Abstract
An efficient procedure for the synthesis of bisindolylmethanes from condensation of indole and aromatic aldehydes or ketones is described. The aromatic electrophilic substitution reactions of indole with aromatic aldehydes and ketones are achieved in the presence of tetrabutylammonium hydrogen sulfate as a mild and efficient solid acid catalyst. This methodology offers several advantages such as good yields, simple procedure, mild and environmentally benign conditions.
Public Interest Statement
The bisindolylmethane derivatives exhibit important biological and pharmaceutical properties such as antimicrobial, antibacterial, antiviral and antifungal, antimetastatic, analgesic, and anti-inflammatory activities. Although the reported synthetic protocols are valuable, most of them suffer from one or more drawbacks such as use of expensive and/or toxic catalysts and solvents. It is therefore clear that due to the biological activities and versatile application possibilities of BIMs, there is a continuous quest for more efficient, green, and mild methods for indole derivatives synthesis. In this research, we reported a mild and green procedure for the synthesis of mono- and di-bisindolylmethane derivatives via the reaction of indole and aldehydes or ketones in the presence of TBAHS as an efficient catalyst.
1. Introduction
The indole ring system is present in many natural products, pharmaceuticals, and agrochemicals. These compounds bearing indole moiety have shown many pharmaceutical properties such as antimicrobial, antibacterial, antiviral, antifungal, antimetastatic, radical scavenging, analgesic, and anti-inflammatory activities (Bell, Carmeli, & Sar, Citation1994; Benabadji, Wen, Zheng, Dong, & Yuan, Citation2004; Fahy, Potts, Faulkner, & Smith, Citation1991; Irie et al., Citation1999; Kamal et al., Citation2009; Kobayashi et al., Citation1994; Kuethe, Citation2006; Shiri, Zolfigol, Kruger, & Tanbakouchian, Citation2010; Sivaprasad, Perumal, Prabavathy, & Mathivanan, Citation2006; Sujatha, Perumal, Muralidharan, & Rajendran, Citation2009; Valeria & Ernesto, Citation1998). Numerous inhibitory activities are reported for bisindolylmethanes (BIMs) derivatives in different cancer including bladder cancer, lung cancer cells, colon cancer, prostate cancer, breast tumor cells (Ge, Fares, & Yannai, Citation1999; Gong, Firestone, & Bjeldanes, Citation2006; Gong, Sohn, Xue, Firestone, & Bjeldanes, Citation2006; Ichite et al., Citation2009; Ling & Wan-Ru, Citation2004; Nachshon-Kedmi, Yannai, & Fares, Citation2004; Safe, Papineni, & Chintharlapalli, Citation2008; Sung et al., Citation2007). The oxidized forms of BIMs are utilized as dyes (Novak, Kramer, Klapper, Daasch, & Murr, Citation1976) and colorimetric sensors (He et al., Citation2006; Martínez, Espinosa, Tárraga, & Molina, Citation2008).
A simple and standard method for the synthesis of bis(indolyl)methanes is the Friedel–Crafts reaction between indoles and carbonyl compounds in the presence of an acid or base. Varieties of catalytic reagents used in the synthesis of BIMs have been reviewed (Shiri et al., Citation2010). Various Brönsted acids (Ramesh, Banerjee, Pal, & Das, Citation2003; Zahran, Abdin, & Salama, Citation2008), Lewis acids (Chen, Yu, & Wang, Citation1996; Kundu & Maiti, Citation2008; Qu et al., Citation2011), heterogeneous acidic catalyst (Firouzabadi, Iranpoor, & Jafari, Citation2006), ionic liquid (Kalantari, Citation2015; Veisi, Hemmati, & Veisi, Citation2009), and some other catalysts have been applied for this synthesis (Haghighi & Nikoofar, Citation2014; Hojati, Zeinali, & Nematdoust, Citation2013; Pore, Desai, Thopate, & Wadgaonkar, Citation2006; Xie, Sun, Jiang, & Le, Citation2014). Although these protocols are valuable, most of them suffer from one or more drawbacks including long reaction times, low yields of products, harsh reaction conditions, corrosive reagents, and use of expensive and/or toxic catalysts and solvents. Moreover, many Lewis acids are deactivated or sometimes decomposed by nitrogen-containing reactants. It is clear that due to the biological activities and versatile application possibilities of BIMs, there is a continuous quest for more efficient, mild, and clean procedures for synthesis of these compounds. This prompted us to investigate the feasibility of less hazardous, solvent-free synthesis of aryl-3,3′-bis(indolyl)methane derivatives under modified experimental conditions.
