?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives: Complexation of non-steroidal anti-inflammatory drugs (NSAIDs) with transition metals—chromium and nickel is a unique approach of masking the inherent side effect of gastrointestinal hemorrhage and ulceration of NSAID along with imparting beneficial pharmacological effects. Methods: Chromium and nickel complexes of indomethacin were synthesized followed by characterization of these complexes by FT-IR spectroscopy, UV–Visible spectroscopy, atomic absorption spectroscopy, calorimetric DSC analysis, and melting point analysis. For screening of biological activities to uncover potentially interesting pharmacological properties, the metal complexes were assayed for peripheral analgesic, central analgesic, and anti-inflammatory activity. Results: Nickel-indomethacin complex at dose of 20 mg/kg showed peripheral analgesia of 67.03% by inhibiting writhing and at its dose of 20 mg/kg showed potent central analgesic action at 60 min (92% elongation of tail flicking time). In anti-inflammatory study, nickel-indomethacin and chromium indomethacin complex at its 15 mg/kg dose in the 2 h showed inhibition of paw edema of 78.35 and 73.23%, respectively, which is comparable to the standard indomethacin. Conclusion: Based upon the results, it can be predicted that chromium and nickel complex of indomethacin may show promising pharmacological effects which can be revealed by extensive analysis using pharmacokinetic and pharmacodynamic test model.
Public Interest Statement
Non-steroidal Anti-Inflammatory drugs, commonly known to people as pain killers are some of the most widely used medications. However, they usually come with some severe side effects namely ulceration and possibly perforation in stomach and small intestine, which can limit the use of these medications.
In this report, we showed that if these medicines are combined with metals, then their efficacy remains the same or increases. At the same time, the above-mentioned side effects would also diminish in severity.
1. Introduction
Immune system responds to any infection and injury through inflammatory process, which has apparent implication in the pathogenesis of arthritis, cancer, stroke, neurodegenerative, and cardiovascular disease (Ricciotti & FitzGerald, Citation2011). Generation of inflammatory response is largely mediated by prostaglandins. Prostaglandins are formed from arachidonic acid (AA) that is metabolized by the sequential actions of cyclooxygenase (COX)-a bifunctional enzyme that exists as discrete isoforms referred to as COX-1 and COX-2 (Smith, DeWitt & Garavito, Citation2000). Besides pain reconciliation, prostaglandins have their distinctive role in gastrointestinal (GI) protection.
Non-steroidal anti-inflammatory drugs (NSAID) is a group of drugs that is most frequently used to ameliorate pain and inflammation in acute or chronic conditions such as rheumatoid arthritis, osteoarthritis, mild-to-moderate pain due to inflammation and tissue injury, headache, pyrexia, and many more. NSAIDs act by affecting COX enzymes, thus inhibiting the conversion of AA to intermediate and terminal prostaglandins. Even though non-selective NSAIDs simultaneously inhibits both COX-1 and COX-2 enzymes, but selective COX-2 inhibitors are unique (Gasparini, Rusconi, Xu, Soldato, & Ongini, Citation2004). Non-selectivity of NSAIDs causes the GI toxicities, renal, and hepatic insufficiency associated with widespread long-term use of NSAID (Rao & Knaus, Citation2008). Furthermore, charged NSAIDs, within the gastric epithelial cells, causes uncoupling of oxidative phosphorylation, resulting into the death of these cells (Somasundaram et al., Citation2000).
NSAIDs also interfere with the efficiency of juxtamucosal pH gradient to protect the epithelium by reducing mucus and bicarbonate secretion, (Darling et al., Citation2004; Lichtenberger, Zhou, Dial, & Raphael, Citation2006) thus making the mucosa vulnerable to damage induced by luminal acid. To overcome the long-term complications of NSAID use, several endeavors have been taken. Among them, organometallic complexation of NSAIDs with transition metals has shown promising outcome. Transitional metal ions, e.g. copper, chromium, iron, nickel, zinc, and nickel have remarkable effect on the drugs’ behavior (Khan, Farid, & Khan, Citation2009). Chelation as such causes radical changes in biological properties of ligands as well as metal component and often results in synergistic effect of both.
