Abstract
The treatment of infectious diseases has increasingly become a serious problem as the pathogens are rapidly acquiring resistance against the current antibiotics. The herb Verbena officinalis has a great repute as ethnomedicine against infections. Keeping in view its immense medicinal scope, the present study was designed. Antimicrobial potential of its stems, leaves, and roots was evaluated against 24 strains of Gram-positive and Gram-negative bacteria. Ethanolic extracts of stems, leaves, and roots of V. officinalis and their fractions in various solvents were assessed. The stems proved to be most potent against all the strains. Its activity against Staphylococcus aureus and Pseudomonas aeruginosa was higher than the antibiotic Amoxicillin. The leaves also showed considerable activity against P. aeruginosa, Citrobacter freundii, and S. aureus. The roots turned out to be highly effective against Bacillus subtilis, S. aureus, and P. aeruginosa. The study confirmed the efficacy of V. officinalis against infectious diseases. While all the three parts of the plant were active against the test micro-organisms, stems were most powerful. The plant has great potential to provide exploitable leads for new antimicrobial drugs.
Public Interest Statement
Microbial infections are one of the main causes of disease and death worldwide. But, their treatment has increasingly become a serious problem due to drug resistance. Hence, the present study was designed, and antimicrobial potential of the stems, leaves, and roots of the medicinal herb Verbena officinalis was evaluated against 24 bacterial strains. Ethanolic extract of each part was fractionated into solvents of increasing polarity, and the extracts and fractions were evaluated. The antimicrobial activities were determined in terms of zones of inhibition and minimum inhibitory concentration (MIC). All the three parts of the plant were active against the test micro-organisms, stems being most powerful. The plant has great potential to provide exploitable leads for new antimicrobial drugs.
1. Introduction
Plants have been used worldwide for treatment of various human ailments since antiquity (Citation1, 2). Their use is still quite prevalent, especially in developing countries in the form of traditional medicine (Citation3). Extensive chemical and pharmacological studies on medicinal plants during the last many decades have led to the validation of traditional claims in many cases and have facilitated identification of their pharmacologically active components. These active principles have provided leads in the development of several drugs (Citation4).
The world is facing a complicated emergence of multi-drug-resistant diseases. The bacteria have become resistant to antibiotics due to chromosomal changes and exchange of genetic materials. The ability to cure common infectious ailments has been adversely affected, resulting in higher cost of healthcare as well as increased rate of morbidity and mortality. Consequently, the antimicrobial resistance is a serious hazard to public health and requires immediate efforts to discover more effective antibiotic agents.
The plant kingdom constitutes an unending source of new natural antimicrobial compounds. The screening of plants for their antimicrobial activities is an important area of research for producing new antimicrobial agents (Citation5). Out of the estimated 250,000–500,000 species of the plants, a very small fraction has been systematically studied and the fraction that has been subjected to biological and pharmacological screening is even smaller.
The genus Verbena contains about 250 species, the majority of which are native to Canada to Southern Chile, and found in Himalayas from Kashmir to Bhutan, Pakistan, India, and Europe. Verbena officinalis is a perennial herb. It is erect, 25–100 cm tall and branched above. Its leaves are 3.5–8 cm long and 1.5–3.5 cm broad. It has pale pink or purplish color flowers about 4 mm across (Citation6, 7).
History reveals a number of diversified medicinal aspects of plant V. officinalis. This plant is used in folk medicine as a topical anti-inflammatory agent (Citation8, 9), analgesic (Citation10), anti-depressant, antioxidant and antifungal (Citation11), anti-arthritis (Citation12), renal impairment (Citation13), Alzheimer’s disease (Citation14), common cold (Citation15), liver diseases (Citation16), cancer (Citation17), nephrosis (Citation18), and prostitis (Citation19). Quantitative analysis of the major constituents of V. officinalis revealed the presence of various chemical compounds in the plant, including triterpenoids, ursolic acid, iridoids, and flavonoids and glycosides (Citation20–23).
