Abstract
In this work, two newly prepared Brønsted-acidic ionic liquids, [MPyrrSO3H]Cl (IL1) and [MMorSO3H]Cl (IL2), were efficiently used as catalysts for the synthesis of amidoalkyl naphthols through the one‐pot, three‐component reaction of β‐naphthol, aryl aldehydes, and acetamide under neat conditions. High activity of the catalysts, excellent yields, short reaction times, simple procedure with an easy work-up, and the absence of any volatile and hazardous organic solvents are some advantages of the present methodology. Moreover, the catalysts are simply prepared and can be recovered conveniently and reused such that considerable catalytic activity can still be achieved after the fifth run.
Public Interest Statement
Application of new ionic Liquids in organic transformations is of great interest in recent years. Therefore, in this paper, two newly prepared Brønsted-acidic ionic liquids were efficiently used as catalysts for the synthesis of amidoalkyl naphthols through the one‐pot, three‐component reaction of β‐naphthol, aryl aldehydes, and acetamide under neat conditions. Some advantages of this procedure are high yields, short reaction times, easy work-up, absence of volatile and hazardous solvents, and reusability of catalysts for a number of times without appreciable loss of activity.
1. Introduction
A major challenge in modern chemistry is the design of highly efficient chemical reaction sequences that provide maximum structural complexity with a minimum number of synthetic steps in short reaction times (Dömling, Citation2006; Schreiber, Citation2000). Multicomponent reactions (MCRs) have gained considerable attention as a powerful method in organic synthesis and medicinal chemistry because they involve simultaneous reaction of more than two starting materials to yield a single product through one-pot reaction (Gore & Rajput, Citation2013; Slobbe, Ruijter, & Orru, Citation2012; Tavakoli-Hoseini & Davoodnia, Citation2011). High atom economy, good selectivity, time and energy saving, low cost, minimum waste production, and short reaction time make MCRs suitable for the synthesis of complex molecules with potential biological activity (Chebanov & Desenko, Citation2012; Manjappa, Peng, Jhang, & Yang, Citation2016; Zang, Zhang, Zang, & Cheng, Citation2010). On the other hand, the nature of the catalyst plays a crucial role in the determination of the product and selectivity (Khan, Khan, & Bannuru, Citation2010; Mirzaei & Davoodnia, Citation2012; Shaterian & Mohammadnia, Citation2012). Therefore, development of inexpensive, mild, and reusable catalysts for MCRs such as the synthesis of amidoalkyl naphthols remains of interest to the synthetic organic chemists. It has been reported that amidoalkyl naphthols can convert to important biologically active aminoalkyl naphthol derivatives by amide hydrolysis. Later compounds have been evaluated for the hypotensive and bradycardiac effects (Dingermann, Steinhilber, & Folkers, Citation2004; Shen, Tsai, & Chen, Citation1999). Amidoalkyl naphthols are generally synthesized via the three‐component reaction of β‐naphthol, an aldehyde, and an amide in the presence of various catalysts, such as Sb(OAc)3 (Hakimi, Citation2016), zirconocene dichloride (Cp2ZrCl2) (Khanapure, Jagadale, Salunkhe, & Rashinkar, Citation2016), ZrOCl2·8H2O (Sheik Mansoor, Aswin, Logaiya, & Sudhan, Citation2016), nano Al2O3 (Kiasat, Hemat-Alian, & Saghanezhad, Citation2016), carbon‐based solid acid (Davoodnia, Mahjoobin, & Tavakoli-Hoseini, Citation2014), H3BO3 (Shahrisa, Esmati, & Nazari, Citation2012), iodine (Nagawade & Shinde, Citation2007), nano‐sulfated zirconia (Zali & Shokrolahi, Citation2012), K5CoW12O40·3H2O (Nagarapu, Baseeruddin, Apuri, & Kantevari, Citation2007), copper p‐toluenesulfonate (Wang & Liang, Citation2011), Al(H2PO4)3 (Shaterian, Amirzadeh, Khorami, & Ghashang, Citation2008), Yb(OTf)3 in [bmim][BF4] (Kumar, Rao, Ahmad, & Khungar, Citation2009), and nano silica phosphoric acid (Bamoniri, Mirjalili, & Nazemian, Citation2014). Although each of these individual methods has its own merits, many suffer from limitations such as long reaction times, unsatisfactory yields, and the use of relatively expensive catalysts. Thus, the exploration of novel methodologies using new efficient and reusable catalysts is still ongoing.
