 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Herein, we have developed the solvent extraction method for the selective separation of precious rhodium(III). Various physicochemical parameters like pH, malonate concentration, n-octylaniline concentration, equilibrium time, aq: org phase ratio, and loading capacity of n-octylaniline are optimized for the quantitative recovery of the rhodium(III). The composition of the extracted species was determined by plotting the log–log graph of Log D[Rh(III)] vs. Log C[n-octylaniline] and Log D[Rh(III)] vs. Log C[malonate]; the stoichiometry was found to be (metal: acid: extractant) 1:2:1. The proposed method was successfully applied for the separation of rhodium(III) from various binary and ternary mixtures of associated metal ions. The separation of the rhodium(III) from real samples was also carried out with the proposed method. The proposed method was applied for analysis of synthetic mixture corresponding to alloys such as pseudo-palladium, iron–rhodium alloy, platinum–rhodium alloy, and rhodium–platinum catalyst.
Public Interest Statement
Rhodium is one of the most abundant elements of platinum group metals. The cost of this metal is very high and applications are very tremendous. It is used in the catalytic converter for changing risky unburned hydrocarbons, carbon monoxide, and nitrogen oxide exhaust emission into fewer noxious gases; it is also used as a catalyst in electroplating. Though its sources are limited over its deployment, its separation is most important. In this article, we used liquid–liquid extraction based on high molecular weight amine such as n-octylaniline. The percentage of extracted rhodium was determined spectrophotometrically. The selectivity, extractant concentration, influence of weak organic acid, back extractant, binary mixture separation, and analysis of alloys were also considered in this study.
1. Introduction
At present, there is a growing demand for platinum group metals; the name platinum group metals (PGMs) includes six elements: ruthenium, rhodium, platinum, palladium, osmium, and iridium. In the past few decades, these metals have found new applications in the jewellery and decorative industries due to their excellent physical and chemical properties and are used extensively in electronic devices, catalysis in the chemical and petroleum refining industries, glass industries, pharmaceutical industries, etc. Rhodium is one of the most expensive PGMs and is indispensable to automotive catalytic converters. The high cost of recovery and limited resources of these metals made it necessary to recover the metals from industry waste. Considering the techniques related to the separation and purification of PGMs, it is important to find an effective separation process to recover these metals with high purity (Citation1). According to the published literature, ion exchange and solvent extraction have been widely employed to separate and recover them. Among Pt, Pd, and Rh, extraction of Rh is the most difficult owing to its intricate chemical properties in chloride solution. Rhodium has seven existence forms of aqua-chloro complexes about [Rh (H2O)6]3+ to [RhCl6]3−. The highly charged octahedral complexes are difficult to extract owing to steric effects (Citation2).
The extent to which a metal ion is extracted from an aqueous into an organic phase is the result of many factors. One of these factors is the amount of water which accompanies the metal complex. This water favors the solubility of the metal complex about the aqueous phase and disfavors its solubility in the organic phase. In the case where the metal ion is fully coordinated, i.e. all its coordination sites are occupied by ligand donor atoms, the water will form the outer sphere of the complex by means of solvation (hydration). Though several sophisticated techniques are in use for the determination of trace and ultra trace quantities of rhodium, spectrometric technique still has the advantage in respect to simplicity and low operating costs but suffers due to matrix effects. Hence, separation and pre-concentration of trace-level quantities of rhodium are necessary prior to actual quantitative analysis. Several extraction procedures are available for this purpose; however, most of these are time-consuming and costly; however, liquid–liquid extraction technique is one of the most suitable, selective, efficient, and powerful techniques for the separation and purification of platinum group metals (Citation3).
