?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In the present study, the molecular interaction between ethanolic extract of drug Piper nigrum with (metal ions) ZnCl2 have been investigated by ultrasonic method. The ultrasonic velocity (U), density (ρ), and viscosity (η) of an ethanolic extract of drug with metal ions of different concentration (number of moles of drug = 1.4018, 2.1027, 2.8036, and 3.5045) and different temperature (T = 308.15, 313.15, 318.15, 323.15, and 333.15) K at 2 MHz have been measured through ultrasonic interferometer. From experimental parameters (ultrasonic velocity (U), density (ρ) and viscosity (η)), various thermoacoustical parameters such as adiabatic compressibility (β), intermolecular free length (Lf), specific acoustic impedance (Z) has been calculated. The change in concentration of drug P. nigrum and varying temperature affects compressibility of solution, which gives idea about molecular interactions in liquid mixtures. Obtained experimental and thermoacoustical parameters are used to investigate the solute–solvent interactions between P. nigrum and ZnCl2 ions which is useful to understand the mechanism of their metabolism in living systems. The conclusion drawn from the ultrasonic investigation is the mixture act as structure breaker and there is a complex formation between ZnCl2 and P. nigrum for the system as a function of concentration and temperature. The results obtained from these studies are helpful for pharmacological applications of drugs, transport of drugs across biological membranes.
Public Interest Statement
Synthesis and ultrasonic investigation of herbal drug is an emerging field for the researchers working in the area of pharmaceuticals. The paper deals with the hot extraction synthesis of drug Piper nigrum which belongs to Piperaceae family. Ultrasonic and thermoacoustic parameters are used to investigate the intermolecular interaction between drug and metal ions. Zn++ ions play an important role in digestive system. Therefore, Zn++ ions are selected to study the interaction with P. nigrum as an antioxidant. The influences of drug concentration and temperature have been systematically investigated. The liquid mixture of P. nigrum and metal ion have been investigated using experimental parameters such as (ultrasonic velocity (U), density (ρ), and viscosity (η)), various thermoacoustic parameters such as adiabatic compressibility (β), intermolecular free length (Lf), and specific acoustic impedance (Z) measurements. The results obtained from these studies are helpful for pharmacological applications of drugs, transport of drugs across biological membranes.
1. Introduction
The study of molecular interaction between the components of binary and ternary liquid mixtures, using ultrasonic speed and thermodynamic parameters derived from it, has been the aim of several earlier researchers (Davis, Fjellanger, & Hiland, Citation1997; Hawrylak, Gracie, & Palepu, Citation1998; Pal, Sharma, & Dass, Citation1999; Tamura, Sonoda, & Murakami, Citation1999). Since most of the biochemical processes are hosted in liquid media, the physicochemical investigation of bioactive molecules in such media becomes more necessary. Bioactive molecules like sugars, amino acids, and drug supply gives useful physicochemical information to understand the complex mechanism of molecular interactions. Ultrasonic technique has been employed to investigate the properties of any substance to understand the nature of molecular interactions in pure liquid (Jacobson, Citation1952), liquid mixtures (Kannappan & Santhi, Citation2006), solutions (Nithya, Nithiyanantham, Mullainathan, & Rajasekaran, Citation2009), aqueous (Sarvazyan, Kharakoz, & Hemmes, Citation1979), non-aqueous (Keller, Legendziewicz, Gliński, & Samela, Citation2000), and mixed electrolytic solutions (Murty, Citation1964), and has led to new insights into the process of ion–ion and ion–solvent interactions. These measurements are used to estimate the different elastic properties of the molecules from which the type of molecular interactions can be very well understood. For understanding the solute–solvent and solute–solute interactions in solution, thermodynamic and physicochemical parameters are very much useful and applicable (Nain & Lather, Citation2013; Nain, Lather, & Sharma, Citation2011; Pal & Chauhan, Citation2010; Sadeghi & Gholamireza, Citation2011; Usmani, Citation2012). These physicochemical parameters are used to estimate the different elastic properties of the molecules from which the type of molecular interactions can be very well understood. Ultrasound is regarded as being of low intensity when there no permanent change takes place in the material during propagation of ultrasonic waves. This is the uniqueness of the ultrasonic method over other diffraction methods such as dielectrics (Hill, Citation1969; Pethig, Citation1992), Raman effect (Logan, Citation1989; Tanabe & Hiraishi, Citation1982), IR (Awasthi & Shukla, Citation2003; Wang, Wu, Xuan, & Wang, Citation2002), and ultrasonic (Ali, Hyder, & Nain, Citation1999; Fakruddin, Sarma, Srinivasu, & Narendra, Citation2014). Ultrasonic measurements of acoustic parameters to change the number of moles give an insight into the molecular process (Bhandakkar, Bhat, Chimankar, & Asole, Citation2014). Stabilization of bioactive molecules (drugs) is related to various molecular interactions, further these interactions are influenced by surrounding solute and solvent molecules. Due to such molecular interaction properties of drugs such as solubility, drug activity, and alternation of solvent structure, these are affected by the presence of solutes.
