Abstract
Gold is being used therapeutically since 2500 BC in Chinese medical history. Red colloidal gold is still used in the Indian Ayurvedic medicine for rejuvenation and revitalization during old age under the name of Swarna Bhasma. The present research describes a more reliable method for assaying growth inhibition of some waterborne bacterial pathogens by gold nanoparticles. The gold nanoparticles were synthesized by chemical method using auric chloride as a precursor salt and sodium citrate as a reducing as well as stabilizing agent. Characterization of Au NPs was performed using UV–Vis, X-ray diffraction (XRD) and TEM (transmission electron microscope). Further, these nanoparticles were tested against four well-explored waterborne bacterial pathogens viz., E. coli, V. cholerae, S. typhimurium, S. dysenteriae, using globally accepted broth microdilution method recommended by CLSI, which exhibited a good antibacterial potency demonstrated in the form of IC50 and MIC. Subsequently, a novel phylogenetic approach for reducing the cost of experimentation was also carried out. In future, Au NPs can be used in making a water purifier system or kit.
Keywords:
Public Interest Statement
The emerging infectious diseases caused by drinking water and the development of drug resistance in the pathogenic bacteria at an alarming rate is a matter of serious concern, which is greatly responsible for human loss annually. Despite knowledge of microbial pathogenesis and application of modern therapeutics, the disease occurrence and mortality associated with these microbial infections still remain high. Therefore, there is a pressing demand to discover novel strategies and identify new antimicrobial agents from natural and inorganic substances to develop the next generation of drugs or agents including nanoparticles, to control human loss. Nanoparticles usually ranging in dimension from 1 to 100 nm have properties unique from their bulk equivalent increasing applications as amendments in industrial, medicine and therapeutics, synthetic textiles and food packaging products. Underlining the present circumstances, we have been forced to apply gold nanoparticles for the potability of water.
1. Introduction
The worldwide escalation of microbial resistance to conventional remedy practices is a serious problem for human health. The indiscriminate use of these antibiotics has lead to this serious damage, thereby, increasing clinical problems in the treatment of these microbial infections (Hema, Shiny, & Parvathy, Citation2012). In view of the increased mounting of infections with emerging multidrug resistance, nanotechnology emerges as a hope to treat such infections (Kagithoju, Godishala, Pabba, Kurra, & Rama swamy, Citation2012). To subdue this resistance, assorted approaches have been employed to regulate or avert the proliferation of microbial cells by the use of antibiotic agents. One of the promising approaches for overcoming bacterial resistance is through the use of nanoparticles (Chaloupka, Malam, & Seifalian, Citation2010). Nanomaterials have fascinated contemplation in discipline owing to their unequaled physical and chemical potencies, which differ considerably from their bulk counterparts (Kumar, Citation2006). At present, apart from metal nanomaterials, carbon sheets are immensely fabricated nanomaterials whose implementations include catalysis, sensors, environmental remediation, drug delivery and personal care products (Pandey et al., Citation2011). An important property of metallic nanoparticles is their ability to target action on different entities and on different target sites (Gordon, Vig Slenters, & Brunetto, Citation2010). Numerous reports have centred on antimicrobial pursuits of various nanoparticles, including silver, gold and copper (Kim et al., Citation2008a; Kumar, Shukla, Pandey, Srivastava, & Dikshit, Citation2015). Among the various metallic nanoparticles, gold nanoparticles have wide range of applications in nanoscale devices and other technologies due to its chemical inertness and resistance to surface oxidation (Sugunan, Thanachayanont, Dutta, & Hilborn, Citation2005). Gold nanoparticles also have potential activity against bacterial pathogens which mainly depends on the size and shape of these particles.
In the contemporary probe, the Au NPs were synthesized exercising wet chemical method, and the product was characterized morphologically with the aid of UV–Vis spectrophotometer, X–Rray diffractometer (XRD) and transmission electron microscope (TEM). Further, these nanoparticles were screened against various leading bacterial strains viz., E. coli, V. cholerae, S. typhimurium and S. dysenteriae, using broth microdilution method of antibacterial susceptibility assay and a phylogenetic approach is applied for reducing the cost of experimentation.
2. Materials and methods
2.1. Synthesis of Au NPs
Trisodium citrate-stabilized Au NPs had been synthesized according to the procedure of Kim et al., (Citation2002) with minor modifications (Kim et al., Citation2002). In brief, a composition of trisodium citrate (3.4 × 10–2 M, 4.0 mL), tannic acid (10 g/dL, 1.0 mL) and potassium carbonate (0.025 M, 1.0 mL) were diluted with deionized water (14.0 mL) and gradually heated to 60 ± 1°C. The aforementioned contents were added to a solution of 1.176 g/dL, 80 mL, chloroauric acid (HAuCl4) at the rate of 1 mL/min at 60 ± 1°C with continuous stirring for 30 min to obtain Au NPs.