A variety of high molecular weight amines (HMWA) had been explained for an extractant for rhodium(III) like alamine 336 (A336) (Citation4–7). In order to find an optimum condition to separate rhodium and iridium, solvent extraction experiments were performed from chloride solution using alamine 336 and tri-n-butyl phosphate (TBP) as an extractant. The extraction was dependent on the concentration of hydrochloric acid (Citation7). Tri-octylamine (Citation8–11), tri-iso-octyamine (alamine 308) (Citation12), aliquat 336 (Citation13, 14), N-n-octylaniline (Citation15), and 2-ethylhexyl amino methyl pyridine (Citation16) have been tested for liquid–liquid extraction of rhodium(III). Phosphorous-containing extractant such as TBP (Citation17) has been used for the quantitative extraction and separation of trace amounts of rhodium from nitric acid and sodium trichloroacetate media has been established based on the formation of an ion-association complex of hexahydrated rhodium cation Rh(H2O and the trichloroacetate (TCA) anion in TBP. A systematic study on the solvent extraction of rhodium with TBP in hydrochloric acid in presence of stannous chloride (Citation18, 19) is warranted. Liquid–liquid extraction of rhodium(III) with caynex 921 from aqueous hydrochloric acid media (Citation20), hydrobromic acid (Citation21), and chloride media (Citation22) has been examined in the presence and absence of stannous chloride. The extraction of rhodium(III) from bromide media (Citation23) with cyanex 923 and cyanex 471-X in toluene was studied. The extraction of rhodium(III) from its chloride solution was carried out using cyanex 923, cyanex 271, and cyanex 272 (Citation22, 24, 25) distribution ratios for the metals were determined under the different concentrations of H+ Cl− ions in the aqueous phase.
Previously, we have reported various methods for the extraction of PGMs with the help of number of HMWA (Citation26–33). Here, we have developed a new extraction system for rhodium(III), especially in weak organic acid. n-Octylaniline with an exploring extracting capacity for malonate media belongs to a kind of high molecular weight amine. Its potential advantages are low cost, good physicochemical properties, completely miscible with all common hydrocarbon diluents, low aqueous solubility, good resistance to hydrolysis, and high purity. The present study represents experimental data on extraction of rhodium(III) from malonate solutions and accesses n-octylaniline as an extractant. The mechanism of extraction and nature of extracted complex were sought. The key observation is that it is possible to separate rhodium(III) directly from other associated rare earth and transition elements. The comparison of liquid–liquid extraction separation method of rhodium(III) is given in Table .
Table 1. Comparison of the proposed method with previously reported solvent extraction methods
2. Experimental
2.1. Instruments
The digital UV–Visible spectrophotometer model Optizen-α (Mecasys Co., Ltd Korea) with 10 mm quartz cell was used for the absorbance measurement and pH measurements were carried out using an Elico digital pH-meter model Li-127. All weighing operations were carried out using Tapson’s analytical single pan balance model 200T having 0.0001 g accuracy.
2.2. Chemicals and solutions
2.2.1. Standard rhodium(III) solution
A stock solution of rhodium(III) was prepared by dissolving 1 g of rhodium trichoride hydrate (Johnson Matthey, UK) in dilute analar hydrochloric acid (1 M) and diluting to 25 mL with water and standardized gravimetrically (Citation34). A working solution of 100 μg/mL was made from it by diluting the stock solution with water.
2.2.2. n-Octylaniline
The extractant n-octylaniline was prepared by the method of Pohlandt’s (Citation35) and its 0.1 M solution was prepared in xylene. All other solutions were prepared from A. R. grade reagents and aqueous solutions were prepared using water. Double distilled water was used throughout the experimental study.
2.2.3. General extraction and determination procedure for rhodium(III)
An aliquot of 200 μg rhodium(III) solution was mixed with a sufficient quantity of sodium malonate to make its concentration 0.03 M in a total volume of 25 mL of the solution. The pH of the aqueous solution was adjusted to 9.0 by dilute sodium hydroxide and hydrochloric acid solution. The solution was then transferred to a 125-mL separating funnel and shaken with 10 mL of 0.1 M n-octylaniline in xylene for 3 min. After separating the two phases, the aqueous phase was discarded and the organic phase was stripped with two 10 mL portions of 1 M hydrochloric acid solution. The stripped aqueous phase was evaporated to moist dryness and extracted into water. The residue was dissolved in minimum amount of 1 M hydrochloric acid and transferred into 50-mL volumetric flask, 10 mL of 20% potassium iodide was added, the solution was mixed well, and heated for 15 min in a boiling water bath. To the cooled solution, 10 mL of 10% stannous chloride solution was added and diluted the solution up to the mark with water containing 1 M hydrochloric acid in final concentration. The unstoppered flask was kept in the boiling water bath for development of reddish brown solution which was measured at 445 nm against a reagent blank. The concentration of rhodium(III) was computed from the calibration curve in a similar manner (Citation36).