Herbs (generally called as herbal medicine) can be used as a drug because they are natural products, easily absorbed by our bodies and generally do not have any adverse effects. But herbal medicines are used in their extracted form. Term extraction is used for pharmaceuticals, involves the separation of medicinally active portions of plant or animal tissues from the inactive or inert components using selective solvents in standard extraction procedures. The significance of herbal extract compared to synthetic drugs is that herbal drugs are absorbed by the body very quickly, especially in older adults (Aswale, Aswale, Gowardipe, & Yavatmal, Citation2015). The medicinal plants are an important source of mineral, vitamin, and trace element and regarded as a potentially safe drug. Molecular interaction studies of the metal ions like Na++, K+, Ca++, Zn++, Cu++, and Fe++ with antioxidant drugs (curcumin, Piper longum) and their derivatives will be useful in understanding the mechanism of action of antioxidants in the living system. Identification of interaction between metal ions with drug become more interesting due to the complex structure of drug and their activities involve interaction with biological membranes (Iqbal & Chaudhry, Citation2009). To interpret the physicochemistry of biological systems, it is essential to examine the properties of drug with metal ions. Only a few physicochemical studies of antioxidant herbal drugs have been outlined so far (Pandey, Srivastava, Shukla, & Saksena, Citation2011). Therefore, we have undertaken a systematic study on the ultrasonic investigation of an antioxidant ethanolic extract of P. nigrum with zinc chloride to investigate interactions present in that system. A vital role is played by drug–metal ion interactions in all the metabolic pathways or biological processes occurring inside the body. Studies of such interactions led to modern drug discovery. Zinc is released from food as free ions during digestion and hence plays a major role in the digestive system and P. nigrum has medicinal properties in diseases like chronic indigestion (Roohani, Hurrell, Kelishadi, & Schulin, Citation2013). Therefore, Zn++ ions are selected to study the interaction with P. nigrum as an antioxidant.
1.1. Drug description (P. nigrum)
P. nigrum (C17H19NO3) is an antioxidant used against digestive system diseases which occur commonly nowadays mainly in adults, such as chronic indigestion, colon toxins, various gastric ailments, indigestion, and diarrhea (Davidson & Branden, Citation1981; Mukhopadhyay, Citation2000; Peter, Citation2012). P. nigrum is one of the most popular spice products in oriental countries. P. nigrum has the ability to stimulate digestion and reduces painful swelling caused by tissue injury (Srinivasan, Citation2005). P. nigrum is commonly known as black pepper (Ravindran, Citation2000). Black pepper is the dried fruit of P. nigrum Linn. and belongs to flower vine of piperaceae family (Jirovetz, Buchbauer, Ngassoum, & Geissler, Citation2002). It is native to India and has been a prized spice since ancient times. The main chemical constituent of P. nigrum is the alkaloid piperine (a trans–trans isomer of 1-piperoyl piperidine), which has many pharmacological properties (Nakatani, Inatani, Ohta, & Nishioka, Citation1986). It is used for the treatment of diarrhea and constipation in ancient times till present. Recent studies revealed that it has many other effects of pharmacological importance, including antimicrobial, antioxidant, and mainly as bio-enhancer which promotes the absorption of many drugs and nutritional supplements. The bio-enhancing activity is due to its inhibitory activity of drug metabolizing enzyme (Cytochrome P450 3A4) abbreviated as CYP3A4 is an important enzyme in the body (Gayasuddin & Iqbal, Citation2013).
2. Materials and methods
2.1. Preparation of the extract from Piperine Seeds
The pure dried seeds of Piperine of good quality were purchased from the local market and used as such. Seeds were ground to a coarse powder. Fifty grams of powdered seed were boiled with half liter of ethanol in a conical flask for 30 min, cooled at room temperature, and then it is decanted. In ethanolic extraction of P. nigrum, particles of coarse powder of P. nigrum are removed by filtration process and a clear solution of ethanolic extraction of drug P. nigrum is obtained. The solutions were prepared by adding a known molecular weight of zinc chloride in ethanol for the preparation of one molar solution of zinc chloride in ethanol using a magnetic stirrer until a clear solution was obtained. Then known numbers of moles of drug (Piperine) were added into a fixed volume of solvent.
Proper calibration of each temperature was achieved with double-distilled water as standard. After mixing the sample, the bubble-free homogeneous sample was transferred into the measuring cell of the interferometer which is specially designed as a double-walled vessel with provision to circulate water at required temperature. Ultrasonic velocity of P. nigrum extract with metal ion over the composition range of 1.4018, 2.1027, 2.8036, and 3.5045 number of moles of the drug was measured using a single-frequency interferometer (Model F-81) with a high degree of accuracy operating at a frequency of 2 MHz supplied by Mittle Enterprises, New Delhi at temperatures 308.15, 313.15, 318.15, 323.15, 328.15, and 333.15 K. The high-frequency generator excites a quartz crystal fixed at the bottom of the vessel, at its resonant frequency. A fine micrometer screw at the top of the cell is used to raise or lower the reflector plate in the liquid through a known distance. The measuring cell is connected to the output terminals of the high-frequency generator through a cable. Ultrasonic waves normal to quartz crystal are reflected from the reflector plate. Stationary waves are formed in the region between the reflector plate and the quartz crystal. The micrometer is slowly moved till a number of maximum readings (N) of the anode current passed. The total distance (d) moved by the micrometer is noted. The velocity of ultrasonic waves in the pure liquid and liquid mixture was determined using the relation.(1)
(1)
where = wavelength of the ultrasonic waves in the liquid mixture and f is the frequency of the generator (2 MHz).
The density (ρ) of the liquid mixture was determined by a specific gravity bottle of 25-ml capacity. The specific gravity bottle with the liquid mixture was immersed in a temperature controlled water bath. The density was determined using the relation.(2)
(2)
where w1, w2, ρ1, and ρ2 are the mass of distilled water, mass of liquid mixtures, density of distilled water, and density of liquid mixture, respectively.
The viscosity of (η) of pure liquids and liquid mixture was determined by an Ostwald viscometer containing the liquid, which was immersed in a temperature-controlled water bath, and the time of flow was measured by a stop watch. The viscosity was determined using the relation.(3)
(3)
where η1, η2, t1, and t2 are the coefficient of viscosity of distilled water, coefficient of viscosity of liquid mixtures, time of flow of distilled water, and time of flow of liquid mixture, respectively.
In this way, preparation and ultrasonic investigation of an extract of P. nigrum from its seeds is shown in the flow chart of methodology.