2.2. Characterization studies of Au NPs
2.2.1. UV–Visible spectroscopy
Ten times diluted Au NPs in deionized water were characterized through UV–Visible spectrophotometer and were recorded on Genesis 10 Thermospectronic spectrophotometer at wavelength 200–300 nm using quartz cuvette.
2.2.2. X-ray diffraction
X-ray diffraction (XRD) was performed on dried Au NPs samples with 40 kV voltage, 30 mA current at a wavelength of 1.54 Å on copper grid using Rigaku Smartlab X-ray diffractometer, Japan. Spectra were obtained for the 2θ range of 10–80°.
2.2.3. Transmission electron micrographs
Transmission electron micrographs (TEM) of gold nanoparticles had been recorded by preparing a sample of 50 μg/mL in ethanol followed by applying the drop of suspension over copper grid. The grid was dried at room temperature and used for the TEM analysis. TEM of Au NPs was recorded over JEOL 1011 (Tokyo, Japan) with primary beam voltage of 80 kV at a camera length 290 mm, having exposure time 1.7 s.
2.3. Selection of test pathogens
The bacterial pathogens have been selected on the basis of their virulent nature. They are well known for their pathogenicity causing stomach infections, cholera, typhoid and bloody dysentery; therefore, we have selected them for our studies. All the species except S. dysenteriae were procured from Microbial Type Culture Collection (MTCC), Chandigarh, India. These strains were re-cultured on nutrient agar (NA) medium, and incubated at 30°C for 3 days (Figure ).
2.4. Antibacterial susceptibility assay
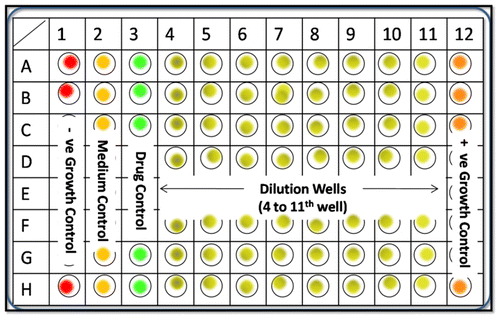
A broth microdilution method protocol was performed according to CLSI guidelines in flat bottom 96-well microtiter plates. Here, Mueller Hinton Broth medium (HiMedia) was used as the assay medium for viability of bacterial pathogen. Two-day-old cultures of pathogen were collected with an inoculating loop and suspended in sterile saline containing 0.1% of saline to make 0.5% McFarland. The McFarland 0.5 standard corresponds approximately to a homogeneous suspension of 1.5 × 106 cells per mL. About 100 μL of medium was transferred to each well, followed by 90 and 80 μL of medium in each well of column numbered 3 and 4, respectively. Then, 10 and 20 μL of Au NPs suspension was added to each well of column number 3 and 4, respectively. Serial dilutions were performed from column 4 to column 11, to obtain the final nanoparticles concentrations which varied from 2.5 mg/mL (4th well) to 0.020 mg/mL (11th well). Bacterial inoculum of 100μL was added to each well except all wells of column 2 and 3, making the final volume of 200μL. Nanoparticles-free well (column 12) containing medium and inoculum served as growth control. Column 1 which contained medium, inoculum and formaldehyde served as negative control and column 2 served as medium control, while column 3 served as drug control containing 200 μL medium and 190 μL medium with10 μL of drug, respectively (Figure ).
2.5. Phylogenetic study
To minimize the cost of experimentation of the waterborne bacterial pathogenic strains of E. coli, V. cholerae, S. typhimurium and S. dysenteriae, a phylogenetic tree of 16S ribosomal RNA was performed. The gene sequence was procured from the gene Bank NCBI database, USA in their FASTA format for further alignment and phylogenetic study. The sequences were aligned in MEGA4 (version 4.0.1) software and gene alignment was obtained for both the DNA sequence and the amino acid sequence by ClustalW program and 1,000 bootstrapped N-J plotting method in MEGA4. Further, the gene sequences were blasted for procurement of more genetic homologues which were aligned in ClustalW, and phylogeny was obtained by N-J plotting (Felsenstein, Citation1985 ;Tamura, Dudley, Nei, & Kumar, Citation2004; Pandey et al., Citation2016).
