Abstract
We describe the syntheses of 3,4-dihydropyrimidinones or analogous thioketones by a one-pot cyclocondensation of acetoacetates, aldehydes and urea or thiourea using gallium (III) chloride as catalyst under solvent-free conditions. The improved Biginelli reaction not only features a simple procedure, high yields and easy purification of production, but also the recycled catalyst could be directly reused for many times while the yields of reaction would not decrease.
Public Interest Statement
A traditional concept in chemistry primarily seeks to increase reaction yield. With the development of chemical industry, people have come to realize chemical hazards to health, community safety and the environment. Now researchers in chemistry are more concerned with benign chemical process, such as solvent-free reaction, recyclable catalyst, aqueous organic reaction and so on.
This article was based on cycling economy and concept of green chemistry, and studied the syntheses of Dihydropyrimidinones or analogous thioketones by using gallium (III) chloride to replace HCl as catalyst under solvent-free conditions. The improved Biginelli reaction not only features a simple procedure, high yields and easy purification of production, but also the recycled catalyst could be directly reused for many times.
Green environment is our goal and we are always trying to come up with new, green and safe ways to resolve the pollution problem of conventional chemical methods..
1. Introduction
Dihydropyrimidinone (DHPM) and its derivatives have attracted considerable interest in recent years due to their diverse pharmacological properties such as calcium channel blockers, antihypertensive agents, neuropeptide antagonists, and α-1a-antagonists (Atwal et al., Citation1990, Citation1991). In 1893, Biginelli first reported the synthesis of DHPMs by a simple one-pot condensation reaction of an aromatic aldehyde, β-ketoester, and urea in ethanol containing a catalytic amount of HCl (Biginelli, Citation1893), however, the yields were low (20–50%).
The acid catalyst is the key in the Biginelli reaction. If there is no acid catalyst, the condensation reaction of aromatic aldehydes can hardly react with urea in the first step. However, it is difficult to freely transmit a protonic acid (H+) catalyst in the non-aqueous medium found in the classical Biginelli reaction, but Lewis acid catalysts can freely bond and separate in non-aqueous medium.
Recently, the Biginelli reaction for the synthesis of DHPMs has gained in popularity again and feature Lewis acid catalysts, such as BF3·OEt2/CuCl (Hu, Sidler, & Dolling, Citation1998), CuI (Kalita & Phukan, Citation2007), CuSO4·5H2O (Gohain, Prajapati, & Sandhu, Citation2004), InBr3 (Fu et al., Citation2002), InCl3 (Ranu, Hajra, & Jana, Citation2000), GaI3 (Li, Mao, An, Zhao, & Zou, Citation2010), GaBr3/GaCl3 (Saini et al., Citation2007), LaCl3·7H2O (Lu, Bai, Wang, Yang, & Ma, Citation2000), LiBr (Maiti, Kundu, & Guin, Citation2003), CeCl3·7H2O (Bose, Fatima, & Mereyala, Citation2003), MgBr2 (Salehi & Guo, Citation2004), CdCl2 (Narsaiah, Basak, & Nagaiah, Citation2004), Al(HSO4)3 (Khodaei, Salehi, Zolfigol, & Sirouszadeh, Citation2004), FeCl3·6H2O/NiCl2·6H2O/HCl (Lu & Bai, Citation2002), SmI2 (Han, Xu, Luo, & Shen, Citation2005), ZnI2 (Jenner, Citation2004), ZnCl2 (El Badaoui, Bazi, Tahir, Lazrek, & Sebti, Citation2005), BiCl3 (Ramalinga, Vijayalakshmi, & Kaimal, Citation2001), Bi(NO3)3 (Slimi, Moussaoui, & ben Salem, Citation2016), which allow to the preparation of DHPM in high yields.
Recently, gallium (III) halides have emerged as a powerful Lewis catalyst for implementing the Biginelli reaction under solvent-free or microwave-assisted conditions (Maiti et al., Citation2003; Bose et al., Citation2003). After considering the gallium (III) halides, gallium (III) chloride, with lower melting point (78°C), is readily melted, and suitable for solvent-free Biginelli reactions.
Herein, we wish to report our study of using gallium (III) chloride for as catalyst for Biginelli reaction under solvent-free conditions (Scheme ).
Scheme 1. Gallium (III) chloride-catalyzed one-pot synthesis of 3,4-dihydropyrimidinones for Biginelli reaction under solvent-free conditions.