Keeping in view the interesting medicinal background of V. officinalis, we initially carried out antibacterial study of the ethanolic extract of its stems, leaves, and roots against the standard strains of a number of bacteria (Citation7). The positive results motivated us to extend our study to 24 clinical isolates of Gram-positive and Gram-negative bacteria. This involved the determination of zones of inhibition and minimum inhibitory concentrations. The efficacy of ethanolic extracts of stems, leaves, and roots of the plant and their fractions was compared. The study established the antimicrobial potential of the plant.
2. Materials and methods
2.1. Chemicals
Mueller-Hinton agar (MHA) was purchased from Scharlau (Spain), normal saline solution (0.9% w/v of NaCl) from Sigma-Aldrich (Germany), McFarland solution from Becton-Dickinson (Canada), phenol (6%), hexane and chloroform from Merck (Germany), ethanol, ethyl acetate, and n-butanol from Riedel-de Haen (Germany), and Amoxicillin from GlaxoSmithKline (Pakistan).
2.2. Preparation of the plant extracts and fractions
The herb V. officinalis was collected from Lahore, Pakistan. It was identified by Prof. C. M. Ashraf, FCC University, Lahore, and a specimen of the plant was kept in the Department of Chemistry. Leaves, stems, and roots were separated, washed with distilled water, and dried under shade for 15 days. Each part was then ground to a fine powder, and macerated in ethanol for 30 days. After filtration, the solvent was evaporated under reduced pressure using rotary evaporator at 45°C to obtain the crude ethanolic extract.
The ethanolic extracts of stems, leaves, and roots were separately subjected to fractionation (Citation24). For this purpose, each extract was suspended in distilled water and extracted with hexane, chloroform, ethyl acetate, and n-butanol, successively. The fractions so obtained were dried in the oven at 45°C for 48 h, and weighed.
2.3. Micro-organisms
In this study, 24 clinical isolates of various bacteria were used, which were identified by standard morphological, and cultural and biochemical profile. The Gram-positive strains used were Staphylococcus epidermidis, Staphylococcus aureus (three strains), Bacillus subtilis, and Bacillus megatherium. The Gram-negative strains used were Achromobacter xylosoxidans, Stenotrophomonas maltophilia, Klebsiella pneumonia, Enterobacter cloacae, Enterobacter aerogenes, Pseudomonas aurantiaca, Pseudomonas aeruginosa (three strains), Escherichia coli (four strains), Salmonella typhi (three strains), Azospirillum lipoferum and Citrobacter freundii.
2.4. Determination of antimicrobial susceptibility and minimum inhibitory concentration (MIC)
To evaluate antimicrobial susceptibility of ethanolic extracts of roots, leaves, and stems of V. officinalis and their fractions, agar well diffusion assay was performed according to a reported method with a minor modification (Citation25, 26). MHA was used for antibacterial testing according to Clinical and Laboratory Standards Institute (CLSI) standards (Citation26). In order to obtain fresh and well-isolated colonies of bacteria, they were inoculated on the nutrient agar (NA) medium (Merck, Germany) poured in Petri dish, which were later placed in the incubator (MJ-70-1, Lilang Scientific Instrument, Shanghai, China) at 37°C for 24 h.
For preparation of bacterial suspension, 5 mL autoclaved normal saline (NS) solution was put into a sterile autoclaved test tube. Then, 4–5 morphologically identical colonies of bacteria were transferred with the help of a wire loop into the test tube having NS solution. The solution was shaken and its turbidity was matched with 0.5% McFarland solution.