Piperine is the major component and it belongs to alkaloids group. In the ethanolic extract of P. nigrum, the presence of piperine is confirmed by Wagner’s test (Firdouse & Alam, Citation2011).
Wagner’s test: 1 ml of extract was added to 2 ml of Wagner’s reagent (2 g of iodine + 6 g of KI in 100 ml of water). A reddish brown (ppt) precipitate was obtained which confirms the presence of alkaloids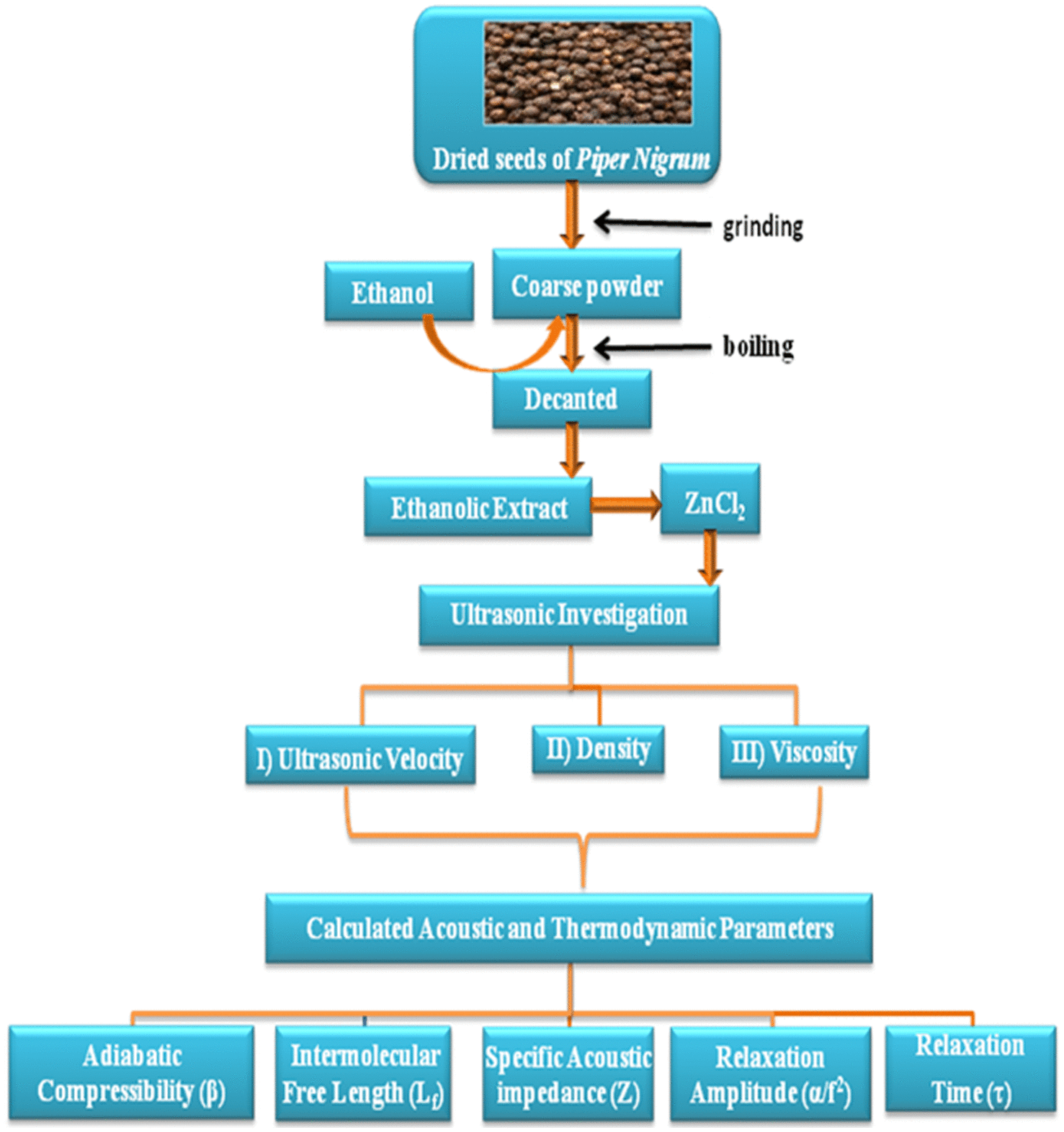 .
.
3. Theory and calculations
3.1. Physical parameters
The various physical parameters were calculated from the measured values using the standard formulae:
3.1.1. Adiabatic compressibility (β)
The adiabatic compressibility (β) has been calculated from the ultrasonic velocity (U) and density (ρ) of the medium using the equation as follows:(4)
(4)
Adiabatic compressibility is a measure of intermolecular association or dissociation or repulsion. The structural arrangement of the molecule affects the adiabatic compressibility.
3.1.2. Specific acoustic impedance (Z)
The specific acoustic impedance is given by following equations, where (U) and (ρ) are the ultrasonic velocity and density of the liquid, respectively.(5)
(5)
Specific acoustic impedance supports the possibility of strong interaction between unlike molecules.
3.1.3. Intermolecular free length (Lf)
(6)
(6)
where K is the temperature-dependent constant called Jacobson constant and it is 2.075 × 10−6 for 303.15 K
Intermolecular free length calculates the distance between the surfaces of the neighboring molecules which gives the molecular distance in terms of free length.
3.1.4. Relaxation time (τ)
The relaxation time can be calculated from the relation as follows:(7)
(7)
where β and η are the adiabatic compressibility and viscosity of the liquid mixture, respectively.
Relaxation time is time taken for the excitation energy to appear as translational energy and it depends on temperature and impurities. So, this parameter gives the idea about impurities present in liquid mixture.
