Abstract
In this work, 1-((benzo[d]thiazol-2-yl)methyl)-4,5-dihydro-3-methyl-N-phenyl-1H-pyrazol-5-imine (3a-h) have been successfully synthesized from the condensation of 2-((Benzo[d]thiazol-2-1-((benzo[d]thiazol-2-yl)methyl)-3-methyl-1H-pyrazol-5(4H)-one (2) with different aromatic amines. On the other hand, in another protocol, cyclization of 2-(benzo[d]thiazol-2-yl) acetohydrazide (4) with different aromatic aldehydes afforded 2-((5-aryl-1H-1,2,4-triazol-3-yl)methyl)benzo[d]thiazoles (5a-h). A perusal of the biological activity studies revealed that the derivatives having chlorine as substituent are more toxic to all six bacteria. Among the chloro group compounds, the compound which has methoxy group is more toxic.
Keywords:
- 2-((benzo[d]thiazol-2-1-((benzo[d]thiazol-2-yl)methyl)-3-methyl-1H-pyrazol-5(4H)-one
- condensation
- 1-((benzo[d]thiazol-2-yl)methyl)-4,5-dihydro-3-methyl-N-phenyl-1H-pyrazol-5-imine
- cyclization
- 2-(benzo[d]thiazol-2-yl) acetohydrazide
- 2-((5-aryl-1H-1,2,4-triazol-3-yl)methyl)benzo[d]thiazoles
- biological activity
Public Interest Statement
Over the years, pyrazole and thiazole derivatives received up surging attention and prominent importance among the family of heterocyclic azoles due to their proven biological, pharmaceutical, and agrochemical applications. The efficacy of clinical use of antiviral, anticancer, and antifungal drugs (Ribavirin, Anastrozole, Fluconazole, Voriconazole etc.) led to intense investigation of 1,2,4-triazole derivatives. A survey of literature depicted a series of many more biologically active heterocyclic compounds with pyrazole, pyrazoline, thiazole, thiazoline, and benzothiazole and benzothiazoline backbone. Stimulated by all such reports, the authors have taken up the synthesis, characterization and biological activity studies of certain 1-((benzo[d]thiazol-2-yl) methyl)-4,5-dihydro-3-methyl-N-aryl-1H-pyrazol-5-amines and 2-((5-aryl-1H-1,2,4-triazol-3-yl) methyl)benzo[d]thiazoles.
1. Introduction
Many of the pyrazole and thiazole derivatives have gained prominent importance among the family of heterocyclic azoles over the years due to their proven biological, pharmaceutical, and agrochemical applications (Eicher & Hauptmann, Citation2003; Joule, Mills, & Smith, Citation1995). The efficacy of clinical use of antiviral, anticancer, and antifungal drugs (Ribavirin, Anastrozole, Fluconazole, Voriconazole etc.) led to intense investigation of 1,2,4-triazole derivatives. 1,2,4-Triazole containing molecules have increasing interest as anticancer, fungicidal, antimicrobial, antitubercular, or anti-inflammatory agents. In addition, they are also significant in agrochemical industry as plant-protecting materials. In recent past, a large number of synthetic protocols have been developed using pyrazole (David et al., Citation2007; Tuan, Tung, & Peter, Citation2007), thiazole benzothiazole as precursors (Ali & Siddiqui, Citation2013). A well-known method to prepare pyrazolines was from the reaction between aliphatic diazo compounds and acetylene derivatives. The most commonly used diazo compounds are diazomethane and ethyl diazoacetate to generate 1H-pyrazole (Reimlinger, Citation1952). A similar method is the reaction of diazo compounds with olefins to generate pyrazolines (Huisgen, Koszinowski, Ohta, & Schiffer, Citation1980). Another popular method to prepare pyrazolines is the addition of hydrazine to α,β-unsaturated carbonyl compounds, which led to the synthesis of a wide variety of pyrazolines (Azarifar & Ghasemnejad, Citation2003). Recent review of Yusuf and Payal Jain provides an excellent bibliography on the synthetic and biological studies of different pyrazoline derivatives (Yusuf & Jain,Citation2014). 1,3-Diphenyl-2-pyrazolines substituted in the 4- and 5-positions with phenyl groups undergo dye-sensitized photo oxidation at widely different rates depending on the substitution pattern. Depending on both the solvent and the substitution pattern, two types of reactions were usually observed: (a) dehydrogenation to the corresponding pyrazoles and (b) incorporation of oxygen with ring opening to yield carbonyl compounds (Evans, Citation1975). A series of pyrazoline derivatives bearing chloro quinolines were synthesized from chalcones and evaluated for antimicrobial activity (Dobaria, Patel, & Parekh, Citation2003). A survey of literature depicted a series of many more biologically active heterocyclic compounds with pyrazole, pyrazoline, thiazole, thiazoline, benzothiazole, and benzothiazoline backbone (Archita, Swati, & Talesara, Citation2008; Bruno et al., Citation1997; Chimenti et al., Citation2004; Hughes et al., Citation2007; Mogilaiah & Rama Sudhakar, Citation2003; Mahesh & Gupta, Citation1974; Thomas, Muller, Ronald, & Markus, Citation1967; Yuhong & Varma, Citation2005, Citation2006). Stimulated by all such reports, the authors have taken up the synthesis, characterization and biological activity studies of certain 1-((benzo[d]thiazol-2-yl) methyl)-4,5-dihydro-3-methyl-N-aryl-1H-pyrazol-5-amines and 2-((5-aryl-1H-1,2,4-triazol-3-yl) methyl)benzo[d]thiazoles.