3. Results and discussion
3.1. Extraction as a function of pH
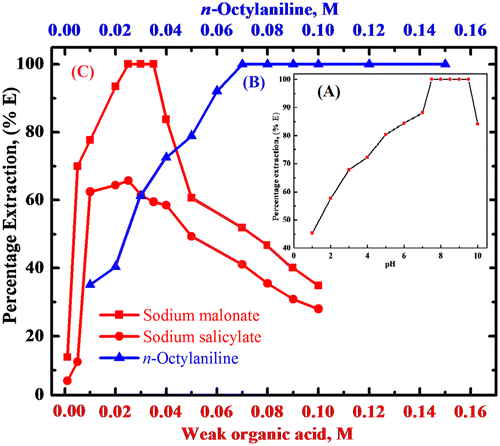
The extraction studies of rhodium(III) were performed at a fixed concentration of 0.03 M sodium malonate and between pH 1–10 with a 0.01 M solution of n-octylaniline in xylene. The pH range observed for the quantitative extraction was 7.5–9.5 with n-octylaniline. Hence, to ensure the complete extraction of rhodium(III), pH 9.0 was adopted for all extraction experiments (Figure (A)).
Figure 1. (A) Plot of pH vs. percentage extraction of rhodium(III). Condition: Rhodium(III) = 200 μg, sodium malonate = 0.03 M, n-octylaniline = 0.1 M in toluene, shaking time = 3 min. (B) Extraction of rhodium(III) as a function of n-octylaniline concentration. Condition: Rhodium = 200 μg, pH = 9.0, sodium malonate = 0.03 M, shaking time = 3 min. (C) Extraction behavior of rhodium(III) as a function of weak organic acid concentration. Condition: Rhodium(III) = 200 μg, pH = 9.0, n-octylaniline = 0.1 M in toluene, shaking time = 3 min.
3.2. Effect of n-octylaniline concentration
Extraction of rhodium(III) was carried out with various concentrations of n-octylaniline in xylene. To optimize the extraction condition, other parameters like pH, period of equilibration, and diluent were kept constant. The extraction was found to be increased with increasing reagent concentration. The extraction of rhodium(III) was quantitative in the range 0.07 M–0.15 M of n-octylaniline in xylene. However, 10 mL of 0.1 M n-octylaniline in xylene was recommended for further extraction studies (Figure (B)).
3.3. Effect of weak organic acid concentration
The extraction of rhodium(III) was studied at pH 9.0 with 0.1 M n-octylaniline in xylene in the presence of varying concentrations from 0.001 to 0.1 M of various weak organic acids. The extraction of ion-pair complex of rhodium(III) was found to be quantitative in the range of 0.025–0.035 M sodium malonate. Hence, 0.03 M concentration of sodium malonate was used for further studies while there was incomplete extraction of rhodium(III) in sodium salicylate and sodium succinate (Figure (C)).
3.4. Effect of diluents
The studies were then performed to find out the most suitable solvent for the extraction of the ion-pair complex of rhodium(III). It was found that a 0.1 M solution of n-octylaniline in benzene, toluene, and xylene provides quantitative extraction of rhodium(III). The extraction of rhodium(III) was incomplete if n-octylaniline is dissolved in chloroform (41.1%) and methyl isobutyl ketone (42.7%), while no extraction in amyl alcohol, 1,2-dichloroethane, n-butyl alcohol, and amyl acetate (Table ). On the safety ground, xylene was preferred to other solvents.
Table 2. Extraction behavior of rhodium(III) as a function of diluents
3.5. Effect of equilibration time
The extraction of rhodium(III) was studied for various time intervals in the range of 10 s–30 min with 0.1 M n-octylaniline (Table ). It was observed that under the optimized experimental conditions, a minimum 1-min time interval was required for attaining equilibrium to extract rhodium(III) quantitatively. But with prolonged shaking over 12 min, there was a decrease in the percentage extraction of rhodium(III) due to the dissociation of ion-pair complex. Hence, in all further studies, both phases were equilibrated for 3 min.