3. Results and discussion
3.1. Characterization of Au NPs
Au NPs were synthesized by a very popular method of citrate reduction and were characterized using UV–Vis spectra, X-ray diffraction pattern, and transmission electron microscopy (Figure ). Initially, average size of nanoparticles determined by Zeta was 55 nm. Further, the formation of Au NPs was observed through the change in colour which was further confirmed by UV–Vis spectra of spectrophotometer which was recorded at 536 nm. The spectrum of HAuCl4 shows absorption spectra of HAuCl4 at 221 nm. Reduction of HAuCl4 under tri-sodium citrate, tannic acid system showed Au NPs with plasmonic absorption spectra at 536 nm which fall in specific wavelength for Au NPs. The absorption spectra obtained from spectrophotometer of gold nanoparticles corresponding to 536 nm refer to the particle size to be around 35–65 nm. This was further ascertained by TEM studies which reflect the particles size ranges between 15 and 45 nm. The TEM shows their confirmed spherical shaped structure also. The observed size of studied AuNPs made it more important because of its size-depended activities. The obtained peaks of XRD demonstrate crystalline nature of nanoparticles which was confirmed by its intensity (1 1 1) on 2 theta obtained at 38–400 (Shukla et al., Citation2015). Since no additional peaks are observed in XRD pattern, therefore, it is also pointing towards purity of nanoparticles.
Figure 3. (A) UV–Vis Spectra of chloroauric acid (a) absorption of gold nanoparticles (b) and characteristic plasmonic peak of gold nanoparticles (c). (B) X-ray diffraction spectra of Gold nanoparticles refer crystalline particles. (C) Transmission electron micrograph (at 1.37 KX) representing the formation of spherical gold nanoparticles.
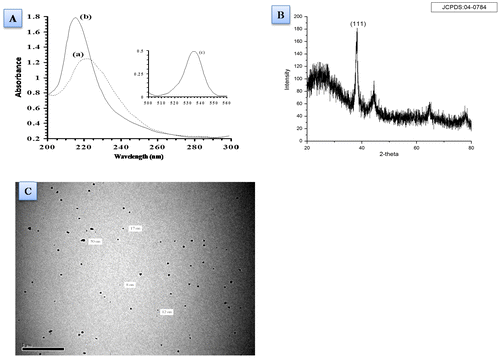
Table demonstrates (based on IC50 and MIC) that gold nanoparticles are very good inhibitors of S. dysenteriae. In the present study, Au NPs was found 50% cidal against all the pathogens with IC50 values i.e. 0.668, 0.688, 0.630 and 0.666 (mg/mL), for E. coli, V. cholerae, S. typhimurium and S. dysneteriae, respectively, whereas MIC values of 1.03 (mg/mL) was recorded only for S. dysneteriae. However, in case of all the four microbial strains, it can be noted that with increasing concentrations of the Au NPs, the optical density decreased, thus growth inhibition has increased (Figure ). The Au NPs, at its highest in vitro concentration, was found to be most effective in inhibiting the viability of bacterium followed by lower dilutions as also supported by other workers. Owing to their small size and higher surface-to-volume ratio, nanoparticles have a greater contact area with micro-organisms. This phenomenon enhances biological and chemical activity of the metal nanoparticles with high antibacterial potency. There are certain evidences which demonstrate that the activity of NPs was much stronger than that of bulk as smaller particles normally have a larger surface-to-volume ratio which provides a more efficient mean for its antibacterial activity (Baker, Pradhan, Pakstis, Pochan, & Shah, Citation2005). The generation of reactive oxygen species like hydrogen peroxide, hydroxyl radicals, superoxide anions presents another elucidation of the antibacterial activity of NPs (Yamamoto, Citation2001). Alternative antioxidant histidine exhibited similar observation in case of viability of Botrytis and Penicillium (He, Liu, Mustapha, & Lin, Citation2001). However, recently, other models have been proposed regarding the mechanism of actions of various compounds in the case of bacteria, fungi and crustaceans (Heinlaan, Ivask, Blinova, Dubourguier, & Kahru, Citation2008; Mishra et al., Citation2016). Apart from different agents like free chlorine bleaching, solar disinfections, coagulants, bios and/ceramic filtration and camphor frequently used as water potability agents, metallic nanoparticles can also be used as a water purifier system. The present study dealing with the growth inhibition of waterborne bacterial pathogens by nanoparticles makes it a suitable replacement for conventional treatment protocols. For the reduction in the experimentation cost and determination of the variability in their susceptibility against the nanoparticles, phylogenetic analysis of the bacterial strains was done. For the selected strains, the gene sequences were procured from GenBank NCBI database in their FASTA format. The gene sequences were aligned in the MEGA 4 (Version 4.0.1) software (Pathak et al., Citation2015; Tamura & Kumar, Citation2004) in the ClustalW format (Figure ). Phylogenetic studies were also done previously in the case of waterborne bacteria using ClustalW computer program and GENETYX MAC 10.1 software. Phylogeny was constructed by the maximum likelihood of the DNA. Similar studies were also done in the reference of different species belonging to Malassezia species and plant growth-promoting rhizobacteria for obtaining the homologous strains (Pandey et al., Citation2013).