2. Results and discussion
2.1. Synthesis and characterization of 1-((benzo[d]thiazol-2-yl) methyl)-4,5-dihydro-3-methyl-N-phenyl-1H-pyrazol-5-imines (3a-h)
Reaction between 2-((benzo[d]thiazol-2-yl)methyl)hydrazine (or 2-(hydrazinylmethyl) benzo[d]thiazole) (1) and ethyl acetoacetate in acetonitrile medium produced 1-((benzo[d]thiazol-2-yl)methyl)-3-methyl-1H-pyrazol-5(4H)-one (2), with 287–289°C M.P. which upon condensation with different amines afforded 1-((benzo[d]thiazol-2-yl)methyl)-4,5-dihydro-3-methyl-N-phenyl-1H-pyrazol-5-imine derivatives (3a-h) under reflux conditions for 2 to 7 h depending on the nature of substituent in amine, as shown in Scheme .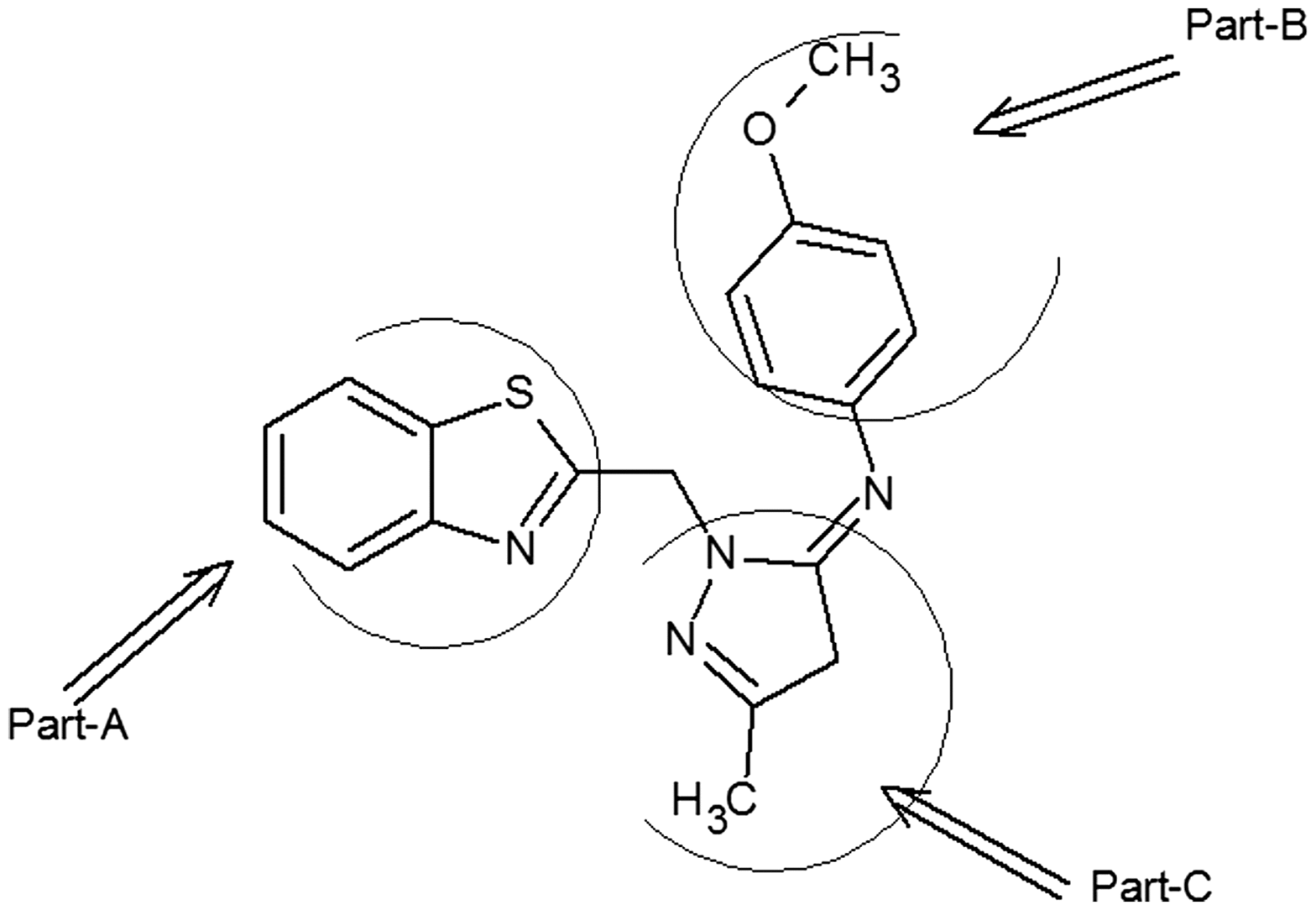
Scheme 1. Synthesis of 1-(benzo[d]thiazol-2-yl)methyl)-4,5-dihydro-3-methyl-N-1H-Pyrazol-5-imines-benzothiazole methyl N-pyrazole imines (3a-h).
![Scheme 1. Synthesis of 1-(benzo[d]thiazol-2-yl)methyl)-4,5-dihydro-3-methyl-N-1H-Pyrazol-5-imines-benzothiazole methyl N-pyrazole imines (3a-h).](/cms/asset/cc2bd28d-f00a-45ef-a8e6-8606bbe5e089/oach_a_1312673_f0001_b.gif)
Structure of 1-(benzo[d]thiazol-2-ylmethyl)-N-(4-methoxyphenyl)-3-methyl-4,5-dihydro-1H-pyrazol-5-imine (3b).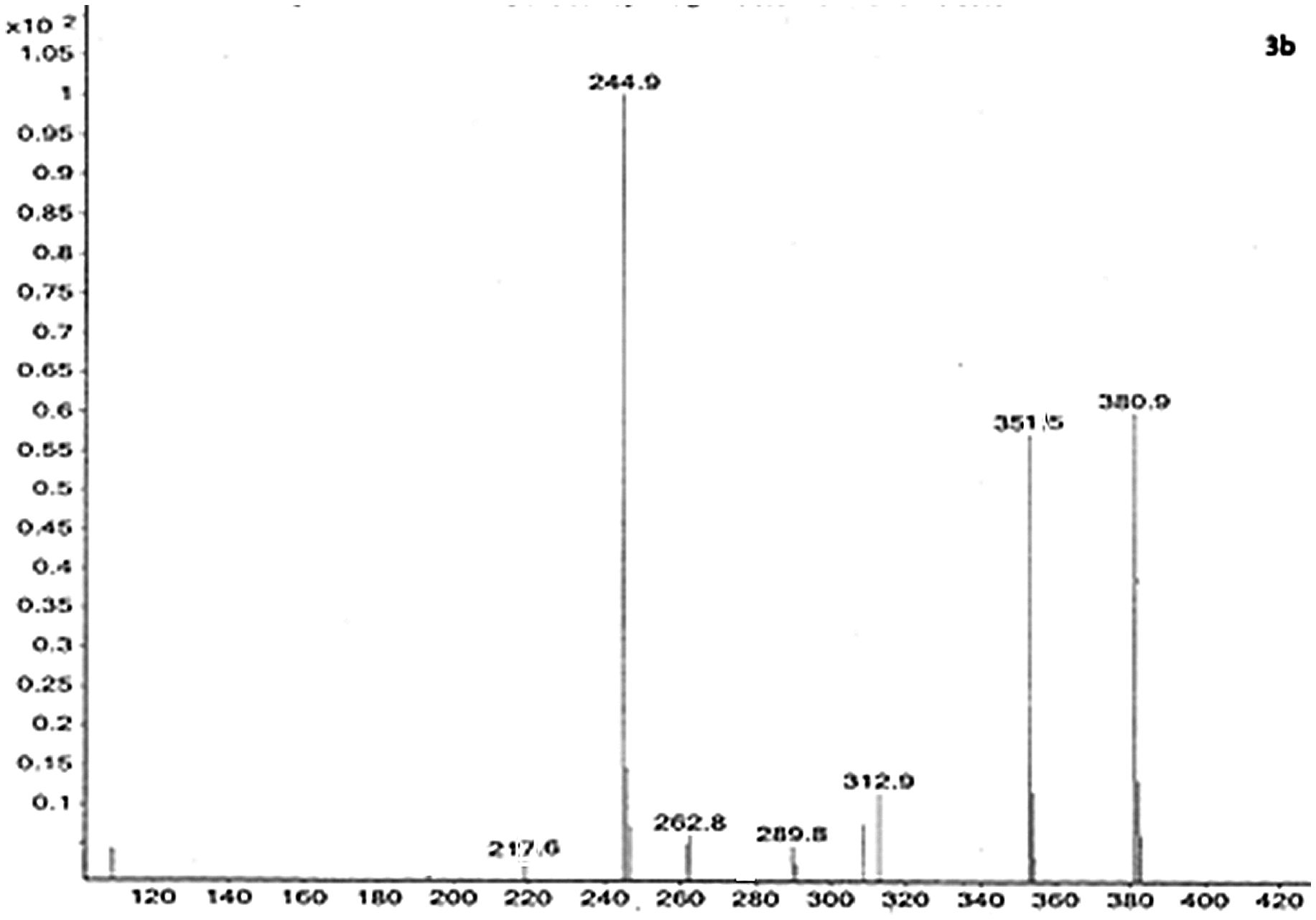
2.1.1. Mass spectrum of 1-(benzo[d]thiazol-2-ylmethyl)-N-(4-methoxyphenyl)-3-methyl-4,5-dihydro-1H-pyrazol-5-amine (3b)
Mass spectrum of (3b) indicated (m/z) peak at 351.5 (351.44) even though the calculated molecular weight of the compound (3b) has come out as 350.44 according to nitrogen rule. The observed value agrees well when the peak is considered as (M + 1) of peak rather than exact (m/z) value. A peak at m/z 217.6 could be explained due to the loss of benzothiozole moiety. Mass spectroscopic data for other the derivatives of (3a-h), are compiled in Table .
