Abstract
Lathyrus sativus L., (grass pea) is an annual plant widely grown as a pulse crop and its dried seeds are harvested and consumed as human food since ancient times. This plant is also commonly grown for animal feed and forage. Grass pea seeds may represent a potential source of several important nutrients for human and animal nutrition. Fatty acid compositions of 173 different grass pea accessions have been studied. Present results indicate that total saturated fatty acids, total monounsaturated fatty acids, total polyunsaturated fatty acids, and total fatty acids ranged from 295.72 to 436.94, 113.19 to 170.78, 127.39 to 179.39, 538.04 to 778.98 mg 100 g−1, respectively. In addition, unsaturated fatty acids, oleic acid, linoleic acid, γ-linolenic acid, and α-linolenic acid that are the main components of fatty acids ranged from 109.22 to 163.95,59.57 to 82.98, 16.18 to 30.38, and 45.56 to 71.59 mg 100 g−1, respectively.
Public Interest Statement
Grasspea, an orphan legume of the arid areas is a good alternative and “insurance crop” in areas that are prone to abiotic stresses. Grass pea has a number of advantages in respect to its properties such as its high protein content, a high yield potential, low fertilization levels, its tolerance to flood or salinity, drought survival. This plant is also commonly grown for animal feed and forage. Grass pea seeds may represent a potential source of several important nutrients for human and animal nutrition. Although the seeds of L. sativus have been consumed for centuries as a legume, the plant is not intensively cultivated in Turkey. Recently, the interest in its cultivation has been increased, but still there is little information on the nutritional value of this legume. In this study, the fatty acid profile of 173 grass pea genotypes was determined. The results show that grass pea is rich in fatty acids and there is a wide variation between genotypes.
1. Introduction
Because of the ever-growing global population, the speedy depletion of natural resources and climate change and the demand–supply gap of foodstuff have been continuously increasing. This problem has become serious day by day as the current yield increases trends that may not be sufficient to cope with the growing foodstuff demand (Hanjra & Qureshi, Citation2010).
To meet foodstuff requirements for the future population is very important to increase the productivity of legumes which is the key sources of protein, minerals, vitamins, iron, zinc, calcium, and magnesium, as well as omega-3 fatty acids. The legume crops play a crucial role in sustaining the farming system by fixing atmospheric nitrogen. Legumes also serve as an excellent source of high-quality and nutritious feed to livestock that increases the animal productivity (Arslan, Citation2016; Hanbury, White, Mullan, & Siddique, Citation2000; Yan et al., Citation2006).
Legumes provide almost all essential minerals and organic nutrients to humans either directly, or indirectly when plants are consumed by animals, which are then consumed by humans (Grusak & DellaPenna, Citation1999). The widespread use of legumes makes this food group an important source of lipid, fatty acids, and protein in animal and human nutrition (Yoshida, Saiki, Yoshida, Tomiyama, & Mizushina, Citation2009). The scientific literature has shown the ability of legume to decrease the glycemic index and cholosterolomy which is due not only to the protective role of dietary fiber, but perhaps also to the favorable content of fatty acids (Pirman & Stibilj, Citation2003). Other research recommendations suggestthat intake of legumes should be increased for better health and management of chronic disease, such as cardiovascular disease, diabetes, and cancer (Chavan, Shahidi, Bal, & McKenzie, Citation1999).
Among legumes, grass pea is a popular drought- and flood-tolerant crop and foodstuff in drought-prone areas of world. Moreover, grass pea is also interesting for the local ecosystems, because it fits well on slopes and in drought periods, reducing soil erosion, and leading to the recovery of unproductive fields. The seeds of grass pea may be the rare food available during periods of famine (Zhao et al., Citation1999).
However, many countries focus on research to develop and improve a narrow range of staple crops, while relatively little attention is given to underutilized or neglected plants. Because, the excessive consumption of seeds of grass pea can cause a neurological disorder (lathyrism), which is believed to be caused by the non-protein amino acid β-N-oxalyl-L-α,β-diaminopropionic acid (β-ODAP) (Lambein, Khan, Kuo, Campbell, & Briggs, Citation1993; Yan et al., Citation2006). This substance, β-ODAP, was known to be primary problem many researchers (Akalu, Johansson, & Nair, Citation1998; Kumar, Bejiga, Ahmed, Nakkoul, & Sarker,Citation2011; Kuo, Bau, Rozan, Chowdhury, & Lambein, Citation2000; Patto et al., Citation2006; Zhao et al., Citation1999). On the other hand, grass pea, like other pulse crops, is valued and cultivated for its high protein content (25–27%) in the seeds (Hanbury et al., Citation2000). It compares favorably with other crops showing many of advantages from the agricultural point of view. Grass pea has a high yielding potential (regarding a pea level) at a low fertilization level (Campbell et al., Citation1994; Patto et al., Citation2006).