To prepare the assay plates for the study of zone of inhibition and for finding minimum inhibitory concentration of each extract against different strains of bacteria, methods were employed in the similar manner as in our previous study (Citation7). Dilutions of the samples were made in MHA. Then, the formulated plates were kept in an incubator at 37°C for about 24 h, after that, they were observed and the growth was noted. The MICs were recorded as the lowest concentrations of the extracts at which visible bacterial growth was completely inhibited. This experiment was performed in triplicate to ensure the reproducibility of the results. In the study, DMSO (dimethyl sulfoxide) was used as a negative control, while Amoxicillin was positive control.
2.5. Statistical analysis
Each antimicrobial activity was determined triplicate in order to ensure reproducibility and statistical mean was calculated with SD.
3. Results
Antimicrobial potential of the ethanolic extracts of leaves, stems, and roots of V. officinalis was determined against 24 bacterial strains in terms of zones of inhibition and the results are exhibited in Table . The MIC of these extracts were determined against 18 bacterial strains and the results are displayed in Tables –. Zones of inhibition of various fractions of the ethanolic extract of each of the plant part against most susceptible stains were also found out and the results are shown in Figures –.
Table 1. Antibacterial activities of ethanolic extracts of stems, leaves, and roots of Verbena officinalis against different strains in comparison with a standard antibiotic (40 mg/ mL)
Table 2. Minimum inhibitory concentrations (MICs, mg/mL) of ethanolic extract of stems of Verbena officinalis and its fractions against different strains
Table 3. Minimum inhibitory concentrations (MICs, mg/mL) of ethanolic extract of leaves of Verbena officinalis and its fractions against different strains
Table 4. Minimum inhibitory concentrations (MICs, mg/mL) of ethanolic extract of roots of Verbena officinalis and its fractions against different strains
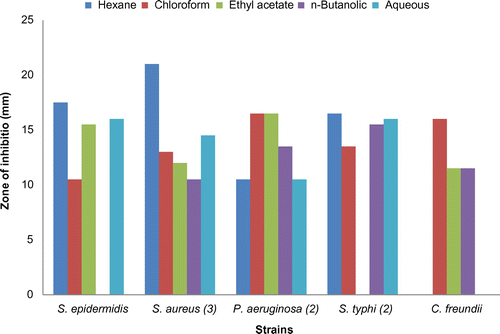
Figure 1. Comparison of antibacterial activities of fractions of stems of Verbena officinalis against different strains at the sample concentration 40 mg/mL (n = 3).
4. Discussion
The plants, as sources of medicinal substances, have played an important role in maintaining the human health since ancient times. Over 50% of all the current drugs are of natural origin (Citation27), and the plant-derived natural products are continuously playing an important role in drug development (Citation28). Therefore, many medicinal plants have been screened for their antimicrobial activity and to treat different diseases caused by the pathogens (Citation29–34). In our previous study (Citation7), we worked on comparison of antimicrobial potential of ethanolic extracts of various parts of V. officinalis against five reference stains and eight clinically isolated bacteria. Keeping in view the positive outcomes of our research, various fractions of crude ethanolic extracts of V. officinalis were tested against 24 different clinical isolates of bacteria for their antimicrobial activity.
4.1. Zones of inhibition (ZOI)
The ethanolic extracts of stems, leaves, and roots of V. officinalis showed a broad range of antimicrobial properties against the tested micro-organisms. The reason for this may be the presence of secondary metabolites, like polyphenols, coumarins, triterpenes, and saponins and cardiac glycosides (Citation6, 21, 23, 35, 36) which have been reported to have considerable antimicrobial activities. Strains of S. aureus displayed zones of inhibition (ZOI) in the range of 31–33 mm for stems, 22–28 mm for leaves, and 16–21 mm for roots. Thus, the stems ethanolic extract showed maximal antibacterial activity against S. aureus. Similarly, various strains of P. aeruginosa showed ZOI in the range of 26–31 mm for stems, 17–23 mm for leaves, and 15–29 mm for roots revealing stems ethanolic extract to be most efficient against the strain. In case of E. coli, ZOI was 13–13.5 mm for stems, 12–17 mm for leaves, and 13–19 mm for roots, displaying roots to be most efficient against E. coli. In case of various strains of S. typhi, ZOI turned out to be 22–26 mm for stems, 15–26 mm for leaves, and 21–22 mm for roots. Thus, the ethanolic extract of stems of V. officinalis was proved to be the most efficient broad spectrum antibacterial agent.