In recent years, ionic Liquids (ILs) have attracted rising interest as eco-friendly solvents, catalysts and reagents in organic transformations due to their advantageous properties, such as non-flammability, negligible vapor pressure, high thermal and chemical stability, and ability to dissolve a wide range of materials (Chowdhury, Mohan, & Scott, Citation2007; Olivier-Bourbigou, Magna, & Morvan, Citation2010; Pârvulescu & Hardacre, Citation2007). ILs are miscible with materials having very wide range of polarities and are simultaneously able to dissolve a wide range of organic, inorganic and organometallic substances. These features offer numerous opportunities for the improvement of organic reactions using ILs as solvents and catalysts. Moreover, their ionic character enhances the reaction rates to a great extent in many reactions. Among them, Brønsted acidic ILs, especially the SO3H-functionalized ones, have designed as environmentally friendly catalysts to replace the traditional mineral liquid acids like sulfuric acid and hydrochloric acid in chemical processes (Greaves & Drummond, Citation2008; Qiu et al., Citation2016; Shirole, Kadnor, Tambe, & Shelke, Citation2017; Vafaeezadeh & Alinezhad, Citation2016; Zolfigol, Khazaei, Moosavi-Zare, & Zare, Citation2010).
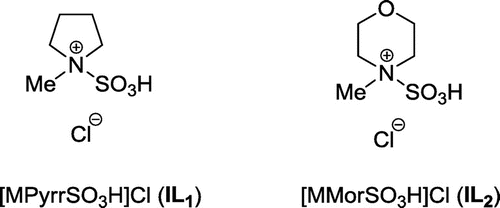
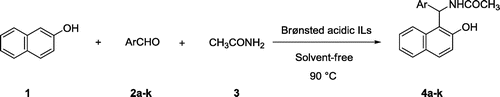
Considering the unique properties of Brønsted-acidic ILs, recently, we have synthesized two sulfonic acid functionalized ILs, including 1-methyl-1-sulfonic acid pyrrolidinium chloride [MPyrrSO3H]Cl (IL1) and 4-methyl-4-sulfonic acid morpholinium chloride [MMorSO3H]Cl (IL2) (Figure ), and successfully applied them as highly efficient catalysts in the synthesis of 1,8-dioxooctahydroxanthenes (Dehghan, Davoodnia, Bozorgmehr, & Bamoharram, Citation2016). These findings encouraged us to explore other applications of these ILs in the synthesis of organic compounds. Therefore, in line with our interest on the development of convenient methods using reusable catalysts (Davoodnia, Citation2011; Davoodnia, Allameh, Fazli, & Tavakoli-Hoseini, Citation2011; Davoodnia, Basafa, & Tavakoli-Hoseini, Citation2016; Davoodnia, Khojastehnezhad, Bakavoli, & Tavakoli-Hoseini, Citation2011; Emrani, Davoodnia, & Tavakoli-Hoseini, Citation2011; Khashi, Davoodnia, & Prasada Rao Lingam, Citation2015; Moghaddas, Davoodnia, Heravi, & Tavakoli-Hoseini, Citation2012; Nakhaei & Davoodnia, Citation2014; Taghavi-Khorasani & Davoodnia, Citation2015), herein, we report the results of our investigation on the application of IL1 and IL2 as catalysts in the synthesis of amidoalkyl naphthols through the one‐pot, three‐component reaction of β‐naphthol, aryl aldehydes, and acetamide under neat conditions (Scheme ).