Therefore, some species of family Leguminosae including Lathyrus species have received considerable attention and their biochemical components (protein, fat, fatty acids, flavonoids) have been investigated (Akpinar, Ali Akpinar, & Türkoğlu, Citation2001; Emre, Şahin, Yilmaz, & Genç, Citation2010).
Among the biochemical components, seed oils with a substantial amount of very long chain of fatty acids have attracted attention because of their value for industrial purposes (Bauman et al., Citation1988). Furthermore, these compounds can be of chemotaxonomic significance. Fatty acids composition of seed lipids can serve as taxonomic markers in higher plants (Aitzetmüller & Tsevegsüren, Citation1994; Bağci, Genc, & Sahin, Citation2001; Harborne & Turner, Citation1984).
Grass pea seeds may represent a potential source of several important nutrients for human and animal nutrition. Therefore, it is necessary to analyze grass pea seeds for their nutrient composition (Chinnasamy, Bal, & McKenzie, Citation2005). This study was conducted to determine the fatty acid compositions of a total of 173 grass pea genotypes.
2. Materials and methods
2.1. Experimental materials
A total of 173 Lathyrus sativus L. genotypes of grass pea was used as a plant material. Among them, 92 genotypes were collected from the natural habitat of Antalya by BATEM (Western Mediterranean Agricultural Research Centre), 10 genotypes were ensured by the Yemen Ministry of Agriculture, 4 genotypes were provided by GAP International Agricultural Research and Education Centre, 20 genotypes were obtained from the Aegean Agricultural Institute Management Gene Center; 43 genotypes were provided by the Bahri Dagdas International Agricultural Research Institute, the Ceora cultivar was ensured by Prof.. Dr. Kadambot Siddique (The University of Western Australia), the İptaş, Karadağ and Eren cultivars were obtained from the Gaziosmanpaşa University Faculty of the Agriculture Department of Field Crops.
This work was conducted on the open field at BATEM in Antalya in Turkey (36°52′ N, 30°50′ E, and altitude 15 m) in 2016. The soil properties of growing area are given in Table . As seen from Table , this area is alkaline, calcareous, and low in organic matter.
Table 1. Soil analyses of experimental area
The seeds of each genotypes were planted on 4 December 2015 and were harvested on 24 May 2016. The seeds, obtained from these plants were analyzed for fatty acids at the Akdeniz University Center for Food and Agricultural Research Laboratory.
2.2. Fatty acids analyses
The clean seeds samples (100 g) of grass pea which are purified from impurities were ground using a blender before analysis.The fatty acids were extracted from 1 g of finely ground samples in accordance with Associaiton of Official Analytical Chemist (AOAC) method (AOAC, Citation2012). In brief, fatty acids were extracted with 10 ml hexane at room temperature in ultrasonic bath. About 1 ml of supernatant hexane was treated with 1 ml of methanolic NaOH at 80°C for 10 min in 15 ml glass tube with Teflon-lined screw cap. The tubes were cooled at room temperature, 2 ml iso-octane and 1 ml saturated sodium chloride were added, shaken vigorously, and left undisturbed forlayers to separate.
The upper hexane layer containing the Fatty Acid Methyl Ester (FAME) was transferred to a vial and stored at −20°C until further analysis by gas chromatography (GC).The FAME standard was obtained from Sigma-Aldrich (Germany). For the FAME analysis, a TRACE TR-Wax GC capillary column (30 m × 0.32 mm i.d.; Thermo Fisher Scientific Inc. Waltham, Massachussetts, USA) was used. The column was connected to a thermo gas chromatograph equipped with a mass selective detector (Thermo Fisher Scientific Inc. Waltham, Massachusetts, USA).The GC oven was kept at 60°C for 3 min, heated at 8°C/min up to 300°C, where it was kept for 1 min, and a total analytical time was 34 min. The carrier gas was helium (1 ml/min). The analysis of a sample by GC was carried out by injecting 2 μl of the sample solution into the GC. The formed methyl ester was identified by comparing its retention time to the retention time of standard methyl ester of fatty acid. Quantitative analysis of the formed methyl esters was determined with five points of FAME calibration curve. Each sample was analyzed in triplicate and the data were presented as mean mg/100 g dry and powdered seed material.