In comparison to Amoxicillin standard, ethanolic extracts of various parts of V. officinalis showed high antibacterial activity against Gram-positive strains, such as S. aureus (one and two), B. subtilis, and B. megatherium as well as Gram-negative strains of K. pneumonia, E. aerogenes, P. aurantiaca, P. aeruginosa (one and two), S. typhi (one and two), and C. freundii. Hence, they can be an effective alternatives for treatment of S. aureus-induced endocarditis, osteomyelitis, respiratory, soft tissue, and endovascular infections, Bacillus-induced pulmonary infections, septicemia, and liver abscission of K. pneumonia, nosocomial infections caused by E. aerogenes, septicemia, typhoid, and bacteremia caused by P. aeruginosa (one and two), S. typhi (one and two) and C. freundii. From data in Table , it can be deduced that ethanolic extracts of all the three parts of the plant contained highly active compounds that are stronger antimicrobial agents even more effective than Amoxicillin against certain strains. Thus, the clinical scope of V. officinalis can be extended as potential treatment option for patients suffering from bronchitis, Lyme disease, ear infections, pneumonia, skin, throat, and urinary tract infections for which Amoxicillin has been considered as treatment of choice.
Based on the antimicrobial activity of the ethanolic extracts of different parts of V. officinalis, various fractions of each extract were analyzed against the most sensitive strains. The fractions that were studied included hexane, chloroform, ethyl acetate, n-butanolic, and aqueous. The hexane fraction of stems showed considerable activities against tested micro-organisms, and proved to be highly effective against S. aureus (19.5 ± 0.707 mm) and S. maltophilia (16.5 ± 0.707 mm). The chloroform fraction of stems showed considerable activity against S. aureus (19.0 ± 0.00 mm). The stems ethyl acetate fraction also showed significant activity against S. aureus (15.5 ± 0.707 mm) equal to Amoxicillin (15.5 ± 1.50 mm). The stems n-butanolic fraction also showed significant activity against S. aureus (28.5 ± 0.707 mm), which was higher than Amoxicillin.
The aqueous fraction also showed antimicrobial activity against S. aureus (20.5 ± 1.414 mm) and B. subtilis (22.0 ± 1.414 mm) more effectively than Amoxicillin.
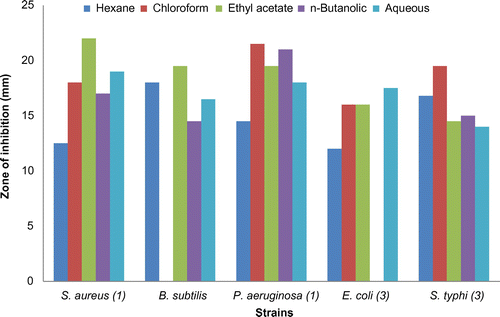
In case of leaves, the hexane fraction showed considerable activities against tested micro-organisms, and proved to be effective against S. aureus (21.0 ± 1.50 mm) but not so much effective as Amoxicillin.
The chloroform fraction was effective against P. aeruginosa and C. freundii with ZOIs at 16.5 ± 1.141 mm and 16.0 ± 1.141 mm, respectively.
The ethyl acetate fraction of leaves showed activity against P. aeruginosa, but was less effective than the antibiotic. The n-butanolic fraction of leaves being the least effective against the strains’. The aqueous fraction was effective against S. epidermidis but not as significantly as the antibiotic does.
