrum (CDCl3, δ-ppm): 4.77 (m, 1H); 4.20 (t, 2H); 3.80 (t, 2H); 3.17 (m, 2H); 2.39 (m, 1H); 1.49 (t, 2H); 1.25 (m, 26H); 0.88 (t, 3H). IR spectrum (KBr νmax cm−1): 3,353 (ν, N–H); 2,951–2,853 (ν, C–H); 1,685 (ν, C=O); 1,541 (δ, N–H).
N-Octyl-O-(2-hydroxyethyl)-carbamate (3c), yield 79.0%, white crystals, m.p. 45–46°C. Found, %: C, 60.75; H, 10.84; N, 6.26. C11H23NO3. Calculated, %: C 60.80; H, 10.67; N, 6.45. 1H NMR spectrum (CDCl3, δ-ppm): 4.87 (m, 1H); 4.19 (t, 2H); 3.79 (t, 2H); 3.16 (m, 2H); 2.42 (m, 1H); 1.48 (t, 2H); 1.28 (m, 8H); 0.87 (t, 3H). IR spectrum (KBr νmax cm−1): 3,311 (ν, N–H); 2,951–2,853 (ν, C–H); 1,699 (ν, C=O); 1,551 (δ, N–H).
4. Conclusion
Accordingly, our results confirm that primary fatty alkyl amines can be very easily converted into corresponding surfactants—alkyl carbamates. Conditions of their syntheses are truly simple—just stirring and heating reagents in an equimolar ratio without any inert gas protection in a common laboratory flask. Obtained surfactants have surface activity comparable to other nonionic surfactants but they are considerably less toxic and highly biodegradable materials. Hence, these surfactants may have a wide exploitation when the simplicity of their preparation is important.
Acknowledgments
Authors are grateful to Dr. O. Muter for determination of toxicity and biodegradability of investigated surfactants and Mr. L. Arbidans for measuring the surface tension.
Additional information
Funding
Notes on contributors
Andris Zicmanis
Sindija Brica, Maris Klavins, and Andris Zicmanis are interested in the development of modern surfactants with better biodegradability than existing ones useful for different applications. Their research is focused on the elaboration of such materials that might be produced in a large scale, including industrial production.
References
- Daphtoxkit F Magna. Crustacean toxicity screening test for freshwater. Standard operational procedure. (2000). Mariakerke: MicroBioTests. Retrieved from http://www.microbiotests.be/SOPs/Daphtoxkit%20magna%20F%20SOP%20-%20A5.pdf
- Friedli, F. E. (Ed.). (2001). Detergency of specialty surfactants. New York, NY: Marcel Dekker. ISBN 978-8793102040.
- Fujita, S., Bhanage, B., Kanamaru, H., & Arai, M. (2005). Synthesis of 1,3-dialkylureas from ethylene carbonate and amines using calcium oxide. Journal of Molecular Catalysis A: Chemical, 230, 43–48. doi:10.1016/j.molcata.2004.12.014
- Hazard, R., Cheymol, J., Charbrier, P., Sekera, A., & Eche-Fialaire, R. (1961). Chemistry and pharmacology of the amino esters of carbamic acids and their quaternary ammonium salts. Bulletin de la Société chimique de France, 11, 2087–2091.
- Hellawell, A., Kone, E., Massey, A., Mead, H. B., Van Zwieten, D., Visser, T., & Wilkinson, R. J. (2014). Process for preparing a urea grease [ WO 2014/122273 A1]. New York, NY: Shell Oil Company.
- Holmberg, K. (Ed.). (2003). Novel surfactants: Preparation, applications and biodegradability (2nd ed.). New York, NY: Marcel Dekker. ISBN 978082474300.
- OECD Guidelines for the Testing of Chemicals. (2014). Test No. 310: Ready biodegradability – CO2 in sealed vessels (Headspace Test). doi:10.1787/2074577x
- Rosen, M. J., & Kunjappu, J. T. (2013). Surfactants and interfacial phenomena (4th ed.). New York, NY: Wiley. ISBN 978-0-470-54194-4.
- Rosenholm, J. B. (2014). Phase equilibriums, self-assembly and interactions in two-, three- and four medium-chain length component systems. Advances in Colloid and Interface Science, 205, 9–47. doi:10.1016/j.cis.2013.08.009
- Sekera, A. (1962). Syntheses and pharmacological studies of a series of basic esters of substituted carbamic acids. Journal mondial de pharmacie, 5, 5–18.
- Somasundaran, P. (Ed.). (2006). Encyclopedia of surface and colloid science (2nd ed.). New York, NY: Taylor & Francis. ISBN 9780849396151.