2. Results and discussion
As a preliminary, we directed our studies toward examination of the effect of various parameters like catalyst composition, effect of solvent, and influence of temperature on the reaction of β-naphthol (1) (1.0 mmol), 4-chlorobenzaldehyde (2d) (1.0 mmol), and acetamide (3) (1.0 mmol) for the synthesis of compound 4d as the model reaction in the absence or presence of IL1 and IL2 as catalysts. A summary of the optimization experiments is provided in Table . First, to illustrate the need for catalyst in the reaction, the model reaction was studied in the absence of catalyst under solvent-free condition. The yield of the product was trace at 90°C after 60 min (Table , entry 1). Next, the reaction was performed in the presence of IL1 or IL2 in different solvents as well as under solvent‐free conditions. Among the solvents tested, those being EtOH, MeOH, CH2Cl2, and MeCN, the reaction proceeded most readily to give the highest yield of the product 4d under solvent‐free conditions. It was observed that the yield of the final product 4d increased with increasing amount of catalyst in the reaction mixture. The best result was obtained with 10 mol% of the catalyst under solvent‐free conditions, which gave the desired product in 95 and 98% yields after 3 and 2 min at 90°C, respectively, for IL1 and IL2 (Table , entry 12). Further increase in temperature and IL1 or IL2 amount were found to have an inhibitory effect on formation of the product (Table , entries 13, 16, 17).
Table 1. Screening of reaction condition for synthesis of compound 4d catalyzed by IL1 or IL2Table Footnotea
With the optimized conditions in hand, β-naphthol was reacted with acetamide and a wide variety of aromatic aldehydes using IL1 or IL2 (Table ). As it can be seen, the reaction is effective with a variety of aromatic aldehydes with electron-donating or withdrawing substituents. Although the kind of aromatic aldehyde had no significant effect on the reaction, in most cases, but not all, aromatic aldehydes substituted with electron‐withdrawing group or none reacted slightly faster than those with electron-donating groups and gave the higher yields of the products. Furthermore, both catalysts were highly efficient, and gave the desired amidoalkyl naphthols in high yields and short reaction times. However, as depicted, IL2 proved to be the better catalyst than IL1 in terms of yield and reaction time.
Table 2. IL1 or IL2 catalyzed synthesis of amidoalkyl naphthols (4a-k)Table Footnotea
We also investigated recycling of the catalysts under solvent-free conditions using the model reaction. After completion of the reaction, the reaction mixture was cooled to room temperature, and warm distilled water was added. The product was collected by filtration, and washed repeatedly with warm distilled water. The combined filtrate was evaporated to dryness under reduced pressure. The residual ionic liquid was repeatedly washed with diethyl ether, dried under vacuum at 60°C, and used for the subsequent catalytic runs. The recovered catalyst worked well for up to five catalytic runs without any significant loss of its activity (95/98, 95/96, 93/95, 92/93, and 91/93% yields for IL1/IL2 catalysts in first to fifth use, respectively).
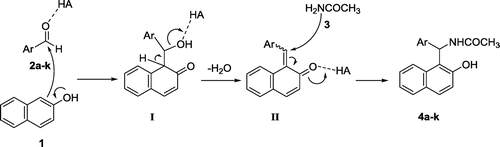
In accordance with the literature (Kiasat et al., Citation2016; Shahrisa et al., Citation2012), the suggested mechanism is described in Scheme . We believe that these ILs can act as Brønsted acids and therefore promotes the reactions by increasing the electrophilic character of the electrophiles in the reaction. At first, ortho-quinone methide (o-QM) intermediate [II] is readily formed in situ by Knoevenagel condensation of β-naphthol (1) and aromatic aldehydes (2a-k) via the intermediate [I]. Subsequent Michael addition of acetamide (3) to the o-QM intermediate [II] afforded the final products 4a-k.