Table 3. Extraction behavior of rhodium(III) as a function of equilibrium time
3.6. Effect of stripping agent
Rhodium(III) from organic phase was stripped with the two 10-mL portions of various stripping agents at different concentrations of mineral acids, buffer solutions, and some bases. Rhodium(III) was quantitatively stripped with hydrochloric acid (1.0 to 3.0 M), nitric acid (1.0 to 3.0 M), sulfuric acid (1.0 to 3.0 M), and hydrobromic acid (1.0 to 3.0 M) from the organic phase (Table ). However, percentage recovery of rhodium(III) from organic phase was found to be incomplete with strippant water and no extraction in ammonia buffer (pH 10), ammonia, and/or sodium chloride. In the recommended procedure, two 10-mL portions of 1.0 M hydrochloric acid were used for the complete stripping of rhodium(III) from loaded organic phase.
Table 4. Extraction behavior of rhodium(III) as a function of stripping agents
3.7. Effect of aqueous to organic volume ratio
The extraction of rhodium(III) was carried out in different aqueous volumes in the range 150–10 mL from 0.03 M sodium malonate medium with 10 mL 0.1 M n-octylaniline in xylene. There was quantitative extraction of rhodium(III) when aq: org phase ratio varied from 10:10 to 50:10. Therefore, in the recommended procedure, the phase ratio 2.5:1 was maintained through the all experimental study.
3.8. Metal loading capacity of n-octylaniline
The influence of the initial rhodium(III) concentration 50–2,500 μg on the extraction by 0.1 M n-octylaniline in xylene was studied. It was observed that varying the initial rhodium(III) concentration in the range of 50–1,500 μg has no significant influence on rhodium(III) extraction with the 10 mL of 0.1 M extractant. The maximum loading capacity of 10 mL 0.1 M solution of n-octylaniline in xylene was found to be 1,500 μg rhodium(III).
3.9. Nature of extracted species
Attempts were made to ascertain the nature of extracted species of rhodium(III) with the extractant using the conventional slope analysis method. The distribution ratio of rhodium(III) was evaluated at different concentrations in molar of sodium malonate at fixed n-octylaniline concentration at pH 6.0 and pH 7.0. A graph of Log D[Rh(III)] vs. Log C[malonate] gave a slope of 2.01 and 1.94, respectively (Figure (A)). Similarly, a plot of Log D[Rh(III)] vs. Log C[n-octylaniline] concentrations at a fixed pH 6.0 and pH 7.0 with 0.03 M malonate gave slope of 1.18 and 1.15, respectively (Figure (B)). This indicates a mole ratio of rhodium(III) to malonate acid as 1:2 and that of n-octyaniline as 1:1. Thus, the extracted species were calculated to be an ion association complex with the probable composition of (metal: acid: extractant) 1:2:1. The probable mechanism of extracted species is as follows:(1)
(1)
(2)
(2)
(3)
(3)
3.10. Effect of diverse ions
The effect of various cations and anions on recovery of rhodium(III) was investigated by applying the proposed method. The tolerance limit was set as the amount of foreign ion causing a change ± 2% error in the recovery of rhodium(III). It was observed that the method is free from interference from a large number of cations and anions. Initially, the foreign ion was added to the rhodium(III) solution in large excess: say 100 mg for anions and 25 mg for cations and extraction of rhodium(III) was carried out. When interference was found to be intensive, the extraction of rhodium(III) was repeated with successively smaller amounts of foreign ion. The amount of foreign ions decreased till the quantitative extraction of rhodium(III) was observed. The only species showing interference of Ir(III) was eliminated by masking with oxalate. The anionic species showing interference in the procedure were ethylenediaminetetra acetic acid (EDTA), succinate, thiocyanate, acetate, tartrate, and bromide due to the formation of strong metal complexes (Table ).