3.1.5. Relaxation amplitude (α/f2)
The relaxation amplitude can be calculated from the relation as follows:(8)
(8)
where (η) and (ρ) are the viscosity and density of the liquid mixture, respectively.
The variation of relaxation amplitude in each curve with concentration strongly supports the presence of strong intermolecular interactions between the constituent molecules of this binary liquid system.
4. Results and discussions
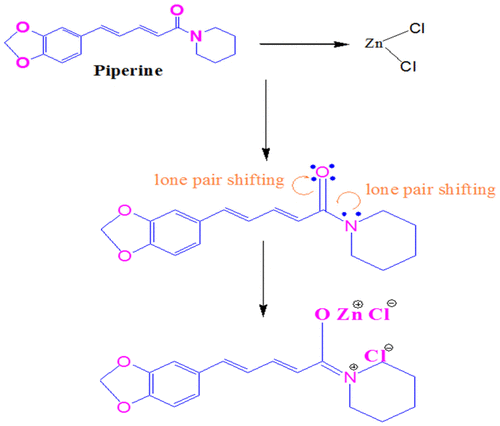
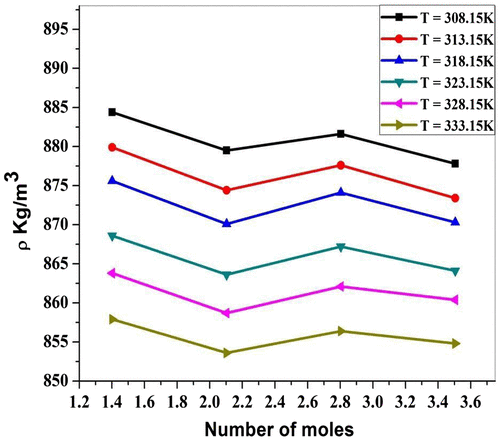
Figure shows the resonating structure of Piperine. When ethanolic extract of P. nigrum is added to ZnCl2 solution, the lone pair of electron on the N atom occurs in the carbonyl group (drug) and further carbon–oxygen bond attaches to Cl− ion and there is formation of salts. Similarly, due to the lone pair shifting on O atom, Zn+ and Cl− ions are attracted toward atom O. The values of ultrasonic velocity for various concentrations (number of moles of drug = 1.4018, 2.1027, 2.8036, and 3.5045) and at various temperatures (T = 308.15, 313.15, 318.15, 323.15, and 333.15) K are listed in Table . It is evident from Table and Figure that ultrasonic velocity increases linearly with an increase in the number of moles of ethanolic extract of P. nigrum in ZnCl2 solution. The increase in ultrasonic velocity can be attributed to the increase in molecular interaction between P. nigrum and zinc metal ion. Similar observations of ultrasonic velocity have been reported in the literature (Awasthi & Shukla, Citation2003). The linear variation of ultrasonic velocity with concentration indicates the occurrence of complex formation between ZnCl2 and P. nigrum. Chemical interaction may involve due to the association between the ion and dipole molecule. It is evident from Table and Figure that there is a decrease in ultrasonic velocity with an increase in temperature. This relation shows the structure breaker behavior of molecules suggesting the decrease in ion–solvent interaction between molecules with the increase in temperature. Thermal energy facilitates the breaking of bonds between associated molecules of the system. This weakens the molecular forces between the metal ion and drug molecules and hence decreases the ultrasonic velocity (Dorman & Deans, Citation2000). The decreasing trend suggests that available thermal energy used for breaking of bonds between the molecules. Thermal energy weakens the molecular forces which tend to decrease in ultrasonic velocity. Density of all concentrations of P. nigrum in ZnCl2 is listed in Table . It is observed from Table that the density decreases with the increase in concentration of P. nigrum in ZnCl2 except at 2.8036 mol concentration. The decrease in density can be attributed to the increase in molecular interaction between P. nigrum and zinc metal ion. It means molecular interaction increases as the concentration of drug increases, but at 2.8036 mol concentration, it decreases for all temperatures. Figure shows the variation of density as a function of concentration. It can be seen from Figure that the density decreases with P. nigrum concentration which suggests that the existed intermolecular interaction becomes weak with increasing concentration. Density is a measure of solute–solvent and ion–solvent interactions. Density of various temperatures of P. nigrum with Zn++ is listed in Table and shown graphically in Figure . It is observed from Table that density decreases with an increase in temperature of P. nigrum with Zn++ ions. The decrease in density can be attributed to the weakness of intermolecular forces between metal ions and drug molecules (Sankarappa, Prashant kumar, & Ahmad, Citation2005).
Table 1. Ultrasonic velocities (U), densities (ρ), and viscosities (η) for ZnCl2 + Piper nigrum system at 308.15 and 313.15, 318.15, 323.15, 328.15, and 333.15 K
Figure 2a. Ultrasonic velocity (U) for ZnCl2 + Piper nigrum at 308.15, 313.15, 318.15, 323.15, 328.15, and 333.15 K.