3. Results and discussion
As a result of fatty acid analysis; Caprylic acid (C8:0), capric acid (C10:0), undecanoic acid (C11:0), myristic acid (C14:0), myristoleic acid (C14:1), cis-10-pentadecenoic acid), Palmitoleic acid (C16:1), cis-10-heptadecenoic acid (C17:1), elaidic acid (C18:1n9t), linolelaidic acid (C18:2n6t), arachidic acid 11,14-eicosadienoic acid (C20:2), cis-8,11,14-eicosatrienoic acid (C20:3n6), cis-11,14,17-eicosatrienoic acid:3n3), arachidonic acid (C20:4n6), cis-5,8,11,14,17-eicosapentaenoic acid (C20:5n3), henicosanoic acid (C21:0), behenic acid 13,16-docosadienoic acid (C22:2), cis-4,7,10,13,16,19-docosahexaenoic acid (C22:6n3), tricosanoic acid (C23:0), lignoceric acid), and nervonic acid (C:24:1n9) have not been able to find at detectable level.
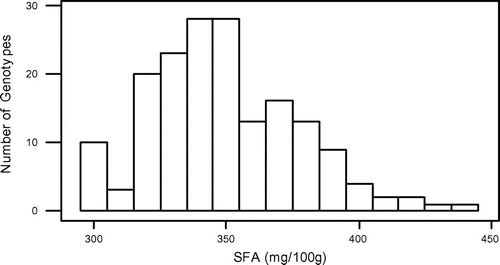
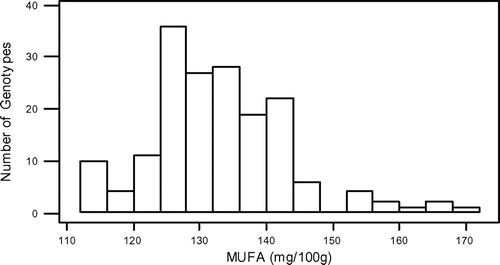
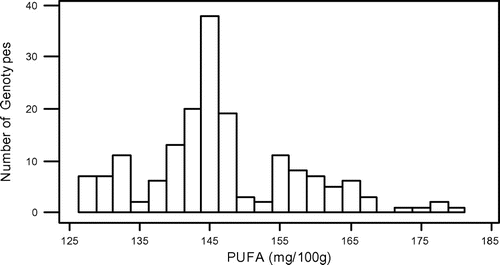
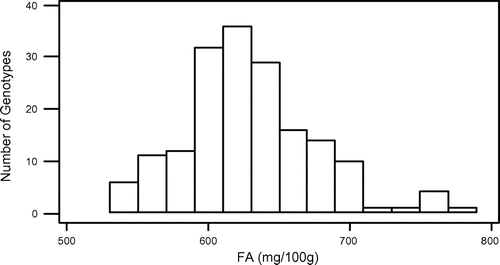
In this study, some fatty acids of 173 grass pea genotypes from Turkey were detected. All results of the saturated fatty acids, unsaturated fatty acids, and total fatty acids are shown in Table S1. Also, the minimum, maximum, and average amounts of 11 major fatty acids which belong to a total of 173 grass pea genotypes are shown in Table . When Table is evaluated, it is seen that there is a large variation in terms of the amount of fatty acids genotypes contain.
Table 2. Minimum, maximum and average fatty acid values of grass pea genotypes (mg 100 g−1 seeds)
In the present study, total fatty acids, SFA, MUFA, and PUFA contents of grass pea genotypes ranged from 538.04 to 778.98, 295.72 to 436.94, 113.19 to 170.78, and 127.39 to 179.39 mg 100 g−1, respectively. Also, in similar way, our results showed that they are inversely correlated between two acids. The mean contents of oleic acid were found to be high (127.76 mg 100 g−1), whereas the mean contents of linoleic acid wrer found to be low (69.11 mg 100 g−1).