Table 1. Synthesis and spectroscopic analysis of 1-((benzo[d]thiazol-2-yl)methyl)-4,5-dihydro-3-methyl-N-phenyl-1H-pyrazol-5-amines (3a-h)
2.1.2. NMR spectrum (3b) of 1-(benzo[d]thiazol-2-ylmethyl)-N-(4-methoxyphenyl)-3-methyl-4,5-dihydro-1H-pyrazol-5-amine(3b)
Proton NMR spectrum of (3b) depicted six singlet peaks at 2.2, 2.5, 3.3, 3.7, 4.5, and 4.7 δ followed by a multiplet at 7–8 δ, respectively. Singlet peak absorbed at 2.2 δ could be due to the methyl group attached to diazole ring in part-C. Peak appeared at 2.5 and 3.7 δ as singlet could be corresponding to d6-DMSO and water. A peak at 4.7 δ is due to protons of –CH2 present between thiazole ring and pyrazole ring of molecule. Peak appeared at 4.5 δ as singlet could be due to the diazole –CH proton in part-C, while the peak appeared at 3.4 δ as singlet could be due to methoxy protons in part-B. Finally the multiplet appeared in the range 7–8 δ might represent all aromatic protons corresponding to the phenyl groups present in part –A and part –B of the molecule. NMR spectroscopic data for other derivatives of (3a-h) are shown in Table .
NMR spectrum (3b) of 1-(benzo[d]thiazol-2-ylmethyl)-N-(4-methoxyphenyl)-3-methyl-4,5-dihydro-1H-pyrazol-5-imine(3b)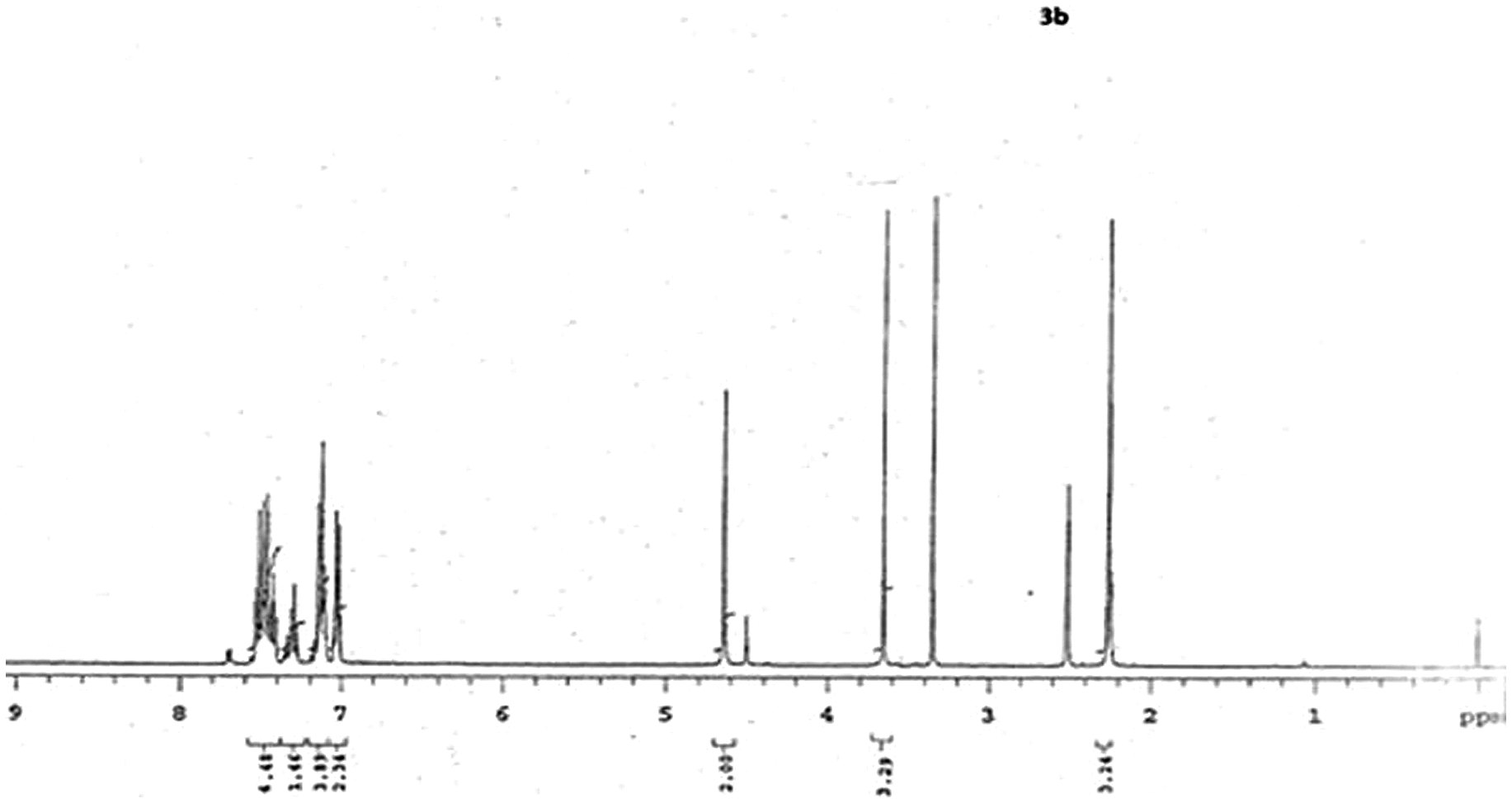
2.1.3. C13 NMR spectrum (3b) of 1-(benzo[d]thiazol-2-ylmethyl)-N-(4-methoxyphenyl)-3-methyl-4,5-dihydro-1H-pyrazol-5-amine
The C13 NMR spectrum for (3b) depicted several peaks at δ 21.34, 38.12, 54.16, 58.12, 115.16, 117.02, 117.34, and 125–167 ppm range, respectively. Peak appeared at 21.34 ppm represents methyl carbon attached to pyrazole ring in part-C, while the peak appeared at 38.12 ppm represents dihydropyrazole ring methylene (–CH2) carbon, peak at 54.16 is due to methoxy carbon in part-B. The peak