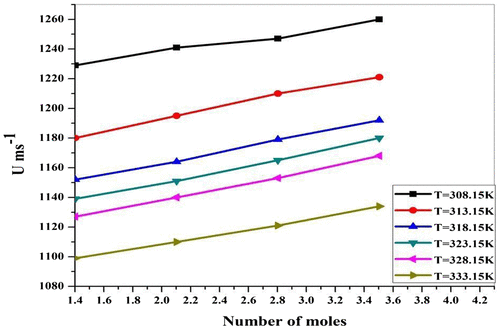
Figure 2b. Density (ρ) for ZnCl2 + Piper nigrum at 308.15, 313.15, 318.15, 323.15, 328.15, and 333.15 K
Viscosity (η) of P. nigrum and zinc chloride has been determined using Ostwald viscometer. It is observed from Table that viscosity increases with an increase in concentration of ethanolic extract of P. nigrum in ZnCl2. From Figure , it is seen that the value of viscosity increases with the increase in concentration of P. nigrum drug except 3.5045 mol concentration. It suggests that the existed intermolecular interaction becomes weak with increasing concentration except at 3.5045 mol value. The same behavior of viscosity observed in molecular interaction of ascorbic acid and aqueous saccharide solution (Nithya et al., Citation2009). Viscosity (η) of P. nigrum with Zn++ ions has been determined using Ostwald viscometer. It is observed from Table and Figure that viscosity decreases with an increase in temperature. (Singh & Quraishi, Citation2012) suggest that as the temperature of the solution increases the ordered structure of molecule breaks and therefore more spacing is formed between the molecules. Hence, the intermolecular forces decreases due to the increase in thermal energy of the system, which causes an increase in the volume expansion and hence viscosity decreases with temperature. Relation concludes the structure-breaking tendency of metal ions for the cluster of drug molecules. From the experimental data as given in Table , various acoustic (concentration-dependent) and thermodynamic parameters (temperature-dependent) such as adiabatic compressibility (β), specific acoustic impedance (Z), intermolecular free length (Lf), relaxation time (τ), and relaxation amplitude (α/f2) were calculated using standard Equations (1, 2, 3, 4, and 5). The adiabatic compressibility decreases with the increase in concentrations of P. nigrum as shown in Figure . It indicates that there is strong solute–solvent interaction. The solution becomes more and more compressed as adding the concentration of ethanolic extract of P. nigrum. The same behavior was observed by (Aswale, Aswale, Dhote, & Tayade, Citation2011) in the ultrasonic investigation of molecular interaction in paracetamol solution at different concentrations. Adiabatic compressibility increases with an increase in the temperature of P. nigrum with Zn++ ions as shown in Table and Figure . As a result, weak ion–dipole interactions exit with the increase in temperature due to structure-breaking nature. The same behavior was observed (Naik & Bawankar, Citation2014).
Figure 2c. Viscosity (η) for ZnCl2 + Piper nigrum at 308.15, 313.15, 318.15, 323.15, 328.15, and 333.15 K.
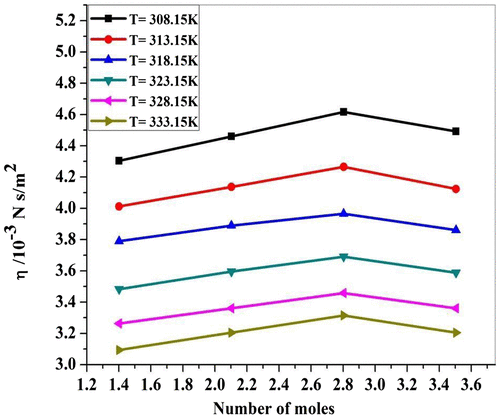
Figure 3a. Adiabatic compressibility (β) for ZnCl2 + Piper nigrum at 308.15, 313.15, 318.15, 323.15, 328.15, and 333.15 K.
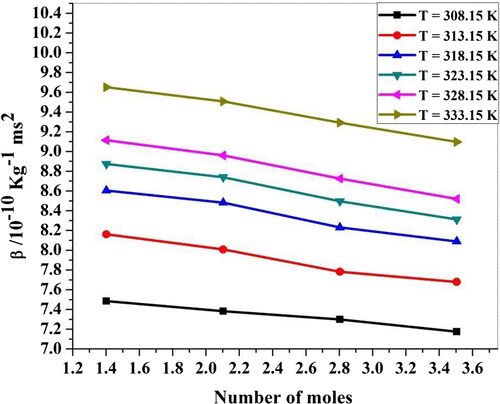
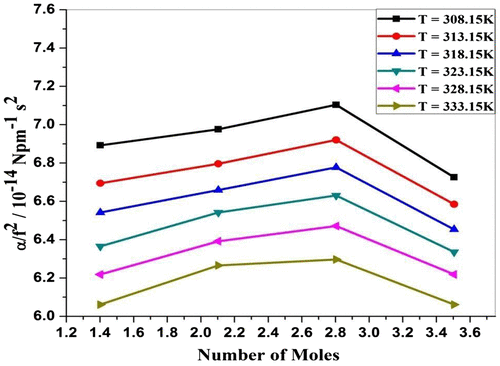
Table 2. Adiabatic compressibilities (β)/10–10 kg−1 ms2, intermolecular free lengths (Lf) /10–11 m, specific acoustic impedance (Z)/105 Kgm−2 s−1, relaxation amplitude (α/f2)/10–14 Npm−1 s2, and relaxation time (τ) / 10–12 s for ZnCl2 + Piper nigrum system at 308.15, 313.15, 318.15, 323.15, 328.15, and 333.15 K
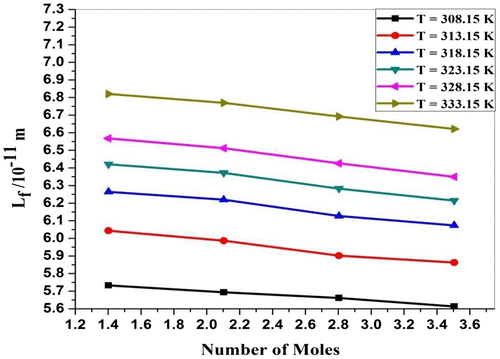
The values of intermolecular free length (Lf) shown in Table and graphically represented in Figure . It is observed that the values of intermolecular free length decrease with an increasing concentration of P. nigrum. The observed behavior shows that there is an enhanced molecular association which is similar for antibiotic drug ampicillin sodium system (Ali, Yasmin, & Nain, Citation2002). It is observed from Figure that the values of intermolecular free length increase with the increase in temperature. This relation is due to the thermal expansion of component molecules of the solutions. This suggests that there is weak molecular interaction between ions and solvent molecules (Aswale, Aswale, & Dhote, Citation2013).