3. Conclusion
In conclusion, we showed that two newly synthesized Brønsted-acidic ILs, IL1 and IL2, efficiently catalyze the synthesis of amidoalkyl naphthols by increasing the electrophilic character of the electrophiles in the reaction β‐naphthol, aryl aldehydes, and acetamide under solvent-free reactions. The kind of aldehyde had no significant effect on the reaction rates and products’ yields. However, in general, electron-poor aldehydes reacted slightly faster than electron-rich ones and gave the higher yields of the products. Also, IL2 proved to be the better catalyst than IL1 in terms of yield and reaction time. Some advantages of this procedure are high yields, short reaction times, easy work-up, absence of volatile and hazardous solvents, and reusability of catalysts for a number of times without appreciable loss of activity.
4. Experimental
The IL1 and IL2 were synthesized according to the our previous report (Dehghan et al., Citation2016). All chemicals were available commercially and used without additional purification. Melting points were recorded on a Stuart SMP3 melting point apparatus. The 1H NMR spectra were recorded with a Bruker 300 FT spectrometer.
4.1. General procedure for the synthesis of amidoalkyl naphthols (4a-k) catalyzed by IL1 or IL2
A mixture of β‐naphthol (1) (1.0 mmol), an aromatic aldehyde (2a‐k) (1.0 mmol), acetamide (3) (1.0 mmol), and IL1 or IL2 (0.1 mmol, 10 mol %) was heated in an oil bath at 90°C for 2–6 min. After completion of the reaction, monitored by TLC, the mixture was cooled to room temperature and warm distilled water was added. This resulted in the precipitation of the product, which was collected by filtration. The crude product was washed repeatedly with warm distilled water and then cold ethanol, and subsequently recrystallized from ethanol to give the pure products 4a-k in high yields. The products were characterized according to comparison of their melting points with those of authentic samples and for some of them by their 1H NMR spectral data.
4.2. Selected 1H NMR data
N-((2-hydroxynaphthalen-1-yl)(phenyl)methyl)acetamide (4a): 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, CH3), 7.10–7.45 (m, 9H, arom-H and CHSP3), 7.76–7.87 (m, 3H, arom-H and NH), 8.52 (d, 1H, J = 8.1 Hz, arom-H), 10.08 (s br, 1H, OH).
N-((2-hydroxynaphthalen-1-yl)(4-nitrophenyl)methyl)acetamide (4b): 1H NMR (300 MHz, DMSO-d6): δ 2.04 (s, 3H, CH3), 7.18–7.33 (m, 3H, arom-H and CHSP3), 7.38–7.46 (m, 3H, arom-H), 7.78–7.88 (m, 3H, arom-H and NH), 8.16 (d, 2H, J = 9.0 Hz, arom-H), 8.62 (d, 1H, J = 7.8 Hz, arom-H), 10.01 (br, 1H, OH).
N-((4-Chlorophenyl)(2-hydroxynaphthalen-1-yl)methyl)acetamide (4d): 1H NMR (300 MHz, DMSO-d6): δ 2.03 (s, 3H, CH3), 7.13–7.44 (m, 8H, arom-H and CHSP3), 7.82 (t, 2H, J = 8.7 Hz, arom-H), 7.87 (br, 1H, NH), 8.54 (d, 1H, J = 8.1 Hz, arom-H), 10.11 (s, 1H, OH).
N-((3-Bromophenyl)(2-hydroxynaphthalen-1-yl)methyl)acetamide (4f): 1H NMR (300 MHz, DMSO-d6): δ 2.00 (s, 3H, CH3), 7.12 (d, 2H, J = 6.9 Hz, arom-H), 7.18–7.45 (m, 6H, arom-H and CHSP3), 7.77–7.90 (m, 3H, arom-H and NH), 8.51 (d, 1H, J = 8.4 Hz, arom-H), 9.69 (br, 1H, OH).