observed at 58.12 ppm might represent –CH2 present between thiazole ring and pyrazole ring in part-C, while the other peak appeared at 115 ppm represents pyrazole ring –CH carbon in part-C. Peak appeared at 117.02 ppm represents pyrazole ring –C = N carbon in part-C, which is connected to methyl group. Peak appeared at 117.34 ppm represents the –C = N carbon in thiazole ring. Finally multiplet appeared in the range 125–167 ppm might represent all aromatic carbons of part-A and part-B.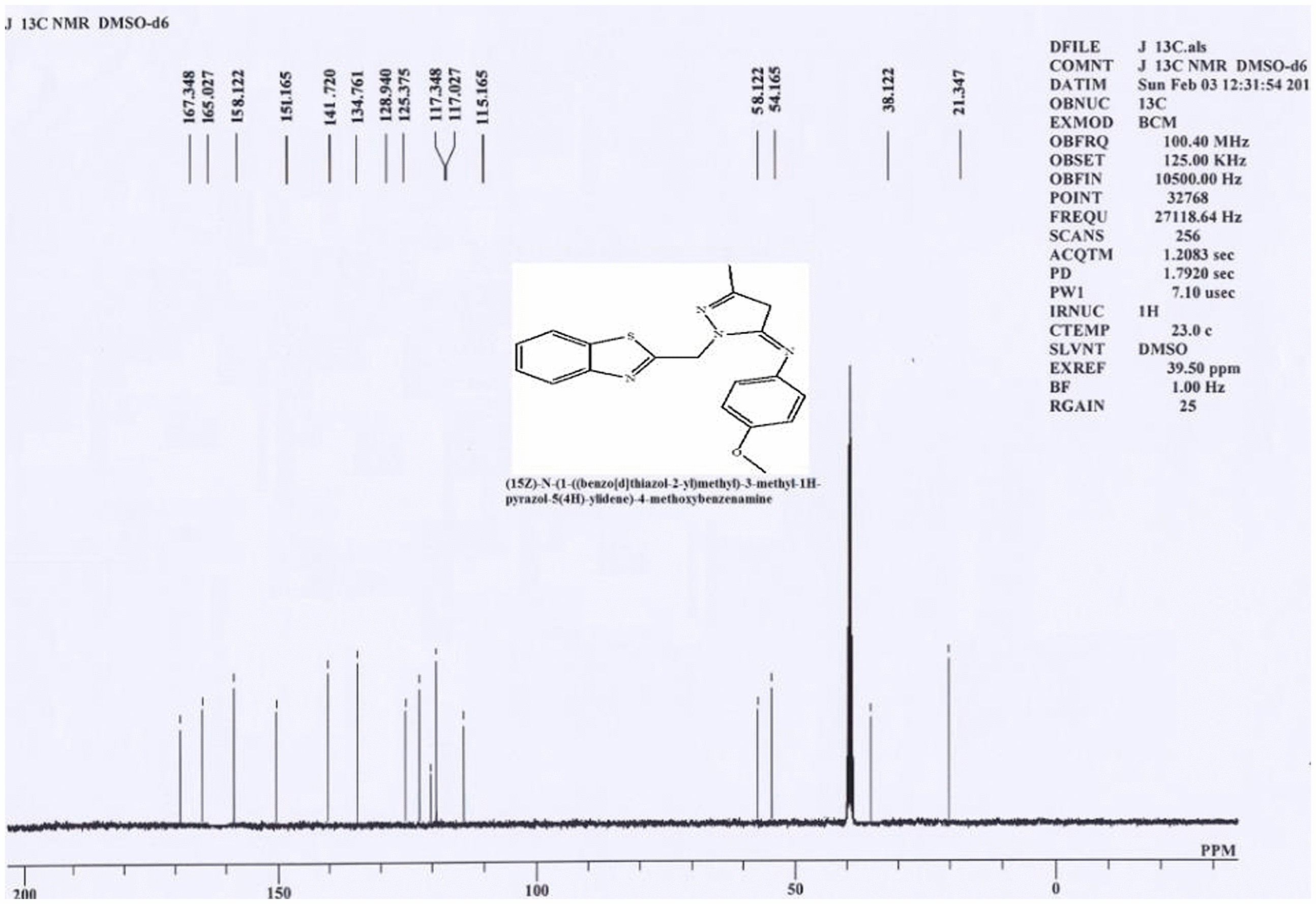
C13 NMR spectrum of 1-(benzo[d]thiazol-2-ylmethyl)-N-(4-methoxyphenyl)-3-methyl-4,5-dihydro-1H-pyrazol-5-amine (3b)
Data presented in Table show that irrespective the amines used in the study the reactions afforded good product yields. However, reaction times surprisingly did not follow a regular trend by changing the substituent (EWG/ED) in the phenyl ring. This trend could be probably due to the cumulative effect of inductive and resonance parameters.
2.2. Synthesis and characterization of 2-((5-phenyl-1H-1,2,4-triazol-3-yl)methyl)benzo[d]thiazoles (5a-h)
Reaction between 2-aminobenzenethiol and 2-cyanoacetohydrazide (0.01 mol), in dry EtOH and triethylamine afforded 2-(benzo[d]thiazol-2-yl) acetohydrazide (4), with 151–152°C M.P under reflux conditions, The product (4) thus obtained upon further treatment with aromatic aldehydes in AcOH and ammonium acetate under reflux conditions gave target compounds 2-((5-phenyl-1H-1,2,4-triazol-3-yl)methyl)benzo[d]thiazole (5a-h), as shown in Scheme . The product obtained was semisolid.
Scheme 2. Synthesis of (IV) 2-(benzo[d]thiazol-2-yl)acetohydrazide [benzothiazole acetohydrazide]; (Va-h) 2-((5-arnyl-1H-1,2,4-triazol-3-yl)methyl)benzo[d]thiazoles[benzothiazole methyl aryl triazoles].
