Abstract
Four Schiff bases, L1, L2, L3 and L4 having –ON– donor system have been synthesised by the condensation of 4-(diethylamino)-2-hydroxybenzaldehyde with 4-nitrobenzohydrazide and 4-methoxybenzohydrazide to form L1 and L2. 4-(dimethylamino) benzaldehyde refluxed in the presence of 4-nitrobenzohydrazide and 4-methoxybenzohydrazide forms L3 and L4, respectively. The four Schiff base complexes (SBC 1A–1D) were synthesised by treatment of the Schiff base ligands with vanadium acetylacetonate and characterised using spectroscopic analysis such as FTIR revealing the presence of azomethine μ (C=N) stretching vibrations around 1612–1623 cm−1, 1H and 13C NMR revealing the presence of azomethine peaks to confirm the formation of the Schiff base ligands. The spectral data of the Schiff base complexes reveal the absence of the azomethine stretching vibrational frequencies and the presence of the V–O stretching vibrational frequency at 993 cm−1. Thermal analysis of the ligands and complexes indicates high thermal stability of 280 and 160°C, respectively. Schiff base complexes 1A and 1B exhibited antimicrobial activities against both species tested with MIC of 305.3 and 378.9 mg/mL, these activities were dependent on the position of the hydroxyl and the length of the alkyl groups on the 4-Methoxy-benzoic acid (4-diethylamino-2-hydroxy-benzylidene)-hydrazide moiety of the Schiff bases complex.
Public Interest Statement
The serendipitous discovery of an antitumor cisplatin’s complex there has been a paradigm shift in pharmaceutical and medicinal researchers. Since then, researchers have been poised in discovering novel metal complexes with medicinal properties to tackle infectious diseases. In this study, vanadium which is known to have moderate toxicity to man but found as a toxin in lower organisms was employed. The efficacy of the toxin against bacterial agents was modified by introducing ligand with activating or deactivating appendages. The minimum inhibitory concentrations and sensitivity of the Schiff base against clinical and resistance strains of Staphylococcus aureus and Enterococcus faecalis were of significance.
Presently, the pharmaceutical industries are finding a market for Schiff base complexes because economically, easy to synthesise the new anti-bacterial agents of vanadium hydrazide Schiff bases compared to the expensive synthetic route of other antibiotics. Scientifically, the interaction of the vanadium compounds with biomolecules is different when compared to other classic drugs.
1. Introduction
Bacterial infections result in about 17 million deaths globally per year, mostly in children and the elderly (Citation1).There are several antibiotic chemotherapeutic drugs available for the treatment of diseases caused by bacteria, however, the growing incidence of antibiotic resistance, due to antibiotics misuse, is posing a significant threat to the control and management of infectious diseases. This problem is exacerbated by the genetic ability of bacteria to acquire and transmit resistance against the drugs used as therapeutic agents making the available antibiotics obsolete (Citation2). Despite advances made in antibiotic chemotherapy, the morbidity and mortality associated with bacterial infections remain significant. The complexities of these diseases necessitate the design and development of a new/novel class of compounds that will exhibit superior potency, selectivity and inferior cytotoxicity compared to existing antibacterial compounds.
A popular drug design strategy involves the inclusion of transition metal ions, often incorporated into compounds of known therapeutic value. A notable example is that of ferroquine, a metal-based drug, in which ferrocene was incorporated into the lateral side chain of chloroquine to enhance its drug activities (Citation3). Motivated by the recorded successes of ferroquine and following the same targeted approach to introducing multifunctionalities: this project aims to incorporate metal ions into the molecular scallop of some Schiff base derivatives with the expectations that their potency may be significantly enhanced as well.
Schiff bases, a class of organic ligands noted for their multiple medicinal benefits such as antibacterial properties, good architectural diversity and excellent coordination capacity is very attractive material for the design of chemotherapeutic agents with potential application for the control of bacterial infections (Citation3–6). Moreover, Schiff bases have numerous applications in drug design, development and enhancement of drug performances (Citation7).
Motivated by the recorded successes of ferroquine, and following the same targeted approach to introducing multifunctionalities: this project aims to incorporate metal ions into some Schiff base derivatives to enhance their biological importance as an alternative replacement to conventional antibiotics which are rapidly becoming ineffective.
2. Experimental
2.1. Chemicals
The following chemicals: methanol, dimethyl sulfoxide. dimethyl formamide, hydrogen peroxide, distilled acetylacetone, acetone, 4-methoxbenzhydrazide, 4-diethyl (amino)-2-hydroxybenzaldehyde, 4-dimethyl(amino)-2-hydroxybenzaldehyde, 4-nitrobenzohydrazide, potassium permanganate, vanadium pentoxide, potassium hydroxide and sodium hydroxide were purchased from LABCHEM. All reagents used in this study are of analytical grade.