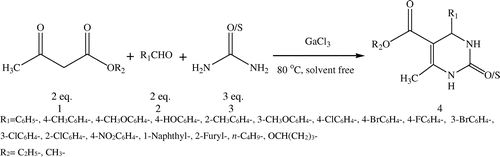
2. Results and discussion
Our initial study was started by reacting benzaldehyde (2 mmol), urea (3 mmol) with ethyl acetoacetate (2 mmol) under different conditions. The results are summarized in Table .
Table 1. The synthesis of dihydropyrimidinone (4a) by reacting benzaldehyde (2 mmol), urea (3 mmol) with ethyl acetoacetate (2 mmol) under solvent-free conditions
As shown in Table , dihydropyrimidinone 4a was observed in low yield at 80 o C with no catalyst (entry 1, Table ). When indium (III) halides, gallium (III) bromide, and gallium (III) iodide were used as catalysts, the yields of dihydropyrimidinone 4a were also lower at 80°C (entries 2–6, Table ). Encouraging, the dihydropyrimidinone 4a was obtained in high yields using gallium (III) chloride as catalyst under the same condition (entry 7, Table ). Increasing the catalyst loading moderately helped to increase yields of 3,4-dihydropyrimidinone 4a (entries 8–10, Table ). The effective reaction time was 6 h (entries 11–12, Table ) and when the reaction temperature was below 75°C, the yields declined sharply (entries 13–14, Table ). Raising the reaction temperature hardly increased the yield of dihydropyrimidinone 4a with respect to 80°C (entry 15, Table ). Gratifyingly, the recycled catalyst could be directly reused and the yields for the four cycles were as high as for the first cycle (entry 16, Table ).
After this successful survey of the reaction conditions, gallium (III) chloride was considered as an appropriate catalyst for the Biginelli reaction, and was used to synthesize a series dihydropyrimidinone 4a-4r by reacting benzaldehydes (2 mmol), urea/thiourea (3 mmol) with ethyl/methyl acetoacetate (2 mmol) at 80°C under solvent-free conditions (Scheme ) and these results are listed in Table . The results are listed in Table .
Table 2. Gallium (III) chloride-catalyzed Biginelli reaction between aldehydes, urea or thiourea and acetoacetate
It was found that benzaldehyde and both electron withdraw groups or electron donating groups could implement Biginelli reaction with urea and ethyl (methyl) acetoacetate in good yields at 80°C (entries 1–13, Table ). The position of the substitution group seldomly had an effect on reaction yields, although it was necessary to prolong the reaction time when ortho- and meta-substituted substrates was used for this reaction (entries 5, 6, 10, 11, and 12, Table ), which may be caused by the increase in steric hindrance around the carbonyl group and the effect of electron withdrawing substituent, respectively. However, aromatic aldehydes substituted by electron-withdrawing group gave higher yields of DHPMs (4) than by electron-donating group at the same position (entries 7–13, Table ). Moreover, naphthaldehyde, furfural also could afford the corresponding DHPMs (4) in high yields under the same conditions smoothly (entries 14–15, Table ). Inspiringly, when an aliphatic aldehyde was used in the present reaction, the corresponding DHPM (4p) also was given the better yield (entry 16, Table ). Finally, a reaction of aromatic aldehydes with ethyl acetoacetate and thiourea also gave high yields of the desired product (entries 17–18, Table ).
3. Conclusions
In summary, we have developed efficient catalyst for the Biginelli reaction using gallium (III) chloride, the condensation of aromatic aldehydes, ethyl acetoacetate, and urea or thiourea generated the corresponding products in excellent yields under free-solvent conditions. The GaCl3 can be recovered by filtration, and untreated GaCl3 was reused directly for Biginelli reaction for at least four cycles. The procedure will find important applications in the synthesis of dihydropyrimidinones to cater the needs of academic and pharmaceutical industries.
4. Experimental section
All melting points were determined on a WT-melting point apparatus. IR (Perkin-Elmer, 2000 FTIR), 1H NMR (DMSO-d6, 400 MHz), 13C NMR (DMSO-d6, 100 MHz) were recorded on a Bruker AC-300 FT spectrometer and MS-GC (HP5890 (II)/HP 5972, EI) spectra were obtained at the Center of Analytical Configuration of Anhui Jianzhu University. Flash chromatographic sheet employed was purchased from Anhui Liangchen Silicon Material Co., Ltd. and all materials from Aldrich and used directly as received.