Table 5. Effect of foreign ions on the extraction of 200 μg rhodium(III) at pH 9.0 in 0.03 M sodium malonate with 0.1 M n-octylaniline in xylene
4. Applications
4.1. Separation and determination of rhodium(III) from binary mixture
The separation of rhodium(III) from some commonly associated metal ions like Pt(IV), Pd(II), Ru(III), Au(III), Os(VIII), Se(IV), Te(IV), Fe(III), Co(II), Ni(II), and Cu(II) using n-octylaniline can be achieved by taking advantage of the difference in the extraction conditions of metal such as the pH of the aqueous phase, reagent concentration, and use of masking agent (Table ). Rhodium(III) was separated from these associated metal ions, under the optimized extraction condition of rhodium(III) where all the added metal ions remained quantitatively in aqueous phase from which they are determined spectrophotometrically by standard methods (Citation36–41). Rhodium(III) from organic phase was stripped and estimated spectrophotometrically by KI + SnCl2 method.
Table 6. Separation of rhodium(III) from binary mixtures
The proposed method was also extended for separation of rhodium(III) from Ir(III) by masking with 25 mg of oxalate. The masked Ir(III) remained in the aqueous phase quantitatively under the optimum extraction conditions of rhodium(III). After demasking Ir(III) with 5-mL concentrated hydrochloric acid with a little boiling solution, it was estimated spectrophotometrically with stannous chloride-hydrobromic acid method. Rhodium(III) was stripped with 1 M hydrochloric acid and determined as described above.
4.2. Separation of rhodium(III) from ternary mixtures
The method was extended for the determination of rhodium(III) in some synthetic ternary mixtures of associated metal ions. The mixture of metal ions was subjected to an extraction condition and separation was carried out. Rhodium(III) was selectively separated from the mixture using the proposed method and the results are presented in Table .
Table 7. Separation of rhodium(III) from ternary mixtures
4.3. Separation of rhodium(III) from synthetic mixtures
A solution of 200 μg of rhodium(III) was taken and known amount of other commonly associated metals was added and extraction of rhodium(III) was carried out using the developed method. The extraction results obtained were in good agreement with the amounts added (Table ).
Table 8. Separation of rhodium(III) from synthetic mixtures
4.4. Analysis of rhodium(III) in synthetic mixtures corresponding to alloys
The proposed method was applied for the analysis of synthetic mixture corresponding to alloys such as pseudo-palladium [equal amount of Rh(III) and Ag(I)] iron–rhodium alloy, platinum–rhodium alloy, and rhodium–platinum catalyst. The real samples of these alloys were not available at the work place, which forced us to use a synthetic mixture with the corresponding composition of alloys; rhodium(III) was extracted under its optimum extraction conditions and determined spectrophotometrically; the results of the analysis are reported in Table .
Table 9. Determination of rhodium(III) from synthetic mixture corresponding to alloys
5. Conclusion
| (1) | Quantitative extraction of rhodium(III) was achieved in 3 min with 0.1 M n-octyaniline in xylene at pH 9.0 | ||||
| (2) | Trace level of rhodium(III) was extracted using low concentration of n-octylaniline. | ||||
| (3) | Extraction reaction occurred through anion exchange mechanism. | ||||
| (4) | The developed method is efficient for quantitative separation of rhodium(III) in the presence of various interfering cations and anions. | ||||
| (5) | The proposed extractive separation method is simple, rapid, selective, reproducible, and suitable for separation and determination of rhodium(III) from associated metal ions and synthetic mixtures. | ||||
Supplementary material
Supplemental material for this article can be accessed here https://doi.org/10.1080/23312009.2017.1339334.
Additional information
Funding
Notes on contributors
Ashwini P. Gaikwad
My research group is mainly involved in the development of easier and cost-effective liquid–liquid extraction technique for the separation and determination of various metal ions with high molecular weight amines. The applicability of the developed method was investigated for the analysis of alloys, minerals, waste water, synthetic mixtures, ayurvedic samples, etc. The group is also occupied in the synthesis of complexing reagent for the invention of spectrophotometric determination method. The research interest is the intention of achieving eco-friendly method to reduce the consumption of chemicals, cost, and time.