Indomethacin is a potent analgesic, anti-inflammatory, and antipyretic agent. However, it is concurrently associated with significant incidence of GI hemorrhage and ulceration. After absorption, membrane permeability of gastric mucosa is increased which promotes efflux of cations like potassium and sodium (Bjarnason, Citation2013; Wallace, Citation2012). As a result, the surface-active phospholipids on the mucosal surface can become injured resulting in GI problems (Lichtenberger, Romero, & Dial, Citation2007; Sukul, Poddar, Saha, & Das, Citation2016).
The present study aims at modifying indomethacin by complexation with divalent transition metals such as nickel and chromium. It has already been established that nickel complexes of several NSAIDs such as mefenamic acid and diclofenac have demonstrated superior activities (Kovala-Demertzi et al., Citation2009). Several organoselenium molecules and chromium (III) complexes: chromium phenylalaninate, chromium histidinate, trinuclear chromium propionate, and chromium nicotinate have shown potential effect to alleviate chronic low-grade inflammation and Type-2 diabetes (Zhou, Xu, & Huang, Citation2016).
Until now, synthesis of indomethacin-chromium has not been reported. Besides, comparative pharmacological study on indomethacin derivatives is scarce. Thus, the present protocol concentrates on the synthesis and chemical characterization of chromium and nickel complexes of indomethacin, as well as the investigation into physicochemical properties, peripheral, central analgesic, and anti-inflammatory activity with the aim of establishing stable chemical complex with enhanced pharmacological profile.
2. Materials and methods
2.1. Chemicals
The raw indomethacin was collected from Ningbo-Smart Pharmaceutical, China. Solvents and chemicals such as chromium chloride hexahydrate and nickel sulfate monohydrate were obtained at highest purity from ACI Pharmaceuticals Ltd., Bangladesh. Morphine, diclofenac, and carrageenan, required for biological evaluation, were collected from Gonoshasthaya Pharmaceuticals Ltd., Beximco Pharmaceuticals Ltd, Bangladesh and Sigma-Aldrich, Germany, respectively. All the apparatus and other reagents were available in Research Lab, Department of Clinical Pharmacy and Pharmacology, Faculty of Pharmacy, University of Dhaka.
2.2. Experimental animals
Swiss-albino mice of either sex, weighed 25 gm each and Wister rats of either sex, having weight of 100–150 gm were collected from the Jahangirnagar University Animal House, Bangladesh. They were housed in standard polypropylene cages and kept under controlled room temperature (24 ± 2°C; relative humidity 60–70%). All experimental procedures involving animals were conducted in accordance to ethical guidelines of the FBS, DU (FBS/14/2015) and approved by the Institutional Ethical Committee of Faculty of Biological Science, University of Dhaka.
2.3. Synthesis of divalent metal complexes of indomethacin
One gram of indomethacin (0.0028 mol) was mixed properly with 11.2-ml acetone. In the meantime, 0.235 g sodium bicarbonate (0.0028 mol) was dissolved in 11.2-ml water. The two newly constituted solutions were mixed together, causing effervescence in the form of bubble. When the effervescence ceased, two other solutions of chromium chloride hexahydrate (0.333 g, 0.0014 mol) and nickel sulfate monohydrate (0.236 g, 0.0014 mol) were prepared separately by dissolving in 11.2-ml water in each case. During the addition, the solutes were mixed slowly and agitated continuously. As a result, pink and white precipitates were formed, respectively. The precipitates were filtered and then washed with water and acetone consequently to remove the inorganic and organic contents, respectively, from the precipitate. At last, the products were dried under vacuum to produce the metal complexes of indomethacin.
2.4. Instrumental measurements
The pH solubility of the metal complexes was determined by dissolving in Britton–Robinson-modified universal buffer solutions within the pH range of 1.75–6.65 followed by spectrophotometric absorption measurement at 318 nm. FT-IR Spectra were recorded with FT-IR 8400S Shimadzu spectrophotometer in the range of 4,000–400 cm−1 (Sukul, Das, Saha, & Rahman, Citation2015). A Shimadzu (Model-UV-1800) spectrophotometer and Varian AA 240FS atomic absorption spectrophotometer were used to record UV–Vis and atomic absorption spectra of metal complexes, respectively. For the detection of thermo-analytical stability, Differential Scanning Calorimetry (DSC) technique was applied, using DSC-60 WS, Shimadzu, Japan, equipped with computer and appropriate software program. Digital Advanced SMP 30, having a maximum temperature of 400°C, was used to determine melting points. Dissolution studies were performed for indomethacin and its divalent complexes in a USP XXI Type 2 Dissorate Dissolution Tester (Model SC-2, Scientific Instruments and Technology Corp., NJ). For measuring the volume of rat’s paw in anti-inflammatory activity assay, Plethysmometer (37140, Ugo Basile, Italy) was used.