4.1. General procedure for the synthesis of 5-alkoxycarbonyl-6-methyl- 4-aryl-3,4-dihydropyrimidin-2(1H)-ones
The mixture of aldehyde (2 mmol), urea or thiourea (3 mmol), ethyl (methyl) acetoacetate (2 mmol), and gallium (III) chloride (0.15 mmol) was heated with stirring at 80°C for the stated tine, and the progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was cooled to reach room temperature, and then was poured into crushed ice and stirred for 10 min. The solid separated was filtered under suction, washed with ice-cold water. The pure products were obtained by recrystallized from hot ethanol or by flash chromatography on silica gel eluting with petroleum ether/EtOAc (4:1, V: V) and identified by IR, 1H, 13C NMR, and HRMS. All compounds obtained were consistent with authentic ones in the literatures (Ahmed et al., Citation2009; Cepanec et al., Citation2005; Fu et al., Citation2002; Ladole et al., Citation2016; Lu & Bai, Citation2002).
4.2. 4-(4-bromophenyl)-5-Ethoxycarbonyl-6-methyl-3,4-dihydropyrimidin-2(1H)-one(4h)
9Mp 212–214°C, lit. 9212–214°C; IR (KBr): υ 3,233, 1,727, 1,659 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ: 9.22 (s, 1H, NH), 7.51 (d, 1H, NH), 7.50–7.18 (m, 4H, C6H4), 5.11 (d, 1H, CH), 3.97 (q, J = 6.9 Hz, 2H, OCH2CH3), 2.23 (s, 3H, CH3), 1.08 (t, J = 6.9 Hz, 3H, OCH2CH3); 13C NMR (DMSO-d6, 100 MHz): δ 165.7, 152.4, 149.2, 144.7, 131.8, 129.0, 120.8, 99.3, 59.7, 54.0, 18.3, 14.6; HRMS: calcd for C14H15N2O3Br: 338.9769, found: 338.9771.
4.3. 5-Ethoxycarbonyl-4-(2-furyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one(4o)
9Mp 211–213°C, lit. 9210–212°C; IR (KBr): υ 3,225, 1,711, 1,640 cm−1; 1H NMR (DMSO-d6, 400 MHz): δ 9.21 (s, 1H, NH), 7.73 (s, 1H, NH), 7.53 (m, 1H, furyl), 6.33 (d, 1H, furyl), 6.07 (d, 1H, furyl), 5.20 (d, J = 3.6 Hz, 1H, CH), 4.00 (q, J = 7.2 Hz, 2H, OCH2CH3), 2.22 (s, 3H, CH3), 1.13 (t, J = 7.0 Hz, 3H, OCH2CH3); 13C NMR (DMSO-d6, 100 MHz): δ 165.4, 156.4, 152.8, 149.7, 142.5, 110.7, 105.7, 97.2, 59.6, 48.2, 18.1, 14.6; MS: m/z 250 (M+), 221, 177; Anal. (%): calcd for C12H14O4N2: C, 57.57; H, 5.64; N, 11.20. Found: C, 57.67; H, 5.67; N, 11.26; HRMS: calcd for C12H14N2O4: 350.7458, found: 350.7452.
4.4. 5-Ethoxycarbonyl-4-(2-furyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-thione(4r)
41Mp 226–227°C, lit. 41225–227°C; IR (KBr): υ 3,244, 1,703, 1,652 cm−1; 1H NMR (DMSO-d6, 400 MHz): δ 9.61 (s, 1H, NH), 7.55 (s, 1H, NH), 7.53 (m, 1H, furyl), 6.35 (d, 1H, furyl), 6.11 (d, 1H, furyl), 5.20 (d, J = 3.2 Hz, 1H, CH), 4.01 (q, J = 4.2 Hz, 2H, OCH2CH3), 2.22 (s, 3H, CH3), 1.11 (t, J = 5.2 Hz, 3H, OCH2CH3); 13C NMR (DMSO-d6, 100 MHz): δ 175.3, 165.4, 155.2, 146.5, 143.2, 111.1, 106.8, 98.8, 60.2, 17.7, 14.6; MS: m/z 250 (M+), 221, 177; Anal. (%): calcd for C12H14O4N2: C, 57.57; H, 5.64; N, 11.20. Found: C, 57.67; H, 5.67; N, 11.26; HRMS: calcd for C12H14N2O3S: 366.4321, found: 366.4317.