In the result, they reported that grass pea seeds may be important for nutritional health and may serve as a valuable nutritional source. Further, in-depth study is necessary to elucidate the nutritional quality and importance of grass pea (Chinnasamy et al., Citation2005). Tamburino et al. (Citation2012), reported that the fatty acid composition showed that saturated acid content was higher (369.32 mg 100 g−1; 53.69%) than that of unsaturated acids (322.44 mg 100 g−1; 46.61%). This finding is similar to our research results.
The normality test for SFA, MUFA, PUFA, and total fatty acids showed that they were normally distributed (Figures –). Also, in our study, the mean SFA content of grass pea genotypes is higher than MUFA + PUFA. However, Kokten, Kaplan, Uzun, and Inci (Citation2015), Chinnasamy et al. (Citation2005) and Chavan et al. (Citation1999) reported that grass pea seeds showed higher MUFA + PUFA than SFA. On the other hand Daulatabad, Hosamani, Desai, and Alagawadi (Citation1987) reported that it can be said that fatty acid amounts of the Lathyrus sp., which are studied in general were similar in point of both SFA and USFA.
Oils rich in oleic and linoleic acids are the most adaptable of all oils and are excellent edible oils. These species might have potential to bea new oilseed crop for the food industry if growth and yield can be improved (Kokten et al., Citation2015). Nine samples of the legume seeds including Lathyrus sativus were analyzed for fatty acids and tocopherol contents. In the result, they reported that the legume seeds had profitable polyunsaturated fatty acids composition, especially from linoleic and linolenic acid point of view (Grela & Günter, Citation1995).
With regard to amounts of saturated and unsaturated fatty acids, a total of 173 grass pea genotypes showed that amounts of saturated fatty acids were slightly higher than unsaturated fatty acids. These findings confirmed data which were reported for Valle Agricola grass pea seeds (Tamburino et al., Citation2012), the polish grass pea seeds (Grela, Jensen, & Jakobsen, Citation1999), and others legumes (Grela & Günter, Citation1995).
Oleic and linoleic acid are the principal component acids (Daulatabad et al., Citation1987). The percentages of these two acids are inversely correlated—some of the legume oils are rich in linoleic acid, whereas in others oleic acid, it is present in larger amounts. In a similar way, our results showed that they are inversely correlated between two acids. The mean contents of oleic acid were found to be high (127.76 mg 100 g−1), whereas the mean contents of linoleic acid were found to be low (69.11 mg 100 g−1).
Linolenic acid was also detected in the seed oil of Lathyrus varieties, but at very low levels in all of the patterns when compared with linoleic and oleic acid. For edible purposes, oil should have a minimal amount of linolenic acid since linolenic acid is commonly thought to be the prime constituent responsible for undesirable flavors in stored oils and in food products containing vegetable oils (Wolff & Kwolek, Citation1971). According to our results, linolenic acid was also detected at low levels when compared with linoleic and oleic acid.
The nutritive value of seeds is determined by both quantity and quality of lipids they contain. Thus, fatty acids present in lipids are playing important role in shelf life, nutrition and flavor of food products (Gaydou, Rasoarahona, & Bianchini, Citation1983). Although lipids constitute a minor portion of many leguminous seeds, their profiles indicate the desirable nature of fatty acid constituents present (Chavan et al., Citation1999).
4. Conclusions
Although the seeds of L. sativus have been consumed for centuries as a legume, the plant is not intensively cultivated in Turkey. Recently, the interest in its cultivation has been increased, but still there are little informations on the nutritional value of this legume. The data obtained from our analyses emphasized Turkey’s grass pea seeds unsaturated fatty acids (TMUFA + TPUFA = 279.66 mg 100 g−1) and saturated fatty acids (349.47) and total fatty acids (629.10) as averagely. This large variation, which is found among the grass pea genotypes in terms of fatty acids, can be used as a selection criterion in future breeding trials.
Funding
The author received no direct funding for this research.
Revised_Table_S1._All_results.docx
Download MS Word (97.3 KB)Acknowledgements
The author gratefully acknowledges the Akdeniz University Scientific Research Project Unit. A special thanks would like to Dr Mehmet Oten from BATEM for providing plant materials and Mr Taner Erkaymaz from Akdeniz University, Food and Agricultural Research Laboratory for the helps in fatty acid analysis.