3. Biological evaluation
3.1. Determination of peripheral analgesic activity
Peripheral analgesic activity was assayed by acetic acid-induced writhing method (Ahmed, Shikha, Sadhu, Rahman, & Datta, Citation2001; Gawade, Citation2012; Rahman et al., Citation2011). To create pain sensation, acetic acid was administered intra-peritoneally to the mice which caused squirming of the body, termed as writhing, at regular intervals and continues to give writhing, until pain prevailed. Compounds having peripheral analgesic activity should counteract the pain sensation and lessen the number of writhing of animals within a given period and with respect to the control group. The writhing inhibition of indomethacin was taken as standard in the present study and then compared with test samples and control.
3.2. Determination of central analgesic activity
For central analgesic activity assessment, the radiant heat tail-flick test was employed (Columbus, OH; Type 812). A constant heat stress was applied on a particular site of the mouse-tail, which acted as pain stimulus. When the stimulus exceeds the threshold, a quick withdrawal of tail was observed (Ahmed, Selim, Das, & Choudhuri, Citation2004). Time taken by the rat to withdraw the tail is termed as tail flicking time. Analgesic response was denoted by % MPE (maximum possible antinociceptive effect) (Pakulska & Czarnecka, Citation2001). Indomethacin at a dose of 10 and 20 mg/kg was used as a standard, and all the standard and metal complexes were given subcutaneously into the tail skin (Martin, Fletcher, Chauvin, & Bouhassira, Citation2007). When the administration of the samples was done, the tail flicking time was tested after 0, 30, 60, and 90 min. The % time elongation of tail flicking was calculated with respect to the control using the following formula. The higher the elongation percentage of the group, the greater the groups’ central analgesic activity.
3.3. Determination of anti-inflammatory activity
Winter’s procedure—carrageenan-induced hind paw edema to influence acute inflammation followed by reducing the volume of the inflamed paw was followed for the determination of anti-inflammatory activity of synthesized complexes (Sukul et al., Citation2017). The aqueous suspensions of test samples were given to overnight-fasted rats by oral route at a dose of 15 mg/kg. Indomethacin was administered as standard drug at a dose of 100 mg/kg body weight, while control groups were given vehicle containing 1% tween-80 in 0.9% saline water as a placebo. After oral administration, 0.1 ml of 1% (w/v) carrageenan in saline solution was injected into the subplanter surface of the right hind paw of each rat through 26 gauge needle. Carrageenan in rat’s hind paw leads to a cascade of biological responses, causing the formation of edema in situ due to localized inflammation. Paw volumes were measured up to a fixed mark on the hind paw at 1–4 h after the administration of the standard and complexes. Percent inhibition of edema was calculated using the formula.
where Vc and Vt represent the average edema volume of control and treated animals, respectively.
3.4. Statistical analysis
All the observed data were represented as means ± S.E.M. Statistical analysis was done using the Statistical Package for Social Science software (SPSS version 16.0), and statistical differences between groups were analyzed by a one-way analysis of variance (ANOVA) which was succeeded by Dunnett’s t-tests.
4. Results
4.1. Characterization of chromium and nickel complexes of indomethacin
In this study, the hydrated chromium and nickel complexes of indomethacin were characterized using an integrated technique of spectroscopic and physicochemical analysis followed by assessing peripheral, central analgesic, and anti-inflammatory activity.
4.2. FT-IR
From the infrared spectra of the synthesized metal complexes, a characteristic band of absorption was observed by which they were identified (Kulaksizoğlu, Gökçe, & Gup, Citation2012). In case of –COOH of indomethacin, C=O shows absorption at (1,725–1,705) cm−1 and single band. –OH group of –COOH shows multiple peaks within (3,400–2,400) cm−1 range (Table ). Indomethacin had five peaks at this region but Cr-indomethacin showed three peaks, and Ni-indomethacin had 2 peaks. Multiple peaks for C=O (which is not present in –COOH) were seen within (1,820–1,660) cm−1 which were absent in the parent compound.
Table 1. FT-IR wave number bands (n) of indomethacin and its metal complexes
4.3. UV–Vis
UV–Visible spectrum of indomethacin and its complexes is shown in Table .