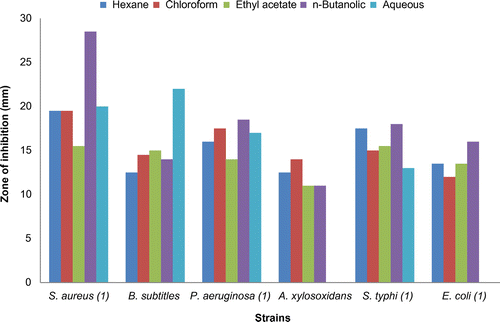
The hexane fraction of roots was significantly effective against B. subtilis (18.0 ± 1.414 mm) which was more effective than Amoxicillin (15.0 ± 1.41 mm). The chloroform fraction was most significant against S. aureus (18.0 ± 1.50 mm) than Amoxicillin and also effective than Amoxicillin against P. aeruginosa (21.5 ± 0.707 mm).
The ethyl acetate fraction was effective against S. aureus (22.0 ± 0.707 mm), P. aeruginosa (19.5 ± 1.414 mm), and B. subtilis (19.5 ± 1.500 mm) more than Amoxicillin.
The n-butanolic fraction of roots showed that this fraction was most effective against P. aeruginosa (21.0 ± 1.414 mm) amongst all the strains and was effective more than Amoxicillin against this strain. The aqueous residue of roots of V. officinalis was effective against S. aureus (19.0 ± 1.141 mm), P. aeruginosa (18.0 ± 0.707 mm), and B. subtilis (16.5 ± 1.500 mm) more than Amoxicillin.
4.2. Minimum inhibitory concentration (MIC)
Based on the results of antimicrobial screening by agar well diffusion assay, it was decided to find out the exact quantity of each sample needed to inhibit the growth of bacterial strains (MIC). The ethanolic extract of stems was found most effective among all the samples. Its MIC values were in the range of 0.8–4.0 mg/mL against different strains. It killed S. aureus at a concentration of 0.8 mg/mL.
The hexane fraction of stems also displayed significant MIC values ranging from 0.8 to 4.0 mg/mL. It killed E. coli, S. typhi, P. aeruginosa, E. aerogenes, and P. aurantiaca at a concentration of 2.8 mg/mL which was higher as compared to the ethanolic extract.
The MIC values of chloroform fraction ranged from 2.0 to 4.4 mg/mL, while those of ethyl acetate and n-butanolic fractions ranged from 1.8 to 4.0 mg/mL and 1.6 to 3.8 mg/mL, respectively. In case of ethyl acetate fraction, the greater number of bacterial strains was inhibited at a concentration of 2.8 mg/mL, while in case of n-butanolic fraction, 2.0 mg/mL and 2.8 mg/mL were found to be the most effective concentration that inhibited the growth of a large number of bacteria. The aqueous fraction also showed considerable activity against the tested micro-organisms and its MIC values ranged from 1.4 to 4.4 mg/mL. The ethanolic extract and its fractions, thus, can be used as a potential source of antibiotics for curing diseases caused by the pathogens.
The ethanolic extract of leaves of V. officinalis was effective against the bacterial strains but not so much as that of the ethanolic crude extract of the stems. The MIC values ranged from 0.8 to 3.8 mg/mL. The hexane and chloroform fractions of leaves also showed significant MICs, whose values ranged from 2.0 to 4.4 mg/mL and 1.8 to 4.4 mg/mL, respectively.
The ethyl acetate and n-butanolic fractions of leaves displayed MICs at 1.6–4.4 mg/mL and 1.6–4.4 mg/mL, respectively, while of aqueous residue MIC values ranged from 1.8 to 4.1 mg/mL.
Like the stems and leaves ethanolic extracts, the roots ethanolic extract was the most effective than all of its fractions but less effective than stems ethanolic extract. Its MIC values ranged from 1.0 to 4.4 mg/mL. However, the MIC values of the hexane and chloroform fractions ranged from 1.4 to 3.6 mg/mL and 0.8 to 4.4 mg/Ml, respectively. In case of ethyl acetate and n-butanolic fractions, the values of MICs ranged from 1.8 to 3.6 mg/mL and 0.8 to 4.4 mg/mL, respectively. The aqueous residue also showed certain range of MICs against the micro-organisms, i.e., from 1.4 to 4.4 mg/mL.