N-((4-Bromophenyl)(2-hydroxynaphthalen-1-yl)methyl)acetamide (4g): 1H NMR (300 MHz, DMSO-d6): δ 2.01 (s, 3H, CH3), 7.08–7.32 (m, 5H, arom-H and CHSP3), 7.40 (t, 1H, J = 8.1 Hz, arom-H), 7.46 (d, 2H, J = 8.4 Hz, arom-H), 7.76–7.88 (m, 3H, arom-H and NH), 8.51 (d, 1H, J = 8.1 Hz, arom-H), 10.04 (s br, 1H, OH).
N-((2-hydroxynaphthalen-1-yl)(4-methoxyphenyl)methyl)acetamide (4j): 1H NMR (300 MHz, DMSO-d6): δ 2.00 (s, 3H, CH3), 3.69 (s, 3H, OCH3), 6.83 (d, 2H, J = 8.7 Hz, arom-H), 7.08–7.31 (m, 5H, arom-H and CHSP3), 7.38 (t, 1H, J = 7.2 Hz, arom-H), 7.75–7.94 (m, 3H, arom-H and NH), 8.49 (d, 1H, J = 8.4 Hz, arom-H), 10.05 (br, 1H, OH).
N-((2-hydroxynaphthalen-1-yl)(pyridin-3-yl)methyl)acetamide (4k): 1H NMR (300 MHz, DMSO-d6): δ 2.02 (s, 3H, CH3), 7.15–7.35 (m, 4H, arom-H and CHSP3), 7.42 (t, 1H, J = 7.5 Hz, arom-H), 7.55 (d, 1H, J = 8.1 Hz, arom-H), 7.78–7.95 (m, 3H, arom-H and NH), 8.37–8.44 (m, 2H, arom-H), 8.57 (d, 1H, J = 8.1 Hz, arom-H), 10.14 (s br, 1H, OH).
Funding
We gratefully acknowledge financial support from the Islamic Azad University, Mashhad Branch, Iran.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/23312009.2017.1312675.
1312675_Supplementary_material.docx
Download MS Word (327.8 KB)Additional information
Notes on contributors
Abolghasem Davoodnia
Abolghasem Davoodnia was born in 1971, Mashhad, Iran. He studied chemistry at Tehran University, Tehran, Iran, where he received BSc in 1994. He received his MSc degree in organic chemistry in 1997 from Ferdowsi University of Mashhad, Mashhad, Iran, under the supervision of prof Majid M. Heravi and completed his PhD in organic chemistry in 2002 under the supervision of prof Mehdi Bakavoli at the same university. Currently, he is working as a professor at the Chemistry Department, Mashhad Branch, Islamic Azad University, Mashhad, Iran. He has published over 140 peer-reviewed articles in ISI journals. His current research interest is on heterocyclic chemistry, catalysis and new synthetic methodologies.
References
- Bamoniri, A., Mirjalili, B. F., & Nazemian, S. (2014). Nano silica phosphoric acid: an efficient catalyst for the one-pot synthesis of amidoalkyl naphthols under solvent-free condition. Journal of the Iranian Chemical Society, 11, 653–658. doi:10.1007/s13738-013-0336-z
- Chebanov, V. A., & Desenko, S. M. (2012). Multicomponent heterocyclization reactions with controlled selectivity (Review). Chemistry of Heterocyclic Compounds, 48, 566–583. doi:10.1007/s10593-012-1030-2
- Chowdhury, S., Mohan, R. S., & Scott, J. L. (2007). Reactivity of ionic liquids. Tetrahedron, 63, 2363–2389. doi:10.1016/j.tet.2006.11.001
- Davoodnia, A. A. (2011). Highly efficient and fast method for the synthesis of biscoumarins using tetrabutylammonium hexatungstate [TBA]2[W6O19] as green and reusable heterogeneous catalyst. Bulletin of the Korean Chemical Society, 32, 4286–4290. doi:10.5012/bkcs.2011.32.12.4286
- Davoodnia, A., Allameh, S., Fazli, S., & Tavakoli-Hoseini, N. (2011). One-pot synthesis of 2-amino-3-cyano-4-arylsubstituted tetrahydrobenzo[b]pyrans catalysed by silica gel-supported polyphosphoric acid (PPA-SiO2) as an efficient and reusable catalyst. Chemical Papers, 65, 714–720. doi:10.2478/s11696-011-0064-8