Acidic tetrabutylammonium hydrogen sulfate (TBAHS) act as a phase transfer catalyst and performs many organic transformations under mild conditions. It has been used for dehydration and cyclization step in Hantzsch reaction (Tripathi, Tewari, & Dwivedi, Citation2004). TBAHS has been applicated for one-pot synthesis of pyrano[2,3-c]pyrazoles via domino/Knoevenagel-hetero-Diels–Alder reaction (Parmar, Teraiya, Patel, & Talpada, Citation2011). It also has been used for synthesis of 3,4-dihydropyrimidin-2(1H)-ones (Shaabani, Bazgir, & Arab-Ameri, Citation2004), 1,8-dioxo-octahydroxanthenes (Karade, Sathe, & Kaushik, Citation2007), 5-substituted 1H-tetrazoles (Wang, Liu, & Cheon, Citation2015), and other heterocyclic compounds (Jończyk & Kuliński, Citation1993; Parmar, Teraiya, Barad, Sharma, & Gupta, Citation2013). TBAHS is an inexpensive, safe-handleable and thermally stable substance.
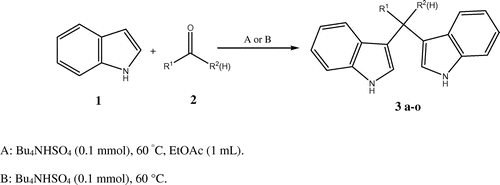
For unique catalyst features of TBAHS and in continuation of our efforts to develop green and mild methodologies for the synthesis of heterocycles (Bodaghifard, Solimannejad, Asadbegi, & Dolatabadifarahani, Citation2016; Mobinikhaledi, Foroughifar, & Bodaghifard, Citation2011), herein we wish to report a mild, efficient, and green procedure for the synthesis of biologically interesting mono- and di-bisindolylmethane derivatives via the reaction of indole and aldehydes or ketones in the presence of TBAHS in solvent-free condition or using a little amount of solvent (Scheme ).
2. Results and discussion
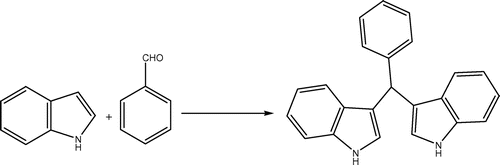
In order to determine the optimum reaction conditions, we examined the influence of the temperature, reaction time, and the amounts of TBAHS upon a model reaction between benzaldehyde (1 mmol) and indole (2 mmol) (Scheme ). The best result was obtained with 0.1 mmol of TBAHS at 60°C using 1 mL EtOAc or in the absence of any solvent (Table , entry 5 and 6). Increasing the amount of catalyst to 0.2 mmol does not affect the product yield (Table , entry 10). Moreover, the catalyst is essential and in the absence of the catalyst, poor yield of the corresponding BIM is produced (Table , entry 7, 11).
Table 1. Optimization of reaction condition for synthesis of 3,3′-bis-indolyl phenylmethaneTable Footnotea
Encouraged by these results, we studied the reaction of various aldehydes and ketones under optimized conditions to better understand the scope and generality of this simple procedure (Scheme ).
A series of aromatic aldehydes and ketones underwent an electrophilic substitution reaction with indole smoothly, to afford a range of substituted bis(indolyl)methanes in good to excellent yields (Table , 3a–o). The results showed that the reaction proceeds very efficiently in all cases. This method is equally effective for aldehydes bearing electron withdrawing or donating groups in the aromatic rings. Furthermore, reactions of ketones with indole were satisfactorily performed by the current pathway but they need longer times and afforded lower yield of products than aldehydes due to lower reactivity of ketones. The products 3i and 3m are novel compounds and have been fully characterized by their elemental analysis, FT-IR and 1H and 13C NMR spectra. The results indicated that the method A need less time that can be related to more and faster interaction of starting materials in the presence of little amount of ethyl acetate. In the absence of ethyl acetate and solid-state manner (method B), the interaction slowed down and in longer time produced the desired products.