2.2. Equipment
IR spectra were recorded using a PerkinElmer 580 Spectrometer (4,000–400 cm−1) in Nujol mull and METTLER TOLEDO TGA with the top-of-the-line ultra-micro-balance with unique built-in calibration weights and NMR spectra on an Agilent VnmrJ3 Spectrometer operating at 500 MHz
2.3. General procedure for the synthesis of Schiff bases
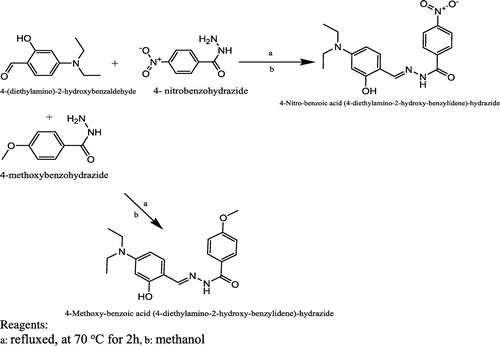
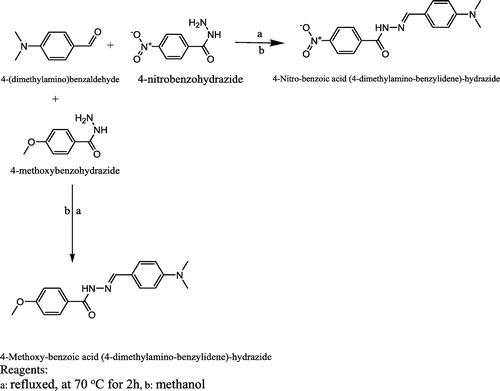
The Schiff base ligands were prepared according to the method described in the literature (Citation8). Stoichiometric quantities of the respective aldehyde (5 mmol) and amine (10 mmol) were dissolved in 100 ml of methanol (Citation8) and refluxed at 70°C for 2 h in a closed system. Consequently, the resultant mixture was cooled to room temperature and then concentrated to a volume of 20 ml. The solid product formed was recovered by filtration, washed with methanol and dried in a desiccator over anhydrous calcium chloride. This procedure was adopted for the synthesis of all the four Schiff base ligands. Scheme present the detail synthetic pathway for L1 and L2 and Scheme present the detail synthetic pathway for L3 and L4
2.4. Synthesis of vanadyl acetylacetonate: VO(acac)2
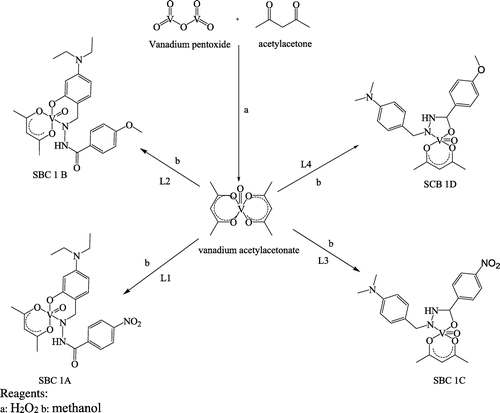
Vanadium pentoxide (5 g, 3.56 mmol) was dissolved in 20 ml of water in a 500-ml beaker. 30% hydrogen peroxide (37.40 ml, 329.90 mmol) was added dropwise under ice-cold condition and stirred till a clear dark solution was formed (Citation9). Distilled acetylacetone (19.80 ml, 192.50 mmol) was added with stirring for 30 min leading to the formation of the brown microcrystalline compound. The reaction mixture was further heated for 15mins at 70°C in a closed with constant stirring. The mixture was concentrated by heating on a steam bath for 30 min and then placed in an ice-water bath for 15 min. However, a green crystal of vanadyl acetylacetonate formed was filtered on Whatman No. 2 filter paper, washed with acetone and placed in a desiccator for 48 h to dry over anhydrous CaCl2.
2.5. Synthesis of Schiff base complexes
A stirred solution of the ligands L1, L2, L3 and L4 were mixed with VO(acac)2 (0.01 mol) to give Schiff base complex of SBC 1A–D, respectively. Equimolar quantities of Schiff base ligands and vanadium acetylacetonate salt were dissolved in 30 ml of methanol in a round-bottom flask fitted with a water condenser. The mixture was stirred vigorously for 20 min and refluxed over a steam bath at 70°C for 1 h in a closed. Consequently, the resultant solution was left overnight in a fume cupboard at ambient temperature until all traces of the solvent had evaporated. The solid formed, washed with methanol and dried over anhydrous calcium chloride in a vacuum desiccator for 48 h. Scheme , details the synthetic pathway for Schiff base complexes.
2.6. Characterisation techniques
The synthesised Schiff base ligands and their metal complexes were characterised using the following techniques: Fourier transform infrared (FTIR), thermogravimetric analysis (TGA) and NMR spectra on an Agilent VnmrJ3 Spectrometer operating at 500 MHz.
3. Antibacterial assay
3.1. Bacterial strains
A total of 10 Staphylococcus aureus; (1RO4) S. aureus, (1RO6) S. aureus, (1RO9) S. aureus, (2RO4) S. aureus, (2RO5) S. aureus, (3RO6) S. aureus, (3RO9) S. aureus, 4R4 S. aureus, 4R5 S. aureus and (15Z1) S. aureus and 5 Enterococcus faecalis; (DEEL4-2) E. faecalis, (STL1-3) E. faecalis, (MP3-1) E. faecalis, (MP3-2) E. faecalis and (V2-2) E. faecalis were used in the bioassay. However, the identities of the isolates used in the study were confirmed using preliminary gram staining, (catalase test, hemolysis on blood agar) and confirmatory serotyping, (specific PCR analysis) and the matrix-assisted laser desorption/ionisation-time of flight (MALDI-TOF) mass spectrometry.