Figure 3b. Intermolecular free length (Lf) for ZnCl2 + Piper nigrum at 308.15, 313.15, 318.15, 323.15, 328.15, and 333.15 K.
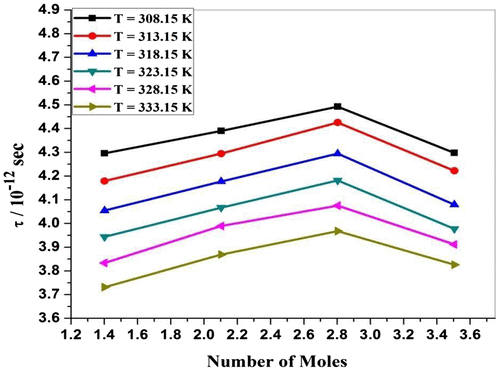
Specific acoustic impedance (Z) is governed by inertial and elastic properties of the medium. Table and Figure show that the value of specific acoustic impedance increases with an increase in concentration. The increase in specific acoustic impedance may be due to the increase in molecular interaction between P. nigrum and zinc metal ion. It shows that there is an increase in interactions within P. nigrum and Zn++ metal ions. The same behavior was observed by Ramteke and group (Ramteke, Chimankar, Mandal, & Tabhane, Citation2015).Specific acoustic impedance (Z) is governed by inertial and elastic properties of the medium. Table and Figure show that the variation of specific acoustic impedance (Z) against temperature is found to be decreased. The decrease in specific acoustic impedance may be due to molecular interaction between P. nigrum with Zn++ ions. This confirms the change in the presence of molecular association between the solvent and solvent molecules (Thakur & Chauhan, Citation2011). From Figure , it is observed that the viscous relaxation time increases with increase in concentration of ethanolic extract of P. nigrum except 3.5045 mol value. This indicates the presence of molecular interaction between Zn++ ions and P. nigrum. From Figure , it is observed that the viscous relaxation time (τ) decrease with the increase in temperature of solution except 3.5045 mol value. This indicates the presence of molecular interaction among ZnCl2 and P. nigrum. This is due to structural relaxation process and in such a situation, it is suggested that, the molecule gets rearranged due to co-operative process (Aswale, Aswale, & Hajare, Citation2012).
Figure 3c. Specific acoustic impedance (Z) for ZnCl2 + Piper nigrum at 308.15, 313.15, 318.15, 323.15, 328.15, and 333.15 K.
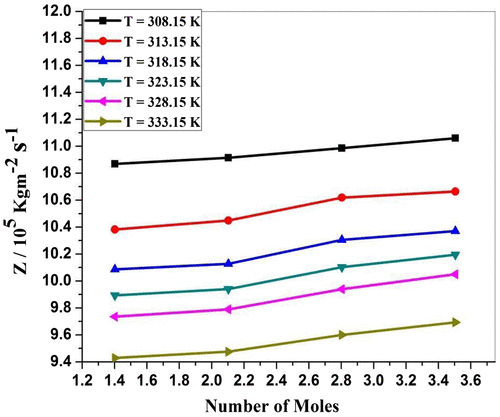
Figure 3d. Relaxation time (τ) for ZnCl2 + Piper nigrum at 308.15, 313.15, 318.15, 323.15, 328.15, and 333.15 K.
The values of relaxation amplitude for various concentrations of P. nigrum are listed in Table . Relaxation amplitude (α/f2) increases with the increase in concentration except 3.5045 mol concentration as shown in Figure , which suggests the existence of interaction between P. nigrum and Zn++ ions. The increase in relaxation amplitude can be attributed to the increase in molecular interaction between P. nigrum and zinc metal ion. The values of relaxation amplitude for various temperatures of P. nigrum with Zn++ ions are listed in Table . Relaxation amplitude (α/f2) decreases with an increase in temperature as shown in Figure , which suggests interaction between P. nigrum and the metal ions. These values are in a decreasing trend in all temperatures (Bhat, Manjunatha, & Varapresad, Citation2001).
5. Conclusion
It can be concluded from the above experimental data of ultrasonic velocity (U), density (ρ), and viscosity (η) that the interferometer technique requires minimum efforts. It is a direct method and has its own identity and significance in material science, which can give an idea about the effectiveness of solvent. The extraction of P. nigrum is successfully prepared and same is used to reconstitute in an ethanolic molar solution of ZnCl2 with different concentration and temperature. As the change in concentration and temperature affects compressibility of solution, this, in turn, affects molecular interactions in liquid mixtures and solutions. Thus, the conclusion drawn from all the studies is that an ethanolic extract of P. nigrum when added to ethanolic Zn++ then drug acts as a structure breaker and there is a complex formation between ZnCl2 and P. nigrum for the system as a function of concentration and temperature. Calculated acoustic and thermodynamic parameters also support the existence of drug–solvent interactions. The results obtained from these studies can thus be helpful for pharmacological applications of drugs as well as to understand pharmacokinetics processes such as transport of drug across biological membranes, drug action, and physicochemical properties.
Additional information
Funding
Notes on contributors
Pallavi B. Nalle
Pallavi B Nalle is working as a research student in physics department at Dr. Babasaheb Ambedkar Marathwada University, Aurangabad. She has published 10 research articles in conferences as well as journals.
K.M. Jadhav
Dr K M Jadhav is working as a professor in physics department at Dr. Babasaheb Ambedkar Marathwada University, Aurangabad (MS), India. He is an Ex-head department of Physics, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, (MS), India. He has 25 years of research experience. He published more than 306 papers in national/international journals. He also completed three research projects. He published three books. Thirty-eight research students have completed their PhD under his supervision. The students are at distinguished posts as principal, registrar, and postdoctoral fellowships. He acted as PhD and MPhil dissertation expert for various universities. He granted two patents: “Innovation Patent by Australian Government” and “Innovation Patent by Government of India.”