2-((5-phenyl-1H-1,2,4-triazol-3-yl)methyl)benzo[d]thiazole (5a):
2-((5-phenyl-1H-1,2,4-triazol-3-yl)methyl) benzo[d]thiazoles [benzothiazole methyl aryl triazoles]
Mass spectrum of 2-((5-phenyl-1H-1,2,4-triazol-3-yl)methyl)benzo[d]thiazole (5a):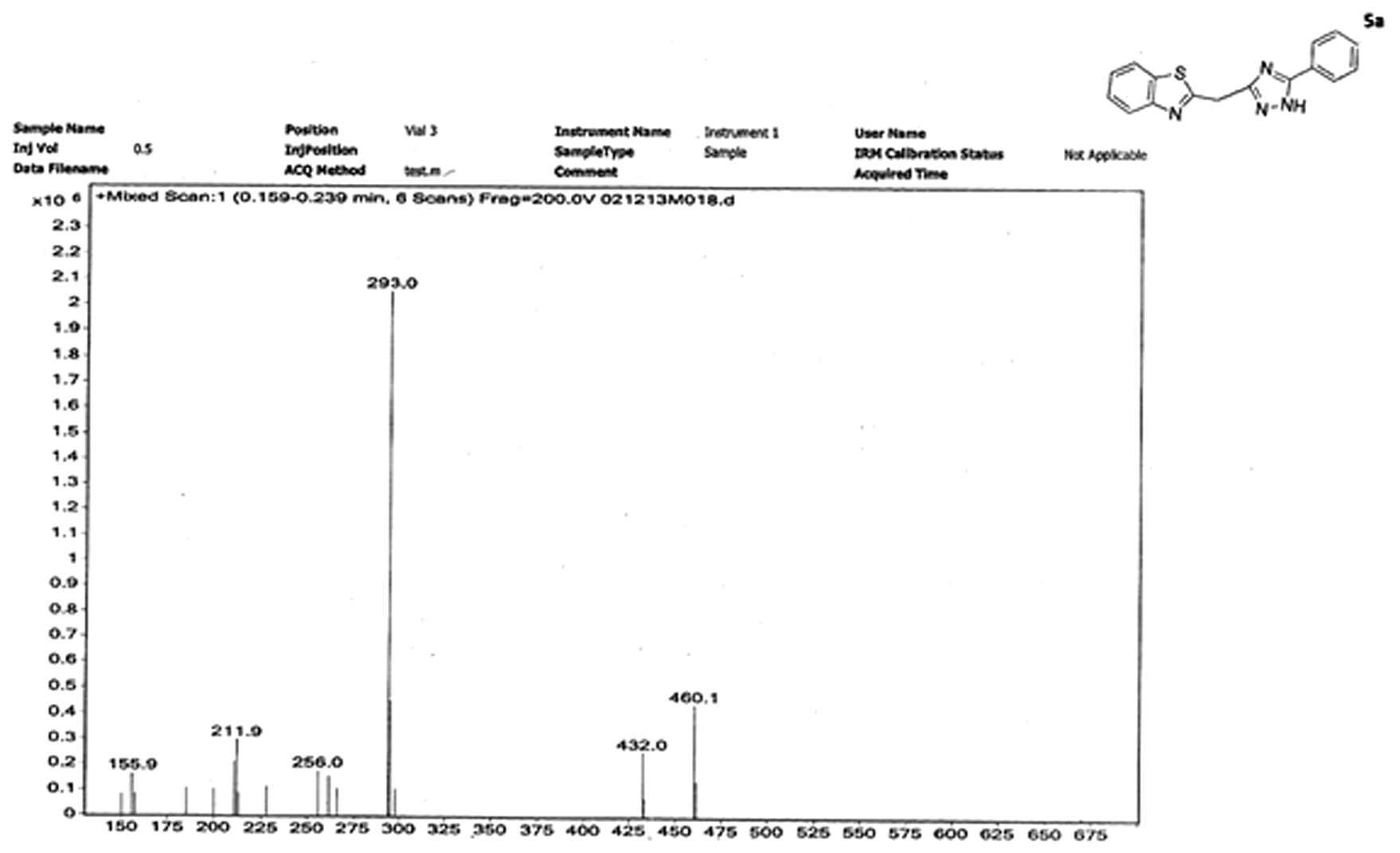
Mass spectrum of (5a) indicated a peak at 293 (m/z), which is in accordance with the calculated with (M + 1) molecular weight of compound (292). It also shows presence of even number of nitrogens present in the compound (5a), according to nitrogen rule. A peak at 228 (m/z) is explained due to lose of a diene moiety from the original molecule. For the other derivatives of compound 5 (i.e. 5a-h), the spectral data are presented in Table .
Table 2. Synthesis and spectroscopic analysis of 2-((5-phenyl-1H-1,2,4-triazol-3-yl)methyl)benzo[d]thiazoles (5a-h)
NMR spectrum of 2-((5-phenyl-1H-1,2,4-triazol-3-yl)methyl)benzo[d]thiazole (5a):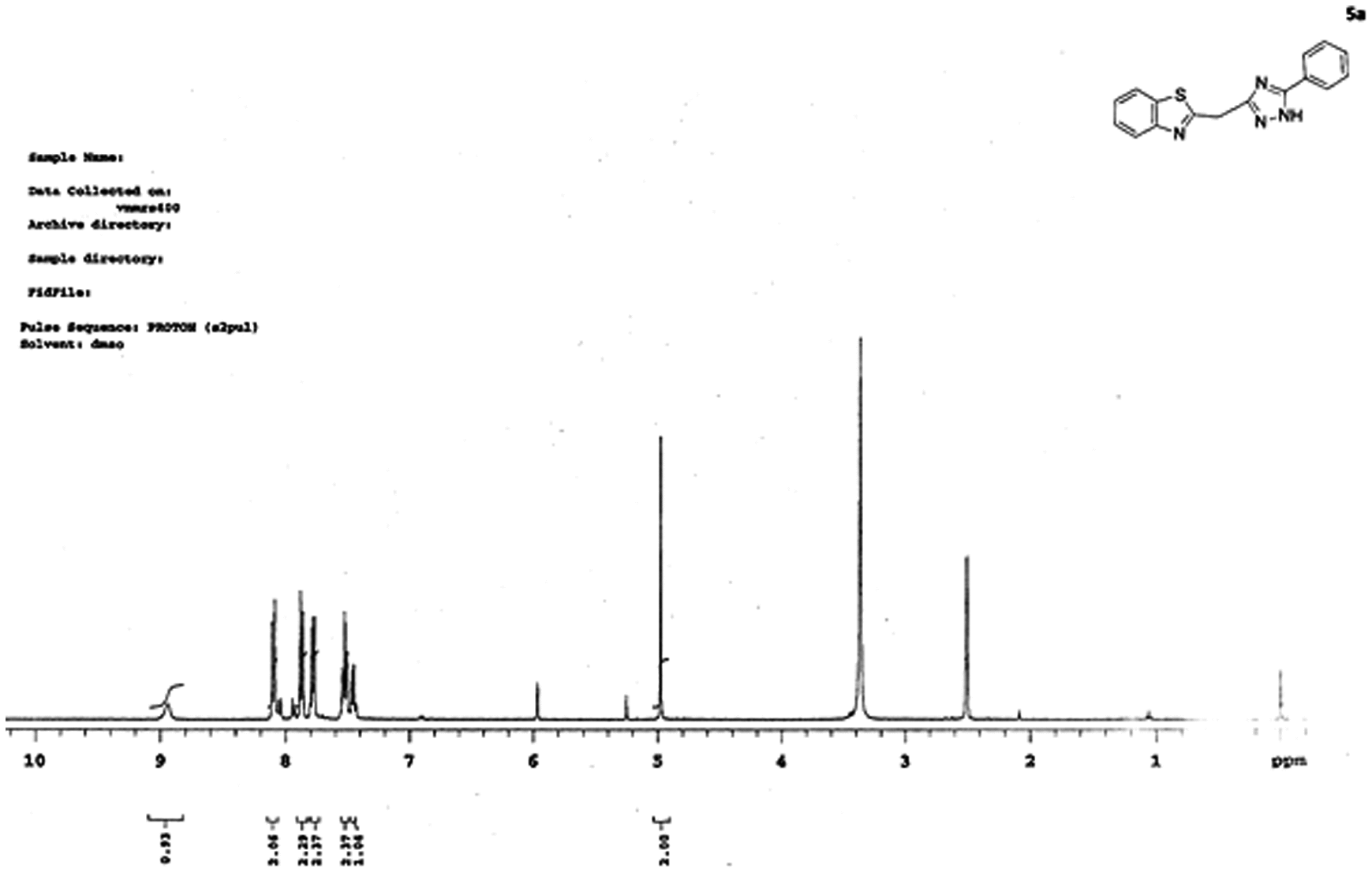
Proton NMR spectrum of (5a) two singlet peaks at 2.5 and 5 δ followed by a multiplet at 7.4–8 δ, respectively. Singlet peak observed at 2.5 δ could be due to the protons which are present on the carbon i.e. the carbon, which is attached to thiazole ring and triazole ring in part-C of the molecule. Peak appeared at 