3.2. Preparation of Schiff base complexes for microbiological assay
About 1 mg of each synthesised Schiff base complex was dissolved in 10 ml of ethanol to obtain a stock solution; serial dilution was carried out to prepare the working solution of 100, 300, 600, 1,000, 2,472, 3,053, 3,789 and 3980 μg/mL. However, to counteract the effect of ethanol in the dissolved solutions a disc that was placed only in ethanol was used as a negative control during the antimicrobial assays.
3.3. In vitro antimicrobial assay using S. aureus and E. faecalis
The method of Vollekovà et al. (Citation10) as modified by Usman et al. (Citation11) was adopted, however, four Schiff base complexes (SBC 1A–D) were tested against 10 S. aureus; 1RO4 S. aureus, 1RO6 S. aureus, 1RO9 S. aureus, 2RO4 S. aureus, 2RO5 S. aureus, 3RO6 S. aureus, 3RO9 S. aureus, 4R4 S. aureus, 4R5 S. aureus and 15Z1 S. aureus and 5 E. faecalis; DEEL4-2 E. faecalis, STL1-3 E. faecalis, MP3-1 E. faecalis, MP3-2 E. faecalis and V2-2 E. faecalis. Consequently, the reference strains E. faecalis ATCC 6569 and S. aureus ATCC® 25923 were used for quality controls during analysis. Three holes were bored in the plates (6 mm diameter) using sterile cork borer, about 20 μl of the Schiff base complexes stock solution was inoculated across the wells and incubated at 37°C for 24 h. Consequently, after incubation, the strains were classified as susceptible, or resistant and for the purposes of analysis, intermediate susceptibility was regarded as susceptible. The average diameter of three readings of the clear zone surrounding the hole was taken as the degree of inhibition against the bacteria and recorded as mean.
4. Minimum inhibitory concentration
To determine the minimum inhibitory concentration of the Schiff base complexes with concentrations of 100, 300, 600, 1,000, 2,472, 3,053, 3,789 and 3,980 μg/mL were used. About 100 μl aliquots prepared bacterial suspensions was spread-plated on solidified Mueller–Hinton agar. Using sterile needles and paper discs different concentrations of the Schiff base complexes were plated on the surface of the agar.
The inoculated plates that contained the antibiotic (ampicillin and ciprofloxacin) and the Schiff base complexes were incubated aerobically at 37°C for 24 h. After the period of incubation at 37°C, the minimum inhibitory concentrations of the Schiff base complexes were recorded.
5. Results and discussion
5.1. Fourier transform infrared spectroscopy (FTIR)
The FTIR results obtained for the bis-(acetylacetonate) oxovanadium(IV), Schiff base ligands (L1, L2, L3 and L4) and Schiff base complexes (SBC 1A–D) are presented in Tables and alongside their individual FTIR vibrational peaks.
Table 1. Characteristic peaks of acetylacetone, VO(acac)2 and Schiff base ligands
Table 2. FTIR absorption bands of Schiff base complexes
The FTIR spectrum of the pure acetylacetone (acac) shows vibrational peaks at 2,877, 2,917 and 2,964 cm−1, however, slightly similar to the observed stretching vibrational frequencies of (2,877 and 2,964 cm−1) in the spectrum of bis-(acetylacetonate) oxovanadium (IV), consequently, due to the methyl and methylene groups. Conversely, this observed difference in the stretching vibrational frequencies of acetylacetone compared to that of bis-(acetylacetonate) oxovanadium (IV) due to the presence of V–O bond (Citation12–15).
Moreover, the peak at 1,704 cm−1 is assigned as a carbonyl stretching, vibration of the acetylacetone molecule (Table ), conversely, this vibrational frequency of the carbonyl is conspicuously absent from the spectrum of bis-(acetylacetonate) oxovanadium(IV), a clear indication of the formation of V–O bond in the synthesis. Ordinarily, the metal–oxygen vibrational frequency is usually observed at 1,020–922 cm−1, however, for bis-(acetylacetonate) oxovanadium (IV) the μ (V–O) was observed at 993 cm−1.
The FTIR spectra of the ligands (L1, L2, L3 and L4) show some interesting bands at 3,189–3,344 cm−1, the vibrations at 3,344, 3,288, 3,189 and 3,224 cm−1 are assigned as the μN–H vibrations of L1, L2, L3 and L4, respectively. The μO–H stretching vibrations are only observed in L1 and L2, however, assigned as 3,558 and 3,508 cm−1, respectively. The weak azomethine bond resulting from the formation of Schiff bases is characteristic vibrational bands for Schiff bases. The absorption bands at 1,623, 1,687, 1,612 and 1,623 cm−1 are characteristic azomethine μ (C = N) stretching vibrational frequencies (Citation16) for L1, L2, L3 and L4, respectively, confirm the formation of Schiff bases. Consequently, the azomethine bond of the Schiff base originates from the reaction of the hydrazide nitrogen and the carbonyl group of the benzaldehyde (Citation17). The other absorption bands observed were basically expected absorption bands due to the benzene rings, the methyl and carbonyl groups stretching and bending vibrations (Table ) (Citation4, Citation15, Citation18).