References
- Rao, C.R.M.; Reddi, G.S. Platinum Group Metals (PGM); Occurrence, Use and Recent Trends in their Determination. Trends Anal. Chem. 2000, 19, 565.10.1016/S0165-9936(00)00031-5
- Benguerel, E.; Demopoulos, G.P.; Harris, G.B. Speciation and Separation of Rhodium (III) from Chloride Solutions: A Critical Review. Hydrometallurgy 1996, 40, 135.10.1016/0304-386X(94)00086-I
- Furman, N.H. Standard of Chemical Analysis; D. Van Norstrand Company: Princenton, NJ, 1963; p 902.
- Goralska, E.; Coll, M.T.; Fortuny, A.; Kedari, C.S.; Sastre, A.M. Studies on the Selective Separation of Ir(IV), Ru(III) and Rh(III) from Chloride Solutions using Alamine 336 in Kerosene. Solv. Extr. Ion Exch. 2007, 25, 65.10.1080/07366290601067820
- Sun, P.; Lee, M.; Lee, M. Separation of Rh(III) from the Mixed Chloride Solutions Containing Pt(IV) and Pd(II) by Extraction with Alamine336. Bull. Korean Chem. Soc. 2010, 31, 1945.10.5012/bkcs.2010.31.7.1945
- Manseung, L.; Jinyoung, L.; Panpan, S. Solvent Extraction of Rhodium(III) and Iridium(IV) from Hydrochloric Acid Solution. J. Korean Inst. Metals Mater. 2010, 48, 430.
- Sun, P.P.; Lee, M.S. Separation of Ir(IV) and Rh(III) from Mixed Chloride Solutions by Solvent Extraction. Hydrometallurgy 2011, 105, 334.10.1016/j.hydromet.2010.11.008
- Levitin, G.; Schmuckler, G. Solvent Extraction of Rhodium Chloride from Aqueous Solutions and its Separation from Palladium and Platinum. React. Funct. Polym. 2003, 54, 149.10.1016/S1381-5148(02)00190-6
- Jaree, A.; Khunphakdee, N. Separation of Concentrated Platinum(IV) and Rhodium(III) in Acidic Chloride Solution via Liquid–Liquid Extraction using Tri-octylamine. J. Ind. Eng. Chem. 2011, 17, 243.10.1016/j.jiec.2011.02.013
- Fedoseev, I.V. Extraction of Carbonyl-chloride Complexes of Rhodium, Ruthenium and Iridium. Tsvetnye Metally; Russian Federation: Moscow, 2009; Vol. 10, p 24.
- Duanghatai, C.; Thumrongrut, M.; Attasak, J. Proceedings of the International Conference on Solid Waste Technology and Management, 22nd, 1229/1- 1229/7, 2007.
- Jin-Young, L.; Kumar, R.; Kim, J.; Soo, J.; Kyu, H.; Park, Y. Liquid–liquid Extraction/Separation of Platinum(IV) and Rhodium(III) from Acidic Chloride Solutions using Tri-iso-octylamine. J. Hazard. Mater. 2009, 168, 424.
- Fontàs, C.; Anticó, E.; Salvadó, V.; Valiente, M.; Hidalgo, M. Chemical Pumping of Rhodium by a Supported Liquid Membrane Containing Aliquat 336 as Carrier. Anal. Chim. Acta 1997, 346, 199.10.1016/S0003-2670(97)00112-8
- Lee, J.Y.; Kumar, J.R.; Kim, J.; Kim, D.; Yoon, H. Extraction and Separation of Pt(IV)/Rh(III) from Acidic Chloride Solutions Using Aliquat 336. J. Ind. Eng. Chem. 2009, 15, 359.10.1016/j.jiec.2008.12.006
- Kolekar, S.S.; Anuse, M.A. Solvent Extraction Separation of Rhodium(III) with N-n-Octylaniline as an Extractant. Talanta 2002, 58, 761.10.1016/S0039-9140(02)00365-X
- Yoshinari, B.; Toshihiro, F. High Selective Solvent Extraction of Palladium(II), Rhodium(III) and Platinum(IV) with 2-Ethylhexyl Amino Methyl Pyridine from Hydrochloric Acid. Chem. Lett. 1992, 254, 727.