Table 2. Absorbance (in chloroform) found in UV–Visible spectrum of indomethacin and its complexes
4.4. AAS
Using atomic absorption spectroscopy, chromium and nickel were found in an amount of 14.87 and 11.23% in its respective metal complexes.
4.5. DSC
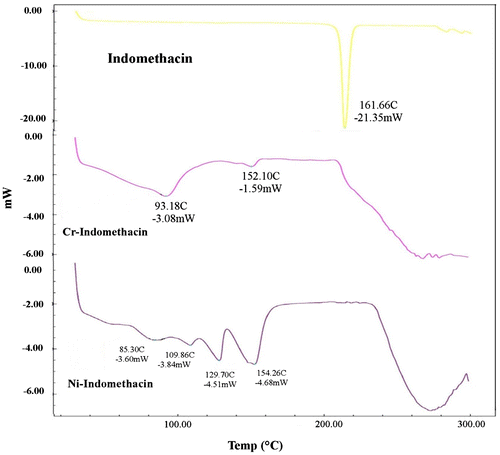
DSC thermogram of indomethacin showed a sharp melting endotherm at 161.66°C, which was easily detectable and corresponded to the melting point of indomethacin at 162°C (Figure ). Nickel complex of indomethacin showed two sharp melting endotherms: one at 129.70°C and another at 154.26°C. Chromium complex endotherm was not sharp enough. It showed two peeks at 93.18 and 152.10°C. The melting points on the DSC thermogram of the samples were supported by the data obtained from digital melting point determination.
4.6. Melting point
The melting point of pure indomethacin was 159.9–163.2°C and chromium and nickel complexes of indomethacin were 154.4–157.2 and 146.5–150.1°C, respectively.
4.7. pH-solubility profile
The pH solubility profile of parent indomethacin and its chromium and nickel complex is given on Table . It is clear that the solubility of indomethacin was highest at pH 7, whereas both the complexes showed better solubility as pH 5.
Table 3. pH-solubility profile of parent indomethacin and its chromium and nickel complex
4.8. Dissolution studies
The dissolution of indomethacin and its complexes was investigated at 37°C in pH 6-phosphate buffer at time 10, 20, 30, 40, 50, and 60 min (Table ). The dissolution of indomethacin-nickel complex (33.3%) in vitro was much higher at 60 min than the indomethacin itself (24%). The release of indomethacin-chromium complex was 9% at that specific time.
Table 4. Dissolution profile of parent indomethacin and its chromium and nickel complex at different time
4.9. Peripheral analgesic activity
Among the synthesized complexes, indomethacin-nickel showed significant (p < 0.001) analgesic activity at a dose of 20 mg/kg with % of writhing inhibition of 67.03 (Table ), while indomethacin-chromium complex showed a significant activity at a dose of 20 mg/kg with 66.48% writhing inhibition. The standard indomethacin showed dose-dependent inhibition of writhing of 35.16 and 64.29% at dose of 10 and 20 mg/kg, respectively.
Table 5. Peripheral analgesic activities of metal complexes of indomethacin
4.10. Central analgesic activity
The test samples elongated the tail-flick time in a dose-dependent manner. At a dose of 10 mg/kg body weight, nickel and chromium complexes showed moderate central analgesic activity when compared to standard morphine at all time intervals and the complexes showed more central analgesic activity than the parent drug indomethacin (Table ). At a dose of 20 mg/kg body weight, nickel and chromium complex and indomethacin itself showed strong central analgesic activity (>80%) after 60 and 90 min when compared to standard morphine. Both complexes showed more activity than indomethacin itself except chromium-indomethacin complex at 20 mg/kg dose after 90 and 120 min.
Table 6. Central analgesic activity of metal complexes of indomethacin
4.11. Anti-inflammatory activity
The anti-inflammatory activity of the standard indomethacin at a dose of 100 mg/kg and metal complexes of indomethacin at their 15 mg/kg doses are demonstrated in Table . The indomethacin-chromium complex at its 15 mg/kg dose in the 2 h showed inhibition of edema of 73.23%, and indomethacin-nickel complex at its 15 mg/kg dose in the 2 h showed inhibition of edema of 78.35%, which was comparable to the standard indomethacin.