5 δ as singlet could be corresponding to the –NH proton in part-C. Finally multiplet appeared in the range 7.4–8.2 δ might represent all aromatic protons corresponding to the phenyl groups present in part-A and part-B of the molecule. For rest of the derivatives (5a-h), the spectral data are given in Table .
(5b) of 2-((5-(4-methoxyphenyl)-1H-1,2,4-triazol-3-yl)methyl)benzo[d]thiazole
C13 NMR spectrum (5b) of 2-((5-(4-methoxyphenyl)-1H-1,2,4-triazol-3-yl)methyl)benzo[d]thiazole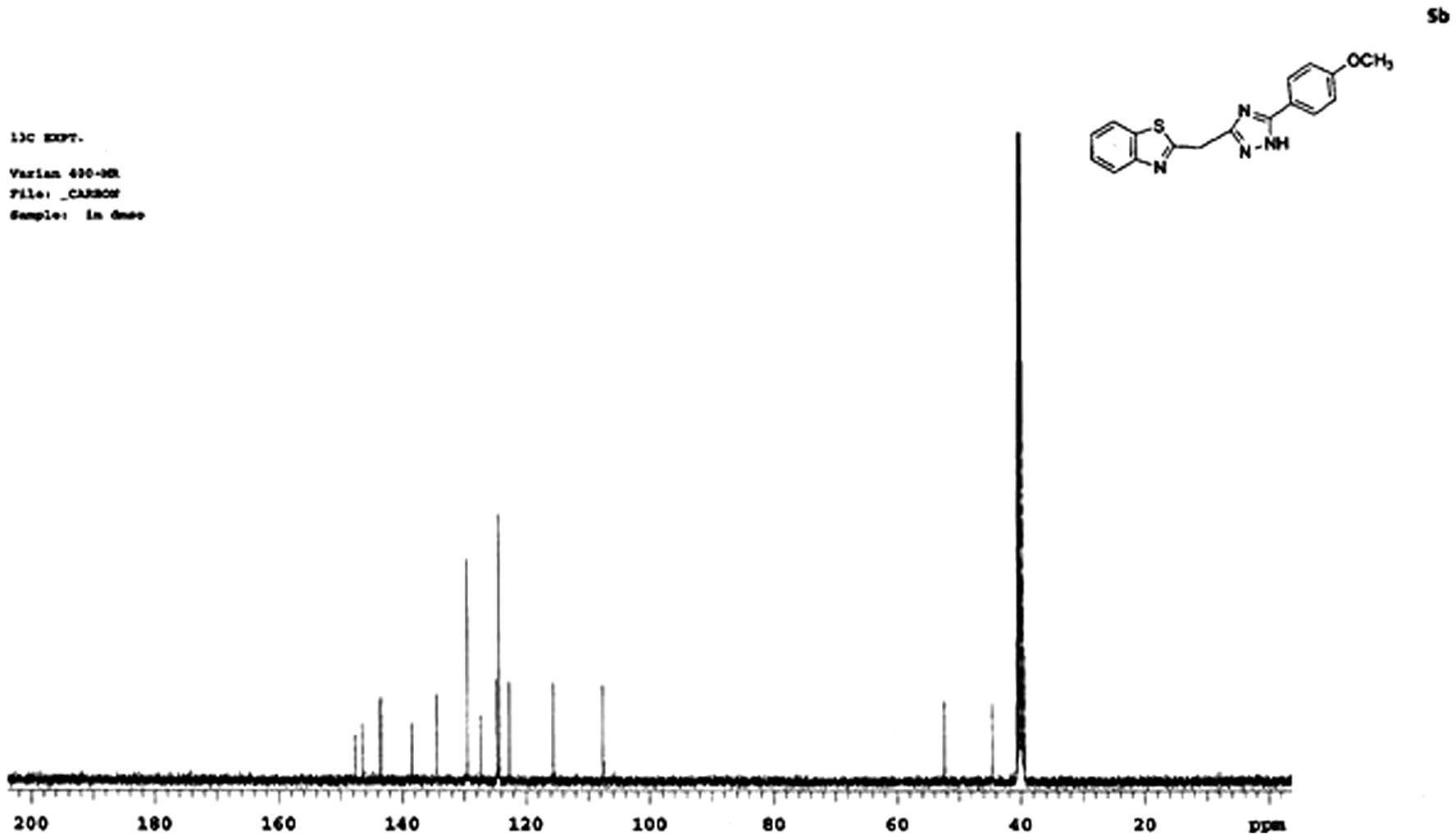
C13 NMR spectrum for (5b) depicted several peaks at 44, 52, 108, 118, 122, and 130–145 ppm range, respectively. Peak appeared at 44 ppm represents the triazole ring carbon in part-C, which is attached to part-B of the molecule, while the peak at 52 ppm represents methoxy carbon in part-B. A peak observed at 108 ppm represents the –CH2 carbon which is attached to part-A and part-C of the molecule. Peak appeared at 118 ppm represents the carbon attached to sulfur and nitrogen of benzothiazole ring (part-A). Peak appeared at 122 ppm represents triazole ring carbon in part-C which is attached to part-A through –CH2 carbon of part-C. The peaks observed in the 132–145 ppm could be attributed to aromatic ring carbons of part-A and part-B, respectively.