Additional information
Notes on contributors
Mehmet Arslan
Mehmet Arslan graduated from Akdeniz University, Faculty of Agriculture, Department of Field Crops in 2000 with a bachelor’s degree, in 2003 with his master’s degree and in 2008 with his PhD in the field of Agronomy and Breeding of Forage Crops. He is still working as a lecturer in Faculty of Agriculture, Department of FieldCrops, Akdeniz University. He deals with species and varieties of forage plants, which are tolerant to the effects of abiotic stress factors such as global climate change, drought, and salinity. In this respect, He is conducting researches which are based on agronomic, morphological, phenological characteristics in Lathyrus sativus genotypes, which are collected from nature. At the same time, he goes on with quality-based research on silage, an important food source for animal nutrition.
References
- Aitzetmüller, K., & Tsevegsüren, N. (1994). Seed fatty acids, “ FRONT – End” desaturases and chemotaxonomy - A case study in the ranunculaceae. Journal of Plant Physiology, 143, 538–543.10.1016/S0176-1617(11)81820-1
- Akalu, G., Johansson, G., & Nair, B. M. (1998). Effect of processing on the content of β-N-oxalyl-α, β-diaminopropionic acid (gb-ODAP) in grass pea (Lathyrus sativus) seeds and flour as determined by flow injection analysis. Food Chemistry, 62, 233–237.10.1016/S0308-8146(97)00137-4
- Akpinar, N., Ali Akpinar, M., & Türkoğlu, Ş. (2001). Total lipid content and fatty acid composition of the seeds of some Vicia L. species. Food Chemistry, 74, 449–453.10.1016/S0308-8146(01)00162-5
- Arslan, M. (2016). Importance and current situation of grass pea (Lathyrus sativus L.) in forage crops production of Turkey. Turkish Journal of Agricultural And Natural Sciences, 3, 17–23.
- Associaiton of Official Analytical Chemist. (2012). Official methods. 13 determination of labeled fatty acids content in milk products and ınfant formula. Retrieved December 15, 2016, from http://stakeholder.aoac.org./SPIFAN/2012.13.pdf
- Bağci, E., Genc, H., & Sahin, A. (2001). Fatty acid patterns of the seed oils of some Lathyrus species L. (papilionideae) from Turkey, a chemotaxonomic approach. Pakistan Journal of Biology, 4, 872–874.
- Bauman, H., Bühler, M., Fochem, H., Hirsinger, F., Zoebelein, H., & Falbe, J. (1988). Natural fats and oils—Renewable raw materials for the chemical industry. Angewandte Chemie, 100, 41–62.
- Campbell, C. G., Mehra, R. B., Agrawal, S. K., Chen, Y. Z., Abd-El-Moneim, A. M., Khawaja, H. I. T., Yadov, C. R., Tay, J. U., & Araya, W. A. (1994). Current status and future strategy in breeding grasspea (Lathyrus sativus). Euphytica, 73, 167–175.10.1007/BF00027192
- Chavan, U. D., Shahidi, F., Bal, A. K., & McKenzie, D. B. (1999). Physico-chemical properties and nutrient composition of beach pea (Lathyrus maritimus L.). Food Chemistry, 66, 43–50.10.1016/S0308-8146(98)00096-X
- Chinnasamy, G., Bal, A. K. & McKenzie, D. B. (2005). Fatty acid composition of grass pea (Lathyrus sativus L.) seeds. Lathyrus Lathyrism Newsletter, 4, 1–4.
- Daulatabad, C. D., Hosamani, K. M., Desai, V. A., & Alagawadi, K. R. (1987). Cyclopropenoid fatty acids in leguminosae oils. JAOCS, 64, 1423.