- Davoodnia, A., Basafa, S., & Tavakoli-Hoseini, N. (2016). Neat synthesis of octahydroxanthene-1,8-diones, catalyzed by silicotungstic acid as an efficient reusable inorganic catalyst. Russian Journal of General Chemistry, 86, 1132–1136. doi:10.1134/S107036321605025X
- Davoodnia, A., Khojastehnezhad, A., Bakavoli, M., & Tavakoli-Hoseini, N. (2011). SO3H-functionalized ionic liquids: Green, efficient and reusable catalysts for the facile dehydration of aldoximes into nitriles. Chinese Journal of Chemistry, 29, 978–982. doi:10.1002/cjoc.201190199
- Davoodnia, A., Mahjoobin, R., & Tavakoli-Hoseini, N. (2014). A facile, green, one-pot synthesis of amidoalkyl naphthols under solvent-free conditions catalyzed by a carbon-based solid acid. Chinese Journal of Catalysis, 35, 490–495. doi:10.1016/S1872-2067(14)60011-5
- Dehghan, M., Davoodnia, A., Bozorgmehr, M. R., & Bamoharram, F. F. (2016). Synthesis, characterization and application of two novel sulfonic acid functionalized ionic liquids as efficient catalysts in the synthesis of 1,8-dioxo-octahydroxanthenes. Heterocyclic Letters, 6, 251–257.
- Dingermann, T., Steinhilber, D., & Folkers, G. (2004). In molecular biology in medicinal chemistry. Weinheim: Wiley-VCH.
- Dömling, A. (2006). Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chemical Preview, 106, 17–89. doi:10.1021/cr0505728
- Emrani, A., Davoodnia, A., & Tavakoli-Hoseini, N. (2011). Alumina supported ammonium dihydrogenphosphate (NH4H2PO4/Al2O3): Preparation, characterization and its application as catalyst in the synthesis of 1,2,4,5-tetrasubstituted imidazoles. Bulletin of the Korean Chemical Society, 32, 2385–2390. doi:10.5012/bkcs.2011.32.7.2385
- Gore, R. P., & Rajput, A. P. (2013). A review on recent progress in multicomponent reactions of pyrimidine synthesis. Drug Invention Today, 5, 148–152. doi:10.1016/j.dit.2013.05.010
- Greaves, T. L., & Drummond, C. J. (2008). Protic Ionic Liquids: Properties and Applications. Chemical Reviews, 108, 206–237. doi:10.1021/cr068040u
- Hakimi, F. (2016). Antimony(III) acetate an efficient catalyst for the synthesis of 1-amidoalkyl-2-naphthols. Journal of Chemical Research, 40, 489–491. doi:10.3184/174751916X14665064359398
- Khan, A. T., Khan, M. M., & Bannuru, K. K. R. (2010). Iodine catalyzed one-pot five-component reactions for direct synthesis of densely functionalized piperidines. Tetrahedron, 66, 7762–7772. doi:10.1016/j.tet.2010.07.075
- Khanapure, S., Jagadale, M., Salunkhe, R., & Rashinkar, G. (2016). Zirconocene dichloride catalyzed multi-component synthesis of 1-amidoalkyl-2-naphthols at ambient temperature. Research on Chemical Intermediates, 42, 2075–2085. doi:10.1007/s11164-015-2136-9
- Khashi, M., Davoodnia, A., & Prasada Rao Lingam, V. S. (2015). DMAP catalyzed synthesis of some new pyrrolo[3,2-e][1,2,4]triazolo[1,5-c]pyrimidines. Research on Chemical Intermediates, 41, 5731–5742. doi:10.1007/s11164-014-1697-3