Almost all the fractions proved to be effective against S. aureus and P. aeruginosa which are causative agents of many infectious diseases (Citation37).
All these results showed that ethanolic extracts of different parts of V. officinalis and its fractions can be used as a potential source of antibiotics for curing diseases caused by the pathogens. Fractions of all the three parts exhibited varied efficacy against different strains. This study provides fundamental knowledge for further study on clinical isolates and pharmacological evaluations.
5. Conclusion
V. officinalis showed notable antimicrobial activity. The ethanolic extracts of its stems, leaves, and roots and their fractions were effective against all the tested Gram-positive and Gram-negative micro-organisms. The stem ethanolic extract was most potent in inhibiting the growth of all the tested micro-organisms, which exhibited significant activity against S. aureus and P. aeruginosa strains, even higher than the antibiotic Amoxicillin. Different fractions of each part were variably active against different strains. Thus, the present study confirmed the ethnomedicinal uses of V. officinalis as a remedy for microbial infections.
Funding
The authors received no direct funding for this research.
Additional information
Notes on contributors
Dildar Ahmed
The chief research interests of our group include natural products, herbal medicine and bioactivities. Over the past many years, the research group of Dr. Dildar Ahmed has been active in isolation of natural products from plants, their characterization and determination of their bioactivities. The targeted bioactivities mainly included antimicrobial, antioxidant, anti-diabetic, and anti-obesity. Our aim is to explore affordable complementary and alternative treatments for human sufferings. We seek and welcome collaborations from researchers working in the same field.
References
- Saslis-Lagoudakis, C.H.; Hawkins, J.A.; Greenhill, S.J.; Pendry, C.A.; Watson, M.F.; Tuladhar-Douglas, W.; Baral, S.R.; Savolainen, V. The Evolution of Traditional Knowledge: Environment Shapes Medicinal Plant Use in Nepal. Proc. R. Soc. B 2014, 281 (1780), 20132768. doi:10.1098/rspb.2013.2768.
- Ncube, N.; Afolayan, A.; Okoh, A. Assessment Techniques of Antimicrobial Properties of Natural Compounds of Plant Origin: Current Methods and Future Trends. Afr. J. Biotechnol. 2008, 7 (12), 1797–1806.10.5897/AJB
- Maroyi, A. Traditional Use of Medicinal Plants in South-Central Zimbabwe: Review and Perspectives. J. Ethnobiol. Ethnomed. 2013, 9 (1), 347–354.
- Maurya, R.; Srivastava, S.; Kulshreshta, D.K.; Gupta, C.M. Traditional Remedies for Fertility Regulation. Curr. Med. Chem. 2004, 11 (11), 1431–1450.10.2174/0929867043365215
- Malik, N.; Malik, Z. Present Status of Subtropical Chir Pine Vegetation of Kotli Hills, Azad Jammu and Kashmir. J. Res. Sci. 2004, 15 (1), 85–90.
- Miraj, S.; Kiani, S. Study of Pharmacological Effect of Verbena officinalis Linn: A Review. Pharm. Lett. 2016, 8 (9), 321–325.
- Ahmed, D.; Chaudhary, M.A.; Raza, A.; Waheed, A.; Khan, S.R.; Ikram, M. Comparative Study of Antibacterial Activity and Mineral Contents of Various Parts of Verbena officinalis Linn. Asian J. Chem. 2012, 24 (1), 68–72.