Table 7. Anti-inflammatory activity of metal complexes of indomethacin
5. Discussion
For the characterization of metal complexes, FT-IR, UV–Vis, AAS, DSC, and melting point were analyzed. In case of FT-IR, as –COOH functional group was absent in the complexes, broad and intense signals for –OH group in 3,700–3,000 cm−1 indicated the presence of hydrogen bonding which might be due to the incorporation of water molecule as ligands in crystal structure. The metal complexes exhibited a definite shift in absorption for the carboxyl group and stretching and bending of carboxyl OH. The shift that associated in the longer wavelength was the indication of the strong involvement of carboxyl group of indomethacin in complexation with chromium and nickel. Donating electrons to the transitional atoms will lower the energy state and therefore longer wavelength shifting arises (Yun et al., Citation2007). Besides FT-IR, bathochromic and hypochromic shift took place in both the cases of chromium and nickel complex (Mazumder, Sukul, & Saha, Citation2016), when UV–Vis spectra were analyzed. In AAS analysis, remarkable amount of metal was found in each sample which was unavailable in pure indomethacin. This indicated the presence of metal in the complexes. In the DSC analysis, both complexes showed different endotherms along with change in melting point less than indomethacin which were representative of the identity of distinctive product and support the complex formation.
The pH-solubility and dissolution profile also showed different characteristics. As indomethacin is a weak acidic drug, it is supposed to show better dissolution at basic medium, thus the reason for maximum solubility at pH 7 (Kumar, Murthy, & Rani, Citation2014). Due to complexation, the indomethacin loses the acidic carboxyl functional group and hence showed maximum solubility at pH 5. The absorption of orally administered compound depends on its solubility (Raman & Rajakumar, Citation2013). As both the complex shows maximum solubility and increased rate of absorption at pH 6 compared to indomethacin, this might indicate a better dissolution of metal complexes in the stomach fluid. The percentage of dissolution pattern of indomethacin and its complex can be explained in terms of their pH-solubility profile. After 60 min, indomethacin-nickel showed better dissolution at pH 6 than indomethacin, followed by indomethacin-chromium complex. This corresponds to the trend of pH-solubility profile of indomethacin and its complexes at pH 6.
In peripheral analgesic activity, both nickel and chromium complex of indomethacin at 20 mg/kg showed significant analgesia than indomethacin. Compared to standard morphine and parent indomethacin, nickel and chromium complexes showed potent central analgesic activity at both 10 and 20 mg/kg dose. The analgesic activity might be due to its interference in the biosynthesis of prostaglandins and leukotrienes from cyclooxygenase and lipooxygenase pathways, which are responsible for blocking of pain sensation. Further bioactivity-guided investigation might reveal potent analgesic compound.
Carrageenan is one form of polysaccharide, which has inherent capability of inducing inflammation. Usually, carrageenan follows a biphasic process to induce inflammation in the animal model. During the initial phase, ranging from 1 to 2 h, carrageenan will cause the release of histamine or serotonin (Georgewill, Georgewill, & Nwankwoala, Citation2010; Saha & Ahmed, Citation2009), which is followed by the release of prostaglandin-like substances, autacoids-like bradykinin, protease, and lisosome in the successive phases, ranging from 3 to 4 h (Saha & Ahmed, Citation2009). The indomethacin demonstrates its anti-inflammatory effect by interfering with second phase, mostly by inhibiting the prostaglandin synthesis. From Table , it is quite evident that the indomethacin-chromium and indomethacin-nickel complex showed prominent anti-inflammatory activity at 2 h. This may be attributed to the capability of inhibition of the histamine or serotonin by both complexes.
6. Conclusion
The experimental protocol mentioned here describes a new pathway to synthesize metal complexes of indomethacin with chromium and nickel. The complexes formed might reduce the dose requirement of indomethacin along with its ulcerogenic side effects. As promising advantage, the therapeutic and pharmacological profile of the parent drug remain unaltered which are supported by the existence of peripheral, central analgesic, and anti-inflammatory activity. For screening the toxicological profile and chronic anti-inflammatory effects of the synthesized complexes, further in-depth studies would be required in future.
Funding
The authors acknowledge the support for this work from Bangladesh Council of Science and Industrial research and Center for Advance Research in Science, University of Dhaka.
Acknowledgments
We are grateful to the Department of Clinical Pharmacy and Pharmacology, University of Dhaka.