- Emre, İ., Şahin, A., Yilmaz, Ô., & Genç, H. (2010). Compositions of seed fatty acids in some Lathyrus taxa from Turkey. Acta Botanica Gallica, 157, 241–246.10.1080/12538078.2010.10516201
- Gaydou, E. M., Rasoarahona, J., & Bianchini, J. P. (1983). A micro-method for the estimation of oil content and fatty acid composition in seeds with special reference to cyclopropenoic acids. Journal of the Science of Food and Agriculture, 34, 1130–1136.10.1002/(ISSN)1097-0010
- Grela, E. R., & Günter, K. D. (1995). Fatty acid composition and tocopherol content of some legume seeds. Animal Feed Science and Technology, 52, 325–331.10.1016/0377-8401(94)00733-P
- Grela, E. R., Jensen, S. K., & Jakobsen, K. (1999). Fatty acid composition and content of tocopherols and carotenoids in raw and extruded grass pea (Lathyrus sativus L). Journal of the Science of Food and Agriculture, 79, 2075–2078.10.1002/(ISSN)1097-0010
- Grusak, M. A., & DellaPenna, D. (1999). Improvıng the nutrıent composıtıon of plants to enhance human nutrıtıon and health. Annual Review of Plant Physiology and Plant Molecular Biology, 50, 133–161.10.1146/annurev.arplant.50.1.133
- Hanbury, C. D., White, C. L., Mullan, B. P., & Siddique, K. H. M. (2000). A review of the potential of Lathyrus sativus L. and L. cicera L. grain for use as animal feed. Animal Feed Science and Technology, 87, 1–27.10.1016/S0377-8401(00)00186-3
- Hanjra, M. A., & Qureshi, M. E. (2010). Global water crisis and future food security in an era of climate change. Food Policy, 35, 365–377.10.1016/j.foodpol.2010.05.006
- Harborne, J.B., & Turner, B.L.. 1984. Plant chemosystematics (pp. 180–191). London: Academic Press.
- Kokten, K., Kaplan, M., Uzun, S., & Inci, H. (2015). Fatty acid and metal composition of the seeds of Lathyrus sativus varities. Chemistry of Natural Compounds, 51, 464–466.
- Kumar, S., Bejiga, G., Ahmed, S., Nakkoul, H., & Sarker, A. (2011). Genetic improvement of grass pea for low neurotoxin (β-ODAP) content. Food and Chemical Toxicology, 49, 589–600.10.1016/j.fct.2010.06.051
- Kuo, Y. H., Bau, H. M., Rozan, P., Chowdhury, B., & Lambein, F. (2000). Reduction efficiency of the neurotoxin b-ODAP in low toxin varieties of Lathyrus sativus seeds by solid state fermentation with Aspergillus oryzae and Rhizopus microspores var. chinensis. Journal of the Science of Food and Agriculture, 80, 2209–2215.10.1002/(ISSN)1097-0010
- Lambein, F., Khan, J. K., Kuo, Y., Campbell, C. G., & Briggs, C. J. (1993). Toxins in the seedlings of some varieties of grass pea (Lathyrus sativus). Natural Toxins, 1, 246–249.10.1002/(ISSN)1056-9014
- Patto, M. C.V, Skiba, B., Pang, E.C.K., Ochatt S.J., Lambein, F., & Rubiales D. (2006). Lathyrus improvement for resistance against biotic and abiotic stresses: From classical breeding to marker assisted selection. Euphytica, 147, 133–147.10.1007/s10681-006-3607-2
- Pirman, T., & Stibilj, V. (2003). An influence of cooking on fatty acid composition in three varieties of common beans and in lentil. European Food Research and Technology, 217, 498–503.10.1007/s00217-003-0784-2
- Tamburino, R., Guida, V., Pacifico, S., Rocco, M., Zarelli, A., Parente, A., & Maro, A. D. (2012). Nutritional values and radical scavenging capacities of grass pea (Lathyrus sativus L.) seeds in Valle Agricola district, Italy. Australian Journal of Crop Science, 6, 149–156.
- Yan, Z. Y., Spencer, P. S., Li, Z. X., Liang, Y. M., Wang, Y. F., Wang, C. Y., & Li, F. M. (2006). Lathyrus sativus (grass pea) and its neurotoxin ODAP. Phytochemistry, 67, 107–121.10.1016/j.phytochem.2005.10.022
- Yoshida, H., Saiki, M., Yoshida, N., Tomiyama, Y., & Mizushina, Y. (2009). Fatty acid distribution in triacylglycerols and phospholipids of broad beans (Vicia faba). Food Chemistry, 112, 924–928.10.1016/j.foodchem.2008.07.003
- Wolff, I. A., & Kwolek, W. F. (1971). Lipids of the Leguminosae. In J. B. Harborne, D. Boulter, & B. L. Turner (Eds.), Chemotaxonomy of the leguminosae (pp. 231–255). London: Academic Press.
- Zhao, L., Chen, X. G., Hu, Z. D., Li, Q. F., Chen, Q., & Li, Z. X. (1999). Analysis of β-N-oxalyl-l-α,β-diaminopropionic acid and homoarginine in Lathyrus sativus by capillary zone electrophoresis. Journal of Chromatography A, 857, 295–302.10.1016/S0021-9673(99)00788-8