- Calvo, M.; Vilalta, N.; San Julián, A.; Fernández, M. Anti-inflammatory Activity of Leaf Extract of Verbena officinalis L. Phytomedicine 1998, 5 (6), 465–467.10.1016/S0944-7113(98)80043-3
- Deepak, M.; Handa, S.S. Antiinflammatory Activity and Chemical Composition of Extracts of Verbena officinalis. Phytother. Res. 2000, 14 (6), 463–465.10.1002/(ISSN)1099-1573
- Calvo, M. Anti-inflammatory and Analgesic Activity of the Topical Preparation of Verbena officinalis L. J. Ethnopharmacol. 2006, 107 (3), 380–382.10.1016/j.jep.2006.03.037
- Casanova, E.; García-Mina, J.; Calvo, M. Antioxidant and Antifungal Activity of Verbena officinalis L. leaves. Qual. Plant. Mater. Veg. 2008, 63(3): 93–97.
- Zhao, L. Traditional Chinese Medicinal Liquid for Treating Rheumatic Arthritis and Rheumatoid Arthritis. Patent# CN101104036(A), App.# CN20071023842 20070621, 2007.
- Grases, F.; Melero, G.; Costa-Bauzá, A.; Prieto, R.; March, J.G. Urolithiasis and Phytotherapy. Int. Urol. Nephrol. 1994, 26 (5), 507–511.10.1007/BF02767650
- Lai, S.-W.; Yu, M.-S.; Yuen, W.-H.; Chang, R.C.-C. Novel Neuroprotective Effects of the Aqueous Extracts from Verbena officinalis Linn. Neuropharmacol. 2006, 50 (6), 641–650.10.1016/j.neuropharm.2005.11.009
- Yu, C.; Caesalpiniae, S. Chinese Medicine Preparation for Treating Cold, It’s Preparing Method and Quality Control Method. Patent# CNI850268(A), App.# CN20061200187 20060301, 2006.
- Li, Y. Chinese Medicinal Herbs for Effectively Treating Cirrhosis, Liver Ascites. Patent# CN101244158. App. # CN20081007413 20080310, 2008.
- Fan, D.; Tian, Y. Medicinal Herb for Treating Liver Cancer and Preparing Process Thereof. Patent# CN101288763 (A), App.# CN20081028695 20080610, 2008.
- Zhang, S.; Gu, H.; Zhang, W.; Li, X.; Li, Y.; Liu, B.; Wang, M.; Zhang, X. Chinese Medicine Composition for Treating Nephropathy. Patent# CN101313971(A), App.# CN20071099974 20070601, 2008.
- Hu, S. Chinese Medicine for Treating prostatitis and Hyperplasia. Patent # CN101195011 (A), App. # CN20071144735 20071128, 2008.
- Deepak, M.; Handa, S.S. Quantitative Determination of the Major Constituents of Verbena officinalis Using High Performance Thin Layer Chromatography and High Pressure Liquid Chromatography. Phytochem. Anal. 2000, 11 (6), 351–355.10.1002/(ISSN)1099-1565
- Tian, J.; Zhao, Y.; Luan, X. Studies on the Chemical Constituents in Herb of Verbena officinalis. Chin. J. Chin. Mater. Med. 2005, 30 (4), 268–269.
- Rehecho, S.; Hidalgo, O.; García-Iñiguez de Cirano, M.G.-I.; Navarro, I.; Astiasarán, I.; Ansorena, D.; Cavero, R.Y.; Calvo, M.I. Chemical Composition, Mineral Content and Antioxidant Activity of Verbena officinalis L. LWT-Food Sci. Technol. 2011, 44 (4), 875–882.10.1016/j.lwt.2010.11.035
- Zhang, T.; Ruan, J.; Lu, Z. Studies on Chemical Constituents of Aerial Parts of Verbena officinalis L. Chin. J. Chin. Mater. Med. 2000, 25 (11), 676–678.
- Ahmed, D.; Zara, S.; Baig, H. In vitro Analysis of Antioxidant Activities of Oxalis Corniculata Linn Fractions in Various Solvents. Afr. J. Trad. Complemen. Altern. Med. 2013, 10 (1), 158–165.