Authors have pursued research on influence of drug Piper nigrum with different metal ions through ultrasonic investigation which is helpful for pharmacological actions.
References
- Ali, A., Hyder, S., & Nain, A. (1999). Studies on molecular interactions in binary liquid mixtures by viscosity and ultrasonic velocity measurements at 303.15 K. Journal of Molecular Liquids, 79, 89–99.10.1016/S0167-7322(98)00105-6
- Ali, A., Yasmin, A., & Nain, A. (2002). Study of intermolecular interactions in binary liquid mixtures through ultrasonic speed measurement. Indian Journal of Pure and Applied Physics, 40, 315–322.
- Aswale, S., Aswale, S., Dhote, A., & Tayade, D. (2011). Ultrasonic investigation of molecular interaction in Paracetamol solution at different concentration. Journal of Chemical and Pharmaceutical Research, 6, 233–136.
- Aswale, S. S., Aswale, S. R., & Hajare, R. S. (2012). Adiabatic compressibility, intermolecular free length and acoustic relaxation time of aqueous antibiotic cefotaxime sodium. Journal of Chemical and Pharmaceutical Research, 4, 2671–2677.
- Aswale, S. S., Aswale, S. R., & Dhote, A. B. (2013). Adiabatic compressibility, free length and acoustic impedance of aqueous solution of Paracetamol. Journal of Natural Sciences, 1, 13–19.
- Aswale, S., Aswale, S. S., Gowardipe, V., & Yavatmal, W. D. (2015). Variation of acoustical parameters of herbal extract pomegranate solutions at frequency 4MHz. International journal of chemical and physical science, 4, 308–313.
- Awasthi, A., & Shukla, J. (2003). Ultrasonic and IR study of intermolecular association through hydrogen bonding in ternary liquid mixtures. Ultrasonics, 41, 477–486.10.1016/S0041-624X(03)00127-6
- Bhandakkar, V., Bhat, V., Chimankar, O., & Asole, A. (2014). Thermo acoustical study of Tetrahydrofuran with ethanol using ultrasonic technique at 323K. Advances in Applied Science Research, 5, 80–85.
- Bhat, J. I., Manjunatha, M., & Varapresad, N. (2001). Acoustic behaviour of citric acid in aqueous and partial aqueous media. Indian Journal of Pure and Applied Physics, 33, 1735–1764.
- Davidson, P., & Branden, A. (1981). Antimicrobial activity of non-halogenated phenolic compounds. Journal of Food Protection®, 44, 623–632.
- Davis, M. I., Fjellanger, I. J., & Hiland, H. (1997). Ultrasonic speeds and volumetric properties of binary mixtures of water with poly (ethylene glycol) s at 298.15 K. Journal of the Chemical Society, Faraday Transactions, 93, 1943–1949.
- Dorman, H., & Deans, S. (2000). Antimicrobial agents from plants: antibacterial activity of plant volatile oils. Journal of Applied Microbiology, 88, 308–316.10.1046/j.1365-2672.2000.00969.x
- Fakruddin, S., Sarma, N., Srinivasu, C., & Narendra, K. (2014). Study of molecular interactions in binary liquid mixture containing quinoline and cresol by using excess thermodynamic parameters at different temperatures. International Journal of Scientific & Engineering Research, 5, 54–59.
- Firdouse, S., & Alam, P. (2011). Phytochemical investigation of extract of Amorphophallus campanulatus tubers. International Journal of Phytomedicine, 3, 32–35.
- Gayasuddin, P., & Iqbal, G. V. (2013). Effect of ethanolic extract of Piper nigrum l. fruits on midazolam induced hypnosis in rats. International Journal of Pharmacology & Toxicology, 3, 5–8.
- Hawrylak, B., Gracie, K., & Palepu, R. (1998). Ultrasonic velocity and volumetric properties of isomeric butanediols plus water systems. Canadian Journal of Chemistry, 76, 464–468.10.1139/v98-032
- Hill, N. E. (1969). Dielectric properties and molecular behaviour. Ann Arbor, MI: Van Nostrand Reinhold.
- Iqbal, M. J., & Chaudhry, M. A. (2009). Thermodynamic study of three pharmacologically significant drugs: Density, viscosity, and refractive index measurements at different temperatures. The Journal of Chemical Thermodynamics, 41, 221–226.10.1016/j.jct.2008.09.016
- Jacobson, B. (1952). Ultrasonic velocity in liquids and liquid mixtures. The Journal of Chemical Physics, 20, 927–928.10.1063/1.1700615
- Jirovetz, L., Buchbauer, G., Ngassoum, M. B., & Geissler, M. (2002). Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction–gas chromatography, solid-phase microextraction–gas chromatography–mass spectrometry and olfactometry. Journal of Chromatography A, 976, 265–275.10.1016/S0021-9673(02)00376-X
- Kannappan, V., & Santhi, R. J. (2006). Ultrasonic investigation of induced dipole-induced dipole interactions in binary liquid mixtures at 298K. Indian Journal of Pure and Applied Physics, 44, 815.
- Keller, B., Legendziewicz, J., Gliński, J., & Samela, S. (2000). Investigation of the optical and ultrasonic properties of praseodymium and cerium chlorides in nonaqueous solutions. Journal of Alloys and Compounds, 300, 334–340.10.1016/S0925-8388(99)00762-8
- Logan, D. E. (1989). The Raman noncoincidence effect in dipolar binary mixtures. Chemical Physics, 131, 199–207.10.1016/0301-0104(89)80169-7
- Mukhopadhyay, M. (2000). Natural extracts using supercritical carbon dioxide. CRC press. Retrieved from http://www.crcnetbase.com/isbn/978142004169910.1201/9781420041699
- Murty, M. (1964). Ultrasonic studies in mixtures of electrolyte solutions (Vol. 33, p. 364). Bangalore: Current science.