- Hossain, K.Z.; Honjo, T. Separation of Trace Amounts of Rhodium(III) with Tri-n-butyl Phosphate from Nitric Acid and Sodium Trichloroacetate Media. Fresenius J. Anal. Chem. 2000, 368, 480.10.1007/s002160000510
- Zou, L.; Chen, J.; Pan, X. Solvent Extraction of Rhodium from Aqueous Solution of Rh(III)–Sn(II)–Cl− System by TBP. Hydrometallurgy 1998, 50, 193.10.1016/S0304-386X(98)00017-6
- Zou, L.; Chen, J.; Huang, Y. An Alternative Way to Separating Ir(IV) and Rh(III) Ions from a Mixed Chloride Solution with Added Stannous Chloride. Hydrometallurgy 2004, 72, 31.10.1016/S0304-386X(03)00133-6
- Mhaske, A.A.; Dhadke, P.M. Extraction Separation Studies of Rh, Pt and Pd using Cyanex 921 in Toluene—A Possible Application to Recovery from Spent Catalysts. Hydrometallurgy 2001, 61, 143.10.1016/S0304-386X(01)00152-9
- Dreher, T.M.; Demopoulos, G.P. The Conversion of Rh(m) Chlorocomplexes to Bromocomplexes and their Solvent Extraction Behaviour. Solv. Extr. Ion Exch. 1999, 17, 1231.10.1080/07366299908934645
- Kedari, S.; Coll, M.T.; Fortuny, A.; Goralska, E.; Sastre, A.M. Liquid–Liquid Extraction of Ir, Ru, and Rh from Chloride Solutions and Their Separation Using Different Commercially Available Solvent Extraction Reagents. Sep. Sci. Technol. 2005, 40, 1927.10.1081/SS-200064551
- Duche, S.N.; Chavan, D.V.; Dhadke, P.M. Extraction Behaviour of Cyanex-923 and Cyanex-471X Towards the Rhodium(III) from Bromide Media and Its Separation from other Platinum Metals. J. Chin. Chem. Soc. 2002, 49, 165.10.1002/jccs.v49.2
- Chavan, D.V.; Dhadke, P.M. Extraction Separation of Ir(III) and Rh(III) with Cyanex 923 from Chloride Media: A Possible Recovery from Spent Autocatalysts. J. Chem. Technol. Biotechnol. 2002, 77, 925.10.1002/(ISSN)1097-4660
- Kedari, C.S.; Coll, M.T.; Fortuny, A.; Goralska, E.; Sastre, A.M. Recovery and Partitioning of Ir(IV) and Ru(III) from Chloride Solutions by Solvent Extraction using Cyanex 923/kerosene. Hydrometallurgy 2006, 82, 40.10.1016/j.hydromet.2006.01.002
- Khogare, B.T.; Kamble, G.S.; Kokare, A.N.; Zanje, S.B.; Suryavanshi, V.J.; Anuse, M.A.; Piste, P.B.; Kokare, B.N. Development of Novel Solvent Extraction Method for Determination of Gold(III) using 4-Heptylaminopyridine: Application to Alloys and Environmental Analysis. J. Environ. Chem. Engg. 2016, 4, 3075.10.1016/j.jece.2016.06.001
- Kokare, A.; Suryavanshi, V.; Zanje, S.; Kore, G.; Anuse, M. Liquid–liquid Extraction and Separation of Lead(II) by Using N-n-Octylcyclohexylamine as an Extractant: Analysis of Real Samples. Anal. Methods 2016, 8, 6158.10.1039/C6AY00887A
- Zanje, S.B.; Kokare, A.N.; Suryavanshi, V.J.; Kore, G.D.; Khogare, B.T.; Anuse, M.A. Extractive Spectrophotometric Determination of Platinum in Cisplatin Injection, Alloys and Catalysts Assisted by 2-Nitrobenzaldehydethiocarbohrdrazone. J. Trace Anal. Food Drug 2016, 24, 1.