The absorption bands of the Schiff base complexes (Table ) show the presence of μN–H of amide and secondary amine, conversely, the disappearance of μO–H stretching vibrations in SBC 1A and 1B. The carbonyl stretching vibration μ(C=O) initially observed in the spectra of the ligands L1, L2, L3 and L4 that were used in the formation of the of the SBC 1A–D, where absence in the spectra of SBC 1C and 1D, showing that the reaction of vanadium acetylacetonate with the ligands L3 and L4 did take place at the carbonyl oxygen unlike the observations of the spectra bands of SBC 1A and 1B. The other absorption bands found are mostly from fingerprint regions (Table ).
5.2. Thermal analysis
The differential and thermogravimetric analyses of Schiff base ligands and complexes were measured in an atmosphere of N2 gas between 0 and 900°C. The thermogravimetric analysis result plotted as percentage weight loss against temperature; provides information on vaporisation, sublimation and phase transition (melting, crystallisation), however, the derivative thermogravimetric analysis provides enthalpy information on the Schiff base ligands and complexes (Citation19).
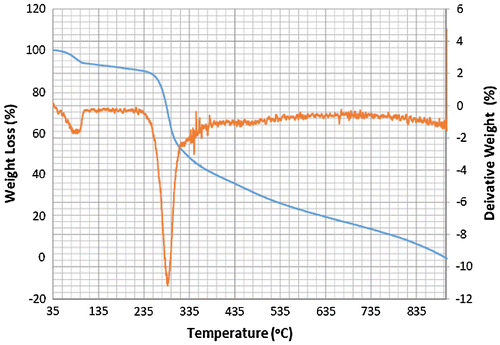
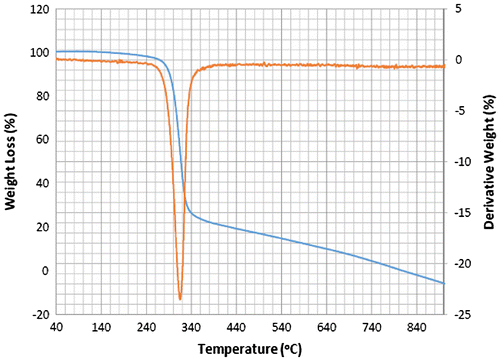
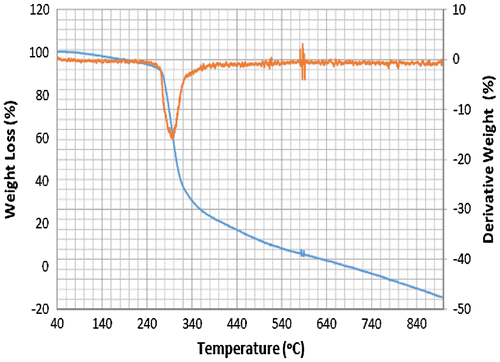
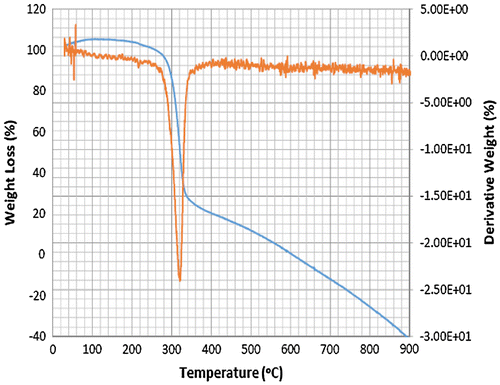
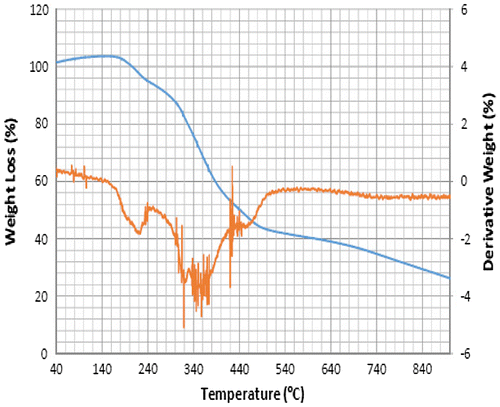
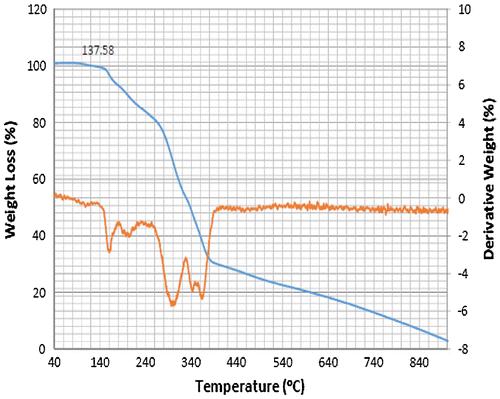
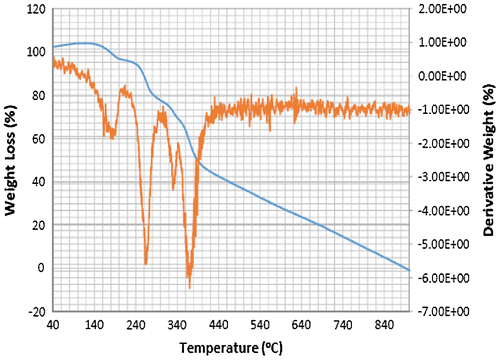
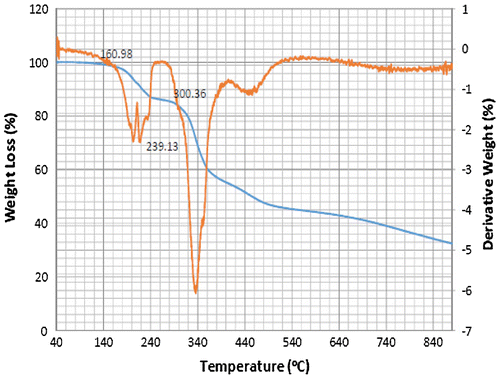
Accordingly, the thermograms of the L2, L3 and L4 reveal a thermal stability of 280°C with single-stage decomposition temperature with a reaction interval of 60°C. Conversely, L1 reveals a thermal stability of 255°C with multi-stage decomposition temperature with a reaction interval of 40°C. The thermograms of SBC 1A–D reveal a multistage decomposition in which the reaction is not resolved. The high thermal stability of the ligands is an indication of it to retaining biological activity, even at high temperature (Citation17).