Spectroscopic data presented in Table revealed that irrespective of the aldehydes used in the study, the reactions afforded very good product yields, even though the reaction times did not follow a regular trend by changing the substituent (EWG/ED) in the phenyl ring. This trend could be probably attributed to the combined contribution of inductive and resonance parameters.
3. Biological screening
3.1. Invitro antimicrobial assay
Standard sterilized filter paper disks (5 mm diameter) impregnated with a solution of the test compound in DMSO (1 mg/ml) was placed on agar plate seeded with the appropriate test organism in triplicate. The tetracycline was used as standard antibacterial agent. DMSO alone was used as control at the same above-mentioned concentration. The plates were incubated at 37°C for 1–5 days. Antimicrobial activity was determined by measuring the diameter of the zone of inhibition surrounding microbial growth. The compounds that showed significant growth inhibition zones were further evaluated for their MIC measurement studies. The zone of inhibition is represented in millimeters (mm).
The bacteria selected to study the antimicrobial activity of the compounds prepared are:
Sp1. Staphylococcus aureus—coccus, gram +ve, causes toxic shock syndrome (TSS).
Sp2. Klebsiella pneumoniae—rod shape or bacilli, gram −ve, causes pneumonia.
Sp3. Salmonella paratyphi A—rod shape, gram −ve, causes typhoid.
Sp4. Salmonella paratyphi B—rod shape, gram −ve, causes typhoid.
From the data presented in Table , it appears that of the many of the compounds prepared in the present study exhibited efficient antibacterial activity. The activity of compounds 3b, 3d, and 3f (against Staphylococcus aureus) are comparable with standard tetracycline drug. However, compounds 3b, 5g, and 5h indicate the maximum activity against Klebsiella pneumonia, while compounds 3f and 3 h depict considerable activity. Against Salmonella paratyphi A, and Salmonella paratyphi B, considerable activity is shown by the compounds 3f, 5g, and 5h.
Table 3. Antibacterial activity of 1-((benzo[d]thiazol-2-yl)methyl)-4,5-dihydro-3-methyl-N-aryl-1H-pyrazol-5-amines and 2-((5-aryl-1H-1,2,4-triazol-3-yl)methyl)benzo[d]thiazoles
4. Experimental
4.1. Synthesis and characterization of 1-((benzo[d]thiazol-2-yl)methyl)-3-methyl-1H-pyrazol-5(4H)-one (2)
A centimolar (0.01 mol) solution of 2-((benzo[d]thiazol-2-yl)methyl)hydrazine (1) and ethyl acetoacetate (0.01 mol) were taken in clean round-bottomed flask and stirred at room temperature in ethanol medium. The reaction afforded 1-((benzo[d]thiazol-2-yl)methyl)-3-methyl-1H-pyrazol-5(4H)-one (2), with 287–289°C M.P. which upon condensation with different amines afforded 1-((benzo[d]thiazol-2-yl)methyl)-4,5-dihydro-3-methyl-N-phenyl-1H-pyrazol-5-amine derivatives (3a-h) under reflux conditions for 2 –7 h depending on the nature of substituent in amine.
4.2. Synthesis of 1-((benzo[d]thiazol-2-yl)methyl)-4,5-dihydro-3-methyl-N-phenyl-1H-pyrazol-5-amines (3a-h)
To a solution of 1-((benzo[d]thiazol-2-yl)methyl)-3-methyl-1H-pyrazol-5(4H)-one (2) (0.01 mol), in dry EtOH (20 mL) amine (0.01 mol) was added and the contents were refluxed for 2 h. The reaction was monitored on TLC. After the completion of reaction, the content was cooled and the separated product was filtered and crystallized from EtOH. Isolated product (1-((benzo[d]thiazol-2-yl)methyl)-4,5-dihydro-3-methyl-N-phenyl-1H-pyrazol-5-amine) was characterized by spectroscopic methods: 1H NMR (DMSO-d6) : δ = 2.20 (s, 3H), 4.78 (s, 2H), 7.01 (d, 2H), 7.18 (m, 4H), 7.31 (t, 1H), 7.40–7.60 (m, 4H). Mass: m/z 322 (M + H).
4.3. Synthesis of 2-(benzo[d]thiazol-2-yl)acetohydrazide (4)
To a solution of 2-aminobenzenethiol (0.01 mol) and 2-cyanoacetohydrazide (0.01 mol), in dry EtOH (20 mL) triethylamine (0.01 mol) was added and the contents were refluxed for 1 h. The reaction was monitored on TLC. After the completion of reaction the content was cooled and the separated product was filtered and crystallized from EtOH. 1H NMR (DMSO-d6) : δ = 8.18 (t, 1H), 7.90 (d, 2H), 7.72 (d, 2H), 7.50 (brs, 2H), 4.96 (s, 2H); Mass: (m/z) 208 (M + H).
4.4. Synthesis of 2-((5-phenyl-1H-1,2,4-triazol-3-yl)methyl)benzo[d]thiazole (5a-h)
To a solution of 2-(benzo[d]thiazol-2-yl) acetohydrazide (0.01 mol) and benzaldehyde (0.01 mol), in AcOH (20 mL) ammonium acetate (0.01 mol) was added and the contents were refluxed for 3 h. The reaction was monitored on TLC. After the completion of reaction, the content was cooled and the separated product was filtered and crystallized from EtOH. These compounds are characterized by spectral data: δ = 4.91 (s, 2H), 7.40 (m, 1H), 7.52 (m, 2H), 7.78 (d, 2H), 7.88 (d, 2H), 8.10 (d, 2H), 8.98 (brs, 1H); m/z = 293.