- Suryavanshi, V.J.; Pawar, R.R.; Anuse, M.A.; Mulik, G.N. 2-Octylaminopyridine Assisted Solvent Extraction System for Selective Separation of Palladium(II) Ion-pair Complex from Synthetic Mixtures and Real Samples. Anal. Methods 2015, 7, 2497.10.1039/C4AY03045A
- Suryavanshi, V.J.; Patil, M.M.; Zanje, S.B.; Kokare, A.N.; Kore, G.D.; Anuse, M.A.; Mulik, G.N. Extraction of Iridium(III) by Ion-pair Formation with 2-Octylaminopyridine in Weak Organic Acid Media. Sep. Sci. Technol. 2016, 51, 1690.10.1080/01496395.2016.1177076
- Suryavanshi, V.J.; Patil, M.M.; Kokare, A.N.; Zanje, S.B.; Pawar, R.R.; Anuse, M.A.; Mulik, G.N. Development of a Liquid–Liquid Extraction System for Rhodium(III) by 2-octylaminopyridine from Weak Malonate Media. J. Chin. Chem. Soc. 2016, 63, 694.10.1002/jccs.201500541
- Zanje, S.B.; Kokare, A.N.; Suryavanshi, V.J.; Waghmode, D.P.; Joshi, S.S.; Anuse, M.A. Development of a Reliable Analytical Method for the Precise Extractive Spectrophotometric Determination of Osmium(VIII) with 2-Nitrobenzaldehydethiocarbohydrazone: Analysis of Alloys and Real Sample. Spectrochim. Acta Part A 2016, 169, 223.10.1016/j.saa.2016.06.051
- Gaikwad, A.; Jagatap, S.; Suryavanshi, V.; Kolekar, S.; Anuse, M. Liquid–Liquid Extraction of Iridium(III) from Malonate Media using Liquid Anion Exchanger. Int. J. Anal. Bioanal. Chem. 2013, 3, 42.
- Beamish, F.E.; Van Loon, J.C. Analysis of Noble Metals: Overview and Selected Methods; Academic Press: London, 1977.
- Pohlandt, C. The Extraction of Noble Metals with n-Octylaniline. Talanta 1979, 26, 199.10.1016/0039-9140(79)80049-1
- Marczenko, Z. Spectophotometric Determination of Elements; Ellis Horwood Limited: Chichester, 1976; pp 229, 370.
- Sandell, E.B. Colorimetric Determination of Traces of Metals, 3rd ed.; Interscience: New York, 1965; pp 503, 519, 702, 774, 781, 524.
- Anuse, M. A.; Chavan, M. B. Studies on Extraction Separation of Platinum Metals and Gold(III) with Pyrimidinethiol: Spectrophotometric Determination of Palladium(II), Osmium(VIII) and Ruthenium(III). Chem. Anal. 1984, 29, 409.
- Kolekar, G.B.; Anuse, M.A. Extractive Spectrophotometric Determination of Selenium(IV) using 1-(4’-bromophenyl)-4,4,6- trimethyl-1,4- dihydropyrimidine-2thiol. Res. J. Chem. Environ. 1998, 2, 9.
- Kolekar, G.B.; Anuse, M.A. Extraction, Separation, and Spectrophotometric Determination of Tellurium(IV) with 1-(4-Bromophenyl)-4,4,6-trimethyl-1,4-dihydropyrimidine-2-thiol. Bull. Chem. Soc. Jpn. 1998, 71, 859.10.1246/bcsj.71.859
- Anuse, M.A.; Kuchekar, S.R.; Chavan, M.B. 1-(4’-Chlorophenyl) - 4, 4, 6-trimethyl-1,4- dihydropyrimidine-2thiol as an Effective Reagent for the Spectrophotometric Determination of Copper after Synergic Extraction. Indian J. Chem. 1986, 25, 1041.

![Figure 2. (A) Log–log plot of Log D[Rh(III)] vs. Log C[malonate] at fixed n-octylaniline concentration. (B) Log–log plot of Log D[Rh(III)]] vs. Log C[n-octylaniline] at fixed malonate concentration.](/cms/asset/83dfe674-9144-453b-aebc-dbec7a324064/oach_a_1339334_f0002_oc.gif)