Table 2. Synthesis of bisindolylmethanes catalyzed by TBAHS
2.1. Experimental
All chemicals and solvents were obtained from commercial sources and used without further purification. All known organic products were identified by comparison of their physical and spectral data with those of authentic samples. Thin layer chromatography (TLC) was performed on UV-active precaoted plates of silica gel (TLC Silica gel60 F254).The FTIR spectrum was recorded on a Shimadzu IR-470 spectrometer using KBr disks. The 1H and 13C NMR spectra were recorded on a Brucker Avance spectrometer operating at 400, 300 and 100, 75 MHz, respectively, in DMSO-d6 or CDCl3 with TMS as an internal standard. Coupling constants, J, were reported in Hertz units (Hz). Spin multiplicities are shown as s (singlet), br s (broad singlet), d (doublet), dd (doublet of doublet), t (triplet), and m (multiplet). Elemental analyses were performed by Vario EL equipment at Arak University.
2.1.1. General procedure for synthesis of bis(indolyl)methanes catalyzed by Bu4NHSO4
Method A: To a mixture of indole (2 mmol), aldehyde, or ketone (1 mmol) ethyl acetate (1 mL) as a solvent, Bu4NHSO4 (0.1 mmol) was added and the mixture stirred magnetically at 60°C. After complete conversion, as indicated by TLC (hexane/ethyl acetate 4:1), the reaction mixture was cooled to room temperature and crushed ice was added. The precipitate was filtered and dried under vacuum. The product was purified by recrystallization hexane–ethyl acetate (Table ).
Method B: To a mixture of indole (2 mmol) and aldehyde or ketone (1 mmol), Bu4NHSO4 (0.1 mmol) was added and the mixture stirred magnetically at 60°C. After complete conversion, as indicated by TLC (hexane/ethyl acetate 4:1), the reaction mixture was cooled to room temperature and crushed ice was added. The precipitate was filtered and dried under vacuum. The product was purified by recrystallization from hexane–ethyl acetate (Table ).
2.1.2. General procedure for synthesis of di-bis(indolyl)methanes
To a mixture of indole (4.5 mmol) and dialdehyde (1 mmol) Bu4NHSO4 (0.1 mmol) was added and the mixture stirred magnetically at 60°C. After complete conversion, as indicated by TLC (hexane/acetone 4:1), the reaction mixture was quenched by adding ice water (10 ml). The precipitate was filtered, evaporated, and the corresponding di-bis(indolyl)methanes were obtained in excellent yields and then recrystallized from hexane–ethyl acetate to afford pure products (Table , entry 9–10).
3. Conclusion
In summary, we have developed a novel, efficient, and environmentally benign method for the synthesis of pharmaceutically important bis(indolyl)methanes and di-bis(indolyl)methanes using tetrabutylammonium hydrogen sulfate in excellent yield. This new protocol has advantages over previously reported procedures such as cleaner reaction profiles, use of inexpensive catalyst, simple experimental and work-up procedure, high conversions, and high yield and chemoselectivity, hence believed to be superior over many existing synthetic methods. Also two novel BIM derivatives have been synthesized and characterized by spectroscopic data.
Additional information
Funding
Notes on contributors
Mohammad Ali Bodaghifard
Mohammad Ali Bodaghifard was born in Hamadan, Iran, in 1978. He received his BSc degree from Bu Ali Sina University, Hamedan, Iran and MSc degree from Shahid Beheshti University, Tehran, Iran, and PhD in organic chemistry in 2010 from Arak University, Arak, Iran. His doctoral thesis was on application of new catalysts in organic transformations and synthesis of heterocycles and spiro-heterocycles. He works as an assistant professor at Arak University from 2012. His current research interest is on green organic synthesis, heterogeneous catalysis, and organic–inorganic hybrid nanostructures.