- Kiasat, A. R., Hemat-Alian, L., & Saghanezhad, S. J. (2016). Nano Al2O3: An efficient and recyclable nanocatalyst for the one-pot preparation of 1-amidoalkyl-2-naphthols under solvent-free conditions. Research on Chemical Intermediates, 42, 915–922. doi:10.1007/s11164-015-2062-x
- Kumar, A., Rao, M. S., Ahmad, I., & Khungar, B. (2009). A simple and facile synthesis of amidoalkyl naphthols catalyzed by Yb(OTf) 3 in ionic liquids. Canadian Journal of Chemistry, 87, 714–719. doi:10.1139/V09-049
- Manjappa, K. B., Peng, Y. T., Jhang, W. F., & Yang, D. Y. (2016). Microwave-promoted, catalyst-free, multi-component reaction of proline, aldehyde, 1,3-diketone: One pot synthesis of pyrrolizidines and pyrrolizinones. Tetrahedron, 72, 853–861. doi:10.1016/j.tet.2015.12.056
- Mirzaei, H., & Davoodnia, A. (2012). Microwave assisted sol-gel synthesis of MgO nanoparticles and their catalytic activity in the synthesis of hantzsch 1,4-dihydropyridines. Chinese Journal of Catalysis, 33, 1502–1507. doi:10.1016/S1872-2067(11)60431-2
- Moghaddas, M., Davoodnia, A., Heravi, M. M., & Tavakoli-Hoseini, N. (2012). Sulfonated carbon catalyzed biginelli reaction for one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones and -thiones. Chinese Journal of Catalysis, 33, 706–710. doi:10.1016/S1872-2067(11)60377-X
- Nagarapu, L., Baseeruddin, M., Apuri, S., & Kantevari, S. (2007). Potassium dodecatungstocobaltate trihydrate (K5CoW12O40·3H2O): A mild and efficient reusable catalyst for the synthesis of amidoalkyl naphthols in solution and under solvent-free conditions. Catalysis Communications, 8, 1729–1734. doi:10.1016/j.catcom.2007.02.008
- Nagawade, R. R., & Shinde, D. B. (2007). Synthesis of amidoalkyl naphthols by an iodine-catalyzed multicomponent reaction of β-naphthol. Mendeleev Communications, 17, 299–300. doi:10.1016/j.mencom.2007.09.018
- Nakhaei, A., & Davoodnia, A. (2014). Application of a Keplerate type giant nanoporous isopolyoxomolybdate as a reusable catalyst for the synthesis of 1,2,4,5-tetrasubstituted imidazoles. Chinese Journal of Catalysis, 35, 1761–1767. doi:10.1016/S1872-2067(14)60174-1
- Olivier-Bourbigou, H., Magna, L., & Morvan, D. (2010). Ionic liquids and catalysis: Recent progress from knowledge to applications. Applied Catalysis A: General, 373, 1–56. doi:10.1016/j.apcata.2009.10.008
- Pârvulescu, V. I., & Hardacre, C. (2007). Catalysis in ionic liquids. Chemical Reviews, 107, 2615–2665. doi:10.1021/cr050948h
- Qiu, T., Guo, X., Yang, J., Zhou, L., Li, L., Wang, H., & Niu, Y. (2016). The synthesis of biodiesel from coconut oil using novel Brønsted acidic ionic liquid as green catalyst. Chemical Engineering Journal, 296, 71–78. doi:10.1016/j.cej.2016.03.096
- Schreiber, S. L. (2000). Target-oriented and diversity-oriented organic synthesis in drug discovery. Science, 287, 1964–1969. doi:10.1126/science.287.5460.1964
- Shahrisa, A., Esmati, S., & Nazari, M. G. (2012). Boric acid as a mild and efficient catalyst for one-pot synthesis of 1-amidoalkyl-2-naphthols under solvent-free conditions. Journal of Chemical Sciences, 124, 927–931. doi:10.1007/s12039-012-0285-6
- Shaterian, H. R., Amirzadeh, A., Khorami, F., & Ghashang, M. (2008). Environmentally friendly preparation of amidoalkyl naphthols. Synthetic Communications, 38, 2983–2994. doi:10.1080/00397910802006396