- George, N.M.; Cutting, K.F. Antibacterial Honey (Medihoney™): in vitro Activity Against Clinical Isolates of MRSA, VRE, and other Multiresistant Gram-Negative Organisms Including Pseudomonas aeruginosa. Wounds 2007, 19 (9), 231–236.
- Ahmed, D.; Mansha, M.; Hannan, A.; Barkaat, M.; Bibi, N. Antibacterial Activity of Ballota limbata Against Potential Multidrug Resistant Human Pathogens. J. Appl. Sci. Res. 2009, 5 (10), 1611–1614.
- Meena, A.K.; Singh, A.; Sharma, K.; Kumari, S.; Rao, M. Physicochemical and Preliminary Phytochemical Studies on the Rhizomes of Glycyrrhiza glabra Linn. Int. J. Pharm. Pharm. Sci. 2010, 2 (2), 48–50.
- Betancur-Galvis, L.; Morales, G.; Forero, J.; Roldan, J. Cytotoxic and Antiviral Activities of Colombian Medicinal Plant Extracts of the Euphorbia genus. Mem. Inst. Oswaldo Cruz 2002, 97 (4), 541–546.10.1590/S0074-02762002000400017
- Akinyemi, K.O.; Oladapo, O.; Okwara, C.E.; Ibe, C.C.; Fasure, K.A. Screening of Crude Extracts of Six Medicinal Plants used in South-West Nigerian Unorthodox Medicine for Anti-Methicillin Resistant Staphylococcus Aureus Activity. BMC Complement. Altern. Med. 2005, 5 (1), 1754.10.1186/1472-6882-5-6
- Nitta, T.; Arai, T.; Takamatsu, H.; Inatomi, Y.; Murata, H.; Iinuma, M.; Tanaka, T.; Ito, T.; Asai, F.; Ibrahim, I. Antibacterial Activity of Extracts Prepared from Tropical and Subtropical Plants on Methicillin-Resistant Staphylococcus aureus. J. Health Sci. 2002, 48 (3), 273–276.10.1248/jhs.48.273
- Bonjar, G.S.; Nik, A.K. Antibacterial Activity of Some Medicinal Plants of Iran Against Pseudomonas aeruginosa and P. fluorescens. Asian J. Plant Sci. 2004, 3 (1), 61–64.
- Nair, R.; Kalariya, T.; Chanda, S. Antibacterial Activity of Some Selected Indian Medicinal Flora. Turk. J. Biol. 2005, 29 (1), 41–47.
- Digrak, M.; Alma, M.H.; Ilçim, A.; Sen, S. Antibacterial and Antifungal Effects of Various Commercial Plant Extracts. Pharm. Biol. 1999, 37 (3), 216–220.10.1076/phbi.37.3.216.6307
- Ali, N.A.; Jülich, W.D.; Kusnick, C.; Lindequist, U. Screening of Yemeni Medicinal Plants for Antibacterial and Cytotoxic Activities. J. Ethnopharmacol. 2001, 74 (2), 173–179.10.1016/S0378-8741(00)00364-0
- Shams, A. M.; M. Mosaddegh; A. Shafaati 2010. Volatile Constituents from the Aerial Part of Verbena officinalis L. (Vervain). Iran. J. Pharm. 1(2): 39–42.
- Sokmen, A.; Jones, B.M.; Erturk, M. The in vitro Antibacterial Activity of Turkish Medicinal Plants. J. Ethnopharmacol. 1999, 67 (1), 79–86.10.1016/S0378-8741(98)00189-5
- Bodey, G.P.; Bolivar, R.; Fainstein, V.; Jadeja, L. Infections Caused by Pseudomonas aeruginosa. Rev. Infect. Dis. 1983, 5 (2), 279–313.10.1093/clinids/5.2.279