Table 1. IC50 and MIC (mg/mL)
Figure 4. Percentage growth inhibition of pathogens in the presence of decreasing concentration of Au NPs.
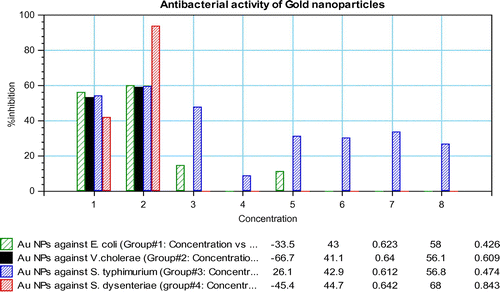
Figure 5. Molecular phylogenetic tree constructed using the 16S rRNA gene sequence of various waterborne bacteria found homologous in the blastx search of the studied bacterial pathogenic strains.
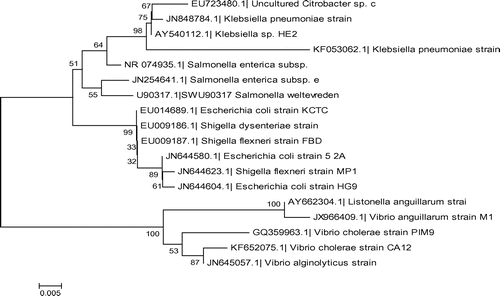
The ClustalW alignment of 16S rRNA gene was subjected to the bootstrapping 1,000 NJ plotting for deciphering the phylogeny of studied bacterial strains. The phylogenetic alignment of the studied bacterial pathogens was in accordance with their susceptibility. Although, E. coli and V. cholerae have been distant in the phylogeny, they have similar susceptibility. The strong homogeneity in the 16S rRNA (phylogenetically conserved region) gene sequence is reflected by their nearness in the tree and also reflected by susceptibility (MICs) to all other pathogens near to tested pathogens, thereby reducing the experimentation cost.
4. Conclusions
From our results, it is proved that chemically synthesized and stabilized gold nanocolloids are effective against most potent bacterial strain. Hence, it is concluded that the stabilized gold nanoparticles as an antibacterial agent due to their inherent elemental properties may be suitable for the formulation of new types of bactericidal materials equivalent to the antibiotics against microbial infections, or they can be utilized in the development of some water purifier system. Moreover, the phylogenetic analysis for reducing the cost of experimentation is a noble approach.
Acknowledgements
The authors are thankful to Head, Department of Botany, University of Allahabad for providing the research facilities; to UGC, New Delhi for financial support; to Dr Rudra Pratap and Dr Sanjeev Kumar Srivastava IISc, Bangalore for characterization facilities under the scheme INUP.
Additional information
Funding
Notes on contributors
Rajesh Kumar
Rajesh Kumar is currently working as a guest faculty (Botany), University of Allahabad, and has expertise in culturing and isolation of genetic material of microbes and in handling instruments like SEM, TEM, Rotary Evaporator, Clevenger Apparatus, Spectramax (384 plus), RT PCR, UV–vis Spectrophotometer, Shaker Incubator, Ultrasonicator, etc. He has 11 publications and 1 award in his credit.
References
- Baker, C., Pradhan, A., Pakstis, L., Pochan, D. J., & Shah, S. S. (2005). Synthesis and antibacterial properties of silver nanoparticles. Journal of Nanoscience and Nanotechnology, 5, 244–249.10.1166/jnn.2005.034
- Chaloupka, K., Malam, Y., & Seifalian, A. M. (2010). Nanosilver as a new generation of nanoproduct in biomedical applications. Trends in Biotechnology, 28, 580–588.10.1016/j.tibtech.2010.07.006
- Felsenstein, J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution, 39, 783–791.10.2307/2408678
- Gordon, O., Vig Slenters, T., & Brunetto, P. S. (2010). Silver coordination polymers for prevention of implant infection: Thiol interaction, impact on respiratory chain enzymes, and hydroxyl radical induction. Antimicrobial Agents and Chemotherapy, 54, 4208–4218.10.1128/AAC.01830-09
- He, L., Liu, Y., Mustapha, A., & Lin, M. (2001). Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiological Research, 166, 207–215.