The thermograms of SBC 1A–D reveal a multi-stage decomposition as a result of mass gain, which is due to oxidation of vanadium from +4 to +5. The DTA thermogram of L1 reveals two endothermal peaks caused by de-nitration (NO2) and dehydroxylation (OH), while the single endothermal peaks on the thermogram of L2, L3 and L4 is as a result of de-nitration and dihydroxylation and de-methoxylation, respectively. The DTA thermogram of the SBC 1B reveals five endothermal peaks and SBC 1A, 1C and 1D reveals four endothermal peaks with the thermal stability of 137–160°C (Figures ). However, the number of decomposition stages is an indication of the degree of purity ligands and complexes synthesised (Citation19).
5.3. 1H and 13C NMR spectra of Schiff base ligands and complexes
1H and 13C NMR spectra in d6-DMSO solution using TMS as an internal standard. 1H NMR spectrum of L1; reveals the presence of a phenolic proton at 6.13 (1H,s) ppm, an amide protons at 8.14 (1H,s) ppm, aromatic protons at 8.46 (1H,d), 8.38 (1H,d), 8.36 (1H,d), 8.16 (1H,d), 7.23 (1H,d), 6.29 (1H,d), 6.29 (1H,s) ppm, azomethine proton at 7.25 (1H,s) ppm, methylene protons at 3.34 (2H,q) ppm, methyl protons at 1.11(3H,t) ppm (supplementary Figure 1.8), however, the 13C spectrum reveals the presence of amide carbon at 161.0 ppm, azomethine carbon at 151.3 ppm aromatic carbons at 160.3, 150.8, 149.7, 139.4, 132.7, 130.0, 124.1, 106.8, 104.3 ppm, methylene carbon at 44.3 ppm, methyl carbon at 13.01 ppm (supplementary Figure 1.9).
Similarly, L2 1H NMR spectrum; reveals the presence of a phenolic proton at 6.13 (1H,s) ppm, an amide nitrogen protons at 8.43 (1H,s) ppm, aromatic protons at 7.42 (1H,d), 7.20 (1H,d), 7.18 (1H,d), 7.16 (1H,d), 7.15 (1H,d), 6.26 (1H,s), 6.28 (1H,d) ppm, azomethine proton at 7.50 (1H,s) ppm, methylene protons at 3.38 (2H,q) and methoxy proton 3.83 (3H,s) ppm (supplementary Figure 1.10), while the 13C spectrum reveals the presence of amide carbon at 162.4 ppm, azomethine carbon at 160.2 ppm, aromatic carbons at 159.7, 150.6, 150.4, 136.0, 132.0, 130.5, 120.1, 119.0,113.2, 108.6, 104.1 ppm, methoxy carbon at 97.4 ppm, methylene carbon at 55.3 and 44.3 ppm, methyl carbon at12.0 ppm (supplementary Figure 1.11).
The 1H NMR spectra of L3 similarly reveals the presence of an amide protons at 8.13 (1H,s) ppm, aromatic protons at 8.38 (1H,d), 8.36 (1H,d), 8.34 (1H,d), 7.69 (1H,d), 7.56 (1H,d), 6.79 (1H,d), 6.78 (1H,d) ppm, azomethine proton at 8.15 (1H,s) ppm and azomethyl proton 3.33, 3.00 (6H,s) ppm (supplementary Figure 1.12), however, the 13C spectrum reveals the presence of azomethine carbon at 162.0 ppm, aromatic carbons at 152.9, 150.2, 149.5, 140.0, 129.5, 129.1, 1,294.0, 121.7, 112.2 ppm (supplementary Figure 1.13).