Funding
We appreciate financial support from the Natural Science Foundation of China [grant number 51573001], the Natural Science Foundation of Anhui Province [grant number 1508085MB36], and the Center of Analytical Configuration of Anhui Jianzhu University.
Additional information
Notes on contributors
Shizhen Yuan
Our research interests are focused on the development of new reactions for building carbon–carbon, carbon–hydrogen, and carbon–heteroatom bonds with particular emphasis on selectivity, cost, and environmental impact. The contents of research include the following areas:
1. Green organic metal reactions: Barbier–Grignard reaction and Pinancol couple reaction in aqueous media, Knoevenagel condensation under solvent-free conditions.
2. Green synthesis of heterocyclic compounds: novel synthetic methods of Pyrroles, Oxazoles, Quinolinones, Dihydropyrimidinones.
3. The asymmetric synthesis of chiral reagent: such as Preparation of L-Proline Chiral Bonded Silica Gels and Their Application in Beer.
4. Chemistry of natural products: extraction and separation of effective components and structure identification.
We hope that research in our lab could help improve some traditional synthetic methods, and in particular provide better solutions to current existing issues related to medicines, materials, and energy.
References
- Ahmed, B., Khan, R. A., & Habibullah, M. (2009). An improved synthesis of Biginelli-type compounds via phase-transfer catalysis. Tetrahedron Letters, 50, 2889–2892.10.1016/j.tetlet.2009.03.177
- Atwal, K. S., Rovnyak, G. C., Kimball, S. D., Floyd, D. M., Moreland, S., Swanson, B. N., … Malley, M. F., (1990). Dihydropyrimidine calcium channel blockers. II. 3-Substituted-4-aryl-1,4-dihydro-6-methyl-5-pyrimidinecarboxylic acid esters as potent mimics of dihydropyridines. Journal of Medicinal Chemistry, 33, 2629–2635.10.1021/jm00171a044
- Atwal, K. S., Swanson, B. N., Unger, S. E., Floyd, D. M., Moreland, S., Hedberg, A., & O’Reilly, B. C. (1991). Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1, 2, 3, 4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agents. Journal of Medicinal Chemistry, 34, 806–811.10.1021/jm00106a048
- Biginelli, P. (1893). Aldehyde-urea derivatives of aceto-and oxaloacetic acids. Gazzetta Chimica Italiana, 23, 360–413.
- Bose, D. S., Fatima, L., & Mereyala, H. B. (2003). Green chemistry approaches to the synthesis of 5-alkoxycarbonyl-4-aryl-3,4- dihydropyrimidin-2(1 H )-ones by a three-component coupling of one-pot condensation reaction: Comparison of ethanol, water, and solvent-free conditions. The Journal of Organic Chemistry, 68, 587–590.10.1021/jo0205199
- Cepanec, I., Litvić, M., Bartolinčić, A., & Lovrić, M. (2005). Ferric chloride/tetraethyl orthosilicate as an efficient system for synthesis of dihydropyrimidinones by Biginelli reaction. Tetrahedron, 61, 4275–4280.10.1016/j.tet.2005.02.059
- El Badaoui, H., Bazi, F., Tahir, R., Lazrek, H. B., & Sebti, S. (2005). Synthesis of 3, 4-dihydropyrimidin-2-ones catalysed by fluorapatite doped with metal halides. Catalysis Communications, 6, 455–458.10.1016/j.catcom.2005.04.003
- Fu, N. Y., Yuan, Y. F., Cao, Z., Wang, S. W., Wang, J. T., & Peppe, C. (2002). Indium(III) bromide-catalyzed preparation of dihydropyrimidinones: Improved protocol conditions for the Biginelli reaction. Tetrahedron, 58, 4801–4807.10.1016/S0040-4020(02)00455-6
- Gohain, M., Prajapati, D., & Sandhu, J. S. (2004). A novel Cu-catalysed three-component one-pot synthesis of dihydropyrimidin-2 (1H)-ones using microwaves under solvent-free conditions. Synlett, 2004, 235–238.