Additional information
Notes on contributors
Abhijit Sukul
Our research group comprises of Abhijit Sukul, Saikat Kumar Poddar, Shahadat Hossain, Sanjana Haque, Kumar Kulldeep Niloy and led by Sajal Kumar Saha. Since 2013, we have published five papers related to pharmacological and toxicological investigation of transition metal complexes of various NSAIDs. So far, we have investigated complexes of indomethacin, tolfenamic acid, and aceclofenac as well as showed that these complexes may be less toxic and more potent than the parent drug molecules.
In our current report, we have described the synthetic procedure as well as pharmacological activities of chromium and nickel complexes of indomethacin. A simple synthetic procedure for chromium and nickel complexes of indomethacin has been described, which may prove to be valuable to aspiring researchers in this field. Furthermore, we have shown that chromium and nickel complexes are more efficacious than indomethacin alone, which may open a window to further research regarding the mechanism of action and large-scale investigation of these complexes.
References
- Ahmed, F., Selim, M. S. T., Das, A. K., & Choudhuri, M. S. K. (2004). Anti-inflammatory and antinociceptive activities of lippia nodiflora Linn. Pharmazie, 59, 329–333.
- Ahmed, M., Shikha, H., Sadhu, S., Rahman, M., & Datta, B. (2001). Analgesic, diuretic, and antiinflammatory principle from Scoparia dulcis. Pharmazie, 56, 657–660.
- Bjarnason. (2013). The impact of recent data on our understanding of the roles of COX-1 and COX-2 in gastrointestinal pathophysiology. Clinical Drug Investigation, 27, 7–13.
- Darling, R. L., Romero, J. J., Dial, E. J., Akunda, J. K., Langenbach, R., & Lichtenberger, L. M. (2004). The effects of aspirin on gastric mucosal integrity, surface hydrophobicity, prostaglandin metabolism in cyclooxygenase knockout mice. Gastroenterology, 127, 94–104.10.1053/j.gastro.2004.04.003
- Gasparini, L., Rusconi, L., Xu, H., Soldato, P. D., & Ongini, E. (2004). Modulation of b-amyloid metabolism by non-steroidal anti-inflammatory drugs in neuronal cell cultures. Journal of Neurochemistry, 88, 337–348.
- Gawade, S. P. (2012). Acetic acid induced painful endogenous infliction in writhing test on mice. Journal of Pharmacology and Pharmacotherapeutics , 3, 348.10.4103/0976-500X.103699
- Georgewill, O. A., Georgewill, U. O., & Nwankwoala, R. N. P. (2010). Anti-inflammatory effects of Morninga oleifera lam extract in rats. Asian Pacific Journal of Tropical Medicine, 3, 133–135.10.1016/S1995-7645(10)60052-1
- Khan, A. H., Farid, K. M., & Khan, T. M. (2009). Effect of tranexamic acid and its derivatives on the chemical and metabolic modulation in aqueous solution. Asian Journal of Pharmaceutical and Clinical Research, 2, 27–33.
- Kovala-Demertzi, D., Hadjipavlou-Litina, D., Staninska, M., Primikiri, A., Kotoglou, C., & Demertzis, M. A. (2009). Anti-oxidant, in vitro, in vivo anti-inflammatory activity and antiproliferative activity of mefenamic acid and its metal complexes with manganese(II), cobalt(II), nickel(II), copper(II) and zinc(II). Journal of Enzyme Inhibition and Medicinal Chemistry, 24, 742–752.10.1080/14756360802361589
- Kulaksizoğlu, S., Gökçe, C., & Gup, R. (2012). Asymmetric BIS (bidentate) azine ligand and transition metal complexes: Synthesis, characterization, DNA-binding and cleavage studies and extraction properties for selected metals and dichromate anions. Journal of the Chilean Chemical Society, 57, 1213–1218.
- Kumar, A. A., Murthy, T. E. G. K., & Rani, A. P. (2014). A concise review on oral ph independent controlled drug delivery system. World Journal of Pharmacy and Pharmaceutical Sciences, 3, 311–324.