1H NMR spectrum of L4; reveals the presence of an amide nitrogen protons at 8.41 (1H,s) ppm, eight aromatic protons at 7.91 (1H,d), 7.90 (1H,d), 7.18 (1H,d), 7.16 (1H,d), 7.07 (1H,d), 7.05 (1H,d), 6.27 (1H,d), 6.25 (1H,d) ppm, azomethine proton at 6.13 (1H,s) ppm, methoxy proton 3.38 (3H,s) at and azomethyl proton 3.84 (6H,s) (supplementary Figure 1.14), while the 13C spectrum reveals the presence of carbonyl carbon at 162.4 ppm, azomethine carbon at 162.1 ppm, aromatic carbons at 160.2,150.5,150.0,132.8, 129.9, 125.7, 114.2, 107.0, 104.1, 100.0 ppm, methoxyl carbon 55.9 ppm and azomethyl carbon at 44.2 ppm. Consequently, the presence of an azomethine and amide nitrogen peaks on the 1H and 13C NMR spectra confirms the formation of a Schiff base (Citation20) (supplementary Figure 1.15).
Accordingly, the complexes where equally subjected to 1H and 13C NMR study, revealing important peaks on the spectra of each complex. The SBC 1A 1H NMR spectrum reveals the presence of an amide nitrogen protons at 8.71 (1H,s) ppm, aromatic protons at 8.32 (1H,s), 8.29 (1H,s), 8.28 (1H,s), 8.11 (1H,s), 7.35 (1H,s), 6.31 (1H,s), 6.29 (1H,s) ppm, methylene protons at 3.59 (2H,q), 3.19(2H,m), 2.17 (2H,q),2.14 (2H,s), 2.03 (2H,q) ppm, methyl protons at 1.24 (3H,d), 1.14 (3H,t) ppm (supplementary Figure 1.16), the 13C spectrum reveals the presence of amide carbon at 167.3 ppm, azomethylene carbon at 24.8 ppm aromatic carbons at 128.2, 123.7, 151.8, 123.7, 128.2, 139.6, 129.4, 105.5, 143.9, 101.9, 156.8, 119.1 ppm, methylene carbon at 58.3, 40.0,44.9, 42.00,31.2 ppm, methyl carbon at 13.2 ppm (supplementary Figure 1.17).
The complex SBC 1B 1H NMR spectrum reveals the presence of an amide nitrogen protons at 8.31 (1H,s) ppm, aromatic protons at 7.91 (1H,d), 7.89 (1H,d), 7.55 (1H,d), 7.53 (1H,d), 7.06 (1H,d), 7.04 (1H,d), 6.78 (1H,s) ppm, methylene protons at 3.83 (2H,q), 3.33 (2H,m), 2.98 (2H,s), 2.51 (3H,t) ppm, methyl protons at 1.24 (3H,t) ppm (supplementary Figure 1.18) however, the 13C spectrum reveals the presence of amide carbon at 167.6 ppm, methoxy carbon at 64.5 ppm, azomethylene carbon at 30.1 ppm aromatic carbons at 167.3, 151.9, 149.5, 131.8, 129.9, 129.0, 126.3, ppm, methylene carbon at 55.9 ppm, methyl carbon at 13.2 ppm (supplementary Figure 1.19).
1H NMR spectrum of SBC 1C reveals the presence of an amine protons at 2.51 (1H,s) ppm, aromatic protons at 8.37 (1H,s), 8.36 (1H,s), 8.34 (1H,s),8.15 (1H,s),8.13 (1H,s), 7.58 (1H,s), 7.56 (1H,s), 6.79 (1H,s) ppm, methylene protons at 3.00 (2H,dd) ppm, methyl protons at 1.24 (3H,s) ppm, methoxyl proton at 3.33(3H,s) (supplementary Figure 1.20), the 13C spectrum reveals the presence of methoxy carbon at 64.5 ppm, azomethylene carbon at 30.1 ppm aromatic carbons at 161.4, 152.2, 150.2, 149.6, 140.0, 135.5, 129.5, 129.1, ppm, methylene carbon at 30.1 ppm and methyl carbon at 13.2 ppm(supplementary Figure 1.21).
1H NMR spectra of SBC 1D reveals the presence of an amine protons at 2.0 (1H,s) ppm, aromatic protons at 6.47 (1H,d), 6.47 (1H,d), 6.88 (1H,d), 6.88 (1H,d), 7.45 (1H,d), 8.12 (1H,d), 8.12 (1H,d), 7.45 (1H,d) ppm, methylene protons at 1.59 (2H,dd) ppm, the azomethyl proton at 2.85 ppm, methyl protons at 1.21 (3H,s) ppm, methoxy proton at 3.33(3H,s) (supplementary Figure 1.22), the 13C spectra reveals the presence of azomethyl carbon at 49.1 and 44.8, ppm aromatic carbons at 130.0, 114.5,114.3,14.1, 105.0 ppm, methane proton at 58.3 ppm methylene carbon at 30.2 ppm and methyl carbon at 13.9 ppm (supplementary Figure 1.23). Consequently, the absence of azomethine (–C=N–) peak in the spectra of all the complexes is a confirmation of the formation of a metal–oxygen bond detected on the FTIR spectral (Table ).
5.4. Antimicrobial activity
The well diffusion and disc diffusion methods were employed in screening the Schiff base complexes 1A–D for their sensitivity and minimum inhibitory concentration, respectively, however, standard antibacterial compounds, ciprofloxacin and ampicillin were used as a control.