References
- Bell, R., Carmeli, S., & Sar, N. (1994). Vibrindole A, a metabolite of the marine bacterium, Vibrio parahaemolyticus, isolated from the toxic mucus of the boxfish Ostracion cubicus. Journal of Natural Products, 57, 1587–1590.10.1021/np50113a022
- Benabadji, S. H., Wen, R., Zheng, J., Dong, X., & Yuan, S. (2004). Anticarcinogenic and antioxidant activity of diindolylmethane derivatives. Acta Pharmacologica Sinica, 25, 666–671.
- Bodaghifard, M. A., Solimannejad, M., Asadbegi, S., & Dolatabadifarahani, S. (2016). Mild and green synthesis of tetrahydrobenzopyran, pyranopyrimidinone and polyhydroquinoline derivatives and DFT study on product structures. Research on Chemical Intermediates, 42, 1165–1179.10.1007/s11164-015-2079-1
- Chen, D., Yu, L., & Wang, P. G. (1996). Lewis acid-catalyzed reactions in protic media. Lanthanide-catalyzed reactions of indoles with aldehydes or ketones. Tetrahedron Letters, 37, 4467–4470.10.1016/0040-4039(96)00958-6
- Fahy, E., Potts, B. C. M., Faulkner, D. J., & Smith, K. (1991). 6-bromotryptamine derivatives from the gulf of California tunicate didemnum candidum. Journal of Natural Products, 54, 564–569.10.1021/np50074a032
- Firouzabadi, H., Iranpoor, N., & Jafari, A. A. J. (2006). Aluminumdodecatungstophosphate (AlPW12O40), a versatile and a highly water tolerant green Lewis acid catalyzes efficient preparation of indole derivatives. Journal of Molecular Catalysis A: Chemical, 244, 168–172.10.1016/j.molcata.2005.09.005
- Ge, X., Fares, F. A., & Yannai, S. (1999). Induction of apoptosis in MCF-7 cells by indole-3-carbinol is independent of p53 and bax. Anticancer Research, 19, 3199–3203.
- Gong, Y., Firestone, G. L., & Bjeldanes, L. F. (2006). 3,3'-diindolylmethane is a novel topoisomerase II catalytic inhibitor that induces s-phase retardation and mitotic delay in human hepatoma HepG2 cells. Molecular Pharmacology, 69, 1320–1327.10.1124/mol.105.018978
- Gong, Y., Sohn, H., Xue, L., Firestone, G. L., & Bjeldanes, L. F. (2006). 3,3'-diindolylmethane is a novel mitochondrial H+-ATP synthase inhibitor that can induce p21Cip1/Waf1 expression by induction of oxidative stress in human breast cancer cells. Cancer Research, 66, 4880–4887.10.1158/0008-5472.CAN-05-4162
- Haghighi, M., & Nikoofar, K. J. (2014). Nano TiO2/SiO2: An efficient and reusable catalyst for the synthesis of oxindole derivatives. Journal of Saudi Chemical Society, Retrieved from : https://scholar.google.com/citations?view_op=view_citation&hl=en&user=llpcGOYAAAAJ&citation_for_view=llpcGOYAAAAJ:ufrVoPGSRksC doi:10.1016/j.jscs.2014.09.002
- He, X., Hu, S., Liu, K., Guo, Y., Xu, J., & Shao, S. (2006). Oxidized bis(indolyl)methane: A simple and efficient chromogenic-sensing molecule based on the proton transfer signaling mode. Organic Letters, 8, 333–336.10.1021/ol052770r
- Hojati, S. F., Zeinali, T., & Nematdoust, Z. (2013). A novel method for synthesis of bis(indolyl)methanes using 1,3-dibromo-5,5-dimethylhydantoin as a highly efficient catalyst under solvent-free conditions. Bulletin of the Korean Chemical Society, 34, 117–120.10.5012/bkcs.2013.34.1.117
- Ichite, N., Chougule, M. B., Jackson, T., Fulzele, S. V., Safe, S., & Singh, M. (2009). Enhancement of docetaxel anticancer activity by a novel diindolylmethane compound in human non-small cell lung cancer. Clinical Cancer Research, 15, 543–552.10.1158/1078-0432.CCR-08-1558
- Irie, T., Kubushirs, K., Suzuki, K., Tsukazaki, K., Umezawa, K., & Nozawa, S. (1999). Inhibition of attachment and chemotactic invasion of uterine endometrial cancer cells by a new vinca alkaloid, conophylline. Anticancer Research, 31, 3061–3066.