- Lichtenberger, L. M., Romero, J. J., & Dial, E. J. (2007). Surface phospholipids in gastric injury and protection when a selective cyclooxygenase-2 inhibitor (Coxib) is used in combination with aspirin. British Journal of Pharmacology, 150, 913–919.10.1038/sj.bjp.0707176
- Lichtenberger, L. M., Zhou, Y., Dial, E. J., & Raphael, R. M. (2006). NSAID injury to the gastrointestinal tract: evidence that NSAIDs interact with phospholipids to weaken the hydrophobic surface barrier and induce the formation of unstable pores in membranes. Journal of Pharmacy and Pharmacology, 58, 1421–1428.10.1211/jpp.58.10.0001
- Martin, F., Fletcher, D., Chauvin, M., & Bouhassira, D. (2007). Constitutive cyclooxygenase-2 is involved in central nociceptive processes in humans. Anesthesiology, 106, 1013–1018.10.1097/01.anes.0000265162.39932.33
- Mazumder, M. M. U., Sukul, A., & Saha, S. K. (2016). Analgesic activities of synthesized divalent metal complexes of tolfenamic acid. Dhaka University Journal of Pharmaceutical Sciences, 15, 89–96.10.3329/dujps.v15i1.29202
- Pakulska, W., & Czarnecka, E. (2001). Effect of Citalopram and Buspirone on the antinoceptive action of analgesic drugs. Acta Poloniae Pharmaceutica, 58, 299–305.
- Rahman, M. A., Sharmin, R., Uddin, M. N., Zaman, M., Rana, S., & Ahmed, N. U. (2011). Antinociceptove and antiinflammatory effect of crinum asiaticum bulb extract. Asian Journal of Pharmaceutical and Clinical Research, 4, 34–37.
- Raman, N., & Rajakumar, R. (2013). Anti-Inflammatory, analgesic and anti-pyretic evaluation of N, N′-Propylenebis(Salicylamide) transition metal complexes in rat. Bulletin of Pharmaceutical and Medical Sciences, 1(1), 1–6.
- Rao, P., & Knaus, E. E. (2008). Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond. Journal of Pharmacy & Pharmaceutical Sciences, 11, 81–110.10.18433/J3T886
- Ricciotti, E., & FitzGerald, G. A. (2011). Prostaglandins and inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology, 31, 986–1000.10.1161/ATVBAHA.110.207449
- Saha, A., & Ahmed, M. (2009). The analgesic and anti-inflammatory activities of the extract of Albizia lebbeck in animal model. Pakistan Journal of Pharmaceutical Sciences, 22, 74–77.
- Smith, W. L., DeWitt, D. L., & Garavito, R. M. (2000). Cyclooxygenases: Structural, cellular, and molecular biology. Annual Review of Biochemistry, 69, 145–182.10.1146/annurev.biochem.69.1.145
- Somasundaram, S., Sigthorsson, G., Simpson, R. J., Watts, J., Jacob, M., Tavares, I. A., … Wrigglesworth, J. M. (2000). Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat. Alimentary Pharmacology & Therapeutics, 14, 639–650.10.1046/j.1365-2036.2000.00723.x
- Sukul, A., Das, S. C., Saha, S. K., & Rahman, S. M. A. (2015). Screening of analgesic, antimicrobial, cytotoxicity and antioxidant activities of metal complexes of indomethacin. Dhaka University Journal of Pharmaceutical Sciences, 13, 175–180.
- Sukul, A., Poddar, S. K., Saha, S. K., & Das, S. C. (2016). Synthesis and characterization of cobalt and manganese complexes of indomethacin and comparative study of local analgesic, anti-inflammatory, and anti-ulcerogenic properties. Russian Journal of General Chemistry, 86, 1935–1943.10.1134/S1070363216080260
- Sukul, A., Poddar, S. K., Haque, S., Saha, S. K., Das, S. C., Mahmud, Z. A., & Rahman, S. M. A. (2017). Synthesis, Characterization and Comparison of Local Analgesic, Anti-Inflammatory, Anti-Ulcerogenic Activity of Copper and Zinc Complexes of Indomethacin. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 16. doi:10.2174/1871523016666170217103402
- Wallace, J. L. (2012). NSAID gastropathy and enteropathy: Distinct pathogenesis likely necessitates distinct prevention strategies. British Journal of Pharmacology, 165, 67–74.10.1111/j.1476-5381.2011.01509.x
- Yun, Y., Chen, P., Zheng, C. L., Yang, Y., Duan, W. G., Wang, L., et al. (2007). Copper-aspirin complex inhibits cyclooxygenase-2 more selectively than aspirin. Yakugaku Zasshi, 127, 1869–1875.10.1248/yakushi.127.1869
- Zhou, J., Xu, H., & Huang, K. (2016). Organoselenium small molecules and chromium(III) complexes for intervention in chronic low-grade inflammation and type 2 diabetes. Current Topics in Medicinal Chemistry, 16, 823–834.