The screening data indicate that Schiff base complexes 1A and 1B, with a 4-diethylamino and 2-hydroxy group on the benzaldehyde rings, showed the best antibacterial activity against E. faecalis and S. aureus among the synthesised Schiff base complexes (Tables and ).
Table 3. Inhibition zone diameter data obtained against S. aureus and E. faecalis
Table 4. Antibacterial activity of the synthesised compounds (MIC in mg/mL)
However, the antibacterial activity against E. faecalis and S. aureus bacteria are due to the effect of the electron-donating group, which activates the Schiff base complex through induction of the benzaldehyde ring. Consequently, this improves the antimicrobial activity of the SBC 1A and 1B. Conversely, the other Schiff base complexes had no valuable inhibitory activity, due to the absence of the electronegative oxygen group at position four of benzaldehyde ring. Nevertheless, an interesting trend is the underlining fact that these two Schiff base complexes 1A and 1B exhibited antimicrobial activities against both species tested with a MIC of 305.3 and 378.9 mg/mL (Table ).
The study reveals that the Schiff base complexes 1A and 1B, with a hydroxyl group at the ortho-position tend to have an affinity for the empty d-orbital metal and increased the antimicrobial activity as the alkyl chain length at the para-position increases. There was a significant difference in antimicrobial activity as the group changes from an electron withdrawing (nitro) to the electron donating (methoxy) group on the benzo hydrazide rings at the para-position (Table ). However, the comparative activities of these two Schiff base complexes with ciprofloxacin and ampicillin make them potential anti-Staphylococcus and anti-Enterococcus lead compounds.
6. Conclusion
In this study, four hydrazide Schiff base ligands were synthesised, L1, 4-(diethylamino)-2-hydroxybenzaldehyde with 4-nitro benzo hydrazide, L2, 4-(diethylamino)-2-hydroxybenzaldehyde with 4-methoxy benzo hydrazide, L3, 4-(dimethylamino) benzaldehyde with 4-nitro benzo hydrazide and L4, 4-(dimethylamino) benzaldehyde with 4-methoxy benzo hydrazide. Consequently, Schiff base complexes were synthesised by the treatment of the ligands with vanadium acetylacetonates.
The choice of the metal was influenced by its biological role. However, vanadium acetylacetonates and hydrazide Schiff base ligands and complexes were successfully synthesised and confirmed by spectroscopic data, such as the FTIR revealing the presence of the characteristic azomethine μ (C=N) stretching vibrational frequencies in the ligands and the formation of the V–O vibrational frequency at 993 cm−1 in the Schiff base complex. In addition, the presence of an azomethine and amide nitrogen peaks on the 1H and 13C NMR spectra confirms the formation of the Schiff bases, however, the absence of the azomethine (–C=N–) peak in the spectra of all the complexes is a confirmation of the formation of a vanadium–oxygen bond around the imine bond.
The thermal analysis reveals a high thermal stability, consequently, an indication that the Schiff base ligands and complexes retain their biological activity even at high temperature. The Schiff base complexes were tested for their antimicrobial potentials against several strains of S. aureus and E. faecalis. The antimicrobial screening data against S. aureus and E. faecalis, however, indicate that Schiff base complexes 1A and 1B showed the best antibacterial activity. Complexes 1A and 1B, with a hydroxyl group at the ortho-position, tend to have increased antimicrobial activity as the alkyl chain length at the para-position increases. Conversely, there was a significant difference in antimicrobial activity as the group changes from an electron withdrawing to the electron donating group on the benzo hydrazide rings. According to the comparative activities of these two Schiff base complexes with the broad spectrum antibiotics, complexes 1A and 1B are potential antimicrobial lead compounds.
Supplemental data
Supplemental data for this article can be accessed at https://doi.org/10.1080/23312009.2017.1336864.
Funding
The authors received no direct funding for this research.
Supplemental_material.docx
Download MS Word (843.6 KB)Acknowledgement
We are thankful to Directorate of research, Vaal University of Technology, Vanderbijlpark, Gauteng, South Africa, for providing all necessary facilities to conduct the experiment.
Additional information
Notes on contributors
Bamidele Joseph Okoli
Our group interest in the synthesis of Schiff bases is motivated by the fact that Schiff bases are one of the important classes of organic compounds with a broad range of biological properties. They are often used as chelating ligands in the field of coordination chemistry, and when complexed with different metals, they exhibit a broad range of biological activities, including antifungal, antibacterial, antimalarial, antiproliferative, anti-inflammatory, antiviral and antipyretic properties. It is these potentials of the Schiff base that drives our research on developing antibacterial agents with novel and more efficient mechanisms of action since currently bacterium and fungi have developed resistance to the current line of treatment.
References
- Iqbal, J.; Siddiqui, R.; Kazmi, S.U.; Khan, N.A. A Simple Assay to Screen Antimicrobial Compounds Potentiating the Activity of Current Antibiotics. Biomed Res. Int. 2013, 1–4.10.1155/2013/927323
- Sathya Bama, S.; Jayasurya, S.; Sankaranarayanan, S.; Bamas, P. Antibacterial Activity of Different Phytochemical Extracts from the Leaves of T. Procumbens. Linn.: Identification and Mode of Action of the Terpenoid Compound as Antibacterial. Int. J. Pharm. Sci. 2012, 4, 557–564.