- Shaterian, H. R., & Mohammadnia, M. (2012). Mild basic ionic liquids catalyzed new four-component synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones. Journal of Molecular Liquids, 173, 55–61. doi:10.1016/j.molliq.2012.06.007
- Sheik Mansoor, S., Aswin, K., Logaiya, K., & Sudhan, S. P. N. (2016). ZrOCl2·8H2O: An efficient and recyclable catalyst for the three-component synthesis of amidoalkyl naphthols under solvent-free conditions. Journal of Saudi Chemical Society, 20, 138–150. doi:10.1016/j.jscs.2012.06.003
- Shen, A. Y., Tsai, C. T., & Chen, C. L. (1999). Synthesis and cardiovascular evaluation of N-substituted 1-aminomethyl-2-naphthols. European Journal of Medicinal Chemistry, 34, 877–882. doi:10.1016/S0223-5234(99)00204-4
- Shirole, G. D., Kadnor, V. A., Tambe, A. S., & Shelke, S. N. (2017). Brønsted-acidic ionic liquid: green protocol for synthesis of novel tetrasubstituted imidazole derivatives under microwave irradiation via multicomponent strategy. Research on Chemical Intermediates, 43, 1089–1098. doi:10.1007/s11164-016-2684-7
- Slobbe, P., Ruijter, E., & Orru, R. V. A. (2012). Recent applications of multicomponent reactions in medicinal chemistry. MedChemComm, 3, 1189–1218. doi:10.1039/c2md20089a
- Taghavi-Khorasani, F., & Davoodnia, A. (2015). A fast and green method for synthesis of tetrahydrobenzo[a]xanthene-11-ones using Ce(SO4)2·4H2O as a novel, reusable, heterogeneous catalyst. Research on Chemical Intermediates, 41, 2415–2425. doi:10.1007/s11164-013-1356-0
- Tavakoli-Hoseini, N., & Davoodnia, A. (2011). Carbon-based solid acid as an efficient and reusable catalyst for one-pot synthesis of tetrasubstituted imidazoles under solvent-free conditions. Chinese Journal of Chemistry, 29, 203–206. doi:10.1002/cjoc.201190053
- Vafaeezadeh, M., & Alinezhad, H. (2016). Brønsted acidic ionic liquids: Green catalysts for essential organic reactions. Journal of Molecular Liquids, 218, 95–105. doi:10.1016/j.molliq.2016.02.017
- Wang, M., & Liang, Y. (2011). Solvent-free, one-pot synthesis of amidoalkyl naphthols by a copper p-toluenesulfonate catalyzed multicomponent reaction. Monatshefte für Chemie - Chemical Monthly, 142, 153–157. doi:10.1007/s00706-010-0429-7
- Zali, A., & Shokrolahi, A. (2012). Nano-sulfated zirconia as an efficient, recyclable and environmentally benign catalyst for one-pot three component synthesis of amidoalkyl naphthols. Chinese Chemical Letters, 23, 269–272. doi:10.1016/j.cclet.2011.12.002
- Zang, H., Zhang, Y., Zang, Y., & Cheng, B.-W. (2010). An efficient ultrasound-promoted method for the one-pot synthesis of 7,10,11,12-tetrahydrobenzo[c]acridin-8(9H)-one derivatives. Ultrasonics Sonochemistry, 17, 495–499. doi:10.1016/j.ultsonch.2009.11.003
- Zolfigol, M. A., Khazaei, A., Moosavi-Zare, A. R., & Zare, A. (2010). 3-Methyl-1-Sulfonic acid imidazolium chloride as a new, efficient and recyclable catalyst and solvent for the preparation of N-sulfonyl imines at room temperature. Journal of the Iranian Chemical Society, 7, 646–651. doi:10.1007/BF03246053