- Heinlaan, M., Ivask, A., Blinova, I., Dubourguier, H. C., & Kahru, A. (2008). Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere, 71, 1308–1316.10.1016/j.chemosphere.2007.11.047
- Hema, T. A., Shiny, M., Parvathy, J. (2012) Antimicrobial activity of leaves of Azima tetracantha against clinical pathogens. International Journal of Pharmacy and Pharmaceutical Sciences, 4 317–319.
- Kagithoju, S., Godishala, V., Pabba, S. K., Kurra, H. B., & Rama swamy, N. (2012). Antibacterial activity of flower extract of Pongamia pinnata Linn—An elite medicinal plant. International Journal of Pharmacy and Pharmaceutical Sciences, 4, 130–132.
- Kim, Y., Robert, C. J., Jiagtian, L., Joseph, T., Hupp, J. C., & Schatz, F. (2002). Synthesis, linear extension and preliminary resonant hyper-Rayligh scattering studies of gold core/silver shell nano particles: Comparison of theory and experiment. Chemical Physics Letter, 325, 421–428.
- Kim, K. J., Sung, W. S., Moon, S. K., Choi, J. S., Kim, J. G., & Lee, D. G. (2008a). Antifungal activity and mode of action of silver nano-particles on Candida albicans. Journal of Microbiology and Biotechnology, 18, 1482–1484.
- Kumar, C. (Ed.). (2006). Antibacterial action of zinc oxide nanoparticles against food borne pathogens Nanotechnology Life Science (pp. 5–14). Weinheim: Wiley-VHC.
- Kumar, R., Shukla, S. K., Pandey, A., Srivastava, S. K., & Dikshit, A. (2015) Copper oxide nanoparticles: An antidermatophytic agent for Trichophyton spp. Nanotechnology Reviews, 4, 401–409.
- Mishra, R. K., Mishra, V., Pandey, A., Tiwari, A. K., Pandey, H., Sharma, S., & Pandey, A. (2016). Exploration of anti-malassezia potential of Nyctanthes arbor-tristis L. and their application to combat the infection caused by Mala s1 a novel allergen. BMC Complementary and Alternative Medicine, 16, 47. doi: 10.1186/s12906-016-1092-2
- Pandey, H., Parashar, V., Parashar, R., Prakash, R., Ramteke, P. W., & Pandey, A. C. (2011). Controlled drug release characteristics and enhanced antibacterial effect of graphene nanosheets containing gentamicin sulfate. Nanoscale, 3, 4104–4108.10.1039/c1nr10661a
- Pandey, A., Mishra, R. K., Tiwari, A. K., Kumar, A., Bajaj, A. K., & Dikshit, A. (2013). Management of cosmetic embarrassment caused by Malassezia spp. with fruticose lichen Cladia using phylogenetic approach. Biomed Research international, 2013, 169794. doi:10.1155/2013/169794
- Pandey, M., Pandey, A., Shukla, S. K., Kumar, R., Pathak, A., Mishra, R. K., & Dikshit, A. (2016) A comparative analysis of in vitro growth inhibition of waterborne bacteria with bioactive plant Lippia nodiflora L. and camphor, Desalination and Water Treatment. doi:10.1080/19443994.2016.1160436
- Pathak, A., Shukla, S. K., Pandey, A., Mishra, R. K., Kumar, R., & Dikshit, A. (2015) In vitro antibacterial activity of ethno medicinally used lichens against three wound infecting genera of Enterobacteriaceae. Proceedings of the National Academy of Sciences, India, Section B: Biological Sciences. doi:10.1007/s40011-015-0540-y
- Shukla, S. K., Kumar, R., Mishra, R. K., Pandey, A., Pathak, A., Zaidi, MGH, … Dikshit, A. (2015). Prediction and validation of gold nanoparticles (GNPs) on PGPR: A step toward development of nano-biofertilizers. Nanotechnology Reviews, 4, 439–448.
- Sugunan, A., Thanachayanont, C., Dutta, J., & Hilborn, J. G. (2005). Heavy-metal ion sensors using chitosan-capped gold nanoparticles. Advanced Materials, 6, 335–340.
- Tamura, K. M., & Kumar, S. (2004). Prospects for inferring very large phylogenies by using the neighbour-joining method. Proceedings of the National Academy of Sciences of the United States of America, 101, 11030–11035.
- Tamura, K., Dudley, J., Nei, M., & Kumar, S. (2004). MEGA 4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596–1599.
- Yamamoto, O. (2001). Influence of particle size on antibacterial activity of zinc oxide. International Journal of Inorganic Materials, 3, 643–646.