- Han, X., Xu, F., Luo, Y., & Shen, Q. (2005). An efficient one-pot synthesis of dihydropyrimidinones by a samarium diiodide catalyzed Biginelli reaction under solvent-free conditions. European Journal of Organic Chemistry, 2005, 1500–1503.10.1002/(ISSN)1099-0690
- Hu, E. H., Sidler, D. R., & Dolling, U. H. (1998). Unprecedented catalytic three component one-pot condensation reaction: An efficient synthesis of 5-alkoxycarbonyl-4-aryl-3, 4-dihydropyrimidin-2 (1H)-ones. The Journal of Organic Chemistry, 63, 3454–3457.10.1021/jo970846u
- Jenner, G. (2004). Effect of high pressure on Biginelli reactions. Steric hindrance and mechanistic considerations. Tetrahedron Letters, 45, 6195–6198.10.1016/j.tetlet.2004.05.106
- Kalita, H. R., & Phukan, P. (2007). CuI as reusable catalyst for the Biginelli reaction. Catalysis Communications, 8, 179–182.10.1016/j.catcom.2006.06.004
- Khodaei, M. M., Salehi, P., Zolfigol, M. A., & Sirouszadeh, S. (2004). Efficient synthesis of 3, 4-dihydropyrimidin-2 (1H)-ones by aluminum hydrogensulfate. Polish journal of chemistry, 78, 385–388.
- Ladole, C. A., Salunkhe, N. G., & Aswar, A. S. (2016). A microwave assisted synthesis of 3,4-dihydropyrimidin-2(1H)-ones and thiones using NiFe2O4 ferrite as an effective and reusable catalys. Journal of Indian Chemical Society, 93, 337.
- Li, D., Mao, H., An, L., Zhao, Z., & Zou, J. (2010). Gallium(III) iodide-catalyzed Biginelli-type reaction under solvent-free conditions: Efficient synthesis of dihydropyrimidine-2(1H)-one derivatives. Chinese Journal of Chemistry, 28, 2025–2032.10.1002/cjoc.201090338
- Lu, J., & Bai, Y. (2002). Catalysis of the Biginelli reaction by ferric and nickel chloride hexahydrates. One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Synthesis, 2002, 466–470.10.1055/s-2002-20956
- Lu, J., Bai, Y., Wang, Z., Yang, B., & Ma, H. (2000). One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using lanthanum chloride as a catalyst. Tetrahedron Letters, 41, 9075–9078.10.1016/S0040-4039(00)01645-2
- Maiti, G., Kundu, P., & Guin, C. (2003). One-pot synthesis of dihydropyrimidinones catalysed by lithium bromide: An improved procedure for the Biginelli reaction. Tetrahedron Letters, 44, 2757–2758.10.1016/S0040-4039(02)02859-9
- Narsaiah, A. V., Basak, A. K., & Nagaiah, K. (2004). Cadmium chloride: An efficient catalyst for one-pot synthesis of 3, 4-dihydropyrimidin-2 (1H)-ones. Synthesis, 2004, 1253–1256.
- Ramalinga, K., Vijayalakshmi, P., & Kaimal, T. N. B. (2001). Bismuth(III)-catalyzed synthesis of dihydropyrimidinones: Improved protocol conditions for the Biginelli reaction. Synlett, 2001, 863–865.10.1055/s-2001-14587
- Ranu, B. C., Hajra, A., & Jana, U. (2000). Indium(III) chloride-catalyzed one-pot synthesis of dihydropyrimidinones by a three-component coupling of 1,3-dicarbonyl compounds, aldehydes, and urea: An improved procedure for the Biginelli reaction. The Journal of Organic Chemistry, 65, 6270–6272.10.1021/jo000711f
- Saini, A., Kumar, S., & Sandhu, J. S. (2007). Aluminium(III) halides mediated synthesis of 5-unsustituted 3,4-dihydropyrimidin-2(1H)-ones via three component Biginelli-like reaction [J]. Indian Journal of Chemistry B, 46, 1690–1694.
- Salehi, H., & Guo, Q. X. (2004). A facile and efficient one‐pot synthesis of dihydropyrimidinones catalyzed by magnesium bromide under solvent‐free conditions. Synthetic Communications, 34, 171–179.10.1081/SCC-120027250
- Slimi, H., Moussaoui, Y., & ben Salem, R. (2016). Synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones via Biginelli reaction promoted by bismuth(III)nitrate or PPh3 without solvent. Arabian Journal of Chemistry, 9, S510–S514.10.1016/j.arabjc.2011.06.010