- Naik, R. R., & Bawankar, S. V. (2014). Acoustical studies of molecular interactions in the solution of methyl cobalamine drug at different temperatures and concentrations. Orbital-The Electronic Journal of Chemistry, 6, 87–92.
- Nain, A. K., & Lather, M. (2013). Probing solute–solute and solute–solvent interactions in (l-arginine+d-xylose/l-arabinose+ water) solutions at different temperatures by using volumetric and viscometric methods. The Journal of Chemical Thermodynamics, 63, 67–73.10.1016/j.jct.2013.04.001
- Nain, A. K., Lather, M., & Sharma, R. K. (2011). Volumetric, ultrasonic and viscometric behavior of l-methionine in aqueous-glucose solutions at different temperatures. Journal of Molecular Liquids, 159, 180–188.10.1016/j.molliq.2011.01.010
- Nakatani, N., Inatani, R., Ohta, H., & Nishioka, A. (1986). Chemical constituents of peppers (Piper spp.) and application to food preservation: Naturally occurring antioxidative compounds. Environmental Health Perspectives, 67, 135–142.10.1289/ehp.8667135
- Nithya, R., Nithiyanantham, S., Mullainathan, S., & Rajasekaran, M. (2009). Ultrasonic investigation of molecular interactions in binary mixtures at 303K. Journal of Chemistry, 6, 138–140.
- Pal, A., & Chauhan, N. (2010). Interactions of diglycine in aqueous saccharide solutions at varying temperatures: A volumetric, ultrasonic and viscometric study. Journal of Solution Chemistry, 39, 1636–1652.10.1007/s10953-010-9620-z
- Pal, A., Sharma, S., & Dass, G. (1999). Ultrasonic speeds and volumetric properties of mixtures containing polyethers and 2-methoxyethanol at the temperature 298.15K. The Journal of Chemical Thermodynamics, 31, 273–287.10.1006/jcht.1998.0449
- Pandey, A., Srivastava, R., Shukla, A. K., & Saksena, A. (2011). Physico-chemical studies on molecular interactions of curcumin with mono and divalent salts at different temperature. International Journal of Smart Home, 5, 7–23.
- Peter, K. V. (2012). Handbook of herbs and spices (1st ed.). Woodhead Publishing. Retrieved from http://store.elsevier.com/Handbook-of-Herbs-and-Spices/isbn-9780857090409/
- Pethig, R. (1992). Protein-water interactions determined by dielectric methods. Annual Review of Physical Chemistry, 43, 177–205.10.1146/annurev.pc.43.100192.001141
- Ramteke, S., Chimankar, O., Mandal, R., & Tabhane, V. (2015). Investigation of acoustical behavior of dichlofenac sodium drug in ethanol at different temperature. International Journal of Science and Research, 1, 289–292.
- Ravindran, P. (2000). Black pepper: Piper nigrum. CRC Press. Retrieved from http://www.crcnetbase.com/isbn/9780203303870
- Roohani, N., Hurrell, R., Kelishadi, R., & Schulin, R. (2013). Zinc and its importance for human health: An integrative review. Journal of Research in Medical Sciences, 18, 144–157.
- Sadeghi, R., & Gholamireza, A. (2011). Thermodynamics of the ternary systems:(water+ glycine, l-alanine and l-serine+ di-ammonium hydrogen citrate) from volumetric, compressibility, and (vapour+ liquid) equilibria measurements. The Journal of Chemical Thermodynamics, 43, 200–215.10.1016/j.jct.2010.08.021
- Sankarappa, T., Prashant kumar, M., & Ahmad, A. (2005). Ultrasound velocity and density studies in some refined and unrefined edible oils. Physics and Chemistry of Liquids, 43, 507–514.10.1080/00319100500192889
- Sarvazyan, A., Kharakoz, D., & Hemmes, P. (1979). Ultrasonic investigation of the pH-dependent solute-solvent interactions in aqueous solutions of amino acids and proteins. The Journal of Physical Chemistry, 83, 1796–1799.10.1021/j100476a021
- Singh, A., & Quraishi, M. (2012). Journal of Chemical and Pharmaceutical Research, 4, 322–325.
- Srinivasan, K. (2005). Spices as influencers of body metabolism: an overview of three decades of research. Food Research International, 38, 77–86.10.1016/j.foodres.2004.09.001
- Tamura, K., Sonoda, T., & Murakami, S. (1999). Thermodynamic properties of aqueous solution of 2-isopropoxyethanol at 25°C. Journal of Solution Chemistry, 28, 777–789.10.1023/A:1021728430543
- Tanabe, K., & Hiraishi, J. (1982). Raman linewidth study of molecular interactions in mixtures of hexafluorobenzene with benzene and with mesitylene. Journal of Raman Spectroscopy, 12, 274–277.10.1002/jrs.v12:3
- Thakur, S. K., & Chauhan, S. (2011). A study of acoustical behavior of drug colimax in aqueous mixture of methanol at 25°C. Advances in Applied Science Research, 2, 208–217.
- Usmani, M. A. (2012). Interactions in (l-alanine/l-threonine+ aqueous glucose/aqueous sucrose) systems at (298.15–323.15) K. Thermochimica acta, 527, 112–117.
- Wang, J., Wu, Y., Xuan, X., & Wang, H. (2002). Ion–molecule interactions in solutions of lithium perchlorate in propylene carbonate+ diethyl carbonate mixtures: an IR and molecular orbital study. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 58, 2097–2104.10.1016/S1386-1425(01)00686-2