5. Conclusions
In summary, the authors have successfully synthesized 1-((benzo[d]thiazol-2-yl)methyl)-4,5-dihydro-3-methyl-N-phenyl-1H-pyrazol-5-amine (3a-h) from the condensation of 2-((benzo[d]thiazol-2-1-((benzo[d]thiazol-2-yl)methyl)-3-methyl-1H-pyrazol-5(4H)-one (2) with different aromatic amines. On the other hand, in another protocol, cyclization of 2-(benzo[d]thiazol-2-yl) acetohydrazide (4) with different aromatic aldehydes afforded 2-((5-aryl-1H-1,2,4-triazol-3-yl)methyl)benzo[d]thiazoles (5a-h). A perusal of the biological activity studies revealed that many of the prepared compounds in the present study exhibited efficient antibacterial activity. The activity of compounds 3b, 3d, and 3f (against Staphylococcus aureus) are comparable with standard tetracycline drug. However, compounds 3b, 5g, and 5h indicate the maximum activity against Klebsiella pneumonia, while compounds 3f and 3h depict considerable activity. Against Salmonella paratyphi A, and Salmonella paratyphi B considerable activity is shown by the compounds 3f, 5g, and 5h.
Funding
The authors received no direct funding for this research.
Supplementary material
Supplementary material for this article can be accessed here http://dx.doi.org/10.1080/23312009.2017.1312673.
1312673_supplementary_data.docx
Download MS Word (1.4 MB)Acknowledgments
The authors sincerely thank the authorities of Osmania University in the Department of Chemistry (main campus), O.U.P.G. College (Saifabad campus) for constant support and timely help.
Additional information
Notes on contributors
Pathki Uma
Prof. Pondichery Kuppuswamy Saiprakash completed his PhD in Physical Chemistry in 1974 from Osmania University, Hyderabad, while Prof. Kamatala Chinna Rajanna has obtained PhD, under the supervision of Saiprakash in 1982 from the same University. Both the professors are still actively involved in green chemistry, catalysis and materials chemistry research, in the Department of Chemistry, Osmania University, Hyderabad, which is celebrating centenary year activities. Over 40 years of teaching and research experience, both of them published about 150 papers in prestigious national and international journals. Under their supervision, several students obtained PhD in chemical sciences. Mrs. Pathki Uma and Firasath Unnisa are working as Assistant Professors in Osmania University Hyderabad and active in research.
References
- Ali, R., & Siddiqui, N. (2013). Biological aspects of emerging benzothiazoles: A short review. Journal of Chemistry, 2013 Article ID 345198. doi:10.1155/2013/345198
- Archita, B., Swati, O., & Talesara, G. L. (2008). Facile synthesis of alkoxypthalimide derivatized benzimidazole assembled pyrazoles, pyrimidines and isoxazoles via common intermediate chalcone. Indian Journal of Chemistry, 47B, 1096–1107.
- Azarifar, D., & Ghasemnejad, H. (2003). Microwave-assisted synthesis of some 3, 5-arylated 2-pyrazolines. Molecules, 8, 642–648.10.3390/80800642
- Bruno, O., Schenone, S., Ranise, A., Bondavalli, F., Falcone, G., Filippelli, W., … Motula, G. (1997). N-arylsubstituted-1-aryl-3,4-diphenyl-5-pyrazole amines with analgesic and antiarrhythmic properties. Farmaco, 52, 615–618.
- Chimenti, F., Bolasco, A., Manna, F., Secci, D., Chimenti, P., Befani, O., … La Torre, F. (2004). Synthesis and selective inhibitory activity of 1-acetyl-3, 5-diphenyl-4, 5-dihydro-(1 H)-pyrazole derivatives against monoamine oxidase. Journal of Medicinal Chemistry, 47, 2071–2074.10.1021/jm031042b
- David, K., Uros, G., Anton, M., Georg, D., Branko, S., & Jurij, S. (2007). A simple synthesis of 4-(2-aminoethyl)-5-hydroxy-1H-pyrazoles. Tetrahedron, 63, 11213–11222.
- Dobaria, A. V., Patel, J. R., & Parekh, H. H. (2003). Synthesis of pyrazolines and isoxazole derivatives bearing chloroquinoline nucleus as potential antimicrobial agents. Indian Journal of Chemistry, Section B, 42B, 2019–2022.
- Eicher, T., & Hauptmann, S. (2003). The chemistry of heterocycles: Structure, reactions, syntheses, and applications. Weinheim, FRG: Wiley-VCH.10.1002/352760183X
- Evans, N. A. (1975). Dye-sensitized photooxidation of some substituted 1, 3-diphenyl-2-pyrazolines. Australian Journal of Chemistry, 28, 433–437.10.1071/CH9750433
- Hughes, T. V., Emanuel, S. L., Beck, A. K., Wetter, S. K., Connolly, P. J., Prabha, K., … Fuentes-Pesquera, A. R. (2007). 4-Aryl-5-cyano-2-aminopyrimidines as VEGF-R2 inhibitors: Synthesis and biological evaluation. Bioorganic & Medicinal Chemistry Letters, 17, 3266–3270.10.1016/j.bmcl.2007.04.021
- Huisgen, R., Koszinowski, J., Ohta, A., & Schiffer, R. (1980). Cycloadditionen der Diazoalkane an 1-Alkene. Angewandte Chemie, 92, 198–199.
- Joule, J. A., Mills, K., & Smith, G. F. (1995). Heterocyclic chemistry (3rd ed.). London: Chapman and Hall.10.1007/978-1-4899-3222-8
- Mahesh, V. K., & Gupta, R. S. (1974). Condensation products of dehydroacetic acid with aromatic aldehydes and pyrazolines derived from them. Indian Journal of Chemistry, 12B, 956–959.
- Mogilaiah, K. & Rama Sudhakar, G. (2003). Synthesis of pyrazoline, pyrimidine and 1,5-benzodiazepine derivatives of 1,8-naphthyridine and evaluation of antibacterial activity. Indian Journal of Chemistry, 42B, 636–640.
- Reimlinger, H. (1952). Bisdiazo‐alkane, I Reaktionen der Bisdiazo‐alkane mit Acetylen. Chemische Berichte, 92, 970–977.
- Thomas, J. J., Muller, Ronald, B., & Markus, A. (1967). A novel three-component one-pot pyrimidine synthesis based upon a coupling− isomerization sequence. Organic letters, 2, 1967–1970.
- Tuan, T. D., Tung, T. D., & Peter, L. (2007). One-pot synthesis of pyrazole-5-carboxylates by cyclization of hydrazone 1,4-dianions with diethyl oxalate. Tetrahedron Letters, 20, 3591–3593.
- Yuhong, J., & Varma, R. S. (2005). Microwave-assisted cyclocondensation of hydrazine derivatives with alkyl dihalides or ditosylates in aqueous media: Syntheses of pyrazole, pyrazolidine and phthalazine derivatives. Tetrahedron Letters, 46, 6011–6014.
- Yuhong, J., & Varma, R. S. (2006). queous N-heterocyclization of primary amines and hydrazines with dihalides: microwave-assisted syntheses of N-azacycloalkanes, isoindole, pyrazole, pyrazolidine, and phthalazine derivatives. The Journal of Organic Chemistry, 71, 135–141.
- Yusuf, M., & Jain, P. (2014). Synthetic and biological studies of pyrazolines and related heterocyclic compounds. Arabian Journal of Chemistry, 7, 553–596.10.1016/j.arabjc.2011.09.013