- Jończyk, A., & Kuliński, T. (1993). A simple synthesis of 2-phenylethynyl- and 2-phenylthioethynyl-2-substituted phenylacetonitriles under phase-transfer catalytic (PTC) conditions. Synthetic Communications, 23, 1801–1811.10.1080/00397919308011280
- Kalantari, M. (2015). Synthesis of 1,8-dioxo-octahydroxanthenes and bis(indolyl)methanes catalyzed by [Et3NH][H2PO4] as a cheap and mild acidic ionic liquid. Arabian Journal of Chemistry, 5, 319–323.Retrieved from: http://www.sciencedirect.com/science/article/pii/S1878535210001802
- Kamal, A., Khan, M. N. A., Reddy, K. S., Srikanth, Y. V. V., Ahmed, S. K., Kumar, K. P., & Murthy, U. S. N. (2009). An efficient synthesis of bis(indolyl)methanes and evaluation of their antimicrobial activities. Journal of Enzyme Inhibition and Medicinal Chemistry, 24, 559–565.10.1080/14756360802292974
- Karade, H. N., Sathe, M., & Kaushik, M. P. (2007). An efficient synthesis of 1, 8-dioxo-octahydroxanthenes using tetrabutylammonium hydrogen sulfate. Arkivoc, xiii, 252–258.
- Kobayashi, M., Aoki, S., Gato, K., Matsunami, K., Kurosu, M., & Kitagawa, I. (1994). Trisindoline, a new antibiotic indole trimer, produced by a bacterium of Vibrio sp. separated from the marine sponge Hyrtios altum. Chemical & Pharmaceutical Bulletin, 42, 2449–2451.10.1248/cpb.42.2449
- Kuethe, J. T. (2006). A general approach to indoles: Practical applications for the synthesis of highly functionalized pharmacophores. CHIMIA International Journal for Chemistry, 60, 543–553.10.2533/chimia.2006.543
- Kundu, P., & Maiti, G. (2008). A mild and versatile synthesis of bis (indolyl)-methanes and tris (indolyl) alkanes catalyzed by antimony trichloride. Indian Journal of Chemistry, 47B, 1402–1406.
- Ling, J., & Wan-Ru, C. (2004). U.S. Patent WO2004018475 A2.
- Martínez, R., Espinosa, A., Tárraga, A., & Molina, P. (2008). Bis(indolyl)methane derivatives as highly selective colourimetric and ratiometric fluorescent molecular chemosensors for Cu2+ cations. Tetrahedron, 64, 2184–2191.10.1016/j.tet.2007.12.025
- Mobinikhaledi, A., Foroughifar, N., & Bodaghifard, M. A. (2011). Simple and efficient method for three-component synthesis of spirooxindoles in aqueous and solvent-free media. Synthetic Communications, 41, 441–450.10.1080/00397911003587507
- Nachshon-Kedmi, M., Yannai, S., & Fares, F. A. (2004). Induction of apoptosis in human prostate cancer cell line, PC3, by 3,3′-diindolylmethane through the mitochondrial pathway. British Journal of Cancer, 91, 1358–1363.10.1038/sj.bjc.6602145
- Novak, T. J., Kramer, D. N., Klapper, H., Daasch, L. W., & Murr, B. L. (1976). Formation of dyes derived from diindolylpyridylmethanes. The Journal of Organic Chemistry, 41, 870–875.10.1021/jo00867a025
- Parmar, N. J., Teraiya, S. B., Barad, H. A., Sharma, D., & Gupta, V. K. (2013). Efficient one-pot synthesis of precursors of some novel aminochromene annulated heterocycles via domino knoevenagel–hetero-diels–alder reaction. Synthetic Communications, 43, 1577–1586.10.1080/00397911.2011.652755
- Parmar, N. J., Teraiya, S. B., Patel, R. A., & Talpada, N. P. (2011). Tetrabutylammonium hydrogen sulfate mediated domino reaction: Synthesis of novel benzopyran-annulated pyrano[2,3-c]pyrazoles. Tetrahedron Letters, 52, 2853–2856.10.1016/j.tetlet.2011.03.108
- Pore, D. M., Desai U. V., Thopate, T. S., & Wadgaonkar, P. P. (2006). A mild, expedient, solventless synthesis of bis (indolyl) alkanes using silica sulfuric acid as a reusable catalyst. Arkivoc, xii, 75–80.