- Muruganandam, L.; Kumar, K.K.; Balasubramanian, K. Synthesis, Characterization, Antibacterial, Antifungal and Anticancer Studies of a New Antimetabolite: N-[(Diphenylamino)methyl]acetamide and Some of its Inner Transition Metal Chelates. Chem. Sci. Trans. 2013, 2, 379–384.10.7598/cst2013
- Girgaonkar, M.V.; Shirodkar, S.G. Synthesis, Characterization and Biological Studies of Cu (II) and Ni (II) Complexes with New Bidentate Shiff’s Base Ligands as 1. Res. J. Recent Sci. 2012, 1, 110–116.
- Sallomi, I.J.; Shaheen, A.J. Complexes of Hydrazide Schiff Bases. Trans Met. Chem. 1994, 19, 275–276.
- Prakash, A.; Adhikari, D. Application of Schiff Bases and their Metal Complexes–A Review. Int. J. ChemTech. Res. 2011, 3, 1891.
- Bal, M.; Ceyhan, G.; Avar, B.; Köse, M.; Kayraldiz, A.; Kurtoğlu, M. Synthesis and X-ray Powder Diffraction, Electrochemical, and Genotoxic Properties of a New Azo-Schiff base and its Metal Complexes. Turkish J. Chem. 2014, 38, 222–241.10.3906/kim-1306-28
- Canpolat, E.; Şahal, H.; Kaya, M.; Gur, S. Synthesis, Characterization, Antibacterial and Antifungal Activities Studies of Copper(II), Cobalt(II) and Zinc(II) Complexes of the Schiff base Ligand Derived from 4,4-diaminodiphenylether. J. Chem. Soc. Pakistan. 2014, 36, 106–112.
- Pouralimardan, O.; Chamayou, A. C.; Janiak, C.; Hosseini-Monfared, H. Hydrazone Schiff Base Manganese(II) Complexes: Synthesis, Crystal Structure, and Catalytic Reactivity. Inorg. Chim. Acta. 2007, 360, 1599–1608.10.1016/j.ica.2006.08.056
- Volleková, A.; Košťálová, D.; Sochorová, R. Isoquinoline Alkaloids from Mahonia Aquifolium Stem Bark are Active Against Malassezia spp. Folia Microbiol. (Praha). 2001, 46, 107–111.10.1007/BF02873586
- Usman, H.; Haruna, A.K.; Akpulu, I.N.; Ilyas, M.; Ahmadu, A.A.; Musa, Y.M. Phytochemical and Antimicrobial Screenings of the Leaf Extracts of Celtis integrifolia Lam. J Trop Biosci. 2005, 5, 72–76.
- Bartyzel, J.; Necki, J.M.; Zieba, D.; Rozanski, K.; Gasiorek, M. Uptake of Atmospheric Hydrogen by Soils: A Case Study from Southern Poland. Eur. J. Soil Sci. 2013, 64, 597–609.10.1111/ejss.2013.64.issue-5
- Melia, D.; Saha, S. Minimising Prescribing Errors in the ICU. Crit. Care. 2014, 18 (Suppl 1), P1.10.1186/cc13191
- Shivhare, S.U.G.A.M.; Mishra, R.A.K.E.S.H.; Gharia, A.N.I.L.; Gautam, M.D. Synthesis, Characterization and Antimicrobial Studies of Cu(II) and Ni(II) Complexes with Schiff Base Derived from N-Amino rhodanine and Salicylaldehyde. Int. J. Pharm. Sci. 2012, 4, 394–397.
- Sam, N.; Md Abu, A.; Md Abdus, S.; Fasihuddin, A.; Mohd Razip, A. Synthesis, Spectral Characterization and Biological Activities of Organotin (IV) Complexes with Ortho-vanillin-2-Hydrazinopyridine (VHP). Open J. Inorg. Chem. 2012, 2012, 22–27.10.4236/ojic.2012.22004
- Jber, N.R.; Abood, R.S.; Al-dhaief, Y.A. Synthesis and Spectral Study of New Azo - Azomethine Dyes and its Copper (II) Complexes Derived from Resorcinol, 4-Aminobenzoylhydrazone and 4-Amino antipyrine. J. Al-Nahrain Univ. 2011, 14, 50–56.
- Cimerman, Z.; Miljanić, S.; Galić, N. Schiff Bases Derived from Aminopyridines as Spectrofluorimetric Analytical Reagents. Croat. Chem. Acta. 2000, 73, 81–95.
- Önal, Z.; Yildirim, I.; Kandemirli, F.; Arslan, T. Experimental and theoretical studies on the reactions of 1-amino-5-benzoyl-4-phenyl-1H-pyrimidine-2-one-thione compounds with ethyl acetoacetate. Struct. Chem. 2010, 21, 809–816.
- Thakar, A.S.; Joshi, K.T. Synthesis, Characterization and Antibacterial Activity of Schiff Bases and their Metal Complexes Derived from 4-Acyl-1-phenyl-3-methyl- 2-pyrazolin-5-ones. J. Chem. 2010, 7, 1396–1406.
- Tai, D.N.; Thanh, N.D.; Nam, P.D.; Duc, H.T. 1H- and 13C-NMR Spectra of some Azomethines of 5-Amino-2-Phenylindole Series. Vietnam J. Chem. 2007, 45, 642.