- Qu, H.-E., Xiao, C., Wang, N., Yu, K.-H., Hu, Q.-S., & Liu, L.-X. (2011). RuCl3·3H2O catalyzed reactions: Facile synthesis of bis (indolyl) methanes under mild conditions. Molecules, 16, 3855–3868.10.3390/molecules16053855
- Ramesh, C., Banerjee, J., Pal, R., & Das, B. (2003). Silica supported sodium hydrogen sulfate and amberlyst‐15: Two efficient heterogeneous catalysts for facile synthesis of bis‐and tris (1H‐indol‐3‐yl) methanes from Indoles and carbonyl compounds [1]. Advanced Synthesis & Catalysis, 345, 557–559.10.1002/adsc.200303022
- Safe, S., Papineni, S., & Chintharlapalli, S. (2008). Cancer chemotherapy with indole-3-carbinol, bis(3′-indolyl)methane and synthetic analogs. Cancer Letters, 269, 326–338.10.1016/j.canlet.2008.04.021
- Shaabani, A., Bazgir, A., & Arab-Ameri, S. (2004). Tetrabutylammonium hydrogen sulfate: An efficient catalyst for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones under solvent-free conditions. Phosphorus, Sulfur, and Silicon and the Related Elements, 179, 2169–2175.10.1080/10426500490474815
- Shiri, M., Zolfigol, M. A., Kruger, H. G., & Tanbakouchian, Z. (2010). Bis- and trisindolylmethanes (BIMs and TIMs). Chemical Reviews, 110, 2250–2293.10.1021/cr900195a
- Sivaprasad, G., Perumal, P. T., Prabavathy, V. R., & Mathivanan, N. (2006). Synthesis and anti-microbial activity of pyrazolylbisindoles—promising anti-fungal compounds. Bioorganic & Medicinal Chemistry Letters, 16, 6302–6305.10.1016/j.bmcl.2006.09.019
- Sujatha, K., Perumal, P. T., Muralidharan, D., & Rajendran, M. (2009). Synthesis, analgesic and anti-inflammatory activities of bis (indolyl) methanes. Indian Journal of Chemistry, 48B, 267–272.
- Sung, D. C., Yoon, K., Chintharlapalli, S., Abdelrahim, M., Lei, P., Hamilton, S., … Safe, S. (2007). Nur77 Agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor–dependent and nuclear receptor–independent pathways. Cancer Research, 67, 674–683.
- Tripathi, R. P., Tewari, N., & Dwivedi, N. (2004). Tetrabutylammonium hydrogen sulfate catalyzed eco-friendly and efficient synthesis of glycosyl 1,4-dihydropyridines. Tetrahedron Letters, 45, 9011–9014.
- Valeria, L., & Ernesto, M. (1998). EP0887348 A1.
- Veisi, H., Hemmati, S., & Veisi, H. (2009). Highly efficient method for synthesis of bis(indolyl)methanes catalyzed by FeCl. Journal of the Chinese Chemical Society, 56, 240–245.10.1002/jccs.v56.2
- Wang, Z., Liu, Z., & Cheon, S. H. (2015). Facile synthesis of 5‐substituted 1H‐tetrazoles catalyzed by tetrabutylammonium hydrogen sulfate in water. Bulletin of the Korean Chemical Society, 36, 198–202.10.1002/bkcs.2015.36.issue-1
- Xie, Z.-B., Sun, D-Zh, Jiang, G.-F., & Le, Zh-G. (2014). Facile synthesis of bis(indolyl)methanes catalyzed by α-chymotrypsin. Molecules, 19, 19665–19677.10.3390/molecules191219665
- Zahran, M., Abdin, Y., & Salama, H. (2008). Eco-friendly and efficient synthesis of bis (indolyl) methanes under microwave irradiation. Arkivok, 11, 256–265.